Abstract
Background
To evaluate the association between blood urea nitrogen (BUN) to creatinine (Cr) (BUN/Cr) ratio and the in-hospital mortality of critically ill patients with cerebral infarction in intensive care unit (ICU).
Methods
In this cohort study, the data of 3059 participants with cerebral infarction were collected from the Medical Information Mart for Intensive Care (MIMIC)-III and the MIMIC-IV database. After propensity score matching (PSM) on age and gender, 2085 people were involved in and divided into the alive group (n = 1390) and the dead group (n = 695) based on the results of follow-up. Multivariate logistic analyses were applied to identify the confounders and the association between BUN/Cr and mortality of cerebral infarction.
Results
The median follow-up time was 10.5 days. Among 2778 participants, 695 were dead at the end of follow-up. Univariate analysis revealed that BUN/Cr [risk ratio (RR) = 1.01, 95% confidence interval (CI): 1.01-1.02] might be associated with the in-hospital mortality of cerebral infarction patients. After adjusting for respiratory failure, malignant cancer, anticoagulation, liver disease, white blood cell (WBC), red cell distribution width (RDW), glucose, bicarbonate, and temperature, BUN/Cr had week correlation with the increased risk of in-hospital mortality of cerebral infarction patients (RR = 1.01, 95% CI: 1.01-1.02).
Conclusion
This study evaluated the association between BUN/Cr and the in-hospital mortality of cerebral infarction patients in ICU and found that BUN/Cr had weak correlation with the increased risk of in-hospital mortality of patients with cerebral infarction in ICU especially in males and those with respiratory failure, malignant cancer, and without liver disease, as well as those receiving anticoagulation.
1. Introduction
Cerebral infarction is a kind of brain injury due to the obstruction of blood supply in the brain, which induces ischemic and hypoxic necrosis of innervation [1]. As one of the most common types of cerebrovascular diseases, cerebral infarction accounts for about 70% of all cerebrovascular diseases and approximately 85% of all strokes [2–4]. Cerebral infarction has high incidence, disability, recurrence rate, and mortality, which has resulted in a substantial burden to the society [5]. In China, the reported 1-month morality rate after cerebral infarction was 2.3%-3.2%, and the 3-month mortality rate was 9%-9.6%, and the mortality or disability rate in patients with acute cerebral infarction was 34.5%-37.1% [6]. The estimated mortality of cerebral infarction was about 6.17 million every year all over the world [7]. Nearly 1/3 patients with acute cerebral infarction suffered poor outcomes [8]. To identify essential biomarkers associated with the prognosis of cerebral infarction patients might be helpful for improving the outcomes of these patients.
The fluid metabolism including dehydration status was reported to be potentially associated with the occurrence of cerebral infarction [9]. Dehydration could lead to increased blood viscosity, reduced cardiac output per stroke, impaired collateral blood flow, and decreased cerebral perfusion, which might increase the risk of cerebral infarction [10]. Blood urea nitrogen (BUN) and creatinine (Cr) were metabolic end products of nitrogen-containing substances in human bodies, which were readily available biomarkers of renal function in clinic [11]. BUN to creatinine (BUN/Cr) ratio was a laboratory biomarker frequently used for determining the dehydration [12]. In previous studies, BUN/Cr was identified as an independent prognostic indicator for poor outcomes of patients with different diseases, including stroke [13] and heart failure [14]. Some other studies identified that low level of BUN/Cr might increase the risks of ischemic stroke [15]. The roles of BUN/Cr on diseases were inconformity. Besides, the potential role of BUN/Cr on the prognosis of patients with cerebral infarction was still unclear. Therefore, this study is mainly to evaluate the association between BUN/Cr and the in-hospital mortality of critically ill patients with cerebral infarction in intensive care unit (ICU).
In the present study, we evaluated the association between BUN/Cr and the in-hospital mortality of critically ill patients with cerebral infarction in intensive care unit (ICU) based on the data from the Medical Information Mart for Intensive Care (MIMIC)-III and the MIMIC-IV. We also stratified the analysis on age, gender, and whether the patient was complicated with respiratory failure, malignant cancers, or liver diseases or whether the patient received anticoagulation treatments.
2. Materials and Methods
2.1. Study Design and Population
In this cohort study, the data of 3059 participants with cerebral infarction were collected from MIMIC database, including 1568 in the MIMIC-III and 1491 in the MIMIC-IV. The MIMIC-III is a large, single-center open database comprising the electronic health records including demographic characteristics, monitoring vital signs, laboratory and microbiological examination, imaging examination, observation and recording of intake and output, drug treatment, length of stay, survival data, and discharge or death records of more than 60,000 individuals admitted to an ICU at the Beth Israel Deaconess Medical Center between 2001 and 2012 [16]. The MIMIC-IV database is an updated version of the MIMIC-III, and improvements have been made including simplifying the structure, adding new data elements, and improving the usability of previous data elements. The MIMIC-IV involves the comprehensive and high-quality electronic health records of patients admitted to the ICU or emergency department of the Beth Israel Deaconess Medical Center from 2008 to 2019 [17]. The database got the approval from the institutional review boards of the Massachusetts Institute of Technology (Cambridge, Massachusetts) and the Beth Israel Deaconess Medical Center (Boston, Massachusetts). Cerebral infarction in patients was diagnosed when admitted to ICUs based on International Classification of Diseases, Ninth Revision (ICD-9) code and the Tenth Revision (ICD-10) code. ICD-9: 43301, 43311, 43321, 43331, 43381, 43391, 43401, 43411, and 43491; ICD-10: I63. In our study, patients who aged <18 years were excluded. Those admitted to ICU <24 h or who had no data on BUN/Cr were also excluded. Finally, the data of 2778 participants were analyzed. At the end of the follow-up, 2083 patients were alive, and 695 patients were dead. After propensity score matching (PSM) on age and gender, 2085 people were involved in and divided into the alive group (n = 1390) and the dead group (n = 695).
2.2. Variables
The main variable investigated was BUN/Cr. Covariables analyzed in the study included comorbidities (congestive heart failure (CHF) (yes or no), atrial fibrillation (AF) (yes or no), diabetes mellitus (yes or no), respiratory failure (yes or no), renal failure (yes or no), malignant cancer (yes or no), hypertension (yes or no), and liver disease (yes or no)), medication use (thrombolytic (yes or no) and anticoagulation (yes or no)), laboratory data (heart rate (time/min), systolic blood pressure (SBP) (mmhg), diastolic blood pressure (DBP) (mmhg), mean arterial pressure (MAP) (mmhg), respiratory rate (time/min), temperature (°C), white blood cell (WBC) (K/μl), platelets (PLT) (K/μl), hemoglobin (g/dL), red cell distribution width (RDW) percent, hematocrit percent, Cr (mg/dl), International Normalized Ratio (INR), BUN (mg/dl), glucose (mg/dl), bicarbonate (mEq/l), sodium (mEq/l), and potassium (mEq/l)), and the Sequential Organ Failure Assessment (SOFA) Score, the Simplified Acute Physiology Score II (SAPSII), the Oxford Acute Severity of Illness Score (OASIS), and Charlson comorbidity index.
2.3. Outcome Variable
The outcome in the present study was the in-hospital death of participants with cerebral infarction in ICU. All subjects in ICU were followed up until death or discharge. The median follow-up time was 10.5 days. Among 2778 participants, 695 were dead at the end of follow-up.
2.4. Sensitivity Analysis
The missing values of all variables were shown in Supplementary Table 1. The results of sensitivity analysis revealed that no statistical difference was observed in the data before and after multi-interpolation. As exhibited in Supplementary Table 2, the age was statistically different between the alive group and the dead group (66.42 years vs. 71.51 years). To make the baseline data equilibrated between the alive group and the dead group, PSM was applied. After PSM, the data of age and gender showed no statistical difference between the two groups.
2.5. Statistical Analysis
The continuous variables were presented in the forms of mean ± standard deviation (SD) if the data were normally distributed or M (Q1, Q3) if the data were not normally distributed. Student's t-test was used to compare the difference between groups. The categorical variables were displayed as n (%), and chi-square and Wilcoxon rank sum test were applied to judge the differences between groups. The data with missing value <10% were multi-interpolated and with missing value ≥10% were excluded. PSM was performed on the data. Sensitivity analysis was performed between the data before multi-interpolation and after multi-interpolation as well as before PSM and after PSM. Multivariate logistic analyses were applied to identify the confounders of the association between BUN/Cr and mortality of cerebral infarction. Model 1 included all variables with statistical difference between the alive group and the dead group. Model 2 included BUN/Cr, respiratory failure, malignant cancer, anticoagulation, liver disease, temperature, WBC, RDW percent, glucose, and bicarbonate. Subgroup analysis was performed to assess the association between BUN/Cr and the mortality of cerebral infarction in different groups of people concerning age, gender, and whether the patient was complicated with respiratory failure, malignant cancer, or liver disease or whether the patient received anticoagulation treatments. The risk ratio (RR) was employed to evaluate the association between BUN/Cr and the mortality of cerebral infarction in ICU. The confidence level was set as α = 0.05. All statistical analysis was conducted via SAS 9.4, and R 4.0.3.
3. Results
3.1. Comparisons of the Baseline Characteristics between the Alive Group and the Dead Group
This study was a cohort study involving the data of 3059 participants with cerebral infarction. Among all participants, patients aged <18 years (n = 14) and those admitted to ICU <24 h (n = 261) were excluded. Also, 6 patients without data on BUN/Cr were excluded. Finally, the data of 2778 participants were analyzed. After PSM on age and gender, 2085 people were finally included in our study and divided into the alive group (n = 1390) and the dead group (n = 695). The detailed screen process was displayed in Figure 1.
Figure 1.
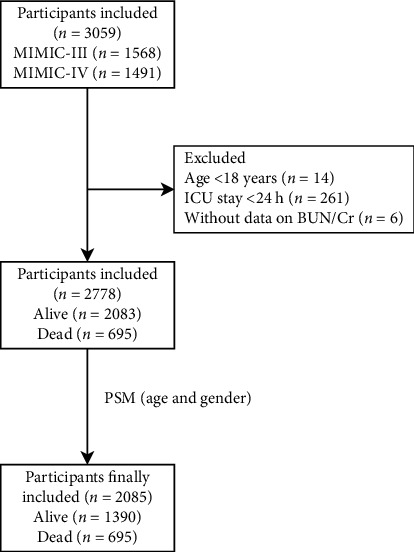
The screen process of participants in this study.
As observed in Table 1, the percentages of patients with CHF (26.12% vs. 30.79%), respiratory failure (31.08% vs. 53.67%), renal failure (19.78% vs. 26.91%), malignant cancer (11.94% vs. 18.27%), and liver disease (4.96% vs. 10.65%) were lower in the alive group than the dead group. The percentage of patients with anticoagulation use in the alive group was higher than the dead group (90.36% vs. 85.47%). The average heart rate (83.56 times/min vs. 88.95 times/min), RDW (14.37% vs. 15.01%), potassium level (4.15 mEq/l vs. 4.24 mEq/l), and OASIS (31.53 vs. 36.17) in the alive group were lower than the dead group. The average temperature (36.59°C vs. 36.26°C), hematocrit (12.03 g/dl vs. 11.55 g/dl), hematocrit (36.05% vs. 34.93%), and bicarbonate (23.91 mEq/l vs. 23.00 mEq/l) were higher in the alive group than in the dead group. The median SOFA score (2 vs. 4), SAPSII (35 vs. 43), Cr (1.00 mg/dl vs. 1.10 mg/dl), BUN (19 mg/dl vs. 22 mg/dl), and BUN/Cr (19.00 vs. 20.00) were lower in the alive group than in the dead group.
Table 1.
Comparisons of the baseline characteristics between the alive group and the dead group.
| Variables | Total (n = 2085) | Alive (n = 1390) | Dead (n = 695) | Statistics | P |
|---|---|---|---|---|---|
| CHF, n (%) | χ 2 = 5.062 | 0.024 | |||
| No | 1508 (72.33) | 1027 (73.88) | 481 (69.21) | ||
| Yes | 577 (27.67) | 363 (26.12) | 214 (30.79) | ||
| AF, n (%) | χ 2 = 0.164 | 0.686 | |||
| No | 1154 (55.35) | 765 (55.04) | 389 (55.97) | ||
| Yes | 931 (44.65) | 625 (44.96) | 306 (44.03) | ||
| Diabetes mellitus, n (%) | χ 2 = 0.192 | 0.662 | |||
| No | 1451 (69.59) | 963 (69.28) | 488 (70.22) | ||
| Yes | 634 (30.41) | 427 (30.72) | 207 (29.78) | ||
| Respiratory failure, n (%) | χ 2 = 99.754 | <.001 | |||
| No | 1280 (61.39) | 958 (68.92) | 322 (46.33) | ||
| Yes | 805 (38.61) | 432 (31.08) | 373 (53.67) | ||
| Renal failure, n (%) | χ 2 = 13.627 | <.001 | |||
| No | 1623 (77.84) | 1115 (80.22) | 508 (73.09) | ||
| Yes | 462 (22.16) | 275 (19.78) | 187 (26.91) | ||
| Malignant cancer, n (%) | χ 2 = 15.376 | <.001 | |||
| No | 1792 (85.95) | 1224 (88.06) | 568 (81.73) | ||
| Yes | 293 (14.05) | 166 (11.94) | 127 (18.27) | ||
| Thrombolytic, n (%) | χ 2 = 3.202 | 0.074 | |||
| No | 1777 (85.23) | 1171 (84.24) | 606 (87.19) | ||
| Yes | 308 (14.77) | 219 (15.76) | 89 (12.81) | ||
| Anticoagulation, n (%) | χ 2 = 11.088 | <.001 | |||
| No | 235 (11.27) | 134 (9.64) | 101 (14.53) | ||
| Yes | 1850 (88.73) | 1256 (90.36) | 594 (85.47) | ||
| Hypertension, n (%) | χ 2 = 3.542 | 0.060 | |||
| No | 793 (38.03) | 509 (36.62) | 284 (40.86) | ||
| Yes | 1292 (61.97) | 881 (63.38) | 411 (59.14) | ||
| Liver disease, n (%) | χ 2 = 23.429 | <.001 | |||
| No | 1942 (93.14) | 1321 (95.04) | 621 (89.35) | ||
| Yes | 143 (6.86) | 69 (4.96) | 74 (10.65) | ||
| Heart rate (time/min), mean ± SD | 85.36 ± 20.10 | 83.56 ± 18.71 | 88.95 ± 22.21 | t = −5.50 | <.001 |
| SBP (mmhg), M(Q1,Q3) | 137 (116, 155) | 138 (118,155) | 134 (113,155) | Z = −2.151 | 0.031 |
| DBP (mmhg), M (Q1, Q3) | 70 (58, 83) | 70 (58, 83) | 70 (58, 82) | Z = −0.187 | 0.851 |
| MAP (mmhg), M (Q1, Q3) | 89 (76, 102) | 89 (77, 103) | 88 (75, 102) | Z = −1.072 | 0.284 |
| Respiratory rate (time/min), M (Q1, Q3) | 18.00 (15.00, 22.00) | 18.00 (15.00, 21.00) | 19.00 (15.00, 24.00) | Z = 4.577 | <.001 |
| Temperature (°C), mean ± SD | 36.48 ± 2.72 | 36.59 ± 1.80 | 36.26 ± 3.96 | t = 2.15 | 0.032 |
| SOFA, M (Q1, Q3) | 2.00 (1.00, 5.00) | 2.00 (0.00, 4.00) | 4.00 (1.00, 6.00) | Z = 7.945 | <.001 |
| SAPSII, M (Q1, Q3) | 38.00 (30.00, 47.00) | 35.00 (28.00, 44.00) | 43.00 (35.00, 53.00) | Z = 12.891 | <.001 |
| OASIS, mean ± SD | 33.08 ± 9.92 | 31.53 ± 9.68 | 36.17 ± 9.67 | t = −10.33 | <.001 |
| WBC (K/μl), M(Q1, Q3) | 10.30 (7.80, 13.80) | 9.90 (7.70, 13.30) | 11.50 (8.00, 15.80) | Z = 5.198 | <.001 |
| PLT (K/μl), M (Q1, Q3) | 215.00 (160.00, 279.00) | 217.00 (164.00, 276.00) | 211.00 (153.00, 285.00) | Z = −1.257 | 0.209 |
| Hemoglobin (g/dl), mean ± SD | 11.87 ± 2.31 | 12.03 ± 2.29 | 11.55 ± 2.32 | t = 4.55 | <.001 |
| RDW percent, mean ± SD | 14.59 ± 1.99 | 14.37 ± 1.88 | 15.01 ± 2.11 | t = −6.79 | <.001 |
| Hematocrit percent, mean ± SD | 35.68 ± 6.51 | 36.05 ± 6.48 | 34.93 ± 6.53 | t = 3.70 | <.001 |
| Creatinine (mg/dl), M (Q1,Q3) | 1.00 (0.80, 1.40) | 1.00 (0.80, 1.30) | 1.10 (0.80, 1.60) | Z = 4.686 | <.001 |
| INR, M (Q1,Q3) | 1.20 (1.10, 1.40) | 1.20 (1.10, 1.30) | 1.20 (1.10, 1.40) | Z = 6.144 | <.001 |
| BUN (mg/dl), M (Q1,Q3) | 20.00 (14.00, 29.00) | 19.00 (14.00, 27.00) | 22.00 (16.00, 34.00) | Z = 6.401 | <.001rep |
| Glucose (mg/dl), M (Q1,Q3) | 130.00 (107.00, 167.00) | 126.00 (106.00, 160.00) | 136.00 (110.00, 184.00) | Z = 4.853 | <.001 |
| Bicarbonate (mEq/l), mean ± SD | 23.61 ± 4.26 | 23.91 ± 3.97 | 23.00 ± 4.72 | t = 4.36 | <.001 |
| Sodium (mEq/l), mean ± SD | 139.24 ± 4.64 | 139.37 ± 4.42 | 138.98 ± 5.03 | t = 1.77 | 0.076 |
| Potassium (mEq/l), mean ± SD | 4.18 ± 0.77 | 4.15 ± 0.73 | 4.24 ± 0.83 | t = −2.49 | 0.013 |
| Charlson comorbidity index, M (Q1,Q3) | 5.00 (3.00, 8.00) | 5.00 (3.00, 7.00) | 6.00 (3.00, 8.00) | Z = 2.891 | 0.004 |
| BUN/Cr, M (Q1, Q3) | 19.38 (15.00, 24.44) | 19.00 (15.00, 23.75) | 20.00 (15.00, 25.71) | Z = 2.756 | 0.006 |
CHF: congestive heart failure; AF: atrial fibrillation; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; WBC: white blood cell; PLT: platelets; RDW: red cell distribution width; INR: International Normalized Ratio; SOFA: the Sequential Organ Failure Assessment; SAPSII: the Simplified Acute Physiology Score II; OASIS: the Oxford Acute Severity of Illness Score; BUN: blood urea nitrogen; Cr: creatinine.
3.2. The Association between BUN/Cr and Mortality of Participants with Cerebral Infarction
The variables with statistical difference between the alive group and the dead group were included in the multivariable regression analysis model 1. The results depicted that BUN/Cr (RR = 1.01, 95% CI: 1.01-1.02), respiratory failure (RR = 2.34, 95% CI: 1.90-2.87), malignant cancer (RR = 1.68, 95% CI: 1.27-2.22), anticoagulation (RR = 0.44, 95% CI: 0.33-0.60), liver disease (RR = 1.63, 95% CI: 1.11-2.37), WBC (RR = 1.01, 95% CI: 1.00-1.03), RDW percent (RR = 1.08, 95% CI: 1.03-1.14), glucose (RR = 1.01, 95% CI: 1.01-1.01), bicarbonate (RR = 0.97, 95% CI: 0.95-0.99), and temperature (RR = 0.95, 95% CI: 0.92-0.99) might be associated with the in-hospital mortality of cerebral infarction patients. After adjusting for respiratory failure, malignant cancer, anticoagulation, liver disease, WBC, RDW, glucose, bicarbonate, and temperature, BUN/Cr was associated with increased risk of in-hospital mortality of cerebral infarction patients (RR = 1.01, 95% CI: 1.01-1.02) (Table 2).
Table 2.
The association between BUN/Cr and mortality of participants with cerebral infarction.
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | |
| BUN/CR | 1.01 (1.01-1.02) | 0.045 | 1.01 (1.01-1.02) | 0.025 |
| CHF | 0.98 (0.78-1.24) | 0.884 | ||
| Respiratory failure | 2.34 (1.90-2.87) | <0.001 | 2.50 (2.04-3.05) | <0.001 |
| Renal failure | 1.10 (0.86-1.41) | 0.438 | ||
| Malignant cancer | 1.68 (1.27-2.22) | <0.001 | 1.72 (1.31-2.26) | <0.001 |
| Anticoagulation | 0.44 (0.33-0.60) | <0.001 | 0.46 (0.34-0.61) | <0.001 |
| Liver disease | 1.63 (1.11-2.37) | 0.012 | 1.72 (1.19-2.49) | 0.004 |
| Heart rate | 1.00 (1.00-1.01) | 0.086 | ||
| SBP | 1.00 (1.00-1.01) | 0.259 | ||
| Respiratory rate | 1.02 (1.00-1.03) | 0.088 | ||
| Temperature | 0.95 (0.92-0.99) | 0.007 | 0.95 (0.93-0.97) | <0.001 |
| WBC | 1.01 (1.00-1.03) | 0.023 | 1.01 (1.01-1.03) | 0.023 |
| Hemoglobin | 0.91 (0.76-1.08) | 0.270 | ||
| RDW percent | 1.08 (1.03-1.14) | 0.004 | 1.11 (1.06-1.16) | <0.001 |
| Hematocrit percent | 1.03 (0.97-1.10) | 0.281 | ||
| INR | 0.98 (0.89-1.09) | 0.740 | ||
| Albumin | 0.94 (0.79-1.11) | 0.451 | ||
| Glucose | 1.01 (1.01-1.01) | 0.008 | 1.01 (1.01-1.01) | 0.002 |
| Bicarbonate | 0.97 (0.95-0.99) | 0.033 | 0.96 (0.94-0.99) | 0.001 |
| Sodium | 0.99 (0.98-1.00) | 0.248 | ||
| Potassium | 1.05 (0.93-1.19) | 0.402 | ||
| Charlson comorbidity index | 1.01 (0.98-1.05) | 0.552 | ||
Mode l included variables with statistical difference between the alive group and the dead group. Model 2: multivariable logistic regression analysis adjusting respiratory failure, malignant cancer, anticoagulation, liver disease, temperature, WBC, RDW percent, glucose, and bicarbonate. CHF: congestive heart failure; SBP: systolic blood pressure; WBC: white blood cell; RDW: red cell distribution width; INR: International Normalized Ratio; BUN: blood urea nitrogen; Cr: creatinine.
3.3. The Association between BUN/Cr and Mortality of Patients with Cerebral Infarction in Different Subgroups
According to the data in Table 3, no significant effect of BUN/Cr on the in-hospital mortality was observed in patients aged ≥65 years or <65 years (all P > 0.05). In terms of gender, BUN/Cr was associated 1.02-fold risk of in-hospital mortality in male patients with cerebral infarction in ICU (RR = 1.02, 95% CI: 1.01-1.04), but had no statistical effect on female patients (P > 0.05). BUN/Cr was correlated with the increased risk of in-hospital mortality in cerebral infarction patients with respiratory failure (RR = 1.02, 95% CI: 1.01-1.03) or malignant cancer (RR = 1.03, 95% CI: 1.01-1.05). In cerebral infarction patients that received anticoagulation, the risk of in-hospital morality was increased by 0.01 as one-unit increase of BUN/Cr (RR = 1.01, 95% CI: 1.01-1.02). As in patients without liver disease, BUN/Cr was a risk factor for the in-hospital mortality of cerebral infarction patients in ICU (RR = 1.01, 95% CI: 1.01-1.02).
Table 3.
The association between BUN/Cr and mortality of patients with cerebral infarction in different subgroups.
| Subgroup | Model | |
|---|---|---|
| RR (95% CI) | P | |
| Age | ||
| ≥65 | 1.01 (1.00-1.02) | 0.099 |
| <65 | 1.01 (0.99-1.03) | 0.273 |
| Gender | ||
| Male | 1.02 (1.01-1.04) | 0.023 |
| Female | 1.01 (0.99-1.02) | 0.445 |
| Respiratory failure | ||
| Yes | 1.02 (1.01-1.03) | 0.041 |
| No | 1.01 (0.99-1.03) | 0.286 |
| Malignant cancer | ||
| Yes | 1.03 (1.01-1.05) | 0.032 |
| No | 1.01 (1.00-1.02) | 0.126 |
| Anticoagulation | ||
| Yes | 1.01 (1.01-1.02) | 0.028 |
| No | 1.02 (0.98-1.05) | 0.341 |
| Liver disease | ||
| Yes | 1.02 (0.99-1.05) | 0.242 |
| No | 1.01 (1.01-1.02) | 0.040 |
Multivariable logistic regression analysis adjusting respiratory failure, malignant cancer, anticoagulation, liver disease, temperature, WBC, RDW percent, glucose, and bicarbonate; BUN: blood urea nitrogen; Cr: creatinine.
4. Discussion
In the current study, the effect of BUN/Cr on the in-hospital mortality of cerebral infarction patients in ICU was assessed. The results delineated that BUN/Cr had weak correlation with the increased risk of in-hospital mortality of patients with cerebral infarction in ICU. In addition, the increased risk of in-hospital mortality of cerebral infarction patients was also observed in males and those with respiratory failure, malignant cancer, and without liver disease, as well as those receiving anticoagulation. The findings of our study might highlight the role of BUN/Cr in the prognosis of patients with cerebral infarction, remind the clinicians to be concerned about the BUN/Cr level of patients, and provide some interventions for those with a high level of BUN/Cr.
Herein, we found that BUN/Cr had weak association with the increased risk of in-hospital mortality of patients with cerebral infarction in ICU. BUN/Cr was a composite index of BUN and Cr, and the added value in addition to BUN or Cr was explored before. In a study of Akimoto et al., BUN/Cr was disproportionately increased in patients with cerebral infarction, which might be the contributor for the occurrence of cerebral infarction [18]. Previously, Kim et al. depicted that BUN/Cr was an independent risk factor for venous thromboembolism in patients with acute ischemic stroke [19]. In addition, the increased BUN/Cr ratio was associated with poor outcomes in patients with ischemic stroke [20]. BUN/Cr ≥15 was reported to be an independent risk factor for predicting the long-term outcome of thrombolyzed patients with acute ischemic stroke [21]. The mechanisms underlying the association between BUN/Cr and the in-hospital mortality of cerebral infarction in ICU might be due to that BUN/Cr was an important indicator for dehydration, which was indicated by an increased BUN/Cr ratio and served a clinical maker for deterioration in acute stroke [22]. Dehydration could reduce the brain perfusion and impair the neuroplasticity [23, 24]. Previous studies also indicated that dehydration might increase the event of venous thromboembolism after acute infarction [25]. In addition, BUN/Cr levels were also associated with abnormal inflammation [26], oxidative stress [27], and endothelial dysfunction [28] in patients, which might be other potential mechanisms.
In this study, the association between BUN/Cr and the increased risk of in-hospital mortality of cerebral infarction patients was observed in subgroups including males and those with respiratory failure, malignant cancer, and without liver disease, as well as those receiving anticoagulation. Patients with respiratory failure and malignant cancer might be associated with a higher infection and inflammation status [29, 30]. Anticoagulation was applied for the treatment of venous thrombosis and prophylaxis of postoperative venous thrombosis [31]. Anticoagulation was reported to be an influencing factor of hemorrhagic transformation in nonthrombolysis patients with cerebral infarction [32]. In patients receiving the anticoagulation, abnormal BUN/Cr ratio might be a more reliable indicator associated with the mortality in cerebral infarction patients. For male patients and those with respiratory failure, malignant cancer, and without liver disease, as well as those receiving anticoagulation, BUN/Cr level should be paid some attention. For those with high BUN/Cr levels, appropriate interventions should be provided.
Our study evaluated the role of BUN/Cr on the in-hospital mortality of cerebral infarction patients in ICU. BUN/Cr was identified as an indicator for in-hospital mortality of these patients. BUN and Cr are easily obtained biomarkers and can be widely applied in clinic. We also performed subgroup analyses and found the associations of BUN/Cr with specific cerebral infarction patients. The limitation in the present study was that the data were extracted from MIMIC-III and MIMIC-IV database, and some important variables including the detailed data on the treatments during ICU stay were not included, which might affect the outcomes of patients. In the future, more randomized controlled trials were required to verify the findings of the current study.
5. Conclusion
This study evaluated the association between BUN/Cr and the in-hospital mortality of cerebral infarction patients in ICU and found that BUN/Cr had weak correlation with the increased risk of in-hospital mortality of patients with cerebral infarction in ICU especially in males and those with respiratory failure, malignant cancer, and without liver disease, as well as those receiving anticoagulation. The findings of our study reminded the clinicians to pay attention on the level of BUN/Cr in cerebral infarction patients in ICU.
Acknowledgments
The present study data was based on the MIMIC-III and MIMIC-IV database. We would like to thank all staff and patients involved in the construction of the MIMIC-III and MIMIC-IV database. And the preprint version by doi:10.21203/rs.3.rs-1462345/v1 could be found on Research Square [33].
Abbreviations
- MIMIC:
The Medical Information Mart for Intensive Care
- ICU:
Intensive care unit
- RR:
Risk ratio
- CI:
Confidence interval
- CHF:
Congestive heart failure
- AF:
Atrial fibrillation
- SBP:
Systolic blood pressure
- DBP:
Diastolic blood pressure
- MAP:
Mean arterial pressure
- WBC:
White blood cell
- PLT:
Platelets
- RDW:
Red cell distribution width
- INR:
International Normalized Ratio
- SOFA:
The Sequential Organ Failure Assessment
- SAPSII:
The Simplified Acute Physiology Score II
- OASIS:
The Oxford Acute Severity of Illness Score
- BUN:
Blood urea nitrogen
- Cr:
Creatinine
- ICD-9:
International Classification of Diseases, Ninth Revision (ICD-9).
Contributor Information
Zhi-Wen Zhou, Email: 15409979@qq.com.
Wen-Sheng Zhou, Email: zhouwensheng2004@163.com.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
TC contributed to the conceptualization, literature search, data collection, data curation, data interpretation, data analysis, writing original draft, and writing review and editing. AL contributed to the conceptualization, writing original draft, and writing review and editing. QG contributed to the writing original draft and data curation. LZ contributed to the literature search and data interpretation. YZ contributed to the data collection and data curation. ZZ contributed to the conceptualization, writing review and editing, data curation, and data analysis. WZ contributed to the conceptualization, writing review and editing, data curation, and data interpretation.
Supplementary Materials
Supplementary Table 1: the missing values of all variables and sensitivity analysis before and after manipulation. CHF: congestive heart failure; AF: atrial fibrillation; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; WBC: white blood cell; PLT: platelets; RDW: red cell distribution width; INR: International Normalized Ratio; SOFA: the Sequential Organ Failure Assessment; SAPSII: the Simplified Acute Physiology Score II; OASIS: the Oxford Acute Severity of Illness Score; BUN: blood urea nitrogen; Cr: creatinine. Supplementary Table 2: the data before and after PSM. PSM: propensity score matching. The original data has been uploaded to http://figshare.com. doi:10.6084/m9.figshare.19518868
References
- 1.Sun W., Li G., Zeng X., et al. Clinical and imaging characteristics of cerebral infarction in patients with nonvalvular atrial fibrillation combined with cerebral artery stenosis. Journal of Atherosclerosis and Thrombosis . 2018;25(8):720–732. doi: 10.5551/jat.43240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence E. S., Coshall C., Dundas R., et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke . 2001;32(6):1279–1284. doi: 10.1161/01.STR.32.6.1279. [DOI] [PubMed] [Google Scholar]
- 3.Wen H., Lv M. Correlation analysis between serum procalcitonin and infarct volume in young patients with acute cerebral infarction. Neurological Sciences . 2021;42(8):3189–3196. doi: 10.1007/s10072-020-04856-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou M., Wang H., Zhu J., et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet . 2016;387(10015):251–272. doi: 10.1016/s0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 5.Roth G. A., Johnson C. O., Nguyen G., et al. Methods for estimating the global burden of cerebrovascular diseases. Neuroepidemiology . 2015;45(3):146–151. doi: 10.1159/000441083. [DOI] [PubMed] [Google Scholar]
- 6.Seners P., Baron J. C. Revisiting ‘progressive stroke’: incidence, predictors, pathophysiology, and management of unexplained early neurological deterioration following acute ischemic stroke. Journal of Neurology . 2018;265(1):216–225. doi: 10.1007/s00415-017-8490-3. [DOI] [PubMed] [Google Scholar]
- 7.Johnson C. O., Nguyen M., Roth G. A., et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology . 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T., Xiang L. Elevated plasma haptoglobin level as a potential marker for poor prognosis in acute cerebral infarction. European Neurology . 2018;79(3-4):154–160. doi: 10.1159/000487648. [DOI] [PubMed] [Google Scholar]
- 9.Chang S. W., Huang Y. C., Lin L. C., et al. Effect of dehydration on the development of collaterals in acute middle cerebral artery occlusion. European Journal of Neurology . 2016;23(3):494–500. doi: 10.1111/ene.12841. [DOI] [PubMed] [Google Scholar]
- 10.Tsai Y. H., Yang J. L., Lee I. N., et al. Effects of dehydration on brain perfusion and infarct core after acute middle cerebral artery occlusion in rats: evidence from high-field magnetic resonance imaging. Frontiers in Neurology . 2018;9:p. 786. doi: 10.3389/fneur.2018.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murata A., Kasai T., Matsue Y., et al. Relationship between blood urea nitrogen-to-creatinine ratio at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart and Vessels . 2018;33(8):877–885. doi: 10.1007/s00380-018-1135-3. [DOI] [PubMed] [Google Scholar]
- 12.Gao B., Gu H., Yu W., et al. Admission dehydration is associated with significantly lower in-hospital mortality after intracerebral hemorrhage. Frontiers in Neurology . 2021;12, article 637001 doi: 10.3389/fneur.2021.637001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng L., Wang C., Qiu S., et al. Association between blood urea nitrogen-to-creatinine ratio and three-month outcome in patients with acute ischemic stroke. Current Neurovascular Research . 2019;16(2):166–172. doi: 10.2174/1567202616666190412123705. [DOI] [PubMed] [Google Scholar]
- 14.Qian H., Tang C., Yan G. Predictive value of blood urea nitrogen/creatinine ratio in the long-term prognosis of patients with acute myocardial infarction complicated with acute heart failure. Medicine . 2019;98(11, article e14845) doi: 10.1097/md.0000000000014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng R., Liu K., Li W., et al. Blood urea nitrogen, blood urea nitrogen to creatinine ratio and incident stroke: the Dongfeng-Tongji cohort. Atherosclerosis . 2021;333(333):1–8. doi: 10.1016/j.atherosclerosis.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Johnson A. E., Pollard T. J., Shen L., et al. MIMIC-III, a freely accessible critical care database. Scientific data . 2016;3(1, article 160035) doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao L., Zhou S., Chang P., An S. Effects of ondansetron use on outcomes of acute kidney injury in critically ill patients: an analysis based on the MIMIC-IV database. Journal of Critical Care . 2021;66:117–122. doi: 10.1016/j.jcrc.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Akimoto T., Ito C., Kato M., Ogura M., Muto S., Kusano E. Reduced hydration status characterized by disproportionate elevation of blood urea nitrogen to serum creatinine among the patients with cerebral infarction. Medical Hypotheses . 2011;77(4):601–604. doi: 10.1016/j.mehy.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Kim H., Lee K., Choi H. A., Samuel S., Park J. H., Jo K. W. Elevated blood urea nitrogen/creatinine ratio is associated with venous thromboembolism in patients with acute ischemic stroke. Journal of Korean Neurosurgical Association . 2017;60(6):620–626. doi: 10.3340/jkns.2016.1010.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrock J. W., Glasenapp M., Drogell K. Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clinical Neurology and Neurosurgery . 2012;114(7):881–884. doi: 10.1016/j.clineuro.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Li S. S., Yin M. M., Zhou Z. H., Chen H. S. Dehydration is a strong predictor of long-term prognosis of thrombolysed patients with acute ischemic stroke. Brain and Behavior: A Cognitive Neuroscience Perspective . 2017;7(11, article e00849) doi: 10.1002/brb3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L., Qiu S., Wang C., et al. Effects of the blood urea nitrogen to creatinine ratio on haemorrhagic transformation in AIS patients with diabetes mellitus. BMC Neurology . 2019;19(1):p. 63. doi: 10.1186/s12883-019-1290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Font M. A., Arboix A., Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Current Cardiology Reviews . 2010;6(3):238–244. doi: 10.2174/157340310791658802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillis A. E., Ulatowski J. A., Barker P. B., et al. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovascular Diseases . 2003;16(3):236–246. doi: 10.1159/000071122. [DOI] [PubMed] [Google Scholar]
- 25.Liu X., Feng Y., Zhu X., et al. Serum anion gap at admission predicts all-cause mortality in critically ill patients with cerebral infarction: evidence from the MIMIC-III database. Biomarkers . 2020;25(8):725–732. doi: 10.1080/1354750x.2020.1842497. [DOI] [PubMed] [Google Scholar]
- 26.Giribabu N., Karim K., Kilari E. K., Salleh N. _Phyllanthus niruri_ leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. Journal of Ethnopharmacology . 2017;205:123–137. doi: 10.1016/j.jep.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Vaziri N. D., Dicus M., Ho N. D., Boroujerdi-Rad L., Sindhu R. K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney International . 2003;63(1):179–185. doi: 10.1046/j.1523-1755.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 28.Legrand M., Rossignol P. Cardiovascular consequences of acute kidney injury. The New England Journal of Medicine . 2020;382(23):2238–2247. doi: 10.1056/NEJMra1916393. [DOI] [PubMed] [Google Scholar]
- 29.Boopathi E., Thangavel C. Dark side of cancer therapy: cancer treatment-induced cardiopulmonary inflammation, fibrosis, and immune modulation. International Journal of Molecular Sciences . 2021;22(18):p. 10126. doi: 10.3390/ijms221810126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X., Wang H., Xie G., Lin S., Ji C. Increased systemic immune-inflammation index can predict respiratory failure in patients with Guillain-Barré syndrome. Neurological Sciences . 2022;43(2):1223–1231. doi: 10.1007/s10072-021-05420-x. [DOI] [PubMed] [Google Scholar]
- 31.Patel S., Hossain M. A., Ajam F., et al. Dabigatran-induced acute interstitial nephritis: an important complication of newer oral anticoagulation agents. Journal of Clinical Medical Research . 2018;10(10):791–794. doi: 10.14740/jocmr3569w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiao Y., Li G., Xing Y., Nie D., Liu X. Influencing factors of hemorrhagic transformation in non-thrombolysis patients with cerebral infarction. Clinical Neurology and Neurosurgery . 2019;181:68–72. doi: 10.1016/j.clineuro.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Chen T., Li A. P., Gong Q., et al. The association of blood urea nitrogen to creatinine ratio and the prognosis of critically ill patients with cerebral infarction: a cohort study. 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: the missing values of all variables and sensitivity analysis before and after manipulation. CHF: congestive heart failure; AF: atrial fibrillation; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; WBC: white blood cell; PLT: platelets; RDW: red cell distribution width; INR: International Normalized Ratio; SOFA: the Sequential Organ Failure Assessment; SAPSII: the Simplified Acute Physiology Score II; OASIS: the Oxford Acute Severity of Illness Score; BUN: blood urea nitrogen; Cr: creatinine. Supplementary Table 2: the data before and after PSM. PSM: propensity score matching. The original data has been uploaded to http://figshare.com. doi:10.6084/m9.figshare.19518868
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


