Abstract
Background and objective
Anterior cruciate ligament (ACL) reconstruction calls for artificial ligaments with better bioactivity, however systematic reviews regarding bioactivity enhancement strategies, technologies, and perspectives of artificial ligaments have been rarely found.
Methods
Research papers, reviews, and clinical reports related to artificial ligaments were searched and summarized the current status and research trends of artificial ligaments through a systematic analysis.
Results
Having experienced ups and downs since the very first record of clinical application, artificial ligaments differing in material, and fabrication methods have been reported with different clinical performances. Various manufacturing technologies have developed and realized scaffold- and cell-based strategies. Despite encouraging in-vivo and in-vitro test results, the clinical results of such new designs need further clinical examinations.
Conclusion
As the demand for ACL reconstruction dramatically increases, novel artificial ligaments with better osteoinductivity and mechanical performance are promising.
The translational potential of this article
To develop novel artificial ligaments simultaneously possessing excellent osteoinductivity and satisfactory mechanical performance, it is important to grab a glance at recent research advances. This systematic analysis provides researchers and clinicians with comprehensive and comparable information on artificial ligaments, thus being of clinical translational significance.
Keywords: Cruciate ligaments reconstruction, Artificial ligament, Osteoinductivity, Mechanical properties
Nomenclature
Abbreviations
- ACL
anterior cruciate ligament
- bFGF
Basic fibroblast growth factor
- bMSC
bone mesenchymal stem cell
- CHI
chitosan
- CL
cruciate ligaments
- ECM
extracellular matrix
- ELD
electrolytic deposition
- GDF
growth and differentiation factor
- GO
graphene oxide
- HAP
hydroxyapatite
- HPC
hydroxy-propyl cellulose
- LAD
ligament augment device
- LARS
ligament advanced reinforcement system
- MSC
mesenchymal stem cell
- PCL
poly(ε-caprolactone)
- PEUU
poly (ester urethane) urea
- PGA
polyglycolic acid
- PLA
polylactic acid
- PLAGA
polylactide-co-glycolide
- PLCL
copoly(lactic acid-co- (ε-caprolactone))
- PLGA
poly(lactic-co-glycolic acid
- PNaSS
poly(sodium styrene sulfonate)
- PP
polypropylene
- PTFE
polytetrafluoroethylene
- rhBMP-7
recombinant human bone morphogenetic protein 7
- TCP
tricalcium phosphate
1. Introduction
CL is crucial for keeping the knee joint mechanically stable and balanced. Damage to CLs is one of the commonest knee damage forms. Indeed, severe CL injuries like tear or rupture may occur during traffic accidents as well as athletic training, during which excessive stress gets transferred to CL, thus patients can be found within a quite wide age range [1]. Unrepaired CL damage may lead to irreversible articular cartilage defects and osteoarthropathy [2]. Since the poor vascularization of natural ligament tissue and the existence of synovial fluid within the knee joint, it is hard for natural ligaments to regenerate after such severe damage. Surgical intervention has proven to be an ideal approach for ligamentous treatment.
The earliest ligamentous injury surgeries of ACL focused on restoring the original native injured ligament, which is termed ACL repair. Generally, ACL repair allows the natural anatomy of the ligament to be maintained, which prevents from loss of native tissue that provides the proprioceptive protective function to the knee [3]. ACL repair became the “golden standard” for ACL treatment in the 1970s and 1980s [4]. However, such satisfying initial clinical outcomes were followed by concerning rates of re-rupture and reoperation, in midterm follow-ups [5]. Recognition of the above-mentioned 1poor outcomes led to a paradigm shift away from ACL repair in the 1980s and 1990s. What is worthy to note recently is that, a few studies dig into ACL repair for potential advantages, in a wish to avoid surgeries by adopting viable conservative options also known as “biologic augmentation”. Such biologic augmentation includes enhancement of the ligamentous healing process by modulating the inflammatory response, downregulating the production of matrix degradative enzymes after injury, and maximizing the regenerative potential of ACL in the cellular population [6,7]. In fact, a growing interest in novel augmentation techniques has recently emerged, including both biologic agents and biomaterials, which could be even combined, inspiring designing novel ligamentous grafts with biomimetic physiochemical performances [8].
Despite attempts to improve the outcome of ligament repair, primary arthroscopy-assisted ACL reconstruction has emerged as the premier treatment strategy and is now considered the new “gold standard” [9]. ACL reconstruction can be attained by combined uses of several types of equipment, including interference screws, adjustable fixation devices, ligamentous grafts, etc. Interference screws fixation is practically adopted in tendon transfer and is reported with high satisfaction rates and low complication rates [10]. However, as commercial interference screw products like Biosure Regenesorb™ are economically costly, research regarding organic implant-less techniques for ACL reconstruction, like Bone and Site Hold Tendon Inside (BASHTI) technique, are considered as a substitute for interference screw fixation, especially where fast recovery is expected [11]. Adjustable fixation devices like Ultrabutton™, on the other hand, focus on providing fixation for ligamentous implantation by string-loops and a metal button. Graft fixation by such devices demonstrates higher failure stress than interference screw fixation, yet the risk of exaggeration of the bungee-cord effect is undeniable following inaccurate measurements during graft or tunnel preparation [12]. Moreover, despite some research in favor of the clinical biomechanical outcome of ACL reconstruction via adjustable fixation devices, systematic biomechanical investigations into clinical outcomes are still lacking from the stage [[13], [14], [15]]. As for ligamentous graft, the artificial ligament has been considered ideal for ACL reconstruction, if compared with autografts and allografts for being free from concerns of disease transferring and lacking ligament supply.
The interest in artificial ligaments that emerged no later than the 1970s has been booming in the 1990s, as displayed in Fig. 1. The first clinical application of artificial ligament on CL reconstruction can be traced back to the 1970s when Food and Drug Administration (FDA) approved the first series of artificial ligament products such as Proplast™, which were soon withdrawn from the market due to severe complications. Following were novel artificial ligaments, e.g., Leeds-Keio™, Gore-Tex™, Stryker Dacron™, and LARS™, that varied in materials, constructions, or weaving methods. Indeed, though generally regarded as candidates for clinical applications, most of them failed to survive through long-term prognosis evaluations due to high complication and failure rates. Increasing evidence indicated that the poor bioactivity of artificial ligaments may be the cause of implant failure [16,17]. For example, PET, one of the most popular materials for artificial ligaments, is considered the most adopted and reliable among different polyester materials for its outstanding biomechanical properties and biocompatibility [2]. However, the lack of osseointegration strongly restricts the prognosis effect and clinical application of such candidates, as in most cases it is fibrous scar structure, rather than desired autogenetic ligament tissue, that forms between the prosthesis and the host organization [18]. Although facing a dispute over long-term prognoses, artificial ligaments are still available and popular in clinical applications, free from donor-site morbidity and disease transmission if compared with autografts and allografts [2,19]. That is to say, if mechanical and bioactive performance should be significantly enhanced, artificial ligaments would be granted in potential clinical translation and applications.
Fig. 1.
The published papers were searched on the Web of Science Core Collection, using the phrases (ARTIFICIAL LIGAMENT∗) as a topic on 1 July 2022. (A) The number of publications and (B) the number of citations from 1990 to 2022 are presented.
Recently, several reviews have managed to discuss the clinical outcome of artificial ligaments. For example, Zhang et al. and Sun et al. systematically reviewed the postoperative performance of synthetic grafts in ACL reconstruction by comparing autografts and synthetic grafts in terms of postoperative knee stability and function [20,21]. Novel materials with potential clinical application in ACL reconstruction are also discussed [22]. As mentioned above, bioactivity is also a key property for the clinical applications of artificial ligaments but systematic summarizations on this point are still far from enough.
This article is expected to fill the gap and offer research fellows insights into the novel design of artificial ligaments with better osseointegration performance. In this paper, the root causes of postoperative complications of artificial ligaments are elaborated on based on a review of the current clinical performance of artificial ligaments. In addition, methods to enhance the osseointegration of artificial ligaments are discussed based on categorized strategies and corresponding manufacturing technologies.
2. Current artificial ligaments and their clinical performance
As it is expected to limit joint movement within a proper range, artificial ligament should demonstrate excellent superior service durability. Therefore, artificial ligaments should perform excellent mechanical and biological reliabilities throughout the autologous ligament reconstruction process. Some representative artificial ligament products and their properties were summarized and listed in Table 1. In this section, an overall review of the clinical performance of current artificial ligaments is provided in terms of mechanical and biological performance.
Table 1.
Basic information of some representative artificial ligament products.
| Commercial name | Manufacturer | Material | Ultimate tensile strength (N) | Stiffness (N/mm) | Elongation at break (%) | Limitation | Clinical outcome | Legend |
|---|---|---|---|---|---|---|---|---|
| Gore-Tex | WL core &Associates, Arizona, USA | PTFE | 5300 [23,24] | 322 [23,24] | 9 [23,24] | Limited issue ingrowth wear debris | The complication rate of 76% Market exit in 1993 |
 [25] [25] |
| LAD | 3M, Minnesota, USA | PET | 1730 [23] | 36 [23] | Cannot solely replace ACL | Stability and functional outcome improved, however not recommended as an ACL substitute |
 [26] [26] |
|
| Stryker Dacron | Stryker, Michigan, USA | PET | 3600 [27] | 420 [27] | 18.7 [27] | Prone to anteroposterior | 60% rapture rate Market exit in 1994 |
 [25] [25] |
| Leeds-Keio | Neoligamnet Ltd, UK | PP | 2200 [28] | 280 [5,28] | Degenerative changes | 28% rapture rate after 10 years unsatisfactory guarantee |
 [29] [29] |
|
| LARS | Structural instruments and Devices, Arc-sur-Tille, France. | PET | 4700 [5,16] | 200 [16] | Bone tunnel enlargement | Excellent functional outcomes, with higher knee stability, yet long-term results are still required |
 [30] [30] |
2.1. Biomechanical performance
Mechanical properties of materials such as strength, elongation, creep performance, and fatigue behavior are of great significance for the assessment of the clinical behavior of artificial ligaments, thus being prior in prosthesis design. However, according to corresponding current standards, the service duration of the ligament prosthesis is evaluated based on in vitro tests and cannot completely present the synergetic effect of the complex physiological environments under daily cyclic loading conditions, thus the designed service duration of artificial ligaments cannot be reached [31].
Natural ligaments consist of numerous fibrous bundles wrapped by synovial membranes, and each bundle demonstrates a different secondary structure so that ligaments can move freely along different surfaces of the joints. Such an elaborate structural hierarchy shaped the mechanical response of natural ligaments into a non-linear form. ACL is loaded throughout the bend and stretch process of knee joints. The maximum loading, stiffness, and elongation of ACL are 1775 ± 269 N, 182 ± 33 N/mm, and 15.9 ± 3.5 mm, respectively. The stress–strain curve of the ligament is generally characterized by a J-shaped dependence with a toe region. From the perspective of anatomy, the non-linear behavior of natural ligaments is effective in maintaining normal knee kinematics [30,32]. When a progressive load is applied on the knee, alignment of collagen fibers provides increased stiffness to limit excessive motions and results in the typical J-shaped convex stress–strain curve. When all the straightened collagen fibrils participate in the mechanical response, a linear region occurs [2,[33], [34], [35]]. Apart from the J-shaped stress–strain curve, another important characteristic is the viscoelastic behavior of ligaments, which allows the relaxation of a certain amount of stress during dynamic loading and is considered important in maintaining the mechanical stability of the knee [36]. It is indicated that such dissipating effects are due to modulus loss by interactions between collagen fibers and the hydrated matrix of proteoglycans and glycoprotein [32,33]. Thus, it is expected that artificial ligaments should demonstrate various mechanical responses at different phases of autologous ligament recovery.
The design of artificial ligaments should adequately emulate the mechanical and viscoelastic features to improve biomechanical compatibility. Improvements in novel biomaterial manufacturing have made available a new generation of artificial ligaments with better biocompatible designs. Take LARS™ artificial ligament as an example, potential machining residues and oils that could elicit synovitis have been removed in advance, while a middle part consisting of a bunch of pre-twisted free fibers was adopted to increase resistance to torsional fatigue and wear and tear. Even though LARS™ is the most clinically applied prosthesis for ACL reconstruction, a biomechanical evaluation of LARS™ in a sheep model of ACL replacement claimed that LARS™ is not suitable for ACL replacement as none of the reconstructions approached the mechanical performance of the normal ACL in the ovine model. A significant decrease in ultimate tensile strength was caused by partial tearing of the artificial ligaments observed in 40% of the cases [19,37].
Aside from mechanical response for sheltering autologous ligament recovery, the postoperative biomechanical interaction between the prosthesis and the bone tunnel should also be taken into consideration in biomechanical performance investigation. Bone tunnel enlargement is one of the most witnessed complications associated with LARS™. According to clinical records, the bone tunnel enlargement rate is 7% due to incorrect femoral tunnel replacement [38]. In some short- and middle-term follow-ups, such incorrect replacement gave rise to knee synovitis and indicated the poor remodeling of artificial grafts. Researchers believed that it was the poor osseointegration between PET graft and bone tunnel inwall that caused the “wiper effect”, leading to enlargement of the bone tunnel. However, long-term follow-ups are required for further evaluation. Another biomechanical complication is associated with tibial and femoral screw loosening. The concern lies in the cutting effect of the screw-on artificial ligament. Mostly, artificial ligaments are fixed by interface screws at the end of bone tunnels. Swinging or swiping of artificial ligaments in bone tunnels, also known as the “wiper effect”, leave the prosthesis vulnerable to be cut by screws, which causes fiber fracture and fabric loosening [30,39,40].
Inappropriate postoperative biomechanical interaction between the prosthesis and the bone tunnel results in not only prosthesis failure but also secondary damage to the host tissue. Wang et al. reported two cases of knee synovitis out of 26 cases of ACL reconstruction with LARS after a mean follow-up of 11.2 years, concluding that the loosening of the fixation system is one of the heightened causes [41]. Meanwhile, Glezos et al. studied a case with knee synovitis after LARS implantation with preservation of the native ACL stump. No obvious rupture and infection have been proven absent; however, the presence of associated focal degenerate change was seen in the subsynovial layer on histological analysis, indicating the initiation of an arthritic process resulting from the foreign body synovitis [42]. One of the effective methods to tackle these is by promoting the bioactivity of the implantation material.
2.2. Biological performance
Aside from biomechanical performance, satisfying biological performance is also crucial to artificial ligaments for successful ACL reconstruction. A biocompatible biomaterial must meet the requirement that physiological reactions of host tissue be restricted within a clinically reasonable range. Different applications call for different biocompatibilities. For artificial ligaments, synovial liquid and bone tunnel compose the working environment for ligamentous grafts after ACL reconstruction. In ACL reconstruction, autograft and allograft demonstrate the best biocompatibility with excellent after-implantation strength and firm osseointegration. However, complications including hip ligament defect, local scar, hamstring deterioration, and postoperative infection have been reported. Meanwhile, known as being free from cross-infection and source shortage, most failures of artificial ligaments are caused by aseptic loosening according to follow-up studies [43,44]. Thus, synthetic ligament grafts are clinically not recommended for primary ACL reconstruction but could be used for revision procedures if other options are not available, which leaves the bioactivity deficiency problem as the most focus in the designing of artificial ligaments [45].
The earliest stages of artificial ligament design have witnessed the exploration of bioactivity enhancement. The earliest reported case of ligament reconstruction with artificial material was silver strings being applied to ACL reconstruction, however, failed due to a strong rejection reaction within two months [46]. Clinical application of artificial ligament was suspended until the invention of novel polymer materials in the 1950s. During this time, new artificial ligament products like PTFE net-shape prosthesis, multi-strand nylon-PET hybrid wire, and Proplast™ artificial ligaments [[47], [48], [49], [50]]. However, these products were reported with poor biomechanical performance. Later when came the 1970s, the clinical application of artificial ligaments in ligament reconstruction reached its peak with the presence of novel products like Gore-Tex™, Leeds-Keio™, 3M Kennedy LAD™, and LARS™, among which LARS™ is by far the most reliable [19].
Clinical results after long-term follow-ups confirmed LARS™ artificial ligament with efficacy in fast functional recovery of ACL [44,45,51]. In most cases, the complication of ACL reconstruction with LARS is attributed to biological failure, which induces mechanical instability, instead of mere mechanical failure [17]. It is worth pointing out that, biological performance and mechanical performance are practically interlocked as the promotion of either side cast brightness on the other. In the early phases after implantation, a mechanical stable graft shelters autologous ligament recovery, which is crucial for firm osseointegration with the host bone. In return, a bony connection between the graft and host bone provides a stable biomechanical work environment for the graft.
3. Strategies for promotion of artificial ligament bioactivity
Based on the above discussion, current clinical available artificial ligaments have been known as biomechanically reliable within a certain duration, however likely to induce complications due to biological inertia. Thus, different strategies have been proposed to enhance the bioactivity of artificial ligaments (Fig. 2) and are reviewed in this section.
Fig. 2.
Strategies to enhance bioactivity of artificial ligament categorized as graft-based (A, B) and cell-based (C, D). (A) Biodegradable material implanted into a bone tunnel gets adhered by fibroblasts secreting ECM, and with time lapsing the original material gets replaced by autologous tissue that creeps back. (B) Bioactive additives like TCP crystal and bioglass fiber get coated onto the fabric and contribute to osteointegration formation. (C) Fibers with different radii of curvature result in different cell polarization tendencies. A large radius of curvature results in circumferential polarization while the small one results in axial polarization. (D) 4-arm-PEG hydrogel network demonstrating different physicochemical properties that mediate fibroblast behavior by cellular signals.
3.1. Scaffold-based strategy
Traditional synthetic grafts made for ligament treatment are expected to stay biomechanically and biochemically stable throughout the service life, leaving no chance for ligamentization. However, the latest research shed light on the transition of ligamentous graft from synthetic material to autologous tissue by introducing biodegradable material or bioactive additives to the scaffold system.
In the initial stages of artificial ligament design, the graft was simply regarded as a replacement for natural ligament tissue and thus remains permanent after implantation. After the first few attempts, artificial ligaments have been widely clinically accepted in the 1980s and 1990s, during which artificial ligaments showed quite encouraging primary outcomes with low complication rates and good postoperative recovery [48]. These permanent artificial ligaments are composed of non-degradable materials, like carbon fiber, PET, or PTFE. Carbon fiber artificial ligament was one of the first-generation products in the early 1980s [36]. It populated for a while for satisfying tensile strength and was considered qualified in short-term follow-ups. However, later reports claimed poor biocompatibility of carbon fiber that host organizations fail to grow into pores among fibers. In long-term clinical observations, carbon fiber artificial ligaments were found to be vulnerable to abrasion, slack even fracture, leading to severe synovitis. Similarly, PTFE was also once popular as artificial ligament materials however ended up being eliminated from the market [52]. Gore-Tex™ is representative of PTFE artificial ligaments. The root cause for implant failure was believed to be poor biocompatibility of PTFE as host organizations completely failed to grow into the prosthesis [53,54]. Compared with carbon fiber and PTFE, PET possesses satisfying mechanical properties but also biocompatibility thus considered the most promising in artificial ligament fabrication. The most applied artificial ligaments today, such as LARS™ and Leeds-Keio™, take PET as raw material. However, poor bioactivity of PET artificial ligaments has been massively reported. Thus, novel prosthesis materials development and corresponding modification strategies have been a hot spot in the artificial ligament field.
3.1.1. Biodegradable material
Rather than non-degradable materials to compose permanent scaffolds, biodegradable materials degenerate in surgically created bone tunnels after implantation and leave space for natural tissue to creep back, thus have been employed in artificial ligament fabrication in recent years. Biodegradable materials used in artificial ligament composition, including natural polymers like collagen, silk, CHI, HA, synthetic polymers such as PLA, PGA, PLGA, PCL, and biodegradable based polymeric composites, have been used to produce scaffolds for biodegradable tendon and ligament tissue engineering as gels, membranes, or three-dimensional fibrous scaffolds [[55], [56], [57]]. Although these biodegradable scaffolds have been confirmed with good histocompatibility and osteoinductivity by previous research claiming that these scaffolds could promote healing between the graft and bone tunnels by leaving space for autologous tissue creep replacement, poor biomechanical properties have restricted them from further clinical application. Such a mechanical deficiency owes to the inherent strength of the material itself, but also inappropriate in-vivo degradation speed. As it takes weeks for autologous tissue to complete creeping replacement, a fast in-vivo degradation rate causing significant loss in graft strength would lead to the absence of effective mechanical support, which will eventually fail ACL reconstruction operation. Thus, the process of biodegradable ligament graft losing rigidity and newly formed autologous ligaments gaining rigidity must keep pace with each other.
However, the fabrication of slowly degradable biomaterial with satisfying biomechanical performance has always been significantly challenging. In spite, some researchers attempted to fabricate mechanically available artificial ligaments with biodegradable materials. Okada et al. [54] prepared CHI-HAP composite mono-fiber for ACL reconstruction using the coagulation method. The results showed that the addition of HAP inhibits swelling of the fibers compared with chitosan fiber, thus an improvement in bone-bonding of PET rope could be expected. However, HAP lowered the overall toughness. Meanwhile, Rangel et al. designed PNaSS/PCL-based graft material with a four-year degradation time and obtained suitable mechanical properties for native ACL replacement after surface functionalization [33]. Kawakami et al. fabricated artificial grafts using wet electrospinning processes using PEUU and evaluated them in vitro and in vivo in a rat model of ACL reconstruction with an emphasis on cellular filtration and remodeling along with any changes in the tensile strength of the graft [58]. According to the results, the synthetic wet PEUU grafts promoted cellular infiltration and neovascularization while also alleviating inflammation and increasing tensile strength. Rather than fiber modification, Yang et al. applied HPC in synthesizing coating for PET artificial ligament, reporting that graft osseointegration in the bone tunnel was enhanced without obvious loss in mechanical performance [59]. Li et al. put efforts into accessories by applying partially biodegradable TCP/PEEK anchors to assist a silk-based ACL graft to shelter from prompt mechanics loss [60]. According to biomechanical evaluations and histological observations in a porcine model, firm incorporation of the silk graft to the bone tunnel was identified as well as naturally similar histological transitions from the silk graft to bone.
3.1.2. Bioactive additive
Bioactive additives being incorporated with structural mesh materials have also been proven effective to endow artificial ligaments with bioactive properties. Such materials include polymers and bio-ceramics. Bioactive additives join the artificial ligament system in the forms of comonomer, and coating, being either injectable or rigid depending on the intended use [61,62].
Bioactive additives provide the matrices with the ability to stimulate bioreactions between materials and host tissue. One of the most used additives is bioactive glasses first discovered by Hench in the early 1970s [63]. Bioactive glasses, also known as bioglasses, mainly composed of silica tetrahedron, tend to form HAP when immersed in body fluid, thus considered promising in promoting graft-bone osseointegration. Chen et al. modified the PET sheet by coating bioglass on the surface. According to in vivo tests, bioglass-coated PET sheets behave significantly better in the max load-to-failure test, and distinct new bone formation was observed only in the bioglass-PET group [63]. Apart from osteogenesis additives like bioglass, HAP, and TCP, cell-oriented bioactive additives seek to mediate the cellular behavior of human MSCs. Human MSCs are multipotent cells that are known to be recruited to bone fracture sites and play a significant role in bone regrowth, thus intriguing researchers about recruiting endogenous human MSCs to promote the healing of bone tunnels. Anseth et al. fabricated thiol–ene photopolymerized hydrogels and governed human MSC migration within the in vivo microenvironment [64]. Such hydrogels can be coated onto artificial ligament fabrics by electrospinning or 3D printing [65,66]. Rather than non-organic gels, the physiologically active substance has also been enveloped to function as bioactive additives and reported with a bright prospect of improving graft-bone osseointegration [67]. Chen et al. designed an ECM/GelMA hydrogel scaffold via 3D printing with bMSC exosome as the bioactive additive. Such a scaffold enhanced chondrocyte migration and significantly facilitated cartilage regeneration in the animal model [68]. Most recently, living cells encapsulated or enveloped as additives to form the so-called “bioink” for 3D printing have been catching researchers’ eyes. Such a method is especially effective in bioactivity enhancement of artificial ligaments as newly formed tissues can be self-assembly and self-organizing owing to cellular microenvironmental cues provided by cells in the bioink, which will be discussed in the next part [[69], [70], [71]].
3.2. Cell-based strategy
Scaffold-based strategies discussed above have been experiencing tremendous progress and have proven effective in artificial ligament fabrication for bioactivity promotion. Meanwhile, some researchers relate bioactivity promotion to the postoperative behavior of cells surrounding the ligamentous grafts after implantation, providing that, based on tissue engineering principles, some cell-based strategies are supposed to efficiently enhance postoperative follow-ups of scaffolds [72,73].
Cell-based strategies are established on the fact that ample bMSCs in the bone tunnel possess the differentiation potential to meet the inadequacy of the healing capacity of the natural tendon and ligament tissues. Biomechanical cues function by adjusting cellular adherence and matrix formation, which further interferes with ECM secretion, and the mutual shape effect between ECM and scaffold will work together to meet tissue engineering demands for ligamentization as cells demonstrate morphological transformation according to the external stimulus, including biomechanical cues and biological cues, provided by the ECM in the organism [74]. Such cues provided by biomaterials may facilitate the restoration of the structure and function of damaged or dysfunctional autologous tissues, both in cell-based therapies and acellular therapies [75]. In this section, cell-based strategies are categorized as biophysical cues and biological cues are then discussed.
3.2.1. Biophysical cue
Biophysical signals include hardness, ductility, topographical factors, etc., which have been reported with the ability to motivate ligament healing and relieve osteoarthritis [76,77]. Thus, in artificial ligament design, these signals are supposed to be converted to bMSCs by proper ECM construction.
Stiffness: materials with different hardness and ductility mediate cellular behaviors by altering the cellular skeleton, thus being efficient in promoting osseointegration between the graft and host bone. Generally speaking, cells are prone to migrate towards hard regions, characterized by relatively higher young's module, which has been well-reviewed in some works [[78], [79], [80]]. Ito et al. reported a strengthening effect of elastin coating on the artificial ligament, resulting from enhancement of cartilage formation and bone formation around bone tunnels [81].
Topography: in terms of topographical factors, two-dimensional contact between the cell and matrix is the dominant contact form for artificial ligaments without postprocessing like coating or blending. Like the traditional cell culture method, artificial ligaments get implanted and work to provide a substrate for surrounding cells to adhere to. Researchers managed to fabricate micropatterns including grooves or humps arranged in spatial orders, which contributes to changes in hydrophilicity, surface energy, and consequently cell interactions [82]. Meanwhile, topographical variations are found in fibers that compose ligamentous grafts. The radius of curvature of fibers must also be taken into consideration. Ji et al. set up a series of research works on collective cell polarization and alignment, claiming that the nematic order parameter of substrate generates a spatial distribution of mechanical forces that function as a stress-driven mechanism to mediate the polarization–contraction relation of cells by developing direction- and position-dependent behaviors [[83], [84], [85]]. Also, artificial ligaments with postprocessing like coating or sol–gel process usually generate a relative layer, rather than merely a substrate, for cells to penetrate, providing cells with 3D contacts on a micrometer scale. Such a penetrable layer works in the same way as a culture medium in 3D cell culture.
3.2.2. Biochemical cue
Analogic to bone fracture, biochemical signals regulate histological morphology during graft healing [86]. Such biochemical signals, usually referring to cell growth factors, integrins, or pharmaceuticals, guide the different early healing phases including the proliferation phase, and maturation phase, throughout the graft healing process [87].
Integrins: integrins like Arginyl-glycyl-aspartate (RGD) sequence is known as the integrin-binding site of ECM proteins such as fibronectin and collagen, thus considered contributive in founding osseointegration. Khorolsuren et al. investigated periodontal ligament cells (PDLCs) on RGD-synthetic polypeptide conjugate coatings through cell surface characteristics and concluded that cyclic RGD conjugates can be adopted as a biocompatible material to strengthen the cell adhesion inducer effect of artificial cell-substrate coating, thus might be suitable for therapeutic applications when adopting orthopaedic scaffolds [88].
Growth factors: The growth factor is important for the smart incorporation of tissue regeneration and repairing process to regulate cellular behaviors, which can be used for promoting the formation of ligamentous tissue [89]. Furumatsu et al. conducted a combined use of bFGF and GDF-5 to enhance the healing of medial collateral ligament injury. Peptide hydrogels were adopted to fabricate a self-assembling biodegradable scaffold to provide delivery of growth factors to injured tissue. Physiological characterization indicated that not only proliferation and migration of ligament fibroblast got enhanced, but also collagen synthesis and fiber alignment got regulated [90].
Pharmaceuticals: Some researchers managed to promote the bioactivity of the artificial ligament by the application of pharmaceuticals to restrain postoperative inflammations. Conventional pharmacological agents with a relatively short duration of action results in vulnerability to non-adherence, thus long-acting preparations have been proposed such as long-term implants with active substances with a sustained-release rate [91]. Bishop et al. [92] adopted vancomycin as an addition to orthopedic bone cement and reported that vancomycin contributed to eliminating the most common causative orthopedic implant pathogens without obvious mechanical property loss. Yet, little research is reported concerning pharmaceuticals application on artificial ligament design.
4. Manufacturing technologies for artificial ligament bioactivity promotion
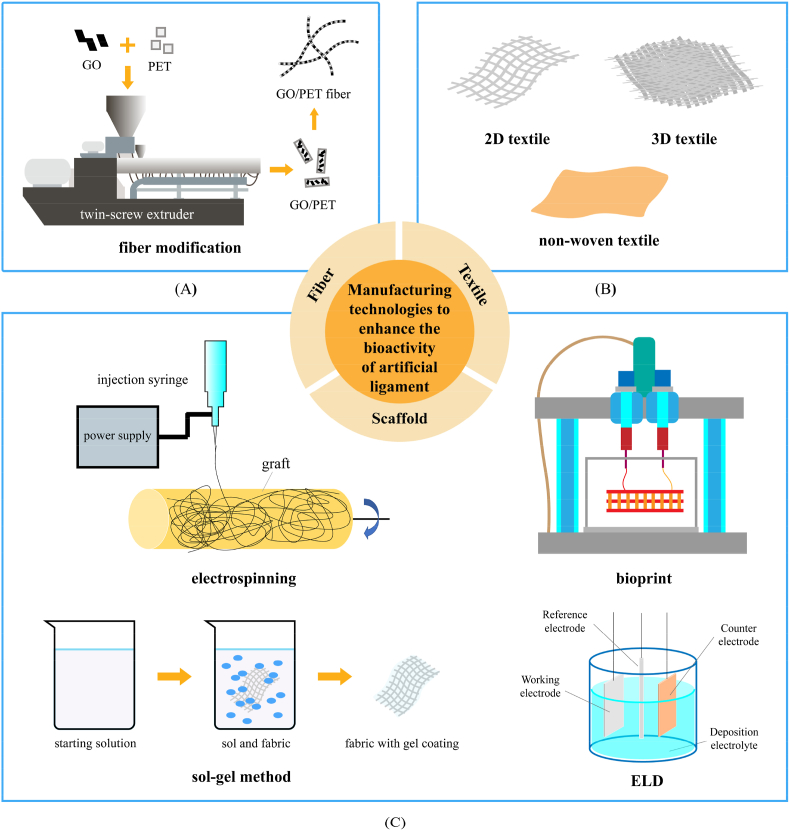
Based on the strategies discussed above, various manufacturing technologies for artificial ligament bioactivity promotion have been developed focusing on different aspects (Fig. 3). In this section, manufacturing technologies involved in recent research works will be reviewed according to a fiber-textile-scaffold category.
Fig. 3.
Manufacturing technologies to enhance bioactivity of artificial ligament categorized as fiber (A), textile (B), and scaffold (C). (A) Bioactive additives and matrix aggregates are put into the charging barrel of a twin-screw extruder and produced blended composite aggregates, which enables the production of composite fiber with bioactivity. (B) Diagram of 2D textile, 3D textile, and non-woven textile. (C) Diagram of coating forming on rolling graft via electrospinning, artificial tissue being bioprinted, sol–gel method and ELD process.
4.1. Fiber modification
Fiber modification, which alters the chemical composition of fabrics from the fiber level by stem grafting or blending modification, has been adopted to enhance the bioactivity of ligament grafts. Han et al. [93] investigated the effects of graphene modification on the bioactivation of PET-based artificial ligaments and claimed that graphene exhibits considerable potential for enhancing the surface bioactivation of materials. Mei et al. [94] managed to blend GO with PET aggregates to fabricate 2% GO/PET fiber via a twin-screw extruder and melt spinning. The as-fabricated fiber was reported with ease of weaving, and the fabrics demonstrated better hydrophily without scarifying mechanical strength compared to the control group. Meanwhile, human bMSCs were co-cultured with GO/PET fiber woven fabrics, and enhancement in cell growth, proliferation, and differentiation was observed.
4.2. Textile design
Textile designs cast an influence on the bioactivity of artificial ligaments by varying topographical cues like the fiber orientation and pore size of scaffolds [55]. Popular artificial ligaments are woven, knitted, or braided in various ways and possess different textiles, which largely accounts for prostheses' overall behavior. For artificial ligament prostheses, an interconnected pore network, in an order to allow the adhesion and migration of cells and growth of autologous tissue that creeps back, has been considered the essential morphological requirement aside from mimicking mechanical responses of native ACL [57,94].
Two-dimensional textile: Two-dimensional textile is by far the most industrially adopted and efficient method to fabricate artificial ligaments. Two-dimensional textile is to arrange the fibers in the warp and weft directions according to a certain law with a crisscross textile structure is obtained to meet different use purposes. At present, artificial ligament products used in ACL reconstruction in the world, such as Neoligaments™, Ligastic™, and LARS™, are prepared by two-dimensional weaving methods [95]. LARS™ is the most adopted in China among commercial artificial ligaments as mentioned above with two-dimensional textiles. According to initial clinical cases of artificial ligaments, friction caused by uneven stress between meridional fibers and parallel fibers was reported in joint flexion and extension, which eventually led to fatigue wear. To shelter from such a tribological problem, LARS™ adopted a three-stage design: parallel fibers are taken away from the middle stage and pre-twisted for 90° (i.e., free fibers), while both ends are normally woven [96]. Follow-up reports indicate satisfying therapeutic effect in early and middle terms as patients were able to regain athletic ability, confirming that LARS™ works even better than autologous hamstring graft at stabilizing the knee joint. Though regarded as clinically effective with such a bionic free-fiber structure, it proved hard to maintain the tensile property of LARS™ due to weaving method limitations [19,36].
Three-dimensional textile: Three-dimensional textile introduced the z-axis into the textile system by employing special three-dimension weaving machines to produce integral fabrics spreading in three dimensions without additional tailoring or sewing by simply configuring corresponding parameters as illustrated in Fig. 4. Cooper et al. [98] designed a fiber-based tissue-engineered scaffold for ligament replacement and conducted in vitro evaluation. Biodegradable PLAGA fibers were adopted to fabricate 3D-construct artificial ligaments with different predesigned braiding angles. The final product was a 48-yarn 3D circular braid and 48/60-yarn 3D rectangle braids with a construct. According to the observation, changes in braiding angle can alter the mode pore diameter, porosity, mechanical properties, and geometry. The maximum and ultimate tensile strength of the scaffolds was found to be a function of scaffold geometry, fiber number, and yarn density, and the stress–strain profile was found to be similar to natural ligament tissue in the 48-yarn rectangle group. Following was in vitro evaluation, during which the attachment morphology of ACL fibroblasts indicates that the optimal pore parameter for cells to grow into is 100–300 μm. As a 3D braided flexible interconnected network helps to transport oxygen and nutrients throughout the implant site, the scaffold demonstrates better bioactivity than previous ligament prostheses in intriguing cell proliferating [58]. However, as the number of knots greatly increases with the participation of the Z-axis, the risk of fabric disintegration also rises. Model simulation of 3D textiles contributes to optimizing braiding parameters design towards mechanical performance enhancement, yet research related to bioactivity enhancement is rarely found [[99], [100], [101]].
Fig. 4.
PGA three-dimensional woven scaffolds (A) Microstructure of a 3D orthogonally woven structure. 3D structures were woven by interlocking multiple layers of two perpendicularly oriented sets of in-plane fibers with the third set of fibers in the through-plane direction; (B) SEM image from the top surface of the construct [97].
Non-woven textile: Other than investigations of weaving procedure, non-woven fabrication methods like electrospinning, low-temperature sintering method, and model casting have been reported to introduce geometrical topography to the surface of polymer biomaterials like PET, PLA, and PLGA. Expected is that non-woven fabric should, however, studies concerning non-woven artificial ligament fabrication, interaction with biology systems, toxicity, and in-vivo studies are still in their infancy [66,102,103].
Textile design of artificial ligament should consider not only mechanical stability but also cytocompatibility. Studies of novel textiles with satisfying biocompatibility have been extensively reported, however, only a minority investigated biomechanical responses. Elmarzougui et al. investigated the impact of polyurethane yarns on the mechanical properties of braid artificial ligaments and concluded that incorporating elastic fibers leads to mechanical performances very close to those of the natural ACL [104]. Elmarzougui et al. measured hysteresis of dynamic fatigue of textile artificial ligaments and claimed that the preconditioning braided prosthesis is essential for decreasing energy dissipation and residual deformation [105]. Meanwhile, manufacturing technologies other than weaving equipment, such as computer simulation like the Finite Element Method, non-woven fabrication methods like electrospinning, low-temperature sintering method, and model casting. Some researchers tend to take advantage of computational simulation when deciding on optimal weaving parameters, which benefits patient-specific prosthesis design. Laurent et al. [32] studied the biomechanical behavior of a multilayer braided scaffold made of PLCL fibers with braiding parameters set up by using a dedicated Finite Element code. The report claims that the scaffold architecture is predictable with adjustable design parameters while the mechanical properties and biological performance are tailorable by adjusting the braiding angle, number of layers of the scaffold, or the diameter of the braided fibers. Still, further work is highly needed concerning the characterization and modeling of the effect of cyclic loading on the mechanical behavior of the manufactured prosthesis.
4.3. Scaffold modification
Some research seeks to fabricate a coating layer onto ligamentous prosthesis for bioactivity enhancement. Such coating layers contribute to bioactivity enhancement by providing both physical support and chemical cues to evoke tissue–material reactions. Commonly adopted coating technologies will be discussed in this section.
Sol–gel method: The sol–gel process is used to obtain a variety of high-purity inorganic oxides or hybrid inorganic-organic materials, based on the hydrolysis and condensation of metal or silicon alkoxide [106]. The sol–gel method allows for coatings with controlled stoichiometry and particle size, thus considered promising in ligamentous graft coating fabrication. Saito et al. [107] attained titania coating by sol–gel method and investigated the effect of titania-based surface modification of PET on bone-implant bonding and peri-implant tissue reaction. According to the test results, the bone-bonding ability was effectively achieved in PET materials by titania-based surface modification, owing to obvious HAP deposition induced by titania coating.
ELD: ELD is another frequently adopted coating method. Wang et al. [108] fabricated a composite coating consisting of collagen protein and calcium phosphate mineral silicon substrate by ELD. Self-assembly of collagen fibrils and the deposition of calcium phosphate minerals were observed, providing novel methods to coat inert artificial ligament fabrics for osseointegration promotion.
Bioprint: Following principles of tissue engineering, living cells or cell-derived bioactive matters are encapsulated in matrices like hydrogel to establish a cytocompatible composite and maintain mechanical properties by supporting 3D interaction and allowing tissue regeneration, which is called “bioink” [109]. Merceron et al. fabricated a 3D integrated muscle-tendon unite construct by bioprinting. The artificial muscle was fabricated by thermoplastic polyurethane co-printing with C2C12 cell-laden hydrogel-based bioink, while the artificial tendon was fabricated by poly(ε-caprolactone) co-printing with NIH/3T3 cell-laden hydrogel-based bioink [110]. Such a complex structure guaranteed cell viability as well as satisfying stiffness, providing a bright perspective in orthopedic use. Yet, no ligament-specific decellularized ECM bioink has been reported, which also leaves a vacancy for future efforts [111].
Electrospinning: Electrospinning allows the production of long continuous fibers with a controlled diameter ranging from nanometers to microns, mimicking the nanoscale structure of tendon and ligament ECM, providing topographical cues, mechanical cues as well as biochemical cues [22]. Chen et al. designed a “Swiss roll”-like bio bioactive hybrid scaffolds for promoting bone tissue ingrowth and tendon-bone healing after ACL reconstruction consisting of PCL electrospun nanofiber membranes loaded with recombinant rhBMP-7 and PET fiber mesh fabric [112]. Post-implant histological observation confirmed that such scaffolds enhanced osteogenesis at the tendon-bone interface and shortened the time of ligament-bone healing, thus offering better mechanical properties. It's worth noting that even though bioactive coating has been proven efficient in promoting osteointegration of artificial ligament fabrics and bone tunnels, long-term follow-ups for clinical reliability examination.
5. Perspective and outlook
In this mini-review, we have introduced the status of artificial ligament clinical performance and summarized current strategies for the enhancement of the bioactivity of artificial ligaments. Strategies to enhance the bioactivity of artificial ligaments have been categorized into two interpenetrating groups: one is to focus on the prosthesis design towards the creeping back of autologous tissue, and the other one is to focus on the behavior of bone-formation-related cells.
In a word, the final purpose of artificial ligament design is complete ligamentization, both engineers and clinicians are on the same page. Thus, cellular reaction with the ligamentous grafts is the best foothold and the combination of ligament prosthesis design and tissue engineering has been regarded as the most promising direction in developing the next new generation of artificial ligaments. The cell-based strategies, including biomechanical cues and biochemical cues, enable direct cellular reactions with synthetic grafts thus beneficial to osseointegration between the graft and host bone. Specifically, biomechanical cues, like topographical designs and stiffness variation, have been reported with the ability to modify the spatial contribution of cells, thus is expected to solve the problems of the random spatial distribution of collagens in early post-implant phases. Biochemical cues, on the other hand, mediating cellular behavior by interfering with intracellular and intercellular chemical reactions, provide another direct control on cell reactions with synthetic grafts. In fact, current commercial ligamentous graft products are lacking the ability to directly intrigue expected cellular reactions for osseointegration. Hence, deeper investigations and translations are needed to make such cell strategies from bench to applications.
Moreover, the establishment of simulation models for artificial ligaments’ performance under various physiological environments has been considered time- and labor-saving in graft design, thus relevant research fields should also be paid close attention to Refs. [[113], [114], [115], [116]]. It is worth pointing out that despite all the strategies and technologies mentioned above, to obtain an ideal market reception, a good balance of manufacturing costs and clinical benefits should be underlined throughout the design and manufacturing process.
Ethical statement
No human and animal subjects are involved.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
This work is financially supported by grants from Shenzhen Science and Technology Innovation Committee (RCBS20210706092410021) and Sun Yat-sen University (start-up fund). The authors appreciate helpful comments and suggestions from Prof. Dr. Jiali Wang, Sun Yat-sen University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2022.07.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jamil T., Ansari U., Najabat Ali M., Mir M. A review on biomechanical and treatment aspects associated with anterior cruciate ligament. Irbm. 2017;38(1):13–25. [Google Scholar]
- 2.He X., Li Y., Guo J., Xu J., Zu H., Huang L., et al. Biomaterials developed for facilitating healing outcome after anterior cruciate ligament reconstruction: efficacy, surgical protocols, and assessments using preclinical animal models. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120625. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi E.F., Tang K., Grant J.A. Is ACL repair really back? A review of modern techniques. Operat Tech Sports Med. 2021;29(2) [Google Scholar]
- 4.Mahapatra P., Horriat S., Anand B.S. Anterior cruciate ligament repair - past, present and future. J Exp Orthop. 2018;5(1):20. doi: 10.1186/s40634-018-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nwachukwu B.U., Patel B.H., Lu Y., Allen A.A., Williams R.J. Anterior cruciate ligament repair outcomes: an updated systematic review of recent literature. Arthroscopy. 2019;35(7):2233–2247. doi: 10.1016/j.arthro.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Murray M.M. Current status and potential of primary ACL repair. Clin Sports Med. 2009;28(1):51–61. doi: 10.1016/j.csm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J.O., Yasen S.K., Palmer H.C., Lord B.R., Britton E.M., Wilson A.J. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845–1851. doi: 10.1007/s00167-016-4150-x. [DOI] [PubMed] [Google Scholar]
- 8.Kon E., Di Matteo B., Altomare D., Iacono F., Kurpyakov A., Lychagin A., et al. Biologic agents to optimize outcomes following ACL repair and reconstruction: a systematic review of clinical evidence. J Orthop Res. 2022;40(1):10–28. doi: 10.1002/jor.25011. [DOI] [PubMed] [Google Scholar]
- 9.Taylor S.A., Khair M.M., Roberts T.R., DiFelice G.S. Primary repair of the anterior cruciate ligament: a systematic review. Arthroscopy. 2015;31(11):2233–2247. doi: 10.1016/j.arthro.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Marsland D., Morris A.M., Gould A.E.R., Calder J.D.F., Amis A.A. Systematic review of tendon transfers in the foot and ankle using interference screw fixation: outcomes and safety of early versus standard postoperative rehabilitation. Foot Ankle Surg. 2022;28(2):166–175. doi: 10.1016/j.fas.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Borjali A., Nourani A., Moeinnia H., Mohseni M., Korani H., Ghias N., et al. Comparison of mechanical properties in interference screw fixation technique and organic anterior cruciate ligament reconstruction method: a biomechanical study. BMC Muscoskel Disord. 2021;22(1):1047. doi: 10.1186/s12891-021-04788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onggo J.R., Nambiar M., Pai V. Fixed- versus adjustable-loop devices for femoral fixation in anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2019;35(8):2484–2498. doi: 10.1016/j.arthro.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Noonan B.C., Dines J.S., Allen A.A., Altchek D.W., Bedi A. Biomechanical evaluation of an adjustable loop suspensory anterior cruciate ligament reconstruction fixation device: the value of retensioning and knot tying. Arthroscopy. 2016;32(10):2050–2059. doi: 10.1016/j.arthro.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Born T.R., Biercevicz A.M., Koruprolu S.C., Paller D., Spenciner D., Fadale P.D. Biomechanical and computed tomography analysis of adjustable femoral cortical fixation devices for anterior cruciate ligament reconstruction in a cadaveric human knee model. Arthroscopy. 2016;32(2):253–261. doi: 10.1016/j.arthro.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Schroven I.T.J., Geens S., Beckers L., Lagrange W., Fabry G. Experience with the Leeds-Keio artificial ligament. Knee Surg Sports Traumatol Arthrosc. 1994;2:214–218. doi: 10.1007/BF01845590. [DOI] [PubMed] [Google Scholar]
- 16.Wang C.L., Hsiao C.K., Hsu A.T., Dung C.Z., Chang C.H. Biocompatibility and mechanical property of LARS artificial ligament with tissue ingrowth. J Mech Med Biol. 2012;12(1) [Google Scholar]
- 17.Li H., Yao Z., Jiang J., Hua Y., Chen J., Li Y., et al. Biologic failure of a ligament advanced reinforcement system artificial ligament in anterior cruciate ligament reconstruction: a report of serious knee synovitis. Arthroscopy. 2012;28(4):583–586. doi: 10.1016/j.arthro.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Banoriya D., Purohit R., Dwivedi R.K. Advanced application of polymer based biomaterials. Mater Today. 2017;4(2):3534–3541. [Google Scholar]
- 19.Roe J., Bourke H., Glezos C., Salmon L., Waller A., Pinczewski L. Revision ACL reconstruction after use of the LARS ligament: a case series. Med Sci Sports Exerc. 2012;15:S142. [Google Scholar]
- 20.Fan D., Ma J., Zhang L. Patellar tendon versus artificial grafts in anterior cruciate ligament reconstruction: a systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):478. doi: 10.1186/s13018-021-02624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J., Wei X.C., Li L., Cao X.M., Li K., Guo L., et al. Autografts vs synthetics for cruciate ligament reconstruction: a systematic review and meta-analysis. Orthop Surg. 2020;12(2):378–387. doi: 10.1111/os.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva M., Ferreira F.N., Alves N.M., Paiva M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J Nanobiotechnol. 2020;18(1):23. doi: 10.1186/s12951-019-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlstedt L., Dalen N., Jonsson U. Goretex prosthetic ligament vs. Kennedy ligament augmentation device in anterior cruciate ligament reconstruction. A prospective randomized 3-year follow-up of 41 cases. Acta Orthop Scand. 1990;61(3):217–224. doi: 10.3109/17453679008993504. [DOI] [PubMed] [Google Scholar]
- 24.Johnson D. Gore-Tex synthetic ligament. Operat Tech Sports Med. 1995;3(3):4. [Google Scholar]
- 25.Noyes F.R., Barber-Westin S.D. In: Noyes' knee disorders: surgery, rehabilitation, clinical outcomes. 2nd ed. FR N., SD B.-W., editors. Elsevier; 2017. Anterior cruciate ligament revision reconstruction: graft options and clinical outcomes; pp. 221–257. [Google Scholar]
- 26.Saragaglia D., Leroy J.M., De Sousa B., Tourne Y., Abu Ai Zahab M. Medium-term results of 173 ligamentoplasties of the anterior cruciate ligament using the Macintosh technique reinforced by the Kennedy ligament augmentation device (LAD) Knee Surg Sports Traumatol Arthrosc. 1995;3:68–74. doi: 10.1007/BF01552377. [DOI] [PubMed] [Google Scholar]
- 27.Gür E., Başbozkurt M., Baydar M., Ertür N. The use of dacron synthetic ligament prosthesis in the reconstruction of anterior cruciate ligament. Acta Orthop Traumatol Turcica. 1991;25:380–384. [Google Scholar]
- 28.Matsumoto H., Fujikawa K. Leeds-Keio artificial ligament: a new concept for the anterior cruciate ligament reconstruction of the knee. Keio J Med. 2001;50(3):161–166. doi: 10.2302/kjm.50.161. [DOI] [PubMed] [Google Scholar]
- 29.Houck D.A., Kraeutler M.J., McCarty E.C., Bravman J.T. Fixed- versus adjustable-loop femoral cortical suspension devices for anterior cruciate ligament reconstruction: a systematic review and meta-analysis of biomechanical studies. Orthop J Sports Med. 2018;6(10) doi: 10.1177/2325967118801762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao K., Chen S., Wang L., Zhang W., Kang Y., Dong Q., et al. Anterior cruciate ligament reconstruction with LARS artificial ligament: a multicenter study with 3- to 5-year follow-up. Arthroscopy. 2010;26(4):515–523. doi: 10.1016/j.arthro.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Iannace S., Sabatini G., Ambrosio L., Nicolais L. Mechanical behaviour of composite artificial tendons and ligaments. Biomaterials. 1995;16(9):675–680. doi: 10.1016/0142-9612(95)99693-g. [DOI] [PubMed] [Google Scholar]
- 32.Laurent C.P., Durville D., Mainard D., Ganghoffer J.F., Rahouadj R. A multilayer braided scaffold for anterior cruciate ligament: mechanical modeling at the fiber scale. J Mech Behav Biomed Mater. 2012;12:184–196. doi: 10.1016/j.jmbbm.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Rangel A., Colaço L., Nguyen N.T., Grosset J.F., Egles C., Migonney V. Adapting mechanical characterization of a biodegradable polymer to physiological approach of anterior cruciate ligament functions. Irbm. 2020:39–48. [Google Scholar]
- 34.Lei T., Zhang T., Ju W., Chen X., Heng B.C., Shen W., et al. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact Mater. 2021;6(8):2491–2510. doi: 10.1016/j.bioactmat.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egbo M.K. A fundamental review on composite materials and some of their applications in biomedical engineering. J King Saud Univ Eng Sci. 2020:557–568. [Google Scholar]
- 36.Jadeja H., Yeoh D., Lal M., Mowbray M. Patterns of failure with time of an artificial scaffold class ligament used for reconstruction of the human anterior cruciate ligament. Knee. 2007;14(6):439–442. doi: 10.1016/j.knee.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Brydges N.M., Argyle D.J., Mosley J.R., Duncan J.C., Fleetwood-Walker S., Clements D.N. Clinical assessments of increased sensory sensitivity in dogs with cranial cruciate ligament rupture. Vet J. 2012;193(2):545–550. doi: 10.1016/j.tvjl.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Pereira H., Correlo V.M., Silva-Correia J., Oliveira J.M., Reis R.L., Espregueira-Mendes J. Migration of "bioabsorbable" screws in ACL repair. How much do we know? A systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):986–994. doi: 10.1007/s00167-013-2414-2. [DOI] [PubMed] [Google Scholar]
- 39.Shiwaku K., Suzuki T., Matsumura T., Takashima H., Otsubo H., Yamashita T. Bioabsorbable interference screws can be used with less tunnel widening in anatomic rectangular tunnel anterior cruciate ligament reconstruction with a bone-patellar-tendon-bone graft. Knee. 2020:1293–1299. doi: 10.1016/j.knee.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Cheng P., Han P., Zhao C., Zhang S., Zhang X., Chai Y. Magnesium inference screw supports early graft incorporation with inhibition of graft degradation in anterior cruciate ligament reconstruction. Sci Rep. 2016;6 doi: 10.1038/srep26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C.L., Hsiao C.K., Ku M.C., Chang C.H. Arthroscopic anterior cruciate ligament reconstruction with lars artificial ligament: an 8–15-year follow-up. J Mech Med Biol. 2013;13(2) [Google Scholar]
- 42.Glezos C.M., Waller A., Bourke H.E., Salmon L.J., Pinczewski L.A. Disabling synovitis associated with LARS artificial ligament use in anterior cruciate ligament reconstruction: a case report. Am J Sports Med. 2012;40(5):1167–1171. doi: 10.1177/0363546512438510. [DOI] [PubMed] [Google Scholar]
- 43.Yang W., Huang X., Wang S., Wang H., Huang W., Shao Z. The long-term outcomes of different grafts in anterior cruciate ligament reconstruction: a network meta-analysis. J Orthop Translat. 2021;26:16–30. doi: 10.1016/j.jot.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parchi P.D., Gianluca C., Dolfi L., Baluganti A., Nicola P., Chiellini F., et al. Anterior cruciate ligament reconstruction with LARS artificial ligament results at a mean follow-up of eight years. Int Orthop. 2013;37(8):1567–1574. doi: 10.1007/s00264-013-1917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X.G., Wang F., He X., Feng J.T., Hu Y.C., Zhang H., et al. Network meta-analysis of knee outcomes following anterior cruciate ligament reconstruction with various types of tendon grafts. Int Orthop. 2020;44(2):365–380. doi: 10.1007/s00264-019-04417-8. [DOI] [PubMed] [Google Scholar]
- 46.Li H., Li J., Jiang J., Lv F., Chang J., Chen S., et al. An osteogenesis/angiogenesis-stimulation artificial ligament for anterior cruciate ligament reconstruction. Acta Biomater. 2017;54:399–410. doi: 10.1016/j.actbio.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Li H., Chen S. Biomedical coatings on polyethylene terephthalate artificial ligaments. J Biomed Mater Res. 2015;103(2):839–845. doi: 10.1002/jbm.a.35218. [DOI] [PubMed] [Google Scholar]
- 48.Mertsching H., Walles T., Hofmann M., Schanz J., Knapp W.H. Engineering of a vascularized scaffold for artificial tissue and organ generation. Biomaterials. 2005;26(33):6610–6617. doi: 10.1016/j.biomaterials.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 49.Gray V.P., Amelung C.D., Duti I.J., Laudermilch E.G., Letteri R.A., Lampe K.J. Biomaterials via peptide assembly: design, characterization, and application in tissue engineering. Acta Biomater. 2022;140:43–75. doi: 10.1016/j.actbio.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin W., Cai J., Sheng D., Liu X., Chen J., Chen S. Establishment of near and non isometric anterior cruciate ligament reconstruction with artificial ligament in a rabbit model. J Orthop Translat. 2021;29:78–88. doi: 10.1016/j.jot.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parchi P.D., Ciapini G., Paglialunga C., Giuntoli M., Picece C., Chiellini F., et al. Anterior cruciate ligament reconstruction with LARS artificial ligament-clinical results after a long-term follow-up. Joints. 2018;6(2):75–79. doi: 10.1055/s-0038-1653950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eskew J.R., Miles D., Davis F., Bruce J. Graft tunnel mismatch in anterior cruciate ligament reconstruction with bone-patellar tendon-bone grafts. J Orthop Surg. 2020;3(1):123–129. [Google Scholar]
- 53.Ramos D.M., Dhandapani R., Subramanian A., Sethuraman S., Kumbar S.G. Clinical complications of biodegradable screws for ligament injuries. Mater Sci Eng C. 2020;109(110423) doi: 10.1016/j.msec.2019.110423. [DOI] [PubMed] [Google Scholar]
- 54.Okada T., Nobunaga Y., Konishi T., Yoshioka T., Hayakawa S., Lopes M.A., et al. Preparation of chitosan-hydroxyapatite composite mono-fiber using coagulation method and their mechanical properties. Carbohydr Polym. 2017;175:355–360. doi: 10.1016/j.carbpol.2017.07.072. [DOI] [PubMed] [Google Scholar]
- 55.Senel-Ayaz H.G., Har-El Y.E., Ayaz H., Lelkes P.I. In: Functional 3D tissue engineering scaffolds. Deng Y., Kuiper J., editors. Woodhead Publishing; Cambridge: 2018. Textile technologies for 3D scaffold engineering; pp. 175–201. [Google Scholar]
- 56.del Hoyo-Gallego S., Perez-Alvarez L., Gomez-Galvan F., Lizundia E., Kuritka I., Sedlarik V., et al. Construction of antibacterial poly(ethylene terephthalate) films via layer by layer assembly of chitosan and hyaluronic acid. Carbohydr Polym. 2016;143:35–43. doi: 10.1016/j.carbpol.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Laflamme M., Lamontagne J., Guidoin R. In: Biomedical textiles for orthopaedic and surgical applications. Blair T., editor. Woodhead Publishing; Cambridge: 2015. Anterior cruciate ligament prostheses using biotextiles; pp. 145–190. [Google Scholar]
- 58.Kawakami Y., Nonaka K., Fukase N., Amore A., Murata Y., Quinn P., et al. A cell-free biodegradable synthetic artificial ligament for the reconstruction of anterior cruciate ligament in a rat model. Acta Biomater. 2021;121:275–287. doi: 10.1016/j.actbio.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 59.Yang J., Jiang J., Li Y., Li H., Jing Y., Wu P., et al. A new strategy to enhance artificial ligament graft osseointegration in the bone tunnel using hydroxypropylcellulose. Int Orthop. 2013;37(3):515–521. doi: 10.1007/s00264-012-1723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X., He J., Bian W., Li Z., Zhang W., Li D., et al. A novel silk-based artificial ligament and tricalcium phosphate/polyether ether ketone anchor for anterior cruciate ligament reconstruction - safety and efficacy in a porcine model. Acta Biomater. 2014;10(8):3696–3704. doi: 10.1016/j.actbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 61.Mathew A.P., Oksman K., Pierron D., Harmand M. Fibrous cellulose nanocomposite scaffolds prepared by partial dissolution for potential use as ligament or tendon substitutes. Carbohydr Polym. 2012;87(3):2291–2298. [Google Scholar]
- 62.Fu Z., Cui J., Zhao B., Shen S.G., Lin K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J Orthop Translat. 2021;28:118–130. doi: 10.1016/j.jot.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y., Chen S., Jiang J., Li H., Gao K., Zhang P. The effect of 58S bioactive glass coating on polyethylene terephthalates in graft-bone healing. J Bionic Eng. 2012;9(4):470–477. [Google Scholar]
- 64.Kyburz K.A., Anseth K.S. Three-dimensional hMSC motility within peptide-functionalized PEG-based hydrogels of varying adhesivity and crosslinking density. Acta Biomater. 2013;9(5):6381–6392. doi: 10.1016/j.actbio.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z., Wang Y., Yan J., Zhang K., Lin F., Xiang L., et al. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504–534. doi: 10.1016/j.addr.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Brown T.D., Dalton P.D., Hutmacher D.W. Melt electrospinning today: an opportune time for an emerging polymer process. Prog Polym Sci. 2016;56:116–166. [Google Scholar]
- 67.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 68.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levit R.D., Landazuri N., Phelps E.A., Brown M.E., Garcia A.J., Davis M.E., et al. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. 2013;2(5) doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Vos P., Lazarjani H.A., Poncelet D., Faas M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev. 2014;67-68:15–34. doi: 10.1016/j.addr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Jakab K., Norotte C., Marga F., Murphy K., Vunjak-Novakovic G., Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2(2) doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu L., Hindieh J., Leong D.J., Sun H.B. Advances of stem cell based-therapeutic approaches for tendon repair. J Orthop Translat. 2017;9:69–75. doi: 10.1016/j.jot.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui L., Xiang S., Chen D., Fu R., Zhang X., Chen J., et al. A novel tissue-engineered bone graft composed of silicon-substituted calcium phosphate, autogenous fine particulate bone powder and BMSCs promotes posterolateral spinal fusion in rabbits. J Orthop Translat. 2021;26:151–161. doi: 10.1016/j.jot.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yilgor C., Yilgor Huri P., Huri G. Tissue engineering strategies in ligament regeneration. Stem Cell Int. 2012;2012 doi: 10.1155/2012/374676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lutolf M.P., Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 76.Yao S.Y., Cao M.D., He X., Fu B.S.C., Yung P.S.H. Biological modulations to facilitate graft healing in anterior cruciate ligament reconstruction (ACLR), when and where to apply? A systematic review. J Orthop Translat. 2021;30:51–60. doi: 10.1016/j.jot.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benazzo F., Zanon G., Pederzini L., Modonesi F., Cardile C., Falez F., et al. Effects of biophysical stimulation in patients undergoing arthroscopic reconstruction of anterior cruciate ligament: prospective, randomized and double blind study. Knee Surg Sports Traumatol Arthrosc. 2008;16(6):595–601. doi: 10.1007/s00167-008-0519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hong Y., Huber A., Takanari K., Amoroso N.J., Hashizume R., Badylak S.F., et al. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials. 2011;32(13):3387–3394. doi: 10.1016/j.biomaterials.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep Phys Sci. 2021;2(8) doi: 10.1016/j.xcrp.2021.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elosegui-Artola A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr Opin Cell Biol. 2021;72:10–18. doi: 10.1016/j.ceb.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Ito N., Hasegawa M., Unno H., Suzuki Y., Miura Y., Matsui Y., et al. Usefulness of elastin for ligament-bone junction healing in rabbits. J Orthop Translat. 2016;7:77–78. [Google Scholar]
- 82.Karp J.M., Yeo Y., Geng W., Cannizarro C., Yan K., Kohane D.S., et al. A photolithographic method to create cellular micropatterns. Biomaterials. 2006;27(27):4755–4764. doi: 10.1016/j.biomaterials.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 83.He S., Green Y., Saeidi N., Li X., Fredberg J.J., Ji B., et al. A theoretical model of collective cell polarization and alignment. J Mech Phys Solid. 2020;137 doi: 10.1016/j.jmps.2019.103860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He S., Su Y., Ji B., Gao H. Some basic questions on mechanosensing in cell–substrate interaction. J Mech Phys Solid. 2014;70:116–135. [Google Scholar]
- 85.Li X., He S., Xu J., Li P., Ji B. Cooperative contraction behaviors of a one-dimensional cell chain. Biophys J. 2018;115(3):554–564. doi: 10.1016/j.bpj.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donahue H.J. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26(5):417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 87.Yao S., Fu B.S., Yung P.S. Graft healing after anterior cruciate ligament reconstruction (ACLR) Asia Pac J Sports Med Arthrosc Rehabil Technol. 2021;25:8–15. doi: 10.1016/j.asmart.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khorolsuren Z., Lang O., Pallinger E., Foldes A., Szabolcs G.G., Varga G., et al. Functional and cell surface characteristics of periodontal ligament cells (PDLCs) on RGD-synthetic polypeptide conjugate coatings. J Periodontal Res. 2020;55(5):713–723. doi: 10.1111/jre.12760. [DOI] [PubMed] [Google Scholar]
- 89.Yang Y., Rao J.D., Liu H.Q., Dong Z.F., Zhang Z., Bei H.P., et al. Biomimicking design of artificial periosteum for promoting bone healing. J Orthop Translat. 2022;36:18–32. doi: 10.1016/j.jot.2022.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saiga K., Furumatsu T., Yoshida A., Masuda S., Takihira S., Abe N., et al. Combined use of bFGF and GDF-5 enhances the healing of medial collateral ligament injury. Biochem Biophys Res Commun. 2010;402(2):329–334. doi: 10.1016/j.bbrc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 91.Wojcik-Pastuszka D., Krzak J., Macikowski B., Berkowski R., Osinski B., Musial W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials. 2019;12(8) doi: 10.3390/ma12081202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bishop A.R., Kim S., Squire M.W., Rose W.E., Ploeg H.L. Vancomycin elution, activity and impact on mechanical properties when added to orthopedic bone cement. J Mech Behav Biomed Mater. 2018;87:80–86. doi: 10.1016/j.jmbbm.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 93.Wang C.H., Guo Z.S., Pang F., Zhang L.Y., Yan M., Yan J.H., et al. Effects of graphene modification on the bioactivation of polyethylene-terephthalate-based artificial ligaments. ACS Appl Mater Interfaces. 2015;7(28):15263–15276. doi: 10.1021/acsami.5b02893. [DOI] [PubMed] [Google Scholar]
- 94.Mei H. Tianjin Medical University; Tianjin: 2017. The study on the preparation and properties of GO/PET artificial liagment scaffold. [Google Scholar]
- 95.Gad K. In: Encyclopedia of toxicology. 3rd ed. P W, editor. Academic Press; Oxford: 2014. Medical textiles; pp. 182–190. [Google Scholar]
- 96.Ambrosio L., Gloria A., Causa F. In: Biomedical composites. L A, editor. Woodhead Publishing; Cambridge: 2010. Composite materials for replacement of ligaments and tendons; pp. 234–254. [Google Scholar]
- 97.Moutos F.T., Freed L.E., Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6(2):162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 98.Cooper J.A., Lu H.H., Ko F.K., Freeman J.W., Laurencin C.T. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26(13):1523–1532. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 99.Shikinami Y., Kawarada H. Potential application of a triaxial three-dimensional fabric (3-DF) as an implant. Biomaterials. 1998;19:617–635. doi: 10.1016/s0142-9612(97)00152-x. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y., Sun X. Digital-element simulation of textile process. Compos Sci Technol. 2000;61:311–319. [Google Scholar]
- 101.Green S.D., Long A.C., El-Said B.S.F., Hallett S.R. Numerical modelling of 3D woven preform deformations. Compos Struct. 2014;108:747–756. [Google Scholar]
- 102.Driscoll T.P., Nerurkar N.L., Jacobs N.T., Elliott D.M., Mauck R.L. Fiber angle and aspect ratio influence the shear mechanics of oriented electrospun nanofibrous scaffolds. J Mech Behav Biomed Mater. 2011;4(8):1627–1636. doi: 10.1016/j.jmbbm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agarwal S., Wendorff J.H., Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49(26):5603–5621. [Google Scholar]
- 104.Marzougui S., Kilani L., Ben A.S., Sakli F. Dynamic fatigue of braided textile ligaments. Arabian J Sci Eng. 2013;39(3):2205–2214. [Google Scholar]
- 105.Elmarzougui S., Ben A.S., Sakli F. Hysteresis measurement for characterising the dynamic fatigue of textile artificial ligaments. J Text Inst. 2011;102(2):109–113. [Google Scholar]
- 106.Nassar E.J., Ciuffi K.J., Calefi P.S., Rocha L.A., Faria E.H., Silva M.L.A., et al. In: Pignatello R., editor. vol. 1. InTech; Rijeka: 2011. Biomaterials and sol–gel process: a methodology for the preparation of functional materials. (Biomaterials science and engineering). [Google Scholar]
- 107.Saito T., Takemoto M., Fukuda A., Kuroda Y., Fujibayashi S., Neo M., et al. Effect of titania-based surface modification of polyethylene terephthalate on bone-implant bonding and peri-implant tissue reaction. Acta Biomater. 2011;7(4):1558–1569. doi: 10.1016/j.actbio.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 108.Fan Y., Duan K., Wang R. A composite coating by electrolysis-induced collagen self-assembly and calcium phosphate mineralization. Biomaterials. 2005;26(14):1623–1632. doi: 10.1016/j.biomaterials.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 109.Bagde A., Bansod A., Agrawal A., Raut A., Fulzele P., Quazi S.Z. Short review on formulation of the bioinks for tendon and ligament 3D bioprinting. Mater Today Proc. 2021 doi: 10.1016/j.matpr.2021.06.157. In press. [DOI] [Google Scholar]
- 110.Merceron T.K., Burt M., Seol Y.J., Kang H.W., Lee S.J., Yoo J.J., et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication. 2015;7(3) doi: 10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- 111.Park W., Gao G., Cho D.W. Tissue-specific decellularized extracellular matrix bioinks for musculoskeletal tissue regeneration and modeling using 3D bioprinting Technology. Int J Mol Sci. 2021;22(15) doi: 10.3390/ijms22157837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang P., Han F., Chen T., Wu Z., Chen S. Swiss roll"-like bioactive hybrid scaffolds for promoting bone tissue ingrowth and tendon-bone healing after anterior cruciate ligament reconstruction. Biomater Sci. 2020;8(871–83):871–883. doi: 10.1039/c9bm01703h. [DOI] [PubMed] [Google Scholar]
- 113.Adouni M., Faisal T.R., Dhaher Y.Y. Computational frame of ligament in situ strain in a full knee model. Comput Biol Med. 2020;126 doi: 10.1016/j.compbiomed.2020.104012. [DOI] [PubMed] [Google Scholar]
- 114.Mau J.R., Hawkins K.M., Woo S.L., Kim K.E., McCullough M.B.A. Design of a new magnesium-based anterior cruciate ligament interference screw using finite element analysis. J Orthop Translat. 2020;20:25–30. doi: 10.1016/j.jot.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mok S.W., Nizak R., Fu S.C., Ho K.K., Qin L., Saris D.B.F., et al. From the printer: potential of three-dimensional printing for orthopaedic applications. J Orthop Translat. 2016;6:42–49. doi: 10.1016/j.jot.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li L., Yang L., Zhang K., Zhu L., Wang X., Jiang Q. Three-dimensional finite-element analysis of aggravating medial meniscus tears on knee osteoarthritis. J Orthop Translat. 2020;20:47–55. doi: 10.1016/j.jot.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.