Abstract
Introduction
Bacterial Vaginosis (BV) is the most common cause of vaginal discharge. However, in some cases, side effects and resistance rates have been reported when antibiotics are administered. This problem has prompted several investigations on the administration of probiotics as an adjunct therapy to treat this infection.
Objection
This study aims to conduct a meta-analysis based on evidence to determine the efficacy and safety of probiotic and antibiotic treatments.
Methods
The meta-analysis was performed using PRISMA guidelines. The literature review was conducted in December 2020 using PubMed, Science Direct, Cochrane Library, and RevMan V.5.3.
Result
The results showed a high and significant cure rate from the analysis of 1006 and 528 samples of probiotics and non-probiotics or control in 16 studies. The recurrence rate was statistically significant with probiotic treatment. Furthermore, neither procedures nor therapy failure showed a significantly lower adverse event rate than the control group.
Conclusion
Probiotic shows better results compared to the control group. However, both have the same occurrence of adverse event.
Keywords: Antibiotics, Bacterial Vaginosis, Probiotics
Abbreviations: BV, Bacterial Vaginosis; CDC, Centers for Disease Control; RCTs, Randomized Control Trials; CRISPR, Clustered regularly interspaced short palindromic repeat-associated
1. Introduction
Bacterial Vaginosis (BV) is the most common cause of vaginal discharge characterized by dysbiosis due to Lactobacilli producing lactic acid as well as facultative and anaerobic proliferation (Muzny et al., 2019a). It is common in the reproductive age and occurs with or without symptoms. The symptoms of this infection in 50% of women include an unpleasant vaginal odor, discharge, itching, and an increase in vaginal pH (Makella S Coudray1, 2021).
The prevalence of BV in different parts of the world varies at approximately between 19 and 24%, and its incidence is higher in developing countries. According to national data from the CDC, the prevalence of this infection in women of reproductive age is about 29%, or approximately 21 million persons (Homayouni et al., 2014).
The CDC has approved the administration of antibiotics such as metronidazole and tinidazole in treating BV. However, in some cases, these drugs showed some side effects and resistance rates (Bacterial Vaginosis, 2021). This problem has prompted several investigations on the administration of probiotics as an adjunct therapy to treat BV. These are believed to increase and reduce BV's cure and recurrence rates, respectively. However, its effectiveness and safety need to be further examined since the use of antibiotics is under development (Larsson et al., 2011).
2. Material and methods
2.1. Literature search
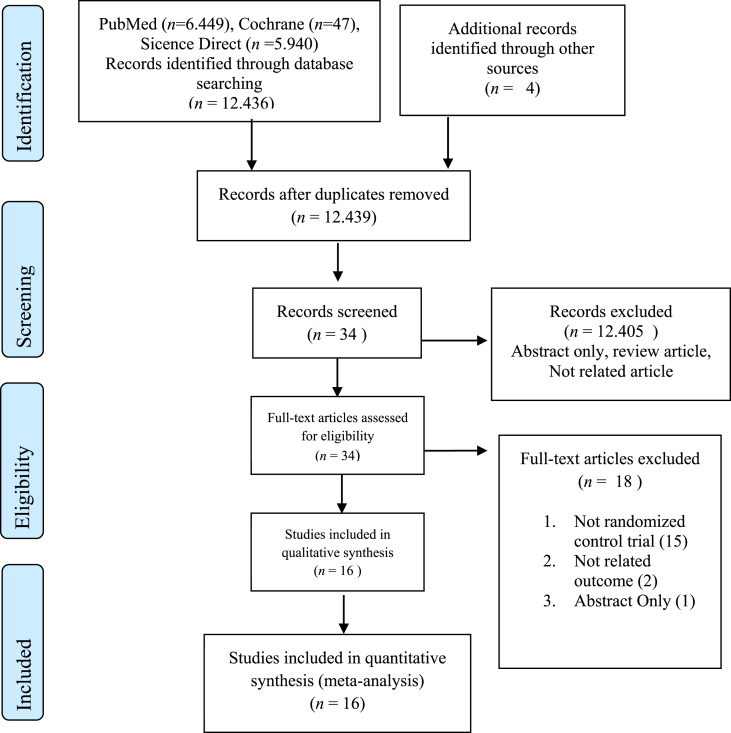
This meta-analysis was conducted following the PRISMA guidelines, as shown in Fig. 1 (Page et al., 2021). The literature search was performed using Cochrane, Sciencedirect, and PubMed. The keywords used are probiotics, antibiotics, and Bacterial Vaginosis. Finally, all randomized control trials from 2006 to 2019 were included.
Fig. 1.
Record screening article with PRISMA flow chart.
2.2. Inclusion and exclusion criteria
The inclusion criteria for this study include (1) randomized control trials (RCTs); (2) comparisons of probiotics with placebo or antibiotics, and (3) a report of at least one of the following outcomes, such as cure rates, failure medication, recurrence rates, and adverse event. Meanwhile, the exclusion criteria are (1) unclear reports of the outcomes as mentioned earlier, (2) unextractable or uncomputable data, and (3) non-RCTs. The authors independently screened the studies, extracted relevant data, and discussed each outcome.
2.3. Outcome and study quality
The assessed results include cure rates, medication failure, recurrence, and adverse events. The quality of the RCTs was determined using a Jadad score with a scale range of 0–5. Furthermore, the score was considered high, moderate, and low quality when it was >4, 3–4, and <3, respectively. Table 1 present the score assessment (Luchini et al., 2021). Finally, the level of evidence was evaluated for each study based on the available criteria of the Oxford Center for Evidence-Based Medicine (OCEBM Levels of Evidence, 2011).
Table 1.
Jadad Score assesment.
| Article | Randomization | Blinding | Withdrawals or Dropouts | Jadad Scale |
|---|---|---|---|---|
| Anukam a 2006 (Anukam et al., 2006a) | 2 | 1 | 1 | 4 |
| Anukam b 2006 (Anukam et al., 2006b) | 2 | 0 | 1 | 3 |
| Bohbot 2017 (Bohbot et al., 2018) | 1 | 2 | 1 | 4 |
| Cohen 2020 (Cohen et al., 2020) | 1 | 2 | 1 | 4 |
| Erikson 2005 (Eriksson et al., 2005) | 1 | 2 | 1 | 4 |
| Happel 2020 (Happel et al., 2020) | 2 | 2 | 1 | 5 |
| Hemmerling 2010 (Hemmerling et al., 2010) | 2 | 2 | 1 | 5 |
| Hummelen 2010 (Hummelen et al., 2010) | 2 | 2 | 1 | 5 |
| Larson 2008 (Larsson et al., 2008) | 1 | 2 | 1 | 4 |
| Laue 2017 (Laue et al., 2018) | 2 | 2 | 1 | 5 |
| Marcotte 2019 (Marcotte et al., 2019) | 2 | 1 | 1 | 4 |
| Martinez 2009 (Martinez et al., 2009) | 2 | 1 | 1 | 4 |
| Mastromarino 2008 (Mastromarino et al., 2009) | 2 | 2 | 1 | 5 |
| Russo 2019 (Russo et al., 2019) | 2 | 1 | 1 | 4 |
| Sgibnev 2009 (Sgibnev & Kremleva, 2020) | 2 | 2 | 1 | 5 |
| Vujic 2013 (Vujic et al., 2013) | 2 | 2 | 1 | 5 |
∗Randomization: 1 point; give additional 1 point if randomization method is appropriate (eg. Computer generated)∗Blinding: 1 point; give additional 1 point if blinding method is appropriate (eg.indetical placebo) ∗Withdrawals: 1 point if the number and the reasons was stated.
2.4. Statistical analysis
The statistical analysis Review Manager version 5.3 was used to calculate each parameter, while Mantel-Hanzel methods combined results across studies. The data type was dichotomous or categorical and expressed as OR with a 95% confidence interval (CI). Furthermore, the Cochrane Chi-squared test and inconsistency (I2) examine the studies' heterogeneity. A P-value <0.05 was considered statistically significant; hence, heterogeneity was significant when I2 >50%. The effect models used were random and fixed, and they were applied when heterogeneity was >50% and <50%, respectively.
3. Result
3.1. Baseline characteristic
A total of 12,436 related articles and four from other sources were analyzed in the form of a bibliography with the final screening results of 16 studies. The total sample obtained was 1534 patients in each treatment group. Furthermore, this includes 1006 and 528 samples of probiotics and probiotics or control, respectively, as shown in Fig. 1.
Table 2 shows the characteristics of the included studies (Anukam, Osazuwa, Ahonkhai, et al., 2006; Anukam, Osazuwa, Osemene, et al., 2006; Bohbot et al., 2018; Cohen et al., 2020; Eriksson et al., 2005; Happel et al., 2020; Hemmerling et al., 2010; Hummelen et al., 2010; Larsson et al., 2011; Laue et al., 2018; Marcotte et al., 2019; Martinez et al., 2009; Mastromarino et al., 2009; Russo et al., 2019; Sgibnev & Kremleva, 2020; Vujic et al., 2013). The evidence base determination level was 1b for 16 RCTs, and the studies attained a significant level of quality, with Jadad scores in the range of 3–5.
Table 2.
Characteristic of study quality and articles.
| Article | Intervention | Country | Study design | LE | Jadad Scale | Case (n) |
|
|---|---|---|---|---|---|---|---|
| Probiotic | Control | ||||||
| Anukam a 2006 (Anukam et al., 2006a) | Antibiotic + Placebo vs Antibiotic | Nigeria | RCT | 1b | 4 | 49 | 57 |
| Anukam b 2006 (Anukam et al., 2006b) | Probiotic vs Antibiotic | Nigeria | RCT | 1b | 3 | 17 | 18 |
| Bohbot 2017 (Bohbot et al., 2018) | Antibiotic + Probiotic vs Antibiotic + Placebo | France | RCT | 1b | 3 | 50 | 48 |
| Cohen 2020 (Cohen et al., 2020) | Probiotics vs Placebo | USA | RCT | 1b | 4 | 152 | 76 |
| Erikson 2005 (Eriksson et al., 2005) | Antibiotic + Probiotic vs Antibiotic + Placebo | Finland | RCT | 1b | 3 | 91 | 96 |
| Happel 2020 (Happel et al., 2020) | Antibiotic + Probiotic vs Antibiotic + Placebo | South Africa | RCT | 1b | 5 | 12 | 18 |
| Hummerling 2010 (Hemmerling et al., 2010) | Antibiotic + Probiotic vs Antibiotic + Placebo | USA | RCT | 1b | 5 | 6 | 18 |
| Hummelen 2010 (Hummelen et al., 2010) | Antibiotic + Probiotic vs Antibiotic + Placebo | Holland | RCT | 1b | 4 | 23 | 28 |
| Larson 2008 (Larsson et al., 2008) | Antibiotic + Probiotic vs Antibiotic + Placebo | Sweden | RCT | 1b | 4 | 37 | 39 |
| Laue 2017 (Laue et al., 2018) | Antibiotic + Probiotic vs Antibiotic + Placebo | German | RCT | 1b | 5 | 18 | 18 |
| Marcotte 2019 (Marcotte et al., 2019) | Antibiotic + Probiotic vs Antibiotic | Sweden | RCT | 1b | 4 | 12 | 14 |
| Martinez 2009 (Martinez et al., 2009) | Antibiotic + Probiotic vs Antibiotic + Placebo | Brazil | RCT | 1b | 4 | 32 | 32 |
| Mastromarino 2008 (Mastromarino et al., 2009) | Probiotics vs Placebo | Italy | RCT | 1b | 3 | 18 | 16 |
| Russo 2019 (Russo et al., 2019) | Antibiotic + Probiotic vs Antibiotic + Placebo | Italy | RCT | 1b | 3 | 24 | 24 |
| Sgibnev 2009 (Sgibnev & Kremleva, 2020) | Antibiotic + Probiotic vs Antibiotic + Placebo | Rusian | RCT | 1b | 5 | 44 | 42 |
| Vujic 2013 (Vujic et al., 2013) | Probiotics vs placebo | Croatia | RCT | 1b | 4 | 394 | 149 |
LE: Level of Evidence Base, 1b: Level of Evidence Randomized control trial.
3.2. Cure rate
Fig. 2 shows eleven articles with 717 patients in the probiotic group and 470 in control. The prebiotic group has a high cure rate (OR 3.30; 95% CI, 1.45 to 7.50; p = 0.004) with a reasonably high heterogeneity as indicated by the chi-Squared test to be I2 = 85%.
Fig. 2.
Forest plot cure rate.
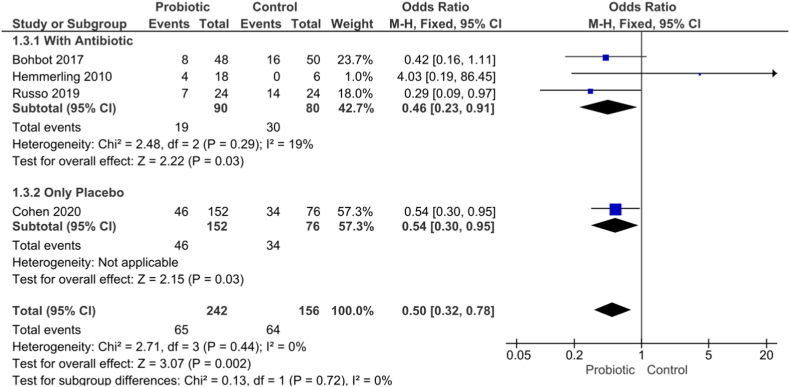
3.3. Recurrence rate
The recurrence rate was compared in four studies, as shown in Fig. 3. The total subgroup reported had a significant difference where the recurrence rate was less in the probiotic group of patients (OR 0.50; 95% CI, 0.32 to 0.078; p = 0.002) without heterogeneity I2 = 0%.
Fig. 3.
Forest plot recurrence rate.
3.4. Adverse event
The five articles in this subgroup showed that abnormalities of itching and burning had no significant difference between the two groups (OR 1.02; 95% CI,0.30 to 3.51; p = 0.97) I2 = 77%, and unpleasant vaginal odor was significantly less in probiotics groups (OR 0.30; 95% CI,0.12 to 0.75; p = 0.01) I2 = 55%. Furthermore, vaginal discharge showed no difference in the two groups (OR 0.54; 95% CI,0.16 to 1.80; p = 0.32) I2 = 79% with an overall analysis (OR 0.57; 95% CI, 0.30 to 0.095; p = 0.03) and had high heterogeneity I2 = 75% as indicated in Fig. 4.
Fig. 4.
Forest plot adverse event.
3.5. Failure of therapy
Six articles in the analyzed studies showed significant differences in the probiotic groups, which have less incidence of therapy failure (OR 0.14; 95% CI, 0.02 to 0.98; p = 0.05) with high heterogeneity of I2 = 76% as presented in Fig. 5.
Fig. 5.
Forest plot failure of therapy.
4. Discussion
Probiotics are believed to be reasonably effective in dealing with cases of vaginal discharge caused by bacteria or BV (Han & Ren, 2021). Meta-analysis was conducted on 16 RCT articles with good quality jaded scores of 3–5 to determine the effectiveness, efficacy, and safety of using probiotics to treat this infection. A high heterogeneity could be due to insufficient samples or different methods in each article.
The cure rate analysis showed a significant difference where its high level is observed in the probiotic group. In treating BV cases, prebiotics has a good effect because exogenous Lactobacilli maintain the pH of the vagina in an acidic state and help colonize endogenous Lactobacilli to restore normal conditions (Chee et al., 2020; Muzny et al., 2019b). The mechanism of these drugs is to decrease increased phosphorylation of nuclear factor-kappa B p65 in vaginal tissue and inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, for restoring the vaginal micro-ecological environment (Zhou et al., 2019). The use of probiotics in the vaginal and orally has the same positive effect. However, it has a fast therapeutic effect when applied to the vaginal than the oral administration (Anukam et al., 2006b; Reznichenko et al., 2020; TesterFarage and Al-Ghazzewi, 2018). Cure rate and diagnosis are determined by Gram stain Nugent scoring (a score of 0–3 was considered optimal, 4–6 intermediate microbiota, and 7–10 BV), or modified Amsel criteria (defined as the presence of at least 2 of the following criteria: vaginal pH > 4.5, positive whiff test, and 20% clue cells) (Amsel et al., 1983; Nugent et al., 1991).
The recurrence rate analysis showed significantly lower results in the probiotic group. These are in accordance with existing research, stating that antibiotics without probiotics have a high recurrence rate (Hillier et al., 2017; Nyirjesy & Schwebke, 2018; Schwebke et al., 2017). Metronidazole is the recommended treatment option for BV (van Schalkwyk et al., 2015; Workowski et al., 2015). However, its recurrence rate is reported to be 21.2%–25.5% compared to probiotics which are 10.6%–11% (Faught & Reyes, 2019). The several factors that play a role in recurrence include the persistence of a BV-associated biofilm, failure to recolonize the vagina with Lactobacilli, reinfection from an untreated partner, and host genetic or immune factors (Vodstrcil et al., 2021).
The treatment failure in BV is significantly high in the control group. Probiotic reduce the inflammatory response in the vagina and inhibits the growth of pathogenic microbiota by restoring pH (Zhou et al., 2019). Clustered regularly interspaced short palindromic repeat-associated (CRISPR)-genes reduce the metronidazole treatment's effectiveness and protect bacteria like G. vaginalis. Furthermore, metronidazole can eliminate low to modest concentrations but cannot make it when a biofilm contains a high G. vaginalis (Deng et al., 2018; Verwijs et al., 2020).
There is no difference between the two groups in the incidence of vaginal discharge, vaginal odor, itching, and burning. This explains the potential of the two groups in reducing these complaints. Zhang et al. reported no serious adverse events due to probiotics and metronidazole therapy (Javed et al., 2019; Zhang et al., 2021).
5. Conclusion
This meta-analysis showed several significant advantages of probiotic treatment regarding cure rate, recurrence rate, and therapy failure. The two groups had a similar adverse event occurrence. However, the insufficient RCT data resulted in a lack of potential outcomes, hence, it appears that extensive follow-up investigations are necessary.
Disclosure
There is no conflict of interests and funding
Ethical approval
Not required.
Patient consent
Not required.
Declaration of competing interest
The authors declare no conflict.
Handling Editor: Dr HE DAIHAI HE
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Sri Adila Nurainiwati, Email: adila@umm.ac.id.
Dwi Nurwulan Pravitasari, Email: Vitha_sabrinaviancha@umm.ac.id.
Probo Yudha Pratama Putra, Email: probo@umm.ac.id.
References
- Amsel R., Totten P.A., Spiegel C.A., Chen K.C., Eschenbach D., Holmes K.K. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. The American Journal of Medicine. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- Anukam K., Osazuwa E., Ahonkhai I., et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic lactobacillus rhamnosus GR-1 and lactobacillus reuteri RC-14: Randomized, double-blind, placebo controlled trial. Microbes and Infection. 2006;8(6):1450–1454. doi: 10.1016/j.micinf.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Anukam K.C., Osazuwa E., Osemene G.I., Ehigiagbe F., Bruce A.W., Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes and Infection. 2006;8(12–13):2772–2776. doi: 10.1016/j.micinf.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Bacterial Vaginosis - STI Treatment Guidelines. Accessed October 23, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm.
- Bohbot J.M., Daraï E., Bretelle F., Brami G., Daniel C., Cardot J.M. Efficacy and safety of vaginally administered lyophilized Lactobacillus crispatus IP 174178 in the prevention of bacterial vaginosis recurrence. Journal of gynecology obstetrics and human reproduction. 2018;47(2):81–86. doi: 10.1016/j.jogoh.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Chee W.J.Y., Chew S.Y., Than L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microbial Cell Factories. 2020;19(1):1–24. doi: 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C.R., Wierzbicki M.R., French A.L., et al. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. New England Journal of Medicine. 2020;382(20):1906–1915. doi: 10.1056/NEJMoa1915254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.L., Gottschick C., Bhuju S., Masur C., Abels C., Wagner-D÷ bler I. Metatranscriptome analysis of the vaginal microbiota reveals potential mechanisms for protection against metronidazole in bacterial vaginosis. mSphere. 2018;3(3) doi: 10.1128/mSphereDirect.00262-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K., Carlsson B., Forsum U., Larsson P.G. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta Dermato-Venereologica. 2005;85(1):42–46. doi: 10.1080/00015550410022249. [DOI] [PubMed] [Google Scholar]
- Faught B.M., Reyes S. Characterization and treatment of recurrent bacterial vaginosis. Journal of Women's Health. 2019;28(9):1218–1226. doi: 10.1089/jwh.2018.7383. [DOI] [PubMed] [Google Scholar]
- Han Y., Ren Q.L. Does probiotics work for bacterial vaginosis and vulvovaginal candidiasis. Current Opinion in Pharmacology. 2021;61:83–90. doi: 10.1016/j.coph.2021.09.004. [DOI] [PubMed] [Google Scholar]
- Happel A.U., Singh R., Mitchev N., et al. Testing the regulatory framework in South Africa - a single-blind randomized pilot trial of commercial probiotic supplementation to standard therapy in women with bacterial vaginosis. BMC Infectious Diseases. 2020;20(1):491. doi: 10.1186/s12879-020-05210-4. Published 2020 Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerling A., Harrison W., Schroeder, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sexually Transmitted Diseases. 2010;37(12):745–750. doi: 10.1097/OLQ.0b013e3181e50026. [DOI] [PubMed] [Google Scholar]
- Hillier S.L., Nyirjesy P., Waldbaum A.S., et al. Secnidazole treatment of bacterial vaginosis: A randomized controlled trial. Obstetrics & Gynecology. 2017;130:379–386. doi: 10.1097/AOG.0000000000002135. [DOI] [PubMed] [Google Scholar]
- Homayouni A., Bastani P., Ziyadi S., et al. Effects of probiotics on the recurrence of bacterial vaginosis: A review. Journal of Lower Genital Tract Disease. 2014;18(1):79–86. doi: 10.1097/LGT.0b013e31829156ec. [DOI] [PubMed] [Google Scholar]
- Hummelen R., Changalucha J., Butamanya N.L., Cook A., Habbema J.D., Reid G. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. International Journal of Gynaecology & Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics. 2010;111(3):245–248. doi: 10.1016/j.ijgo.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Javed A., Parvaiz F., Manzoor S. Bacterial vaginosis: An insight into the prevalence, alternative treatments regimen and it's associated resistance patterns. Microbial Pathogenesis. 2019;127:21–30. doi: 10.1016/j.micpath.2018.11.046. [DOI] [PubMed] [Google Scholar]
- Larsson P.G., Brandsborg E., Forsum U., et al. Extended antimicrobial treatment of bacterial vaginosis combined with human lactobacilli to find the best treatment and minimize the risk of relapses. BMC Infectious Diseases. 2011;11(1):223. doi: 10.1186/1471-2334-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P.G., Stray-Pedersen B., Ryttig K.R., Larsen S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Women's Health. 2008;8:3. doi: 10.1186/1472-6874-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue C., Papazova E., Liesegang, et al. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women - a double-blind, randomised, controlled clinical pilot trial. Beneficial Microbes. 2018;9(1):35–50. doi: 10.3920/BM2017.0018. [DOI] [PubMed] [Google Scholar]
- Luchini C., Veronese N., Nottegar A., et al. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharmaceutical Statistics. 2021;20(1):185–195. doi: 10.1002/pst.2068. [DOI] [PubMed] [Google Scholar]
- Makella S Coudray1 P.M. Bacterial vaginosis - a brief synopsis of the literature makella. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2021;245(305):143–148. doi: 10.1016/j.ejogrb.2019.12.035. (Bacterial) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte H., Larsson P.G., Andersen K.K., et al. An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infectious Diseases. 2019;19:824. doi: 10.1186/s12879-019-4425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Franceschini S., Patta, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), lactobacillus rhamnosus GR-1, and lactobacillus reuteri RC-14: A randomized, double-blind, placebo-controlled trial. Canadian Journal of Microbiology. 2009;55:133–138. doi: 10.1139/w08-102. [DOI] [PubMed] [Google Scholar]
- Mastromarino P., Macchia S., Meggiorini L., Trinchieri V., Mosca L., Perluigi M., Midulla C. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clinical microbiology and infection : The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(1):67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Muzny C.A., Taylor C.M., Swords W.E., et al. An updated conceptual model on the pathogenesis of bacterial vaginosis. The Journal of Infectious Diseases. 2019;220(9):1399–1405. doi: 10.1093/infdis/jiz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzny C.A., Taylor C.M., Swords W.E., Tamhane A., Chattopadhyay D., Cerca N., Schwebke J.R. An updated conceptual model on the pathogenesis of bacterial vaginosis. The Journal of Infectious Diseases. 2019;220(9):1399–1405. doi: 10.1093/infdis/jiz342. 1 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent R.P., Krohn M.A., Hillier S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirjesy P., Schwebke J.R. Secnidazole: Next-generation antimicrobial agent for bacterial vaginosis treatment. Future Microbiology. 2018;13:507–524. doi: 10.2217/fmb-2017-0270. [DOI] [PubMed] [Google Scholar]
- OCEBM Levels of Evidence — Centre for Evidence-Based Medicine (CEBM), University of Oxford. Published 2011. Accessed October 20, 2021. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. International Journal of Surgery. 2021;88:1–11. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznichenko H., Henyk N., Maliuk V., et al. Oral intake of lactobacilli can Be helpful in symptomatic bacterial vaginosis: A randomized clinical study. Journal of Lower Genital Tract Disease. 2020;24(3):284–289. doi: 10.1097/LGT.0000000000000518. [DOI] [PubMed] [Google Scholar]
- Russo R., Karadja E., De Seta F. Evidence-based mixture containing lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Beneficial Microbes. 2019;10(1):19–26. doi: 10.3920/BM2018.0075. [DOI] [PubMed] [Google Scholar]
- van Schalkwyk J., Yudin M.H., Infectious Disease Committee Vulvovaginitis: Screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. Journal of Obstetrics and Gynaecology Canada. 2015;37:266–274. doi: 10.1016/S1701-2163(15)30316-9. [DOI] [PubMed] [Google Scholar]
- Schwebke J.R., Morgan F.G., Jr., Koltun W., Nyirjesy P. A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis. American Journal of Obstetrics and Gynecology. 2017;217(678):e1–e9. doi: 10.1016/j.ajog.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Sgibnev A., Kremleva E. Probiotics in addition to metronidazole for treatment trichomonas vaginalis in the presence of BV: A randomized, placebo-controlled, double-blind study. European Journal of Clinical Microbiology & Infectious Diseases : Official Publication of the European Society of Clinical Microbiology. 2020;39(2):345–351. doi: 10.1007/s10096-019-03731-8. [DOI] [PubMed] [Google Scholar]
- Tester R., Farage H., Al-Ghazzewi Intrinsic and extrinsic carbohydrates in the vagina: A short review on vaginal glycogen. International Journal of Biological Macromolecules. 2018;112:203–206. doi: 10.1016/j.ijbiomac.2018.01.166. ISSN 0141-8130. [DOI] [PubMed] [Google Scholar]
- Verwijs M.C., Agaba S.K., Darby A.C., Janneke H.H., van de Wijgert M. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. American Journal of Obstetrics and Gynecology. 2020;222(2):157.e1–157.e13. doi: 10.1016/j.ajog.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodstrcil L.A., Muzny C.A., Plummer E.L., et al. Bacterial vaginosis: Drivers of recurrence and challenges and opportunities in partner treatment. BMC Medicine. 2021;19:194. doi: 10.1186/s12916-021-02077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujic G., Jajac Knez A., Despot Stefanovic V., Kuzmic Vrbanovic V. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: A double-blind, randomized, placebo-controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2013;168(1):75–79. doi: 10.1016/j.ejogrb.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Workowski K.A., Bolan G.A., Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2015. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lyu J., Ge L., Huang L., Peng Z., Liang Y., Zhang X., Fan S. Probiotic Lacticaseibacillus rhamnosus GR-1 and Limosilactobacillus reuteri RC-14 as an adjunctive treatment for bacterial vaginosis do not increase the cure rate in a Chinese cohort: A prospective, parallel-group, randomized, controlled study. Frontiers in Cellular and Infection Microbiology. 2021;11 doi: 10.3389/fcimb.2021.669901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xu W., Hong K., Li H., Zhang J., Chen X., Zhu Y., Zhang Q., Ding F., Wang F. Therapeutic effects of probiotic Clostridium butyricum WZ001 on bacterial vaginosis in mice. Journal of Applied Microbiology. 2019;127:565–575. doi: 10.1111/jam.14329. [DOI] [PubMed] [Google Scholar]