Abstract
Purpose
To examine whether lung ultrasound prior to prone positioning can predict the resulting gas-exchange response.
Materials and methods
This is a prospective observational study on critically-ill COVID-19 patients with a pilot and confirmation cohort. Lung ultrasound examinations were performed before prone positioning and gas-exchange parameters were recorded before and after position change.
Results
A total of 79 patients, 36 in the pilot cohort and 43 in the confirmation cohort, were included. In the pilot cohort, a moderate correlation between pre-turn lung ultrasound score index (LUSI) and change in PaO2/FiO2 after prone positioning was found. These findings were corroborated and extended upon in the confirmation cohort. The confirmation cohort found that anterior LUSI had the strongest correlation with follow-up time-points 1, 6, 12, and 24 h after prone positioning, with strength of correlation gradually increasing up to 24 h. In a multivariate model anterior aeration loss (odds ratio 0.035; 95%CI 0.003–0.319 for anterior LUSI >50%) and higher pre-turn PaCO2 (odds ratio 0.479 95% CI 0.235–0.979) were negatively predictive of a PaO2/FiO2 increase ≥20 mmHg.
Conclusions
Anterior LUSI, in addition to other clinical parameters, may be used to aid COVID-19 respiratory strategy and a clinician's decision to prone.
Keywords: Lung, Ultrasonography, COVID-19, Respiratory distress syndrome, Prone position
1. Introduction
Invasive mechanical ventilation is the mainstay of treatment for COVID-19 patients with acute respiratory failure. Prone positioning may confer several beneficial respiratory and cardiovascular changes in this patient category: optimizing lung recruitment, ventilation/perfusion matching, more homogeneous distribution of ventilator stress and right ventricular offloading. For patients with severe ARDS, this has been shown to improve outcomes. However, in some patients, prone positioning is ineffective or even detrimental. Moreover, prone positioning may result in accidental extubation, catheter dislodgement, (optic) pressure ulcers, nerve injury, and complicated cardiopulmonary resuscitation [1].
Thus, before deciding to prone, the (procedural) risks should be carefully weighed against the expected respiratory benefits. Unfortunately, reliable tools to predict individual effects of prone positioning do not exist. Response is often defined as a PaO2/FiO2 change ≥20 mmHg [2,3]. Previous investigations on computed tomography failed to identify predictors of response to prone positioning in Acute Respiratory Distress (ARDS) patients [4].
Lung ultrasound score can detect alterations in lung aeration across position changes, and more so, may even predict response to prone positioning [5,6]. Accordingly, lung ultrasound may help guide clinical decisions about respiratory support strategies for COVID-19 patients [7,8]. However, additional evidence is required, as another study did not find an association between LUS and oxygenation response to prone positioning [9].
The aim of this study was to investigate if a lung ultrasound examination prior to position change can predict gas-exchange response. We hypothesized that lung ultrasound score correlates with gas-exchange response after position change.
2. Materials and methods
2.1. Study design and setting
This is a prospective observational study with a pilot and confirmation cohort performed at a tertiary ICU in Amsterdam, The Netherlands. Data for the pilot cohort was collected from November 11th 2020 until February 15th 2021. Data for the confirmation cohort was collected between May 1st 2021 and February 1st 2022. The local institutional review board (METC VUmc) approved the study and necessity for informed consent was waived (2020.011). The STROBE checklist was used in the drafting of this manuscript (EQUATOR network, 2015).
2.2. Participants
All adult (≥18 years) patients admitted to the ICU with COVID-19 that were mechanically ventilated and underwent a lung ultrasound examination within 8 h prior to prone positioning were eligible for inclusion. Patients were only included if they were in supine position for at least the prior 4 h. This interval was selected based on previous research that showed dynamic changes in lung ultrasound-detectable changes within (at maximum) the first 3 h of prone positioning [5]. Patients were not included if they did not undergo prone positioning or no investigator was available.
2.3. Variables
For both cohorts, baseline characteristics including demographics, admission chronology, and ventilator settings were collected from the electronic patient dossier closest to the time of ultrasound examination. The following gas-exchange parameters were collected at time of ultrasound and after prone positioning: arterial oxygen pressure (PaO2), fraction of inspired oxygen (FiO2), and arterial carbon dioxide pressure (PaCO2). These were used to calculate PaO2/FiO2 and A-a gradient. In the pilot cohort, gas-exchange parameters were collected once within the 24 h after prone positioning. In the confirmation cohort, gas-exchange parameters were evaluated at set times in order to more accurately determine the progression of gas-exchange response: 1, 6, 12, and 24 h after prone positioning. Local protocol recommended prone sessions of at least 16 h, which commonly translated to 24 h per session based on logistic experience, safety, and effectivity [10]. Secondarily, parameters on respiratory mechanics (compliance, defined as volume divided by driving pressure; positive end-expiratory pressure, PEEP; ventilatory ratio; and corrected minute ventilation) and hemodynamics (noradrenaline; mean arterial pressure; and pulse frequency) were also collected before prone positioning and at 1 and 12 h after prone positioning [ 11,12]. Prone positioning number and reason for re-supination (if within 24 h) were also collected.
The changes, or response (Δ), across follow-up were calculated for all parameters relating to gas-exchange. Patients with a PaO2/FiO2 change of ≥ + 20 mmHg after prone positioning were classified as responders, whereas those with a PaO2/FiO2 change <20 mmHg were classified as non-responders [2,3]. Secondarily, patients could also be classified according to PaCO2 clearance ≥1 mmHg [13].
2.4. Ultrasound measurements
All examinations were performed or supervised by certified ultrasound physicians using a COVID-19 unit-restricted SonoSite-Edge II ultrasound machine. Measurements were performed in lung setting either using a 10–5 MHz linear transducer (SonoSite L38) or 5–3 MHz curvilinear transducer (SonoSite C60) with multi-beam imaging off and tissue harmonic imaging off [14].
In the pilot cohort, extended lung ultrasound examinations were conducted in 12 zones [9,15]. In the confirmation cohort, only a concise examination of 6 zones was used, as it appears to be a surrogate for 12 zone lung ultrasound score for critically-ill COVID-19 patients in a preliminary analysis [16]. For both cohorts, anatomical regions posterior, lateral, and anterior were also calculated.
Offline analyses of ultrasound images were performed by two investigators (MLAH and RSWS) with extensive ultrasound experience. Investigators were blinded to patient's characteristics. The offline reviewers determined the lung ultrasound score of involvement per zone: normal = 0; well-separated B-lines (>2) = 1; coalescent B-lines, small consolidation = 2; lobar consolidation with tissue-like characteristics = 3 [15]. A lung ultrasound score index (LUSI) was calculated and used for all analyses, where lung ultrasound score was expressed as a percentage of total score achievable, with a higher index representing more aeration loss [17].
2.5. Statistical analysis
Data were collected using SPSS (v26, IBM). All data processing and analyses were performed using Python (v3.8, Jupyter Notebook) language for computing with a statistical suite of libraries. Baseline characteristics and outcome variables for both cohorts were presented and compared using univariate analyses to evaluate for differences.
Pilot cohort. A correlation matrix of LUSI parameters and Δ gas-exchange was estimated using the Spearman's rank correlation coefficient.
2.5.1. Confirmation cohort
A correlation matrix of LUSI and Δ gas-exchange was created across follow-up time-points to validate the findings in the pilot cohort. Furthermore, a clinically useful predictor variable for PaO2/FiO2 response (≥ + 20 mmHg) was identified in the following manner: 1. The LUSI parameter (total, anterior, lateral, or posterior) with the strongest absolute correlation with Δ PaO2/FiO2 was selected. 2. The parameter was used to create Receiver Operating Characteristics (ROC)-curves for PaO2/FiO2 response at all follow-up time-points. 3. The time-point with the largest area under the ROC curve was used to estimate a parameter cut-off using the Youden's J statistic. 4. A categorical LUSI predictor variable was created using this cut-off. Subsequently, the predictor LUSI variable was tested in a multivariate logistic regression model for PaO2/FiO2 response ≥ + 20 mmHg at the respective time-point. The multivariate model also contained other baseline variables that attained a p < .100 for PaO2/FiO2 response at the univariate analysis. Backwards elimination (P > .05) was performed to select clinically relevant variables.
2.5.2. Sample size
No sample size calculation was performed for the statistical analysis of the pilot cohort since it was a hypothesis-generating pilot. For the confirmation cohort, the power analysis was conducted in G-POWER based on the correlation of concise LUSI and PaO2/FiO2 response in the pilot cohort. Thus, an alpha of 0.05, a power of 0.80, and effect size of 0.370 requires a total sample size of 43 subjects [18].
3. Results
A total of 79 patients were included, 36 in the pilot cohort and 43 in the confirmation cohort. Baseline characteristics of both cohorts at are shown in Table 1 . In the pilot cohort, gas-exchange variables were collected at 36 follow-up instances at a median 6 [6.75] hours after prone positioning, none were missing. In the confirmation cohort, gas-exchange variables were collected at 172 follow-up instances (four follow-up time-points across 43 patients). Of these, ten (5.8%) were missing due to early re-supination for the following reasons: imaging requirement (3), facial pressure ulcer (2), prone positioning-induced respiratory failure (2), planned re-supination (2), and thorax pillow malposition (1). Granular LUS and LUSI data for both cohorts is shown in appendix A.
Table 1.
Baseline characteristics.
| Patients (n = 79) | Pilot (n = 36) | Confirmation (n = 43) | P-value | |
|---|---|---|---|---|
| Age | 65.3 ± 9.1 | 68.7 ± 8.7 | 62.6 ± 8.5 | 0.003 |
| Gender | 58 (73.4%) | 27 (75.0%) | 31 (72.1%) | 0.804. |
| BMI | 29.8 ± 6.3 | 28.4 ± 5.4 | 31.0 ± 7.0 | 0.067 |
| Fully vaccinated | 9 (11.4%) | 0 (0%) | 9 (20.1%) | 0.004 |
| SOFA score | 7 [4] | 7.5 [4] | 7 [4] | 0.346 |
| Admission to examination (days) | 4.0 [6.5] | 4.6 [5.8] | 3.7 [8.8] | 0.855 |
| Examination to turn (hours) | 1.8 [3.7] | 1.0 [4.8] | 2.8 [6.9] | 0.206 |
| PaCO2 (kPa) | 7.1 ± 2.0 | 7.0 ± 1.9 | 7.1 ± 2.2 | 0.734 |
| PaO2/FiO2 (mmHg) | 110.9 ± 21.6 | 110.2 ± 22.7 | 111.6 ± 20.8 | 0.595 |
| A-a gradient (kPa) | 44.9 ± 10.9 | 44.4 ± 10.7 | 45.3 ± 11.2 | 0.922 |
| PaO2/FiO2 responders | 47 (59.5%) | 25 (69.4%) | 22 (51.2%) | 0.113 |
| LUSI | 55.5 ± 16.4 | 58.6 ± 16.5 | 52.8 ± 16.1 | 0.136 |
Assessment of PaO2/FiO2 response was selected at six hours after prone positioning in the confirmation cohort. This was based on the interval between turn and PaO2/FiO2 responder assessment in the pilot cohort (median 6.0, mean 8,4). Conversion factor for kPa to mmHg is 7.500, conversion factor for mmHg to kPa is 0.133.
3.1. Pilot cohort: correlation of LUSI and gas-exchange parameters
The Δ gas-exchange parameters before and after prone positioning in the pilot cohort are shown in Appendix B. The average difference in gas-exchange parameters before and after prone positioning was +26.7 ± 46.6 mmHg in PaO2/FiO2 (p = .023), −5.6 ± 14.3 in A-a gradient (p = .102), and + 0.1 ± 1.6 in PaCO2 (p = .915).
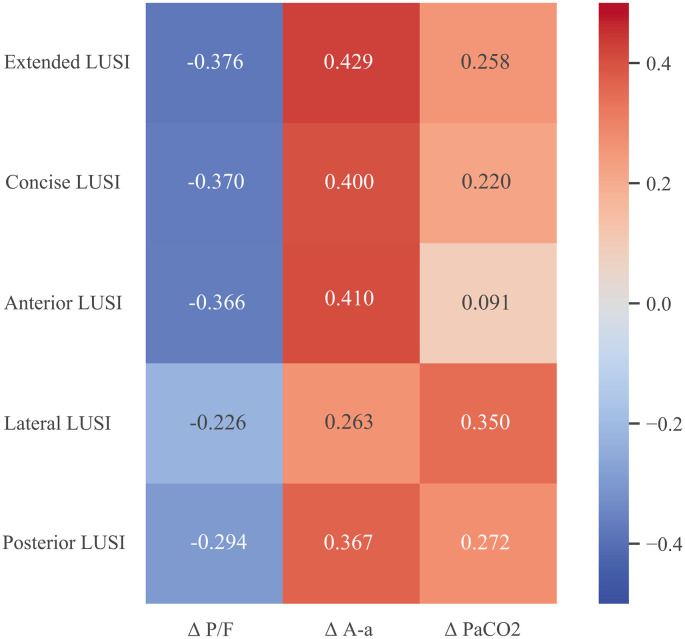
The correlation between LUSI prior to position change and gas-exchange response to position change is shown in Fig. 1 . Extended, anterior, and concise LUSI had positive correlation coefficient with Δ A-a gradient and negative correlation coefficient with Δ PaO2/FiO2 (p < .05) (in order of magnitude). Lateral LUSI had the highest (positive) correlation coefficient with Δ PaCO2 (p < .05).
Fig. 1.
Correlation heat map of LUSI and Δ gas-exchange across prone positioning. Numbers are Spearman’s correlation coefficient. Heat range is from −0.5 to 0.5.
3.2. Confirmation cohort: correlation of LUSI and gas-exchange parameters
The mean PaO2/FiO2 ratio increased gradually throughout the 24 h after proning, with the peak occurring at 24 h after prone positioning (p < .001). Accordingly, the mean A-a gradient gradually decreased across time-points (p < .001). PaCO2 (p = .850), PEEP (p = .838), and compliance (p = .649) did not change across time-points. Appendix C shows the progression of gas-exchange, respiratory mechanics, and hemodynamic parameters preturn and after prone positioning in the confirmation cohort.
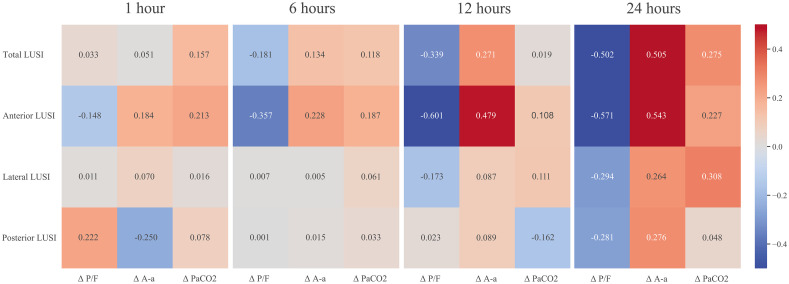
Fig. 2 shows the correlation heat map between LUSI parameters and Δ gas-exchange. Posterior LUSI had the highest (negative) correlation with Δ PaO2/FiO2 one hour after prone positioning. Across all remaining time-points, anterior LUSI had the highest (negative) correlation coefficients for Δ PaO2/FiO2. Lateral LUSI had a (positive) correlation with Δ PaCO2 at 24 h after prone positioning. Appendix D shows progression of correlation across time-points for anterior LUSI and Δ PaO2/FiO2.
Fig. 2.
Correlation heat map of LUSI parameters and gas-exchange parameters across follow-up time-points 1, 6, 12, and 24 hours. Numbers are Spearman’s correlation coefficient. Heat range is from −0.5 to 0.5.
The correlation between LUSI and respiratory mechanics as well as hemodynamic parameters is shown in appendix E and F. All correlations were weak except for the correlation between anterior LUSI and compliance at 12 h after prone positioning (coefficient of −0.419; p = .027).
3.3. Confirmation cohort: identification of a clinically useful predictor
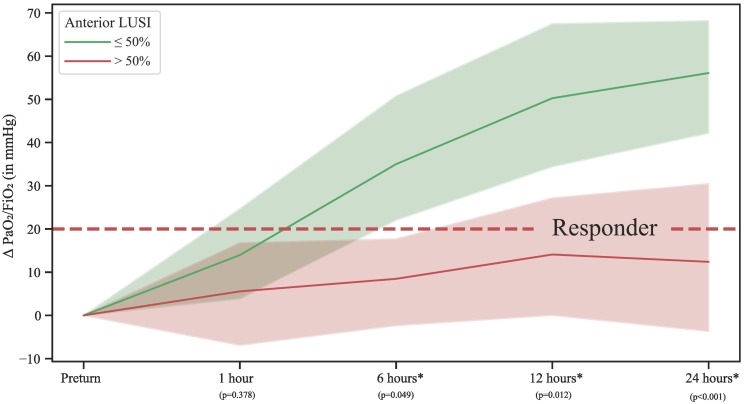
The ROC-curves of anterior LUSI for the prediction of PaO2/FiO2 responders (≥ + 20 mmHg) are shown in appendix G. The area under the curve was estimated as 0.71, 0.67, 0.78, and 0.86 at 1, 6, 12, and 24 h respectively. At 24 h, the Youden's J statistic estimated anterior LUSI cut-off at 50%. Fig. 3 shows differences in gas-exchange benefit for subjects with a LUSI >50% and ≤ 50% across follow-up time-points. A significant difference in Δ PaO2/FiO2 was found from six hours after prone positioning and on; differences were larger when the interval between prone positioning and follow-up time-point was larger. Appendix H shows ROC-curves with area under the curve when dichotomizing patients for PaCO2 clearance ≥1 mmHg, which were all estimated as 0.60 or smaller.
Fig. 3.
Progression of Δ PaO2/FiO2 across all time-points when comparing anterior LUSI >50 and ≤50%. The asterix (*) refers to a significant difference between categories of anterior LUSI. Shaded area is 95% confidence interval. A graph with absolute PaO2/FiO2 is found in appendix I.
3.4. Confirmation cohort: multivariate logistic regression model
Five candidate predictor variables (including the anterior LUSI predictor variable) were selected based on univariate analysis (appendix J). Two variables remained in the model after stepwise backwards elimination in the multivariate model: anterior LUSI >50% and PaCO2 (appendix K). The ORs were 0.035 (95% CI 0.003–0.319) and 0.479 (95% CI 0.235–0.979), respectively. Thus, the odds of a patient being a PaO2/FiO2 responder when having anterior LUSI >50% is 0.035. The accompanying diagnostic accuracy parameters for detecting patients who will not respond using an anterior LUSI>50% cut-off are: sensitivity of 72.7% and a specificity of 87.0%.
4. Discussion
The main findings of this study on lung ultrasound to predict gas-exchange response to prone positioning in critically ill COVID-19 patients were the following: i. A higher LUSI prior to prone positioning correlates with worse gas-exchange response, in particular on anterior examination. This finding was derived and validated in this study; ii. Anterior LUSI >50% (a lung ultrasound score of 4 out of 6 or more) is predictive of lack of PaO2/FiO2 response with an OR of 0.035 (95% CI 0.003–0.319).
This study shows that prone positioning induced greater beneficial gas-exchange response when lung ultrasound found well-aerated pulmonary parenchyma, especially in anterior regions. Immediate evaluation of gas-exchange parameters following prone positioning appears inadequate to fully appreciate the full effect on gas-exchange, as it gradually increased and peaked at 24 h. Gas-exchange changes beyond 24 h were not examined in this study. Differences in correlation coefficients across anatomical regions hint that response is correlated not only to extent but also to localization. These findings are consistent with a previous ARDS study which suggested that a well-aerated anterior lung has greater potential for ventilation-perfusion matching [6]. Interestingly, another investigation suggests that a well-aerated anterior lung simply indicates a focal ARDS phenotype (prone sensitive) as opposed to non-focal ARDS (PEEP sensitive) [19]. Tailoring treatment according to these phenotypes may lead to survival benefit [20]. This study demonstrates that lung ultrasound can play a direct role in tailored treatment of COVID-19 ARDS.
The confirmation cohort identified a useful cut-off of LUSI >50% to predict lack of oxygenation response with a substantial effect size: odds ratio of 0.035 for response ≥ + 20 mmHg. As an example of clinical application: a total anterior lung ultrasound of four or more, corresponding to the presence of anterior bilateral confluent B-lines, subpleural consolidations, or worse is predictive of poor response. Moreover, as evidenced by the correlation coefficients and plots in Appendix D, an even lower anterior LUSI corresponds to substantially improved PaO2/FiO2 response. These results may have clinical implications as a recent studies in COVID-19 patients found higher survival in the gas-exchange responders group [2,21]. Patients with, or at risk for, hemodynamic instability or position-related complications may be evaluated with lung ultrasound to aid decisions and provide an astute respiratory strategy. Additionally, PaCO2 was also predictive of PaO2/FiO2 response, albeit to a lesser degree. A reason may be because inadequate ventilation, with reduced PaCO2 clearance as a consequence, eventually also limits oxygenation.
Lastly, one must be cautious to draw conclusions on patient survival as previous investigations in non-COVID-19 ARDS demonstrate that the beneficial effect of prone positioning cannot be predicted purely based on improved oxygenation parameters [13,22]. Moreover, a general consensus on lung ultrasound's predictive value is lacking; another study did not find an association with oxygenation response after prone positioning [9]. Thus, the decision to prone should not be dictated solely based on prediction of oxygenation response (e.g. by LUSI), but carefully weighed based on integration of several clinical parameters.
This study has several limitations. This is an observational study; total 24 h follow-up was not completed on several patients due to premature re-supination. These missing data therefore bias the results in the direction of PaO2/FiO2 responders. The predictive ability of LUSI may have been even more pronounced had these patients not been re-supinated. Moreover, the current study primarily and robustly investigated the prediction of gas-exchange response; mechanical ventilation settings or hemodynamics were not prospectively controlled. Although our results show no significant differences in respiratory mechanics after prone positioning at one or twelve hours, these variables were not comprehensively standardized with advanced ventilator titration. Future investigations should robustly explore the role of respiratory mechanics parameters such as mechanical power and end-expiratory lung volume considering their potential mediation of survival in ARDS patients. Similarly, (minimally) invasive hemodynamic profiles were not prospectively collected, but their response to prone positioning may be further explored in future literature. There is methodological heterogeneity in lung ultrasound and although this study offers results consistent with the literature, there are other methodological variations that may be worth considering such as protocol type, zone number, transducer type, and machine settings. Lastly, subject age was different between cohorts. This may be caused by the increase in vaccination coverage for elderly patients during ongoing pandemic or by different viral variant predominance. However, slight differences between cohorts occurring during the evolving course of this pandemic do not appear to affects results and may strengthen the general validity of the current study's findings.
Our study also has several strengths. This investigation presents a comprehensive evaluation of gas-exchange response subsequent to position-change and provides a new application of lung ultrasound monitoring in this setting. Observations made in the pilot cohort were replicated and refined in the confirmation cohort, increasing general validity. Finally, our study is adequately powered and is the largest to date on this subject.
5. Conclusions
This study derived and validated that a high lung ultrasound score prior to prone positioning correlates to poor gas-exchange response. In clinical practice a simple anterior LUSI >50% predicts lack of PaO2/FiO2 response ≥ + 20 mmHg with a substantial odds ratio of 0.035. Previous evidence showed that patient survival may not purely depend on gas-exchange response. Thus, prone positioning should be a carefully considered decision based on the integration of several clinical parameters including (anterior) lung ultrasound.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
M.L.A. Heldeweg: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. A. Mousa: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. J. van Ekeren: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. A.W.E. Lieveld: Conceptualization, Data curation, Writing – review & editing. R.S. Walburgh-Schmidt: Data curation, Writing – review & editing. J.M. Smit: Conceptualization, Data curation, Writing – review & editing. M.E. Haaksma: Conceptualization, Data curation, Writing – review & editing. H.J. de Grooth: Formal analysis, Supervision, Writing – original draft, Writing – review & editing. L.M.A. Heunks: Supervision, Writing – original draft, Writing – review & editing. P.R. Tuinman: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2022.154173.
Appendix A. Supplementary data
Supplementary material
References
- 1.Gattinoni L., Taccone P., Carlesso E., Marini J.J. Prone position in acute respiratory distress syndrome, rationale, indications, and limits. Am J Respir Crit Care Med. 2013 Dec 1;188(11):1286–1293. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
- 2.Langer T., Brioni M., Guzzardella A., Carlesso E., Cabrini L., Castelli G., et al. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. 2021 Apr 6;25(1):128. doi: 10.1186/s13054-021-03552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatte G., Sab J.M., Dubois J.M., Sirodot M., Gaussorgues P., Robert D. Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med. 1997;155:473–478. doi: 10.1164/ajrccm.155.2.9032181. [DOI] [PubMed] [Google Scholar]
- 4.Papazian L., Paladini M.H., Bregeon F., Thirion X., Durieux O., Gainnier M., et al. Can the tomographic aspect characteristics of patients presenting with acute respiratory distress syndrome predict improvement in oxygenation-related response to the prone position? Anesthesiology. 2002;97(3):599–607. doi: 10.1097/00000542-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Wang X.T., Ding X., Zhang H.M., Chen H., Su L.X., Liu D.W. Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit Care. 2016;20:1–8. doi: 10.1186/s13054-016-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prat G., Guinard S., Bizien N., Nowak E., Tonnelier J.M., Alavi Z., et al. Can lung ultrasonography predict prone positioning response in acute respiratory distress syndrome patients? J Crit Care. 2016;32:36–41. doi: 10.1016/j.jcrc.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Avdeev S.N., Nekludova G.V., Trushenko N.V., Tsareva N.A., Yaroshetskiy A.I., Kosanovic D. Lung ultrasound can predict response to the prone position in awake non-intubated patients with COVID-19 associated acute respiratory distress syndrome. Crit Care. 2021;25:35. doi: 10.1186/s13054-021-03472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain A., Via G., Melniker L., Goffi A., Tavazzi G., Neri L., et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. doi: 10.1186/s13054-020-03369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haddam M., Zieleskiewicz L., Perbet S., Baldovini A., Guervilly C., Arbelot C., et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42:1546–1556. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 10.Mancebo J., Fernández R., Blanch L., Rialp G., Gordo F., Ferrer M., et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006 Jun 1;173(11):1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 11.Sinha P., Calfee C.S., Beitler J.R., Soni N., Ho K., Matthay M.A., et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019 Feb 1;199(3):333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusina F., Albani F., Bertelli M., Cavallo E., Crisci S., Caserta R., et al. Corrected minute ventilation is associated with mortality in ARDS caused by COVID-19. Respir Care. 2021 Apr;66(4):619–625. doi: 10.4187/respcare.08314. [DOI] [PubMed] [Google Scholar]
- 13.Albert R.K., Keniston A., Baboi L., Ayzac L., Guérin C. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:494–496. doi: 10.1164/rccm.201311-2056le. [DOI] [PubMed] [Google Scholar]
- 14.Touw H.R.W., Tuinman P.R., Gelissen H.P.M.M., Lust E., Elbers P.W.G. Lung ultrasound: routine practice for the next generation of internists. Neth J Med. 2015;73(3):100–107. 25852109 [PubMed] [Google Scholar]
- 15.Bouhemad B., Mongodi S., Via G., Rouquette I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology. 2015;122(2):437–447. doi: 10.1097/ALN.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 16.M.L.A. Heldeweg, A.W.E. Lieveld, H.J. de Grooth, L.M.A. Heunks, P.R. Tuinman; ALIFE study group. Determining the optimal number of lung ultrasound zones to monitor COVID-19 patients: can we keep it ultra-short and ultra-simple? Intensive Care Med 2021;47(9):1041–1043 10.1007/s00134-021-06463-6. [DOI] [PMC free article] [PubMed]
- 17.Heldeweg M.L.A., Lopez Matta J., Haaksma M.E., Smit J., Elzo Kraemer C., de Grooth H.J., et al. Lung ultrasound and computed tomography to monitor COVID-19 pneumonia in critically ill patients: a two-center prospective cohort study. Intens Care Med Exp. 2020;9:1. doi: 10.1186/s40635-020-00367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009 Nov;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 19.Pierrakos C., Smit M.R., Pisani L., Paulus F., Schultz M.J., Constantin J.M., et al. Lung ultrasound assessment of focal and non-focal lung morphology in patients with acute respiratory distress syndrome. Front Physiol. 2021;12:730857. doi: 10.3389/fphys.2021.730857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantin J.M., Jabaudon M., Lefrant J.Y., Jaber S., Quenot J.P., Langeron O., et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7(10):870–880. doi: 10.1016/s2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 21.Park J., Lee H.Y., Lee J., Lee S.M. Effect of prone positioning on oxygenation and static respiratory system compliance in COVID-19 ARDS vs. non-COVID ARDS. Respir Res. 2021;22(1):220. doi: 10.1186/s12931-021-01819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L., Vagginelli F., Carlesso E., Taccone P., Conte V., Chiumello D., et al. Decrease in Paco2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med. 2003;31:2727–2733. doi: 10.1097/01.ccm.0000098032.34052.f9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material