Abstract
Malaria is a life-threatening disease, and Africa is still one of the most affected endemic regions despite years of policy to limit infection and transmission rates. Further, studies into the variable efficacy of the vaccine are needed to provide a better understanding of protective immunity. Thus, the current study is designed to delineate the effect of each dose of vaccine on the transcriptional profiles of subjects to determine its efficacy and understand the molecular mechanisms underlying the protection this vaccine provides. Here, we used gene expression profiles of pre and post-vaccination patients after various doses of RTS,S based on samples collected from the Gene Expression Omnibus datasets. Subsequently, differential gene expression analysis using edgeR revealed the significantly (false discovery rate < 0.005) 158 downregulated and 61 upregulated genes between control vs. controlled human malaria infection samples. Further, enrichment analysis of significant genes delineated the involvement of CCL8, CXCL10, CXCL11, XCR1, CSF3, IFNB1, IFNE, IL12B, IL22, IL6, IL27, etc., genes which found to be upregulated after earlier doses but downregulated after the 3rd dose in cytokine-chemokine pathways. Notably, we identified 13 cytokine genes whose expression significantly varied during three doses. Eventually, these findings give insight into the dual role of cytokine responses in malaria pathogenesis. The variations in their expression patterns after various doses of vaccination are linked to the protection as it decreases the severe inflammatory effects in malaria patients. This study will be helpful in designing a better vaccine against malaria and understanding the functions of cytokine response as well.
Keywords: antibody production, cytokine, gene expression, inflammation, malaria, RNA-seq
Introduction
Malaria remains a well-known and life-threatening disease in many tropical and subtropical countries. Currently, there are near 100 countries and territories where the risk of malaria transmission is present [1]. These countries are visited by more than 125 million international travelers every year [2]. Moreover, Africa has faced 94% of all malaria cases in 2019. There were almost 229 million estimated malaria cases worldwide in the same year. And the number of deaths from malaria stood at more than 400,000. Malaria is known to be caused by Plasmodium parasites. These parasites can infect female Anopheles mosquitoes and spread to people through the biting from these mosquitoes. Among the five parasite species that cause malaria in humans, two species—Plasmodium falciparum and P. vivax bear the highest threat. P. falciparum also accounted for almost 99.7% of malaria cases in the African region and nearly half of World Health Organization South-East Asia Region cases [3].
Most malaria deaths occur in children, and they are dominated by three syndromes: severe anemia, cerebral malaria, and respiratory distress. These syndromes can occur separately, or in combination [1]. One of the elementary features of P. falciparum is the induction of host inflammatory responses that contribute to disease severity and are associated with lethal outcomes [4]. Specifically, systemic levels of some pro-inflammatory cytokines are correlated with severity and death from malaria [5].
Even though the disease appears in documented reports as early as 2700 B.C., malaria vaccine development entered a new milestone only in 2015 [6,7]. The European Medicines Agency positively reviewed the pre-erythrocytic P. falciparum candidate RTS,S vaccine and marked the first human anti-parasite vaccine to pass the regulatory examination [6]. This vaccine provided protection against infection in controlled human malaria infection (CHMI) studies [8-10]. It can also prevent life-threatening malaria and reduce the need for transfusion of blood [11].
Although malaria has been studied in detail, insufficient attention has been paid to how malaria vaccination is associated with several gene expression changes, contributing to increased protection. The efficacy of the malaria vaccine is still not at a desirable level and needs improvement if we want to eradicate malaria [12]. In the current study, we focused on how the various doses of malaria RTS,S/AS01 vaccine facilitate protection and gene expression changes. Though we discussed multiple doses, we were primarily focused on a dataset from the 3rd dose. It could give us a better overview of gene expression changes as delayed doses are found to increase the chance of protection against malaria [13]. RTS,S/AS01 vaccination has been significantly associated with upregulation and downregulation in several gene expressions [14]. Our study would help us conclude how several gene upregulation and downregulation after the vaccination is different from dose to dose and how cytokine's dual role in protection and pathogenicity in malaria is crucial to investigate. Moreover, it will investigate how the absence of negative feedback control in pathophysiologic situations is responsible for impairing cytokine network homeostasis and contribute to local pathogenesis [15]. Overall, this will result in designing a better-performing vaccine against malaria.
Methods
Fig. 1 illustrates the workflow of the research and the methods we used to obtain the results.
Fig. 1.
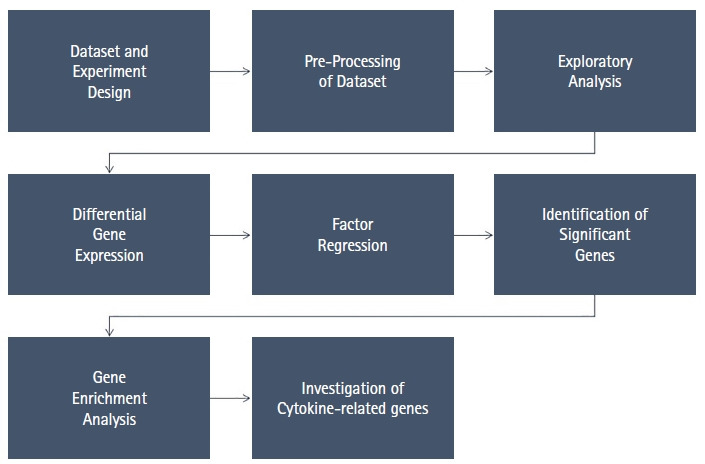
Workflow of the research process.
Dataset and experimental design
Two datasets were obtained from Gene Expression Omnibus with accession numbers GSE102288 and GSE89292. These datasets were published as a BioProject on the National Center for Biotechnology Information with the accession number PRJNA397222 and PRJNA351258. The total number of samples was 275 in GSE102288 and 583 in GSE89292 [16,17].
GSE102288 dataset is obtained from a study at the Center for Infectious Disease Research in the United States. The original research paper by Du et al. [17] demonstrates how the transcript ratio MX2/GPR183, measured 1 day after the 3rd immunization, can differentiate between protected and non-protected individuals. This ratiometric signature can help identify RTS,S/AS01 immunized people with protective immunity suggesting a role for interferon in the RTS,S mode of action.
GSE89292 dataset is obtained from a study at The Jackson Laboratory for Genomic Medicine. The original paper by Kazmin et al. [16] is an analysis of protective immune responses to RTS,S malaria vaccination in humans. The study demonstrated that several peripheral blood mononuclear cells (PBMC) and the circumsporozoite protein (CSP)‒specific antibody titers were highly correlated to protection after vaccination.
Both datasets were necessary for the analysis because GSE102288 did not provide first and second-dose vaccination information but focused on the changes after the 3rd dose and CHMI. On the other hand, GSE89292 dataset provided.
An overview of the datasets is given in Table 1.
Table 1.
Original Dataset source, design, analysis method and sample number
| Dataset | Organism | Experiment type | Sample source | Analysis method | Sample no. |
|---|---|---|---|---|---|
| GSE102288 | Homo sapiens | Expression profiling by high throughput sequencing | PBMC samples | RNA-seq analysis | 275 |
| GSE89292 | Homo sapiens | Expression profiling by array | PBMC samples | RNA-seq analysis | 583 |
PBMC, peripheral blood mononuclear cell; RNA-seq, RNA-sequencing.
Datasets for the identification of differentially expressed genes
We partitioned datasets for two comparative analyses: The GSE102288 dataset was analyzed to compare control vs. CHMI samples, and GSE89292 was analyzed to identify gene expression patterns after the first two doses. Illumina HiSeq 2000 was the used platform (Illumina, San Diego, CA, USA) for GSE102288, and Affymetrix Human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA, USA) was used in GSE89292. All datasets were curated so that only human tissue samples remained in the dataset. Furthermore, Probe ID mapped to gene symbols in the GSE89292 dataset was extracted from the respective platform file. Finally, dataset matrices were prepared for various analyses.
For GSE89292, participants were vaccinated at 28-day intervals. They were subjected to controlled malaria infection 21 days after the final immunization. The CHMI challenge was then administered through five bites by mimicking a natural infection. Parasitemia was monitored for 28 days, and overall monitoring continued for 159 days following the challenge.
For GSE102288, volunteers received either three full doses of RTS,S/AS01 vaccines, or two full doses followed by a delayed fractional third dose. After 3 weeks of the last dose, all the volunteers underwent CHMI. Later on, PBMC samples were collected and analyzed on the day of the 1st vaccination, day of the 3rd vaccination, day 1, 3, 14 post-the 3rd vaccination and day of challenge (CHMI).
Pre-processing of datasets
The GSE102288 dataset contains the FPKM (fragments per kilobase million) value for 15,680 genes. On the other hand, GSE89292 is a microarray data containing RMA normalized value. In the case of the Illumina dataset (GSE102288), FPKM values are converted into log2 values using the T-Bioinfo Server pipeline (Supplementary Fig. 1). In the case of Affymetrix datasets (GSE 89292), the average of multiple probes was computed that correspond to a single gene using the average function in Excel. Ensembl transcript IDs were mapped to the gene symbols using the T-Bioinfo Server's annotation pipeline.
Exploratory analysis
In order to explore the patterns of the data, principal component analysis (PCA) was performed using the T-Bioinfo Server (https://server.t-bio.info/) on all the 275 samples based on the gene expression profiles of the samples. Besides, PCA and Hierarchical Clustering (distance: Euclidean, linkage: ward.D2) were also performed with only significant genes to assess their discriminant potential using the T-Bioinfo Server [18].
Differential gene expression analysis
We performed differential gene expression analysis using the EdgeR [19] tool integrated on the T-Bioinfo Server to select genes that were significantly differentially expressed in pre-vaccination (control) vs. day of challenge (CHMI) samples (Fig. 2A). The T-Bioinfo Server was used for this purpose. Furthermore, p-value and log2 fold change values obtained from edgeR results were used to generate a volcano plot (Fig. 3) for control vs. CHMI samples. Eventually, a heatmap was generated for the top 50 protein-coding upregulated and downregulated genes [20]. Only those genes were considered as significant genes with the false discovery rate (FDR) < 0.005 and log2 fold change at > ±1. We applied this threshold for both datasets.
Fig. 2.
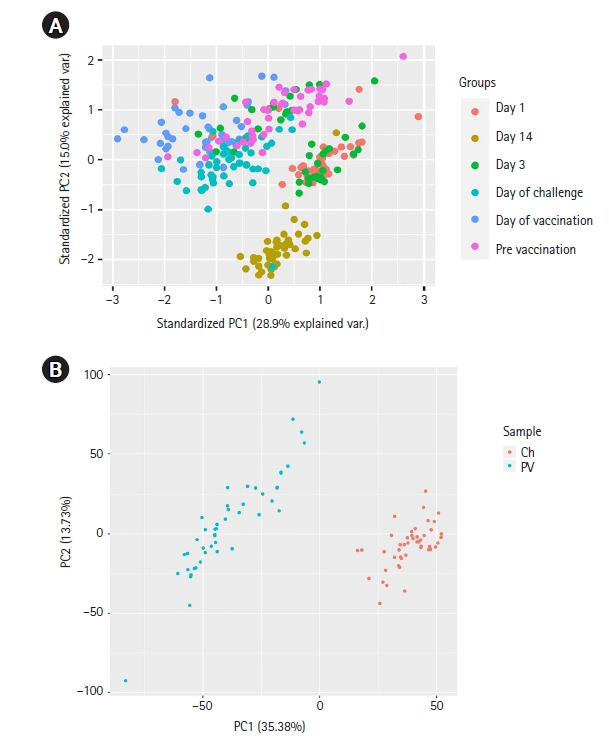
(A) Principal component analysis (PCA) plot for all sample groups in the complete dataset. (B) PCA plot for control vs. controlled human malaria infection samples. PC1, principal component 1; PC2, Principal component 2; Ch, day of challenge; PV, pre-vaccination.
Fig. 3.
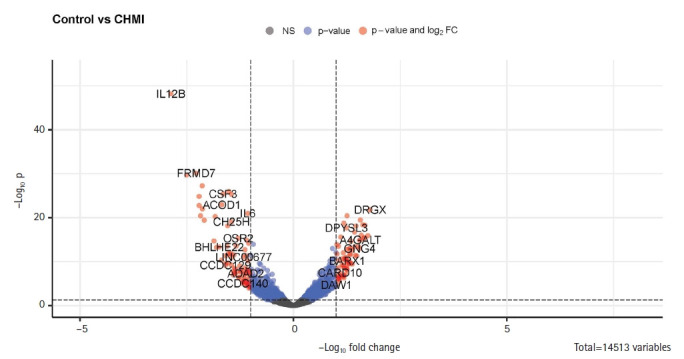
Enhanced volcano plot for control and controlled human malaria infection (CHMI) samples for all annotated genes in R. FC, fold change.
Factor regression analysis
Next, we conducted a factor analysis to interpret the relationship between multiple variables and the malaria vaccine efficacy (Supplementary Fig. 2). Correlation between sex status, protection status, and gene expression was investigated. Our study also helped us determine how other factors are involved in protection status and which genes are significantly expressed in protected samples [21].
Gene enrichment analysis
To understand how the significant genes are related to protection, we assessed the biological and molecular functions of the significant genes with the help of annotation. We used Database for Annotation, Visualization, and Integrated Discovery (DAVID) for annotation [22]. Additionally, we used the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Bioplanet for pathway analysis [23,24]. Literature search was done for the top significant genes, and cytokine or inflammation-associated genes provided a better understanding.
Results
Detection and visualization of variation in data
The exploratory data analysis based on the PCA plot revealed the separate clusters for various sample groups. Principal component 1 (PC1) represents 28.9% variance and PC2 represents 15.0% variance of the data (Fig. 2A). The figure illustrates how various samples fall in different clusters and overlaps as well. Interestingly, here, we observed day 14 samples forming a distinct cluster. Day 1 and day 3 post 3rd vaccination samples remain together in a cluster as they indicate the early expression of genes after the dose. The CHMI (day of the challenge) and control (pre-vaccination) samples also form distinct clusters. So, it is most likely that the gene expression pattern after malaria vaccine doses can indicate how the patient responds to the vaccine. However, the CHMI and pre-vaccination samples do not form distinct clusters like others. While two-thirds of the pre-vaccination samples were taken more than 7 months before CHMI samples, the other one-third was taken 2 months prior. This long gap between samples for these two groups, unlike other groups, might be the reason for not forming distinct clusters. Furthermore, as CHMI occurs more than two months after the first vaccination, the body's immune system was already stronger. It might have contributed to not forming distinct clusters too [25]. Finally, PC1 represents 35.38% variance, and PC2 represents 13.73% variance of the data in control vs. CHMI samples only and falls into different clusters (Fig. 2B).
Identification of differentially expressed significant gene
Since the PCA plot showed clear, distinct clusters for control and CHMI samples, we next identified 219 significantly differentially expressed genes between control and CHMI samples based on EdgeR. Among them, 158 genes were found to be downregulated, and 61 genes were found to be upregulated in control vs. CHMI samples. Further, we generated a volcano plot that included all the genes (Fig. 3). The volcano plot represents significant genes between control and CHMI groups. Furthermore, to identify a manageable subset of genes, we selected only the top 50 differentially expressed genes, including the top 25 upregulated and top 25 downregulated in control vs. CHMI samples (Table 2). Notably, here we have selected only protein-coding and excluded the non-protein-coding genes. However, non-coding RNA genes have been found to help evade human immune attacks by switching expression between variants of var family genes and increasing the severity of malaria infection. but not in inflammation and protection [26,27]. Since our study primarily focused on the human inflammatory gene expression and its association with protection or elevated severity of malaria infection; therefore, we excluded the non-coding genes.
Table 2.
Top 50 significantly expressed protein-coding genes (p < 0.05, FDR < 0.005, and log2 FC > ±1)
| Downregulated genes |
Upregulated genes |
||||
|---|---|---|---|---|---|
| Gene symbol | logFC | FDR | Gene symbol | logFC | FDR |
| IL12B | –2.87295 | 9.85E–45 | DRGX | 1.798081 | 1.35E–19 |
| FRMD7 | –2.27791 | 4.06E–27 | CHST3 | 1.743642 | 4.84E–14 |
| IFNB1 | –2.20904 | 2.05E–22 | KIAA1644 | 1.674141 | 2.76E–16 |
| IL36G | –2.1776 | 2.89E–18 | CACNA1G | 1.669566 | 1.44E–13 |
| CXCL11 | –2.13596 | 1.78E–24 | TNFAIP8L3 | 1.65029 | 1.80E–13 |
| CCL8 | –2.13175 | 9.23E–20 | NECTIN4 | 1.627141 | 2.36E–16 |
| RANBP3L | –1.86129 | 6.74E–13 | RHBDL3 | 1.590967 | 4.04E–14 |
| IL22 | –1.80012 | 1.40E–11 | SMAD6 | 1.567576 | 2.30E–17 |
| BHLHE22 | –1.73922 | 1.23E–11 | A4GALT | 1.565569 | 4.01E–13 |
| ACOD1 | –1.67863 | 1.28E–20 | GNG4 | 1.553002 | 2.61E–11 |
| CSF3 | –1.64862 | 6.61E–23 | HR | 1.550488 | 4.74E–13 |
| CCDC129 | –1.59496 | 7.71E–08 | MAFA | 1.526466 | 9.91E–12 |
| UNC5C | –1.589 | 8.93E–10 | LMX1B | 1.483051 | 9.46E–10 |
| IFNE | –1.58164 | 3.03E–08 | SEMA3F | 1.466303 | 3.83E–16 |
| C1QTNF1 | –1.55235 | 8.45E–12 | CBARP | 1.452397 | 6.85E–10 |
| PLA1A | –1.539 | 3.60E–16 | HOXB6 | 1.435488 | 1.08E–11 |
| GXYLT2 | –1.52936 | 3.14E–10 | RASAL1 | 1.431945 | 8.37E–15 |
| HSD11B1 | –1.52447 | 2.82E–08 | UBE2QL1 | 1.383004 | 4.92E–11 |
| CXCL10 | –1.51384 | 2.10E–23 | CA12 | 1.378161 | 4.43E–08 |
| RHCG | –1.48479 | 2.99E–10 | NCMAP | 1.351544 | 3.31E–08 |
| KCNJ2 | –1.45138 | 7.38E–23 | FAM124A | 1.34904 | 7.86E–13 |
| SLC6A7 | –1.45109 | 6.99E–08 | MMP2 | 1.333238 | 2.41E–11 |
| CH25H | –1.45018 | 4.82E–17 | GAD1 | 1.325456 | 3.36E–10 |
| OR52W1 | –1.40885 | 1.71E–06 | TUBB3 | 1.324794 | 5.41E–10 |
| IL27 | –1.39053 | 5.41E–10 | RGMA | 1.307805 | 2.88E–11 |
FDR, false discovery rate; FC, fold change.
PCA plot based on only significant genes
Next, the PCA plot based on only significant genes between control and CHMI shows a clear distinction among the samples of these groups. PC1 represents 78.74% variance, and PC2 represents 3.64% variance of the data (Supplementary Fig. 3).
Hierarchical clustering and Heatmap for top 50 genes show clear distinctive pattern
Among the significantly expressed genes after the 1st and 2nd dose of vaccination, we identified the same cytokine and inflammation-related genes that were also significantly expressed after the 3rd dose. In order to derive their correlation and visualize the expression pattern, we plotted a line chart (Fig. 4A and 4B). After giving the first dose, there is upregulation of the inflammatory genes and both up and downregulation after the second dose (Fig. 4A). Interestingly, significant downregulation occurs to those same genes after applying the third vaccination dose (Fig. 4B). The gene expression pattern changed through various doses of vaccination. Next, to assess the capability of the top 50 significant genes in distinguishing control and CHMI samples, we performed H-Clustering with this set of genes. The dendrogram represents the two clear, distinct clusters of control and CHMI samples (Fig. 5B). Heatmap represents the expression pattern of the top 50 top genes between control vs. CHMI samples (Fig. 5A), illustrating variation in expression.
Fig. 4.
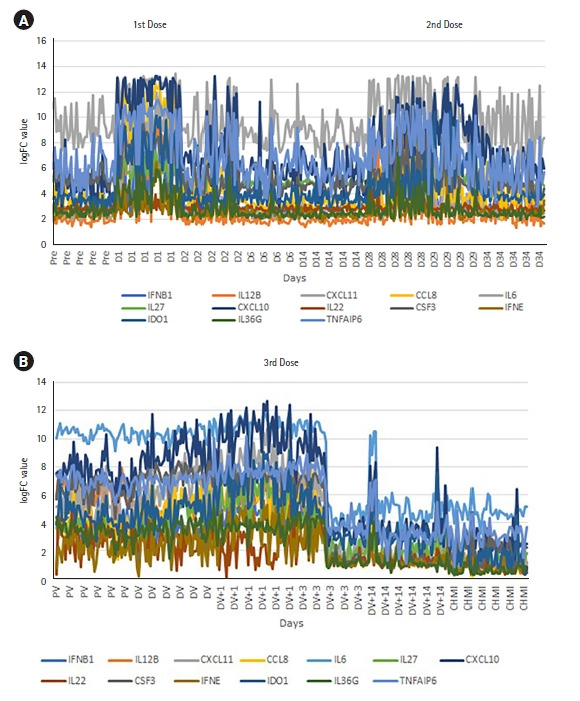
Inflammatory or cytokine gene expression pattern in 1st and 2nd dose (A) and 3rd dose (B) distinct upregulation or downregulation. Pre/PV, pre-vaccination; D1, day of the first dose; D2/D6/D14/D28/D29/D34, 2/6/14/28/29/34 days after first vaccination where the second dose is given on 28th day after the first dose; DV, day of 3rd dose of vaccination; DV+1/DV+3/DV+14, 1/3/14 days after the 3rd dose; CHMI, controlled human malaria infection; FC, fold change.
Fig. 5.
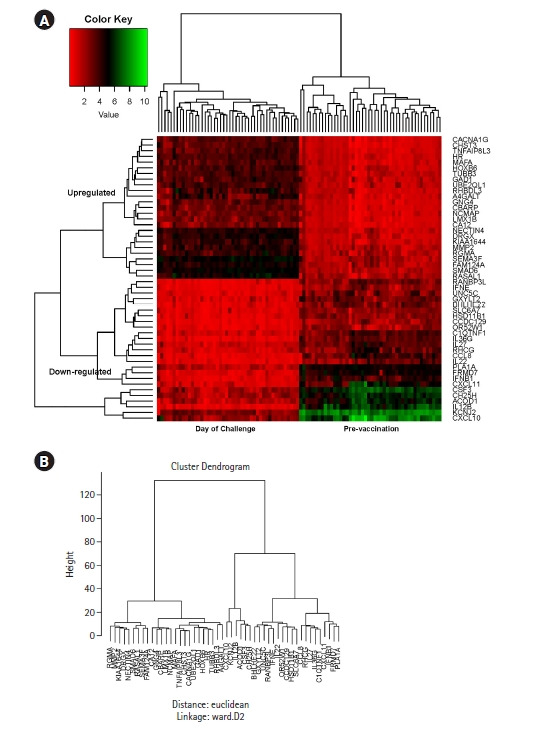
Heatmap (A) and dendrogram (B) generated by Hierarchical clustering based on top 50 significantly differentially expressed genes, illustrating the distinct clusters of control and controlled human malaria infection day samples.
Expression of inflammatory cytokine genes in all doses
Cytokines are the key players of the immune system. Next, to understand the expression pattern of the cytokines during different doses, we investigated the expression pattern for significantly expressed genes with cytokine G.O. function in control vs. CHMI samples. We identified 13 significantly differentially expressed cytokine genes that were expressed after all three doses. These cytokine genes showed a distinctive expression pattern after different doses (Fig. 4A and 4B).
Correlational with other factors of interests
Next, we wanted to see whether the gender of the samples is a significant factor in gene expression regulation. Here, we identified 10 protein-coding genes that were indeed significantly associated with the gender (males and females) on control vs. CHMI comparison (Supplementary Table 1). A PCA plot was also generated to visualize the differences (Supplementary Fig. 4). We further looked for evidence if gender was correlated with protection. No significant genes were found to provide any evidence of gender to be related to protection. Subsequently, we identified13 coding genes significantly differentially expressed in protected vs. non-protected samples on the 3rd vaccination day vs. CHMI samples (Supplementary Table 2). The exploratory data analysis failed to show enough differences between samples while comparing 3rd vaccination vs. CHMI samples, but further analysis is necessary for identifying the importance of the significantly expressed genes after 3rd dose on protected vs. non-protected samples.
Gene set enrichment analysis
As malaria severity is often associated with the overexpression of inflammatory genes, their expression patterns and changes were a major point of interest in our study as we wanted to find out how vaccination doses affect them.
Gene set enrichment analysis was performed for 158 upregulated, 61 downregulated significant genes in CHMI vs. control samples of the 3rd dose on DAVID. Also, functional annotation and clustering for these significantly expressed genes were performed. Top hits with the downregulated genes indicate the enrichment in gene ontology terms associated with inflammatory response, cellular response to lipopolysaccharide, cytokine, chemokine mediated signaling pathway, cell-cell signaling, positive regulation of leukocyte chemotaxis, etc. Functional clustering showed nine clusters with 83 DAVID IDs. The top three clusters were all involved in cytokine activity and had enrichment scores of 4.42, 3.19, and 3.15, respectively. These enrichment scores are measured by the geometric mean of the EASE Scores (modified Fisher Exact) [28]. Here, a higher score for a group is an indication of their more critical (enriched) roles [29]. Similarly, gene enrichment analysis with the upregulated genes for CHMI vs. control showed the gene ontology enriched terms were associated with intramembranous ossification, negative-regulation of calcium ion-dependent exocytosis, epidermis development, angiogenesis, chemical synaptic transmission, positive regulation of calcium ion-dependent exocytosis, cell-cell signaling, etc. Also, functional clustering indicated nine clusters with 68 DAVID IDs, where top ones were associated with glycoprotein, glycosylation site: N linked, and pathways in cancer. Enrichment scores were 1.88, 1.62, and 1.39 for the top three clusters. We also analyzed a dataset from the 1st dose, which showed association with inflammatory response, cytokine, and chemotaxis. Interestingly, CCL7, CXCL1, CXCL11, etc. cytokine genes were upregulated in this case whether they were downregulated after the 3rd dose.
Besides, gene enrichment analysis of significantly differentially expressed genes in protected vs. non-protected showed the enrichment in gene ontology terms, including cell-matrix adhesion and association of signal and glycoprotein [30].
KEGG pathway analysis
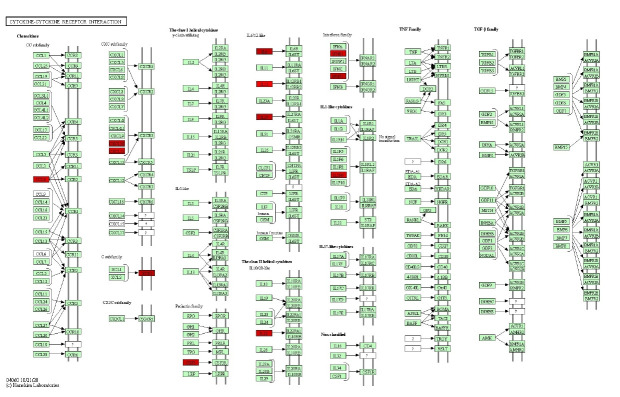
KEGG pathway analysis revealed genes, such as CCL8, CXCL10, CXCL11, XCR1, CSF3, IFNB1, IFNE, IL12B, IL22, IL6, IL36G, IL27, etc. were involved in cytokine-cytokine receptor interaction (Figure 6). Moreover, CSFE, IFNB1, IFNE, IL12B, IL22, IL6 genes were associated with the JAK-STAT signaling pathway (Supplementary Fig. 5).
Fig. 6.
Kyoto Encyclopedia of Genes and Genomes cytokine-cytokine receptor interaction pathway involving significant genes in control and controlled human malaria infection samples.
On the other hand, the upregulated genes, such as GNG12, GNG4, WNT9A, EDNRB, FGF18, LAMA3, MMP2, etc., were found in pathways in cancer (Supplementary Fig. 6).
Discussion
There are two main determinants of severe malaria: sequestration of parasitized red blood cell and surge of pro-inflammatory response [31]. Imbalanced pro and anti-inflammatory immune responses have been found to trigger immune-induced pathology and remain one of the leading causes of cerebral malaria pathogenesis, which might be further amplified by sequestration [32]. Moreover, systemic cytokine levels are correlated with disease severity in malaria as well as sepsis [33]. Thus, it is necessary to examine how multiple vaccination doses change the pattern of inflammatory responses and induce protection upon challenge. Detailed studies in this regard can help increase the efficacy of the vaccine and might be implied to other vaccinations as well. Here, anti-CSP titer levels were analyzed to identify RTS,S/AS01 immunized people who developed protective immunity. It also suggested a role for interferon signaling in the RTS,S mode of action [17].
Towards this end, in the current study, we investigated transcriptomics profiles of pre-and post-vaccination patients after the 1st, 2nd, and 3rd vaccination dose of RTS,S using various bioinformatics techniques, i.e., PCA, differential gene expression analysis, Hierarchical clustering, etc. Exploratory data analysis based on the PCA clearly shows distinct clusters of samples of control and CHMI. Subsequently, differential gene expression analysis using the edgeR scrutinized 219 significantly differentially expressed genes (FDR < 0.005). Eventually, the biological role of significant genes was delineated using gene enrichment analysis.; which reveals the regulation status of chemokines and cytokines in pre-vaccinated and post-vaccinated samples at different doses. Gene enrichment analysis showed that CCL8, CXCL10, CXCL11, XCR1, CSF3, IFNB1, IFNE, IL12B, IL22, IL6, and IL27 were involved in cytokine-chemokine pathways and upregulated after earlier doses but downregulated after the 3rd dose. Analysis of downregulated genes in DAVID and KEGG pathway illustrated how significantly downregulated genes after 3rd vaccination on CHMI is primarily associated with cytokine and inflammation. Overexpression of those inflammation and cytokine gene has been one of the driving forces of death and contribute to pathogenicity [34]. In fact, chemoattractant cytokines or chemokines have proven to be regulators of leukocyte trafficking and potentially contribute to severe malaria [31,35]. Our analysis suggested repeated doses of malaria vaccination help in protection because it is associated with the balanced expression of pro and anti-inflammatory cytokines. SYT4, CBARP, NCS1, CACN1G, RHBDL3, CBARP, etc., significant genes in our analysis have shown functions for calcium ion receptors, calcium gated ion channels, or calcium ion-dependent exocytosis according to gene ontology studies [36-39]. It has been observed that antibody levels to the voltage-gated calcium channels, but not to other ion channels, increase with the severity of malaria infection [40].
Significantly elevated pro-inflammatory IL-6, IL-12 have been observed in the severe-malaria group compared to age-matched healthy children [41]. Also, IL-12 has been found to induce IFN-γ, a key mediator of inflammatory immune responses [42]. IL-12 has shown evidence to play an essential role in the pathogenesis of malaria [42]. Furthermore, levels of IL-6 are elevated in malaria disease and contribute to disease severity [43-45]. Moreover, IFNB1-regulated genes were observed in severe cerebral malaria [46]. IL-12B was the most downregulated gene after the 3rd dose in our study. Furthermore, TNF, IL-1, IL-6, etc., inflammatory genes are over-expressed in falciparum malaria [43,47]. These genes are associated with cardiac insufficiency and myocardial dysfunction [48-50]. Additionally, inflammatory/inducible chemokines CXCL10, CXCL11, and CCL8 suggest involvement in response to the malaria infection [51]. Relative involvement of CXCL10 and CXCL11 has been found to recruit inflammatory leukocytes of malaria-infected mice [31]. Again, studies have indicated that the concentration of CXCL11 was greater in symptomatic than asymptomatic malaria and was upregulated among the fever-positive groups, which identified CXCL11 as a possible biomarker for malarial fever [52]. The activity and capacity of cytokines through directing sequestration and driving anemia restrict oxygen supply to mitochondria and make falciparum malaria primarily a cytokine-driven inflammatory disease [34,52].
Surprisingly, one of the pathways involved with the significant genes in control vs. CHMI samples was the cancer pathway. In Africa, malaria is known to influence genetic variation at several loci in the human genome [53], which might be involved in cancer and impact the biology and epidemiology of both diseases [54]. Moreover, recent evidence suggested that inflammatory cytokines might implicate several cancers [55]. Additionally, cancer–malaria interactions have been reported in the human liver, where malaria parasites attack its life cycle [54]. p53 protein has been observed to play a crucial role in hepatocyte infection by malaria parasite sporozoites. p53 is also the most highly mutated gene in several cancers [55]. This area needs further investigation for better understanding as there is an excellent potential for new novel anti-cancer therapies using anti-malarial drugs [56]. Malaria vaccination can bring new scopes for cancer research and control proto-oncogenes' abnormal expression, thus reducing the risk of cancers [57].
Further, many critical events in the Plasmodium life cycle are regulated by changes in the cellular levels of Ca2+ [58]. Moreover, levels of antibodies to the voltage-gated calcium channels correlate with the increased severity of malaria infection [40]. SYT4, CBARP, NCS1, CACN1G, RHBDL3, CBARP, etc., significant genes in our analysis have shown functions for calcium ion receptors, calcium gated ion channels, or calcium ion-dependent exocytosis according to literature study or gene ontology [36-39]. It has been observed that antibody levels to the voltage-gated calcium channels, but not to other ion channels, increase with the severity of malaria infection [40]. Moreover, P. falciparum protein PfRH1is found to trigger the release of calcium ions. Extensive involvement of calcium signaling has been observed in various crucial pathways of the parasite. Therefore, any interruption would be deleterious for invasion and, ultimately, the growth of the malaria parasite. So, components of calcium signaling are considered for therapeutic interventions [59].
The malaria RTS,S/AS01 vaccine uses the CSP protein as a target antigen against malaria [60]. In earlier stages of vaccine development, higher concentrations of antibodies against CSP were observed on the day of the challenge [61]. Moreover, the control group without vaccination developed malaria earlier than the test group with three doses of vaccination, in which a strong humoral response against CSP was shown [62]. After the initial dose, there are low titers of antibodies against CSP protein. But after later doses, high antibody titers are present, and antibody feedback can further block immunodominant response [56]. Although inflammatory mediators have been repeatedly found to be implicated in the severity of the disease, this evidence gave rise to the widely held belief that severe malaria might be an immune-mediated disease [5,63]. Also, parallels between sepsis and malaria are associated with functionally crucial inflammatory cytokines present in both conditions [34]. Malaria-induced sepsis is related to an intense pro-inflammatory cytokinemia, though the mechanisms behind it are poorly understood [64]. Additionally, a critical illness associated with an inflammatory response is a cause of multifactorial anemia [65]. Anemia could contribute to poor oxygenation of tissues in malaria patients [66].
One of the adverse side effects of this is the overexpression of inflammatory genes, which often have the role of a double-edged sword [67]. As our study suggested, cell-mediated cytokines are less expressed after the 3rd dose, which was not the case after the 1st or 2nd dose, confirming that multiple doses of malaria RTS,S vaccination are crucial in gene expression and control of inflammation in malaria infection. Downregulation of cytokine and inflammation-related genes helps decrease the negative side effects of cytokine storms in malaria as excessive pro-inflammation increases the severity of malaria [34]. Therefore, cytokine overexpression as a part of humoral immunity was reduced after repeated vaccination doses, resulting in protection by reducing the complexities of malaria infection. Additionally, a fractional booster dose initiates high protection upon challenge by increasing antibody somatic hypermutation [13]. Moreover, exploratory data analysis showed evidence that protected-day 14 samples were distinct from other groups, which needs further investigation for early detection of malaria vaccination efficacy before the challenge (Supplementary Fig. 7). Our study also suggests the necessity to further explore passive immunization with monoclonal antibodies as a new approach to prevent and eliminate malaria [47]. As cytokines are associated with various inflammatory diseases, the study of malaria vaccination's control over inflammatory cytokine gene expression might become helpful in other diseases too, where they play a significant role in pathogenesis [68].
This study was able to identify 13 inflammatory genes whose expression in malaria vaccination played a significant role in the cytokine-cytokine receptor interaction pathway, JAK-STAT signaling pathway, and pathways in cancer. Furthermore, we demonstrated a comparatively less focused protection mechanism after vaccination and discussed the gene expression pattern of various vaccination doses. We analyzed the dual role of protection and pathogenicity of cytokines in malaria infection and how multiple doses of vaccination increase protection by influencing these cytokine levels and producing antibodies against the malaria CSP antigen.
Acknowledgments
We thankfully acknowledge Pine Biotech for helping us with their efforts on this project. The groundwork of this project was completed during the Omics Logic Internship Program. The processing and visualization pipelines were generated with the T-BioInfo Server.
Footnotes
Authors’ Contribution
Conceptualization: SD, HK. Data curation: SD. Formal analysis: SD, HK. Funding acquisition: HK, EB, MM. Methodology: SD, HK. Writing - original draft: SD. Writing - review & editing: SD, HK, EB, MM.
Conflicts of Interest
Authors were employed by the company Pine Biotech. However, there was no financial or any other interest present in this research project.
Supplementary Materials
Supplementary data can be found with this article online at http://www.genominfo.org.
Significantly expressed genes in male and female on control vs. controlled human malaria infection samples
Significantly expressed genes in protected and non-protected groups on control vs. controlled human malaria infection samples
Differential gene expression analysis using edgeR Pipeline.
Factor regression pipeline.
Principal component analysis plot for control vs. controlled human malaria infection samples for significant genes.
Significantly expressed genes in males and females on control vs. controlled human malaria infection samples.
JAK-STAT pathway.
Pathways in cancer.
Principal component analysis plot of protected and non-protected samples on various days.
Quantile normalization pipeline.
References
- 1.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 2.World Health Oragnization. WHO International Travel and Health publication 2014, chapter 7: Malaria. Geneva: World Health Oragniazation; 2014. [Google Scholar]
- 3.World Health Oragnization. World malaria report 2019. Geneva: World Health Oragniazation; 2019. [Google Scholar]
- 4.Wassmer SC, Taylor TE, Rathod PK, Mishra SK, Mohanty S, Arevalo-Herrera M, et al. Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am J Trop Med Hyg. 2015;93:42–56. doi: 10.4269/ajtmh.14-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatkowski D, Hill AV, Sambou I, Twumasi P, Castracane J, Manogue KR, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 6.Duffy PE, Patrick Gorres J. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. 2020;5:48. doi: 10.1038/s41541-020-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millet P. Current status and prospects of malaria vaccines. J Travel Med. 1995;2:96–98. doi: 10.1111/j.1708-8305.1995.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 8.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 9.Ockenhouse CF, Regules J, Tosh D, Cowden J, Kathcart A, Cummings J, et al. Ad35.CS.01-RTS,S/AS01 heterologous prime boost vaccine efficacy against sporozoite challenge in healthy malaria-naive adults. PLoS One. 2015;10:e0131571. doi: 10.1371/journal.pone.0131571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rampling T, Ewer KJ, Bowyer G, Bliss CM, Edwards NJ, Wright D, et al. Safety and high level efficacy of the combination malaria vaccine regimen of RTS,S/AS01B with chimpanzee adenovirus 63 and modified vaccinia ankara vectored vaccines expressing ME-TRAP. J Infect Dis. 2016;214:772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora N, L CA, Pannu AK. Towards eradication of malaria: is the WHO's RTS,S/AS01 vaccination effective enough? Risk Manag Healthc Policy. 2021;14:1033–1039. doi: 10.2147/RMHP.S219294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoudi S, Keshavarz H. Efficacy of phase 3 trial of RTS, S/AS01 malaria vaccine: the need for an alternative development plan. Hum Vaccin Immunother. 2017;13:2098–2101. doi: 10.1080/21645515.2017.1295906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214:762–771. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 14.Moncunill G, Scholzen A, Mpina M, Nhabomba A, Hounkpatin AB, Osaba L, et al. Antigen-stimulated PBMC transcriptional protective signatures for malaria immunization. Sci Transl Med. 2020;12:e. doi: 10.1126/scitranslmed.aay8924. aay8924. [DOI] [PubMed] [Google Scholar]
- 15.Zagury D, Burny A, Gallo RC. Toward a new generation of vaccines: the anti-cytokine therapeutic vaccines. Proc Natl Acad Sci U S A. 2001;98:8024–8029. doi: 10.1073/pnas.141224798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A. 2017;114:2425–2430. doi: 10.1073/pnas.1621489114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y, Thompson EG, Muller J, Valvo J, Braun J, Shankar S, et al. The ratiometric transcript signature MX2/GPR183 is consistently associated with RTS,S-mediated protection against controlled human malaria infection. Front Immunol. 2020;11:669. doi: 10.3389/fimmu.2020.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung KY, Ruzzo WL. Principal component analysis for clustering gene expression data. Bioinformatics. 2001;17:763–774. doi: 10.1093/bioinformatics/17.9.763. [DOI] [PubMed] [Google Scholar]
- 19.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W. Volcano plots in analyzing differential expressions with mRNA microarrays. J Bioinform Comput Biol. 2012;10:1231003. doi: 10.1142/S0219720012310038. [DOI] [PubMed] [Google Scholar]
- 21.Tinsley HE, Brown SD. Handbook of Applied Multivariate Statistics and Mathematical Modeling. San Diego: Academic Press; 2000. pp. xxi–xxiii. [Google Scholar]
- 22.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–W175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang R, Grishagin I, Wang Y, Zhao T, Greene J, Obenauer JC, et al. The NCATS BioPlanet: an integrated platform for exploring the universe of cellular signaling pathways for toxicology, systems biology, and chemical genomics. Front Pharmacol. 2019;10:445. doi: 10.3389/fphar.2019.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M, Sato Y, Furumichi M, Morishima K, Tanabe M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019;47:D590–D595. doi: 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clem AS. Fundamentals of vaccine immunology. J Glob Infect Dis. 2011;3:73–78. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broadbent KM, Park D, Wolf AR, Van Tyne D, Sims JS, Ribacke U, et al. A global transcriptional analysis of Plasmodium falciparum malaria reveals a novel family of telomere-associated lncRNAs. Genome Biol. 2011;12:R56. doi: 10.1186/gb-2011-12-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amit-Avraham I, Pozner G, Eshar S, Fastman Y, Kolevzon N, Yavin E, et al. Antisense long noncoding RNAs regulate var gene activation in the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 2015;112:E982–E991. doi: 10.1073/pnas.1420855112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, et al. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 31.Ioannidis LJ, Nie CQ, Hansen DS. The role of chemokines in severe malaria: more than meets the eye. Parasitology. 2014;141:602–613. doi: 10.1017/S0031182013001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark IA, Rockett KA. The cytokine theory of human cerebral malaria. Parasitol Today. 1994;10:410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 33.Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis. 2006;194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 34.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 36.Biankin AV, Kench JG, Colvin EK, Segara D, Scarlett CJ, Nguyen NQ, et al. Expression of S100A2 calcium-binding protein predicts response to pancreatectomy for pancreatic cancer. Gastroenterology. 2009;137:558–568. doi: 10.1053/j.gastro.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Boeckel GR, Ehrlich BE. NCS-1 is a regulator of calcium signaling in health and disease. Biochim Biophys Acta Mol Cell Res. 2018;1865:1660–1667. doi: 10.1016/j.bbamcr.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morino H, Matsuda Y, Muguruma K, Miyamoto R, Ohsawa R, Ohtake T, et al. A mutation in the low voltage-gated calcium channel CACNA1G alters the physiological properties of the channel, causing spinocerebellar ataxia. Mol Brain. 2015;8:89. doi: 10.1186/s13041-015-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saraswati S, Adolfsen B, Littleton JT. Characterization of the role of the Synaptotagmin family as calcium sensors in facilitation and asynchronous neurotransmitter release. Proc Natl Acad Sci U S A. 2007;104:14122–14127. doi: 10.1073/pnas.0706711104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang B, Newbold CI, Williams G, Peshu N, Marsh K, Newton CR. Antibodies to voltage-gated calcium channels in children with falciparum malaria. J Infect Dis. 2005;191:117–121. doi: 10.1086/426512. [DOI] [PubMed] [Google Scholar]
- 41.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naka I, Patarapotikul J, Tokunaga K, Hananantachai H, Tsuchiya N, Ohashi J. A replication study of the association between the IL12B promoter allele CTCTAA and susceptibility to cerebral malaria in Thai population. Malar J. 2009;8:290. doi: 10.1186/1475-2875-8-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kern P, Hemmer CJ, Van Damme J, Gruss HJ, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87:139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 44.Mshana RN, Boulandi J, Mshana NM, Mayombo J, Mendome G. Cytokines in the pathogenesis of malaria: levels of IL-I beta, IL-4, IL-6, TNF-alpha and IFN-gamma in plasma of healthy individuals and malaria patients in a holoendemic area. J Clin Lab Immunol. 1991;34:131–139. [PubMed] [Google Scholar]
- 45.Wenisch C, Linnau KF, Looaresuwan S, Rumpold H. Plasma levels of the interleukin-6 cytokine family in persons with severe Plasmodium falciparum malaria. J Infect Dis. 1999;179:747–750. doi: 10.1086/314630. [DOI] [PubMed] [Google Scholar]
- 46.Boldt ABW, van Tong H, Grobusch MP, Kalmbach Y, Dzeing Ella A, Kombila M, et al. The blood transcriptome of childhood malaria. EBioMedicine. 2019;40:614–625. doi: 10.1016/j.ebiom.2018.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cockburn IA, Seder RA. Malaria prevention: from immunological concepts to effective vaccines and protective antibodies. Nat Immunol. 2018;19:1199–1211. doi: 10.1038/s41590-018-0228-6. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odeh M. Tumor necrosis factor-alpha as a myocardial depressant substance. Int J Cardiol. 1993;42:231–238. doi: 10.1016/0167-5273(93)90053-j. [DOI] [PubMed] [Google Scholar]
- 50.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–209. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 51.Idda ML, Lodde V, Galleri G, Martindale JL, Munk R, Abdelmohsen K, et al. NF90 regulation of immune factor expression in response to malaria antigens. Cell Cycle. 2019;18:708–722. doi: 10.1080/15384101.2019.1580496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Che JN, Nmorsi OP, Nkot BP, Isaac C, Okonkwo BC. Chemokines responses to Plasmodium falciparum malaria and co-infections among rural Cameroonians. Parasitol Int. 2015;64:139–144. doi: 10.1016/j.parint.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nordor AV, Bellet D, Siwo GH. Cancer-malaria: hidden connections. Open Biol. 2018;8:180127. doi: 10.1098/rsob.180127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushansky A, Ye AS, Austin LS, Mikolajczak SA, Vaughan AM, Camargo N, et al. Suppression of host p53 is critical for Plasmodium liver-stage infection. Cell Rep. 2013;3:630–637. doi: 10.1016/j.celrep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNamara HA, Idris AH, Sutton HJ, Vistein R, Flynn BJ, Cai Y, et al. Antibody feedback limits the expansion of B cell responses to malaria vaccination but drives diversification of the humoral response. Cell Host Microbe. 2020;28:572–585. doi: 10.1016/j.chom.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA. A multifactorial role for P. falciparum malaria in endemic Burkitt's lymphoma pathogenesis. PLoS Pathog. 2014;10:e1004170. doi: 10.1371/journal.ppat.1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brochet M, Billker O. Calcium signalling in malaria parasites. Mol Microbiol. 2016;100:397–408. doi: 10.1111/mmi.13324. [DOI] [PubMed] [Google Scholar]
- 59.Soni R, Sharma D, Rai P, Sharma B, Bhatt TK. Signaling strategies of malaria parasite for its survival, proliferation, and infection during erythrocytic stage. Front Immunol. 2017;8:349. doi: 10.3389/fimmu.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Almeida ME, Vasconcelos MG, Tarrago AM, Mariuba LA. Circumsporozoite surface protein-based malaria vaccines: a review. Rev Inst Med Trop Sao Paulo. 2021;63:e11. doi: 10.1590/S1678-9946202163011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon DM, McGovern TW, Krzych U, Cohen JC, Schneider I, LaChance R, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis. 1995;171:1576–1585. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 62.Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 63.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 64.Franklin BS, Parroche P, Ataide MA, Lauw F, Ropert C, de Oliveira RB, et al. Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Natl Acad Sci U S A. 2009;106:5789–5794. doi: 10.1073/pnas.0809742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scharte M, Fink MP. Red blood cell physiology in critical illness. Crit Care Med. 2003;31(12 Suppl):S651–S657. doi: 10.1097/01.CCM.0000098036.90796.ED. [DOI] [PubMed] [Google Scholar]
- 66.English M, Muambi B, Mithwani S, Marsh K. Lactic acidosis and oxygen debt in African children with severe anaemia. QJM. 1997;90:563–569. doi: 10.1093/qjmed/90.9.563. [DOI] [PubMed] [Google Scholar]
- 67.Kumar R, Ng S, Engwerda C. The role of IL-10 in malaria: a double edged sword. Front Immunol. 2019;10:229. doi: 10.3389/fimmu.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Significantly expressed genes in male and female on control vs. controlled human malaria infection samples
Significantly expressed genes in protected and non-protected groups on control vs. controlled human malaria infection samples
Differential gene expression analysis using edgeR Pipeline.
Factor regression pipeline.
Principal component analysis plot for control vs. controlled human malaria infection samples for significant genes.
Significantly expressed genes in males and females on control vs. controlled human malaria infection samples.
JAK-STAT pathway.
Pathways in cancer.
Principal component analysis plot of protected and non-protected samples on various days.
Quantile normalization pipeline.