Abstract
Lentivirus infections in small ruminants represent an economic problem affecting several European countries with important sheep-breeding industries. Programs for control and eradication of these infections are being initiated and require reliable screening assays. This communication describes the construction and evaluation of a new serological screening enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to maedi-visna virus (MVV) in sheep and to caprine arthritis encephalitis virus (CAEV) in goats. The solid phase is sensitized with a combination of the major core protein p25 of MVV produced in Escherichia coli and a peptide derived from the immunodominant region of the viral transmembrane protein gp46. The peptide carries an N-terminal biotin residue and is complexed with streptavidin prior to being coated. The new assay was evaluated with 2,336 sheep serum samples from different European countries with large differences in the levels of prevalence of MVV infections, and the results have been compared to those of the standard agar gel immunodiffusion test. Discrepant samples were analyzed by Western blotting with viral lysate, and most sera could be classified unambiguously. The estimated overall sensitivity of the new ELISA was 99.4% (95% confidence interval [CI], 98.4 to 99.8%) and the specificity was 99.3% (95% CI, 98.7 to 99.6%). A limited set of goat sera (n = 212) was also analyzed, with similar results. These data indicate that the new assay is a reliable tool that can be used in control and eradication programs for small ruminant lentivirus infections.
Maedi-visna virus (MVV, also termed ovine lentivirus) is a nononcogenic, exogenous retrovirus belonging to the Lentiviridae subfamily and related to human immunodeficiency virus (5, 18, 20). MVV infection in sheep is characterized by a relatively long asymptomatic period in which the virus persists in the presence of a strong humoral and cellular response. Following a variable incubation period (2 to 10 years), the virus may cause a chronic, progressive, inflammatory disease in the lungs, joints, and mammary glands of infected animals. In the central nervous system, inflammation and degenerative processes can result from an MVV infection (2).
Caprine arthritis-encephalitis virus (CAEV) is genetically and antigenically closely related to MVV (20). CAEV infection in goats is widespread and may cause important economic losses. The main clinical symptoms are arthritis, chronic subclinical mastitis, and interstitial pneumonia. Encephalitis is often diagnosed in young goats and in adult animals, and encephalitis is seen more frequently than the arthritis observed in MVV-infected sheep.
MVV-infected sheep produce high titers of serum antibodies against viral capsid and envelope proteins. Several serological diagnostic tests to detect these antibodies have been described (6, 7, 9, 11, 17, 21), but only two methods, the agar gel immunodiffusion test (AGIDT) and the whole-virus enzyme-linked immunosorbent assay (WV-ELISA) are used routinely today (11, 15). Accurate estimations of the sensitivities and specificities of routine assays are not easy to obtain, largely due to the lack of an adequate gold standard for diagnosis of MVV infection (4). The laboratory diagnosis of CAEV infection—like that of MVV infection in sheep—is based on the demonstration of the presence of antiviral antibodies by AGIDT or ELISA (8).
Apart from the poor reproducibility and low sensitivity of the AGIDT, which makes it unsuitable as a gold standard for serology of small ruminant lentivirus infections, AGIDT as well as WV-ELISA suffers from the variable quality (low purity and heterogeneous composition) and the high production cost of the viral antigens used. Furthermore, the AGIDT and WV-ELISA are not suitable for large-scale automated testing, which is required for an economic and efficient processing of large numbers of samples, a prerequisite for control and eradication schemes.
Recently, recombinant viral proteins have been widely used for detection of antiviral antibodies in human and veterinary virology. Recombinant DNA techniques can efficiently produce large quantities of highly purified viral proteins. Among the recombinant antigens used for detection of anti-MVV antibodies are those derived from the Gag proteins (more particularly the p25 protein), the transmembrane (TM) glycoprotein gp46, and the external envelope glycoprotein (9, 11, 21). Boshoff and coworkers recently described the use of a glutathione S-transferase–TM protein–p25 fusion protein for detection of antibodies against MVV (1). The results presented, while encouraging, should be regarded as preliminary given the limited number of animals tested and the specificity problems caused by the glutathione S-transferase fusion partner.
Kwang and Torres described an oligopeptide-based immunoassay for the detection of antibodies against MVV in sheep (10). According to computer prediction models, those authors identified three antigenic sites in the N-terminal segment of the MVV TM protein, on the basis of which three peptides were selected. The synthetic peptides showed very high specificities when they were tested with a collection of known seropositive and seronegative sheep sera. However, the sensitivity was lower than that of the corresponding recombinant TM protein, even when a combination of the three peptides was used. They concluded that recombinant proteins are the preferred reagents for the development of MVV immunodiagnostics.
It is therefore apparent that there is still room for more sensitive and specific serodiagnostic methods whose results are reproducible for the detection of lentivirus infections in small ruminants. There is also a need for tests which can be produced with consistent quality on a large scale and which can be automated to allow high throughput of samples.
We herein describe the construction and performance of an MVV ELISA based on recombinant proteins and synthetic peptides which has been evaluated on a large scale with samples obtained from different European countries and from experimentally infected as well as from field-infected animals.
MATERIALS AND METHODS
Animals and sera.
Sera from animals obtained from three European countries with large variations in the levels of prevalence of MVV infection were used (Table 1). Most animals were from the Rasa Aragonese meat breed and were 6 months of age or older, except where otherwise noted. Sera from MVV-free certified Icelandic sheep and from flocks considered MVV free after several years of repeated testing were used as negative controls. All sera were stored at −20°C.
TABLE 1.
Origins of the samples analyzed
| Country | Breed(s) | Sample size |
|---|---|---|
| Spain (Aragon) | Rasa | 1,312 |
| Spain (Basque) | Latxa | 339 |
| Italy | Langhe, Massese, Sarda | 264 |
| Scotland | Texel | 195 |
| France | Not known | 39 |
| Multicentera | Rasa | 187 |
Sera tested by AGIDT in five different locations.
Experimental infection of eight adult ewes was performed in Pisa (Italy). A flock of nine ewes was maintained in isolation for 8 months. The animals were bled at regular intervals, and all serum samples were found negative by AGIDT and ELISA. The animals were then infected with MVV strain 130 (14) by intratracheal inoculation with 1.02 × 106 50% tissue culture infective doses. One animal was kept as an uninfected control. At regular intervals, over a period of 466 days postinfection, serum was collected and tested by AGIDT and ELISA.
Natural vertical transmission from ewe to lamb was studied in Scotland. Lambs born to MVV-infected ewes were sampled for the first time when they were 6 months of age and subsequently at a 2- to 3-month interval. The sera were analyzed by AGIDT and ELISA.
Classical serological tests.
Standard serology by AGIDT was performed as described previously (3, 19). Reagents (Maeditec) were obtained from the Veterinary Laboratory Agency (Weybridge, United Kingdom), and interpretation was carried out according to the manufacturer’s instructions. Sera were classified as positive for gp135 and/or p25, weakly positive for gp135, doubtful (or indeterminate), and negative.
Virus culture and Western blotting.
The EV-1 strain of MVV was passaged in sheep fibroblast culture maintained in Dulbecco’s modified Eagle’s medium (Gibco, Uxbridge, United Kingdom) containing 8% fetal calf serum. Virus containing supernatant was harvested by centrifugation for 30 min (10,000 × g at 4°C) to remove the cell debris, when the culture displayed extensive cytopathic effect. The clarified supernatant was stored in aliquots at −70°C until use.
MVV antigen for use in Western blots was prepared as follows. Cultured sheep skin fibroblasts or chondrocytes at 75% confluence were infected with MVV. The cells were harvested when large syncytia became visible. The monolayer was first washed three times with phosphate-buffered saline (PBS), and cells were then scraped off into 10 ml of PBS. After another two rounds of washing in PBS, cells were collected by centrifugation. The cell pellet was then resuspended in a small volume of sodium dodecyl sulfate (SDS) sample buffer containing β-mercaptoethanol. The sample was then heated in a boiling water bath for 5 min and centrifuged at 10,000 × g for 10 min. From this solution, 5 μl (or 50 μl for a preparative gel) was used per track. After electrophoresis and blotting onto nitrocellulose, the sheets were blocked for 1 h in PBS containing 3% bovine serum albumin and 0.5% Tween 80. Sera were diluted 1/100 in blocking solution. Bound antibodies were detected by alkaline phosphatase-coupled rabbit anti-sheep immunoglobulin G (Jackson, West Grove, Pa.), and nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (BCIP) was used for color development.
Subcloning of the major core protein (p25) gene.
The gene encoding the viral major core protein p25 was isolated by PCR from unintegrated proviral DNA, which was extracted from EV-1-infected sheep fibroblasts (16), grown in vitro by the modified Hirt method (13). The PCR was performed with Taq DNA polymerase. Sense primer (5′-TCCCGGGATCCATAGAAGGTAGAATTGTAAATCTGCAAGCAGG-3′) and antisense primer (5′-ACACCCGGGATCCCTTCTGATCCTACATCTC-3′) (italicized nucleotides indicate BamHI restriction site) were used in a standard PCR as described before (13). The DNA fragment was cloned into pTZ (Pharmacia, Uppsala, Sweden) by established procedures and sequenced. The fragment was recovered by BamHI digestion and cloned into the BamHI site of plasmid pBluescript SK (Stratagene, Leusden, The Netherlands).
Expression of the gene encoding p25 in Escherichia coli.
In the pIGRHISA expression vector used in this work, the initiation codon is followed by a codon for alanine, which is immediately followed by six consecutive histidine codons, and a sequence encoding the cleavage site for enterokinase. The protein produced carries the sequence MAHHHHHHVDDDK at its amino terminus. The enterokinase cleavage site allows the removal of the hexahistidine sequence from the purified protein if necessary.
For cloning in the pIGRHISA vector, the p25 gene was isolated from the pBluescript vector by SmaI and XbaI digestion, cloned into the expression vector pIGRHISA digested with NsiI, blunted with T4 DNA polymerase, and treated with XbaI. This process resulted in plasmid pIGRHISAMVVp25, where the SmaI-NsiI fusion, created in this way, restored the reading frame between the hexahistidine region of the vector and the p25 gene. A translation stop codon was engineered at the end of the p25 gene, as can be seen from the antisense primer used for PCR amplification.
In the expression vector pIGRHISA, transcription of the heterologous gene is controlled by the leftwardly oriented promoter of phage lambda (12). This promoter is controlled by a temperature-sensitive repressor (CIts) provided by a compatible plasmid present in the same cell. This system is tightly controlled and allows the cells to grow under repressed conditions (28°C) in which no (or very limited amounts of) heterologous protein is produced. Upon induction at higher temperatures (37 and 42°C) the protein of interest is produced during a limited period, which can be optimized for each heterologous protein.
To induce expression of the p25 gene, the plasmid was transformed into different E. coli strains (SG4044, MC1061, and UT5600), all containing the compatible plasmid bearing the gene for the CIts repressor. Subsequently the culture was started by inoculation from an overnight saturated culture at a 1/100 dilution. The culture was grown at 28°C until an optical density (OD) (measured at 600 nm) of 0.2 was reached. Then the temperature was shifted to 42°C and the culture was grown for up to 4 h. At regular intervals, aliquots were removed from the culture and the cells were spun down and lysed by boiling them in SDS-mercaptoethanol buffer. Lysates were analyzed by SDS-polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue to reveal the proteins. The highest yield was obtained with strain SG4044, which was chosen for large-scale fermentation.
Purification of the recombinant protein.
The cell pellet obtained by low-speed centrifugation of the bacteria after fermentation was resuspended in lysis buffer (10 mM Tris [pH 6.8] containing 150 mM KCl and 5 mM EDTA) supplemented with phenylmethylsulfonyl fluoride (2 mM) and ɛ-aminocaproic acid (25 mM). Five milliliters of buffer per g of wet cells was used, and the suspension was passed through a French press twice. The lysate was then cleared by centrifugation, and both pellet and supernatant were used for further processing. The pellet fraction was solubilized in buffer containing 6 M guanidinium chloride (50 mM phosphate, 0.05% Triton X-100 [pH 7.2]). The solution was cleared by centrifugation at 30,000 × g and then applied to a preactivated Ni2+-imihodiacetic acid column (Pharmacia) equilibrated with the same buffer containing 20 mM imidazole. The equivalent of 4 liters of fermentation broth was loaded on a 12-ml gel bed. The column was then washed with the loading buffer until the absorption at 280 nm reached the basal level. Subsequently, washings with loading buffer containing 35 mM imidazole followed by washing with buffer containing 50 mM imidazole were performed. The bound protein was then eluted with 200 mM imidazole in the same buffer. Fractions were analyzed via SDS-polyacrylamide gel electrophoresis and Western blotting to evaluate the purity and the yield of the purification.
For the purification of the recombinant protein from the soluble fraction, the supernatant obtained after clearing of the French press lysate was supplemented with solid guanidinium chloride to a 6 M final concentration. Further processing was done as described above.
The purity of the protein eluted with 200 mM imidazole was evaluated by Western blotting and probing with rabbit serum raised against total E. coli protein. From this analysis, the purity of the protein was estimated to be higher than 95%. Silver staining confirmed these results. Total yield of the purified protein was estimated by the micro bicinchoninic acid method (Pierce, Rockford, Ill.) with bovine serum albumine as the standard. Fractions containing the recombinant p25 protein were pooled and dialyzed against phosphate buffer (50 mM phosphate [pH 7.2], 150 mM NaCl) to remove the imidazole. The purified protein was stored in aliquots at −70°C.
Synthetic peptides.
Three synthetic peptides were used in this work: TM1 (B-GGQELDCWHYQHYCVTSTKS), TM2 (B-GGRYRDNCTWQQWEEEIE), and TM3 (B-GGQRDAQRIPDVWTALQG), where the single-letter amino acid code is used and the N-biotin residue (B) is indicated.
The peptides were synthesized on Tentagel S resin (Rapp Polymere GmbH, Tübingen, Germany) with a Rainin Symphony/Multiplex synthesizer by standard 9-fluorenylmethoxycarbonyl chemistry and N-hydroxybenzotriazole–2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tertafluoroborate (HOBt-TBTU) in situ activation. Standard double couplings were performed with a fourfold excess of amino acids in the presence of equimolar amounts of HOBt and TBTU twice for 20 min each time. The group protecting 9-fluorenylmethoxycarbonyl was removed by mild base treatment with 2% piperidine–2% 1,8-diazabicyclo-(5.4.0)undec-7-ene in dimethylformamide.
Biotinylation was performed by dissolving biotin in 30% dimethyl sulfoxide–70% dimethylformamide with in situ activation. After completion of the synthesis, the peptide was cleaved off the resin by incubation for 2.5 h with 90% trifluoroacetic acid (TFA)–5% thioanisole–3% ethanedithiol–2% anisole.
The peptide was precipitated from the reaction mixture with t-butylmethylether. After centrifugation, the pellet was washed three times with t-butylmethylether and dried overnight in vacuo. The purity of the crude peptide was analyzed by reverse-phase high-pressure liquid chromatography (HPLC).
Disulfide bridge formation in the TM1 peptide was performed by oxidation with K3[Fe(CN)6]. A suspension of 50 mg of the peptide in 242 ml of 20% acetonitrile–0.1% TFA was sonicated to dissolve the peptide. K3[Fe(CN)6] was then added to a final concentration of 0.24 mM. The pH was raised to 11 with 3 M sodium hydroxide, and the solution was stirred for 150 min at room temperature in the dark. Finally, the pH was lowered to 1.5 with TFA and the solution was stored at −20°C.
After the oxidation reaction, the oxidized cyclic peptide (TM1c) was separated from the remaining linear peptide by HPLC. Briefly, the sample was loaded on the HPLC column (Nucleosil 10 μm; Alltech) in 0.1% TFA. Elution was performed with a gradient of acetonitrile in 0.1% TFA. The oxidized peptide eluted at 28 to 30% acetonitrile, while the linear peptide eluted as a separate peak at 31 to 32% acetonitrile.
ELISA procedures.
Microwell plates (Maxisorp F96; Nunc, Roskilde, Denmark) were incubated with a solution of recombinant p25 at different concentrations to determine the saturation level. Coating at 5 μg/ml in 50 mM carbonate buffer (pH 9.6) was found to be optimal.
Synthetic peptides (containing N-terminal biotin) were coated either directly as free peptides or as complexes with streptavidin. For the direct coating, the peptide stock solution was diluted to 1 μg/ml of peptide in carbonate buffer (pH 9.6). Preformed peptide-streptavidin complexes were prepared by mixing a solution of streptavidin (9 μl of stock solution containing 5 mg/ml) with the peptide solution (12 μl containing 1 mg/ml) and dilution with 129 μl of carbonate buffer (pH 9.6). After incubation (1 h at 37°C), the complex mixture was diluted to 1 μg/ml of complex in carbonate buffer (pH 9.6) for coating.
When streptavidin complexes were coated together with the recombinant p25 protein, a mixture of the streptavidin complex (3 μg of complex per ml) and the recombinant protein (1 μg/ml) was prepared in carbonate buffer (pH 9.6). All coatings were done with 100 μl of solution per well and incubation for 1 h at 37°C.
After coating, the wells were saturated with blocking buffer (0.1% casein in PBS containing 0.25 M glycine) for 1 h at 37°C and subsequently washed three times with PBS containing 0.05% Tween 20 (washing buffer). Serum samples were diluted 1/500 (in the two-step dilution, 1/100 and subsequently 1/5) in sample diluent (blocking buffer containing 2.86 g of Triton X-705 per liter) and incubated at 100 μl/well for 1 h at 37°C.
After extensive washing with wash buffer, detection of the bound sheep antibodies was performed with a peroxidase-conjugated rabbit anti-sheep immunoglobulin G (Jackson) diluted 1/40,000 in blocking buffer (100 μl/well for 1 h at 37°C). After additional washing, the peroxidase activity was measured by adding 100 μl of substrate-chromogen solution (phosphate-citrate buffer [pH 4.3] containing 0.006% hydrogen peroxide and 3,3′,5,5′-tetramethyl benzidine).
Color development was terminated after 30 min by addition of 100 μl of a 0.5 M solution of sulfuric acid per well, and the color intensity was measured at 450 and 595 nm in an ELISA reader (BioTek Instruments). The results were expressed either as OD values or as signal-to-cutoff ratios (S/CO). Cutoff was determined in each individual experiment with a negative and a positive control sample provided in the kit, and the signal value was the mean of duplicate values for each serum sample. The cutoff value was determined as the sum of the values for the negative control sample plus the positive control sample divided by 4. ELISA results were considered positive when the S/CO was >1.
Milk and serum samples from 40 sheep (from three different Italian commercial MVV-infected flocks) were collected and tested in ELISA. Milk samples were appropriately diluted in the sample buffer employed for serum dilution and processed as described above for serum.
When goat sera were analyzed, the procedure was exactly the same as for the sheep sera, including the conjugate used.
RESULTS
ELISA reactivity of the recombinant MVV core protein and gp46-derived peptides.
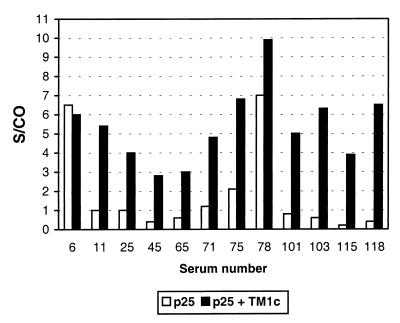
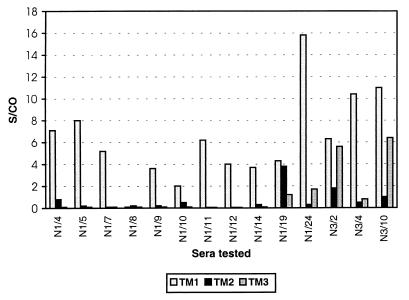
The purified recombinant p25 protein was used to coat microwells, and sera from MVV-infected (AGIDT-positive) and control (AGIDT-negative) sheep were analyzed. Under the experimental conditions used, the sensitivity obtained with the p25 antigen was only 58% (n = 43) and the S/CO values were generally low (<2) (Fig. 1). These results indicate that this antigen alone is not suitable for sensitive detection of MVV antibodies. On the other hand, synthetic peptides derived from the gp46 TM glycoprotein were also studied in a similar experiment. Three different peptides derived from the immunodominant region of the gp46 protein were used (Fig. 2). This experiment showed that of the three peptides tested, the TM1 peptide was the most reactive and that TM3 had intermediate sensitivity (Fig. 2). The S/CO obtained with the TM1 peptide was often higher than 10. A comparison of the results of direct coating of the free peptide and coating of the peptide complexed with streptavidin showed clearly that direct coating was far inferior to coating of the complex for detection of MVV antibodies (not shown).
FIG. 1.
Reactivities of MVV-positive sera in ELISA where the solid phase was sensitized with recombinant p25 alone or with p25 combined with TM1c peptide as described in Materials and Methods. S/CO are given for 12 positive sera, and the cutoff was set at an S/CO of 1. For the p25-only ELISA, the cutoff was determined as the mean of results for negative controls plus 3 standard deviations.
FIG. 2.
ELISA results of MVV-positive sera on a solid phase coated with peptide TM1, TM2, or TM3 and complexed with streptavidin as described in Materials and Methods. The S/CO are given, with cutoff being defined as the mean value for the negative control sera plus 3 standard deviations.
During the development of the test, the reactivity of the freshly dissolved TM1 peptide was lower than that of the TM1 peptide which had been stored in solution for some time. This phenomenon may indicate that intramolecular disulfide bridge formation occurred during storage, resulting in better reactivity. To avoid the change in reactivity as a function of storage time in solution, chemical oxidation and HPLC purification of the peptide were performed as described in Materials and Methods. Subsequent analysis showed a very sharp unique peak in HPLC (not shown), and the molecular mass was verified by mass spectrometric analysis (matrix-assisted laser desorption-ionization–time of flight; 2,564 Da). The oxidized purified peptide (TM1c) had good initial reactivity and stability in solution. Therefore, the TM1c peptide was used in all further assays.
Serological studies.
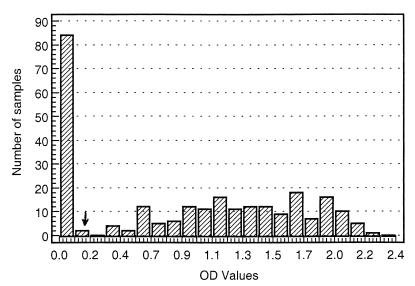
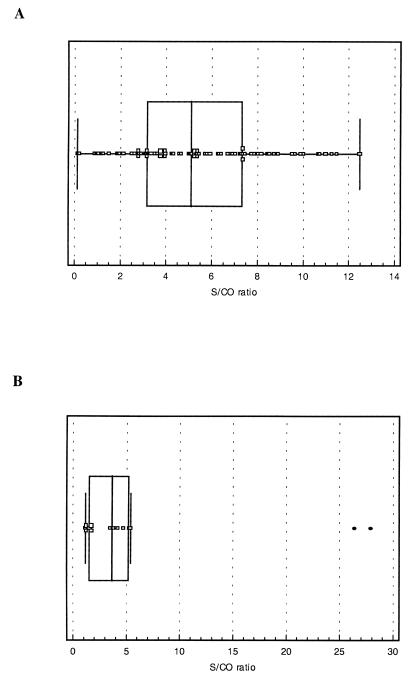
The prototype assay described above was used to study a large set of sera from different European countries (Table 1). For most of these sera, the results of the AGIDT were available for comparison with the results of the ELISA. Table 2 shows the overall outcome of this study, and Fig. 3 illustrates the distribution of the signals for a set of 255 randomly chosen sera. The relative sensitivity (98.4%) and specificity (97.1%) of the ELISA determined from these data are minimum values, especially since the specificity cannot reliably be judged based on the AGIDT results because it is known that the sensitivity of this assay is suboptimal. In an attempt to clarify this situation, other alternative serological assays were performed on 56 sera, with discrepant results (see below). On the other hand, an important number of AGIDT-indeterminate samples were clearly resolved by the ELISA and most of them were scored as positive (Table 2), which again illustrates the relative insensitivity of the AGIDT. The distribution of the S/CO of the AGIDT-indeterminate samples is shown in Fig. 4A. There was no correlation between the indeterminate AGIDT result and the ELISA signal, i.e., some AGIDT-indeterminate samples scored high in ELISA and 51 of 90 sera had an S/CO exceeding 5.
TABLE 2.
Overall results of the AGIDT and ELISA analyses and corrected scoresa
| ELISA result | No. of AGIDT results (n =
2,336) that were:
|
No. of consensus scores
(n = 2,240) that were:
|
|||
|---|---|---|---|---|---|
| Positive | Negative | Indeterminate | Positive | Negative | |
| Positive | 620 | 46 | 90 | 655 | 11 |
| Negative | 10 | 1,564 | 6 | 4 | 1,570 |
See the text for details.
FIG. 3.
Distribution of the ELISA signals (OD values) for 255 sheep sera. The minimum value in this series was 0.014, and the maximum value recorded was 2.2. The cutoff was calculated as described in Materials and Methods and is indicated by the arrow (0.175).
FIG. 4.
(A) Box-and-whisker plot of the ELISA data (S/CO) for the sheep sera with AGIDT-indeterminate results. Median and 25th, and 75th percentiles (boxes), as well as the minimum and maximum values, are indicated. (B) Box-and-whisker plot of ELISA results (S/CO) for 14 goat sera that were AGIDT negative. The two outliers with S/CO between 25 and 30 were not included in the calculations of the median and the percentiles.
To investigate the specificity of the ELISA assay, sera from herds considered MVV free were analyzed. These herds have been regularly tested by AGIDT for several years, and no positive animals were identified during this follow-up, which was continued during the present study. From the 246 animals in three herds (two in Spain and one in Italy), only one animal was found weakly positive in ELISA (S/CO of 1.26), resulting in a specificity of 99.6%, which is in good agreement with the specificity calculated with the entire serum set (99.3%).
The interplate and intraplate variations were also assessed on different occasions. The same serum dilution was tested 50 times in two different plates, and the coefficient of variation was always below 10%, for interplate as well as intraplate variations.
Discrepant results between ELISA and AGIDT.
In an attempt to resolve the discrepancies between both routine tests as much as possible, Western blot analysis with concentrated viral lysate and purified recombinant p25 antigen was performed on most of the discrepant samples. The 46 samples with an AGIDT-negative and ELISA-positive score were analyzed in detail. Of these 46, only 11 were retained as being negative after Western blot analysis, indicating that 35 sera were probably falsely negative in the AGIDT. On the other hand, the AGIDT-positive and ELISA-negative samples (n = 10) (Table 2) were also investigated in detail by Western blot analysis. For five of them, AGIDT was repeated in an independent institute. The positive AGIDT result could not be confirmed for the latter samples, and Western blot analysis showed them to be negative. In addition, two of the samples were from follow-up bleedings of the same animal taken at a 1-year interval, and no indication of a rising titer in ELISA was seen in the second bleeding sample. For a third sample, which was taken in 1995, serum from the same animal drawn in 1996 was completely negative in ELISA and in AGIDT.
Apart from the five samples discussed above, a sixth serum was negative by Western blotting and, hence, also considered to be negative. This left us with four samples for which we had insufficient data to confirm the MVV-negative status indicated by the ELISA. These samples were considered false negatives. When the above data are taken into account, the numbers of true positives and true negatives are as shown in Table 2 (consensus scores). These data result in corrected sensitivity and specificity scores of 99.4 and 99.3%, respectively, with 95% confidence intervals of 98.4 to 99.8% (sensitivity) and 98.7 to 99.6% (specificity).
Seroconversions.
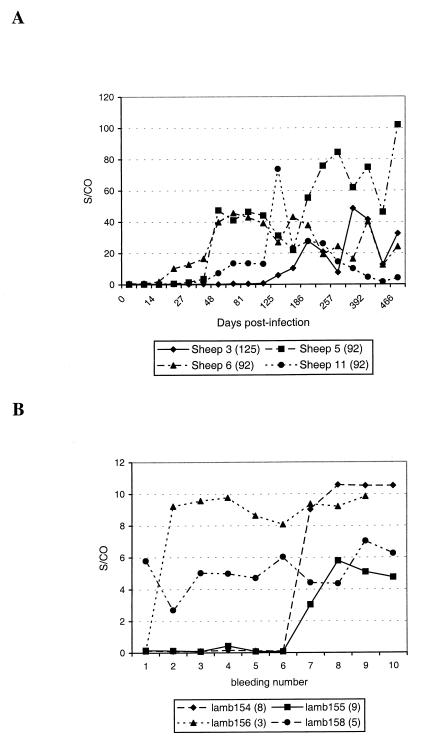
The experimental infection of eight adult animals resulted in the seroconversions of three animals being recorded at the same times in both the AGIDT and the ELISA (125, 44, and 44 days postinfection). For four other animals, the ELISA detected seroconversion much earlier, as illustrated in Fig. 5A. In one case, seroconversion was detected only 125 days postinfection by both tests (Fig. 5A, sheep 3).
FIG. 5.
(A) Seroconversion of four ewes after experimental infection with MVV. For each animal, the time of first AGIDT-positive bleeding is indicated between parentheses (days postinfection). For each sample, ELISA as well as AGIDT analysis of the serum was performed. The ELISA result is considered positive when the S/CO is >1. The values at time zero are the means of results for four control bleedings taken during a period of 200 days prior to infection. Bleeding samples of an uninfected control sheep were analyzed in parallel over a period of 303 days (11 bleedings) and the S/CO was always below the cutoff (between 0.21 and 0.78). (B) Vertical transmission of MVV infection in four lambs born to MVV-infected dams. For each lamb, the first AGIDT-positive bleeding (bleeding number) is indicated after the animal identification number. For each sample, ELISA as well as AGIDT analysis was performed. An S/CO of >1 is considered a positive result.
In another study, 14 lambs born to MVV field-infected dams were sampled 10 times over a period of 23 months at regular intervals. For three animals, no seroconversion was recorded by AGIDT even after 10 bleedings. In one of these animals, the ELISA indicated seroconversion from the seventh bleeding on. Furthermore, four animals were positive in both assays from the first bleeding on and remained so for all subsequent samples. For three other lambs, the AGIDT and ELISA detected seroconversion at the same bleeding. Finally, the ELISA was able to detect seroconversion earlier than AGIDT in four animals, one of which showed a positive ELISA result four bleedings earlier than the AGIDT (Fig. 5B, lamb 158).
Analysis of milk samples.
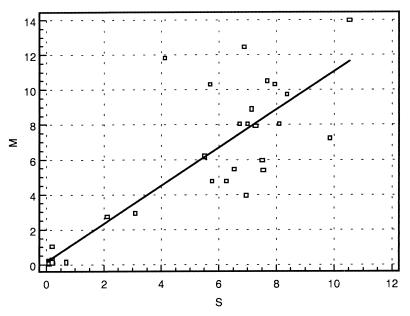
To evaluate the performance of the ELISA with milk, a set of 40 milk samples was analyzed in parallel with the serum taken at the same time. Different dilutions of the milk were assessed (between 1/10 and 1/640), and a 1/50 dilution was chosen as giving the best sensitivity with good specificity. In Fig. 6, the correlation between serum and milk data is shown. For 39 animals a good correlation was found between both body fluids (correlation coefficient, 0.89). For one animal, both values were close to the cutoff (milk, 0.91, and serum, 1.03). Hence, this lack of correlation is not very significant.
FIG. 6.
Correlation between ELISA results obtained with serum and milk samples from the same animals (n = 40). S/CO were plotted on the x axis for serum (S) and on the y axis for milk (M). Cutoff was at an S/CO of 1 in both cases. The linear regression line is also shown (r = 0.89). Influence analysis has not shown significant bias on the correlation by one or more data points.
Analysis of goat sera.
As the etiological agent of CAEV in goats is genetically as well as serologically closely related to the MVV virus, the MVV ELISA was evaluated for the detection of antibodies in goats infected with CAEV. For 103 goat serum samples (from Spain), the AGIDT results (Capriclear, CVL, Weybridge, United Kingdom) were available. Of this sample set, 25 were AGIDT negative and 78 were positive to various degrees. In this set, a perfect correlation between AGIDT and ELISA was found (not shown). Another set of 109 goat sera from Brazilian goat herds was also analyzed. In this case, 31 samples were negative in both AGIDT and ELISA, 64 samples were positive in both assays, and 14 samples were positive in ELISA but negative in AGIDT. The distribution of the signals in the last set is shown in Fig. 4B. These data show that, while some of the AGIDT-negative–ELISA-positive sera were only borderline positive in ELISA, most of these samples had an S/CO over 2 and hence were clearly positive in ELISA. ELISA-negative–AGIDT-positive samples were not found. In this limited set of 212 goat samples the overall sensitivity of the ELISA relative to the AGIDT was 100% whereas the specificity was 86%. However, the latter figure has to be considered with care since the sensitivity of the AGIDT is too low to be used as the gold standard (see the data on discrepant results with the sheep sera). When only the six borderline positive sera are considered as potential false positives, the specificity rises to 94.2%.
DISCUSSION
Infection with ovine lentiviruses is still a major concern in a large part of the European sheep industry, and control programs are only now being initiated. To set up such a control and eradication program, a sensitive and specific diagnostic assay which allows high throughput at a reasonable cost is needed. The AGIDT which is presently used in the field is hardly suitable for this work. Herein, we describe the development and the evaluation of an ELISA which allows the sensitive and reliable detection of ovine lentivirus infection in small ruminants. In setting up the assay, it was soon obvious that the sensitivity which was obtained when we used recombinant major core protein p25 as the sole antigen was largely insufficient compared with the sensitivity of the standard AGIDT. Therefore, a combination of this p25 core protein with a peptide derived from the immunodominant region in the TM protein was investigated. However, when the polystyrene was directly coated with the peptides, hardly any reactivity with MVV-positive sera could be detected. On the other hand, when the same peptides were N biotinylated and coated as streptavidin complexes, very high reactivities were recorded. This result indicated either that passive adsorption of these peptides to the solid phase was highly inefficient or that the antigenic reactivities of the directly coated peptides were severely compromised by the interaction with the plastic. The observation that, for some sera, a good reactivity with the peptides was found may indicate that the peptides bound to the microwells but that for most sera, the relevant epitopes were no longer accessible.
The combined coating of the p25 antigen and the streptavidin-peptide complex resulted in an assay with a high sensitivity (>98%), a somewhat lower specificity (97.1%) than that of the standard AGIDT, and an acceptable distribution of the signals relative to the cutoff point. However, the AGIDT cannot be considered a gold standard since it is known to be rather insensitive and since the test outcome may be influenced by the interpretation of the individual reading the results. For this reason, alternative serological assays were used to investigate the discrepant samples. Western blot analysis with viral lysate or recombinant purified p25 protein was able to resolve most of the discrepant cases. With these results taken into account, both sensitivity and specificity were higher than 99%.
The relative insensitivity of the AGIDT versus the ELISA was also illustrated when the AGIDT-indeterminate samples were analyzed and when the results of the seroconversion studies were considered. Indeed, from the 96 AGIDT-indeterminate samples present in this study, only six were negative in ELISA whereas 90 scored positive with large variations in the S/CO between individual samples. Furthermore, the studies of seroconversion upon experimental infection and natural vertical transmission clearly demonstrated that the ELISA was able to detect the presence of MVV antibodies before the AGIDT. In some animals this time difference was found to be more than 2 months.
The specificity of the assay was further documented by analysis of herds kept in relative isolation and shown to be free of MVV infection based on AGIDT results of consecutive bleedings over several years for each individual animal. In this set of samples, the ELISA reached a specificity of 99.6%, which is in close agreement (within the 95% confidence interval) with the 99.3% specificity calculated from the entire data set after correction (Table 2).
Finally, the performance of the ELISA for the detection of CAEV infection in goats was also evaluated on a limited set of samples, and results comparable to those of the sheep serology were obtained. Also, in this case, about 14% of AGIDT-negative sera were found to be ELISA positive. Although we did not analyze these samples in more detail, it is likely that we would have encountered a situation similar to that for sheep sera, where only 11 of 46 AGIDT-negative–ELISA-positive samples were considered negative, since positivity could not be confirmed upon additional testing. When only the six borderline reactive goat samples are considered false positives, the specificity rises to 94.2%, which is probably still an underestimation, given the relatively small number of samples analyzed.
In addition, preliminary studies of sheep milk samples have also shown that the ELISA can favorably be used to detect MVV antibodies in the milk of infected ewes. The use of milk samples for large epidemiological studies has certain advantages (ease of sampling and storage). This is an additional benefit for the use of this assay in eradication programs.
In conclusion, the combination of a recombinant core protein and a synthetic peptide derived from the TM protein of MVV has resulted in a useful serological assay in the ELISA format for the detection of ovine lentiviral infections. This assay will allow the large-scale monitoring of sheep and goat populations and, consequently, may contribute to the establishment of eradication programs for this infection.
ACKNOWLEDGMENTS
We thank L. Dickson (Edinburgh, Scotland) for excellent technical assistance and C. Vidal (Concordia, Brazil) for providing goat sera. The goat sera from Spain were obtained from A. Contreras (Murcia, Spain). M. Garcia-Goti and N. Cortabarria are acknowledged for bleeding and AGIDT analysis of the sheep from the Basque region (Spain); C. Pacheco and R. Bolea are acknowledged for sample collection and help with the AGIDT in Zaragoza. We also thank B. Van Der Perre for synthesis and purification of the peptides and J.-C. Crispyn for purification of the recombinant protein. F. Shapiro is thanked for editorial comments on the manuscript.
We acknowledge the European Union for financial support of this work (grant AIR3 CT94-1492).
REFERENCES
- 1.Boshoff C H, Dungu B, Williams R, Vorster J, Conradie J D, Verwoerd D W, York D F. Detection of maedi-visna virus antibodies using a single fusion transmembrane-core p25 recombinant protein ELISA and a modified receiver-operating characteristic analysis to determine the cut-off values. J Virol Methods. 1977;63:47–56. doi: 10.1016/s0166-0934(96)02114-3. [DOI] [PubMed] [Google Scholar]
- 2.Clements J E, Zink M C. Molecular biology and pathogenesis of animal lentivirus infection. Clin Microbiol Rev. 1996;9:100–117. doi: 10.1128/cmr.9.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutlip R C, Jackson T A, Laird G A. Immunodiffusion test for ovine progressive pneumonia. Am J Vet Res. 1977;38:1081–1084. [PubMed] [Google Scholar]
- 4.Dawson M, Biront P, Houwers D J. Comparison of serological tests used in three state veterinary laboratories to identify maedi-visna virus infection. Vet Rec. 1982;111:432–434. doi: 10.1136/vr.111.19.432. [DOI] [PubMed] [Google Scholar]
- 5.Doolittle R F, Feng D F, McClure M A, Johnson M S. Retrovirus phylogeny and evolution. Curr Top Microbiol Immunol. 1990;157:1–18. doi: 10.1007/978-3-642-75218-6_1. [DOI] [PubMed] [Google Scholar]
- 6.Houwers D J, Gielkens A L J, Schaake J., Jr An indirect enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to maedi-visna virus. Vet Microbiol. 1982;7:209–219. doi: 10.1016/0378-1135(82)90035-9. [DOI] [PubMed] [Google Scholar]
- 7.Keen J, Kwang J, Rosati S. Comparison of ovine lentivirus detection by conventional and recombinant serological methods. Vet Immunol Immunopathol. 1995;47:295–309. doi: 10.1016/0165-2427(94)05394-8. [DOI] [PubMed] [Google Scholar]
- 8.Knowles D P., Jr Laboratory diagnostic tests for retrovirus infections of small ruminants. Vet Clin North Am Food Anim Pract. 1997;13:1–11. doi: 10.1016/s0749-0720(15)30361-3. [DOI] [PubMed] [Google Scholar]
- 9.Kwang J, Cutlip R. Detection of antibodies to ovine lentivirus using a recombinant antigen derived from the env gene. Biochem Biophys Res Commun. 1992;183:1040–1046. doi: 10.1016/s0006-291x(05)80295-5. [DOI] [PubMed] [Google Scholar]
- 10.Kwang J, Torres J V. Oligopeptide-based enzyme immunoassay for ovine lentivirus antibody detection. J Clin Microbiol. 1994;32:1813–1815. doi: 10.1128/jcm.32.7.1813-1815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConnell I, Peterhans E, Zanoni R G. Concordance with reference sera of a recombinant protein ELISA for maedi-visna antibody detection. Vet Rec. 1998;142:431–433. doi: 10.1136/vr.142.16.431. [DOI] [PubMed] [Google Scholar]
- 12.Remaut E, Stanssens P, Fiers W. Plasmid vectors for high-efficiency expression, controlled by the PL promoter of coliphage lambda. Gene. 1981;15:81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- 13.Reyburn H T, Roy D J, Blacklaws B A, Sargan D R, McConnell I. Expression of maedi-visna virus major core protein, p25: development of a sensitive p25 antigen detection assay. J Virol Methods. 1992;31:305–320. doi: 10.1016/0166-0934(92)90031-8. [DOI] [PubMed] [Google Scholar]
- 14.Rosati S, Tolari F, Bollo E. Identification of lentiviruses isolated from outbreaks of ovine maedi and caprine arthritis encephalitis. Proc Ann Meet Ital Soc Vet Sci. 1992;46:883–886. [Google Scholar]
- 15.Rosati S, Kwang J, Tolari F, Keen J. A comparison of whole virus and recombinant transmembrane ELISA and immunodiffusion for detection of ovine lentivirus antibodies in Italian sheep flocks. Vet Res Commun. 1994;18:73–80. doi: 10.1007/BF01839262. [DOI] [PubMed] [Google Scholar]
- 16.Sargan D R, Bennet I D, Cousens C, Roy D J, Blacklaws B A, Dalziel R G, Watt N J, McConnell I. Nucleotide sequence of EV-1, a British isolate of maedi-visna virus. J Gen Virol. 1991;72:1893–1903. doi: 10.1099/0022-1317-72-8-1893. [DOI] [PubMed] [Google Scholar]
- 17.Simard C L, Briscoe M R. An enzyme-linked immunosorbent assay for detection of antibodies to Maedi-Visna virus in sheep. I. A simple technique for production of antigen using sodium dodecyl sulfate treatment. Can J Vet Res. 1990;54:446–450. [PMC free article] [PubMed] [Google Scholar]
- 18.Sonigo P, Alizon M, Staskus K, Klatzmann D, Cole S, Danos O, Retzel E, Tiolais P, Haase A, Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985;42:369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- 19.Winward L D, Leendertsen L, Shen D T. Microimmunodiffusion test for diagnosis of ovine progressive pneumonia. Am J Vet Res. 1979;40:564. [PubMed] [Google Scholar]
- 20.Zanoni R G. Phylogenetic analysis of small ruminant lentiviruses. J Gen Virol. 1998;79:1951–1961. doi: 10.1099/0022-1317-79-8-1951. [DOI] [PubMed] [Google Scholar]
- 21.Zanoni R G, Nanta I M, Pauli V, Peterhans E. Expression in Escherichia coli and sequencing of the coding region for the capsid protein of Dutch maedi-visna virus strain ZZV 1050: application of recombinant protein in enzyme-linked immunosorbent assay for the detection of caprine and ovine lentiviruses. J Clin Microbiol. 1991;29:1290–1294. doi: 10.1128/jcm.29.7.1290-1294.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]