Abstract
Progressive obstructive pulmonary disease is the primary life-shortening complication in people with Cystic Fibrosis (CF); improvement in life expectancy has led to increased prevalence of non-pulmonary complications. Patients with CF are considered to be at low risk for coronary artery disease (CAD). We report here a case series of six patients with CF with and without known cystic fibrosis related diabetes (CFRD) who had acute myocardial infarction (AMI) requiring coronary stent placement. This was a heterogeneous group of patients, without a clear pattern of consistent risk factors. Interestingly, most patients in this cohort had low LDL. In this review, we discuss risk factors of cardiovascular disease (CVD) that may apply to the CF population. While CAD is rare in people with CF, it does occur. We postulate that the risk will grow with increased longevity and the increased prevalence of co-morbidities such as obesity and dyslipidemia.
Keywords: Cystic fibrosis, Cystic fibrosis related diabetes, Coronary artery disease, Hyperlipidemia, Low cholesterol level, Risk factors
Introduction:
Cystic Fibrosis (CF) is an autosomal recessive disorder, caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Abnormalities in CFTR channel function lead to multisystem disease [1]. Pulmonary complications are the primary life-shortening complication in people with CF, but improvement in life expectancy has led to increased recognition and prevalence of non-pulmonary complications. Although there have been a few isolated case reports of coronary artery disease (CAD) in people with CF, patients with CF have long been considered to be at a low risk for CAD and death from atherosclerotic cardiovascular disease in this population has yet to be reported in the published literature.
Type 1 and type 2 diabetes are associated with increased risk of CAD, which is a frequent cause of death in people with those forms of diabetes. Cystic fibrosis related diabetes (CFRD), one of the most common complications of CF, is found in up to 50 % of adult patients and 80 % of those with classical severe mutations who are over the age of 50 years [2]. While CFRD is known to be associated with microvascular complications like retinopathy, neuropathy and nephropathy [3], macrovascular complications related to CFRD are considered to be rare [4].
We queried 29 adult endocrinologists from CF centers across the USA through email to collect cases of known CAD in people with CF to gain insights about the prevalence and potential risk factors of this problem. These physicians are part of the CF Foundation’s Emerging Leaders in CF Endocrinology program (EnVision). Twenty physicians responded (69 % response rate) and known CAD cases were provided from 4 centers, other respondents were not aware of any case of CAD at their respective CF centers. These centers serve approximately 5,521adult patients with CF (only counting those registered in the CF registry).
In this case series, we describe six patients with CF with CAD and AMI requiring coronary stent placement (Table 1) that were identified through this query. In addition, we performed an extensive literature review for potential reports of CAD in people with CF and identified few additional isolated cases [7], [8], [9], [10]. Thus, while we report a case series of CAD requiring treatment in patients with CF, this complication is still quite rare. Clinical characteristics of the cases included in this series are summarized in Table 1.
Table 1.
Clinical characteristics of patients before their AMI presentation.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age | 52 | 54 | 31 | 34 | 68 | 57 |
| Ethnicity | White | White | Black | White | White | White |
| Gender | Male | Male | Female | Female | Female | Male |
| CF gene mutation | F508del homozygous 3272-26A->G |
F508del homozygous |
F508del homozygous 3120 + 1G->A |
F508del homozygous | F508del/R117C | DelF508/R117C |
| FEV1 at time of MI | 41 % | 26 % | Not Available | 45 % | 60 % | 93 % |
| CFRD, A1c at time of the MI /Diabetes treatment |
A1c 6.2 %, no OGTT or official diagnosis | Yes, 6.9 % On repaglinide |
Yes, A1c 5.5 % On insulin |
Yes, A1c 10.5 % On insulin |
Yes, A1c 6.2 % Diet |
Yes, A1c 7.1 % On insulin + metformin + dulaglutide |
| CFRD related microvascular complications | No | No | retinopathy, nephropathy | retinopathy, neuropathy, peripheral vascular disease | No | retinopathy, neuropathy |
| Duration of CFRD (Years) |
N/A | 7 | 7 | 25 | 9 | 28 |
| BMI kg/m2 | 21 | 23 | 35 | 22 | 16 | 41 |
| Lipid labs | LDL 73 mg/dL, HDL 59 mg/dL, Triglyceride 90 mg/dL | LDL 53 mg/dL, HDL 52 mg/dL, and Triglyceride 132 mg/dL | LDL 55 mg/dL, HDL 56 mg/dL, Triglyceride 119 mg/dL | LDL 55 mg/dL, HDL 45 mg/dL, Triglycerides 96 mg/dL | LDL 52 mg/dL, HDL 45 mg/dL, Triglyceride 43 mg/dL | LDL 116 mg/dL. HDL 41 mg/dL Triglycerides 450 mg/dL |
| Pancreatic insufficiency | yes | yes | yes | Yes | Yes | Yes |
| Hypertension | yes | yes | yes | No | No | Yes |
| CAD presentation and outcome | Acute chest pain, NSTEMI, s/p stent placement |
Acute chest pain, NSTEMI, s/p stent placement | Acute chest pain, NSTEMI, s/p stent placement | Acute chest pain, NSTEMI and ischemic cardiomyopathy s/p stent placement | Acute dyspnea and hypoxia, NSTEMI, s/p stent placement | Acute left arm and neck pain, + hypertensive emergency, NSTEMI s/p stent placement |
| Smoking history | No | No | yes | No | No | Yes |
| Family history of CVD disease | Yes, Stroke in both parents | No | No | Unknown | unknown | No |
| Organ Transplant | No | No | Yes – lungs | No | No | No |
| Statin use | No, started post MI | No, started post MI | No, started post MI | No, started post MI | No | Yes, intensified post MI |
| Aspirin use | No, started post MI | No, started post MI | No, started post MI | No | No | Yes |
Case Presentation
Case 1
A 52-year-old white male with moderately severe lung disease (FEV1 41 %), pancreatic insufficiency (PI) and hypertension presented with chest pain thought initially to be due to anxiety. He was found to have non-ST elevation myocardial infarction (NSTEMI). He underwent coronary angiogram and stent placement in the left circumflex coronary artery (LCX). In the months prior to this presentation, he had experienced episodes of pulmonary exacerbations with a decline in lung function and weight. He did not have a known history of CFRD. His BMI was 21 and HbA1c was 6.2 % during this admission. Lipid panel showed LDL level 73 mg/dL, HDL 59 mg/dL and triglyceride (TGL) 90 mg/dL (Table 1). He never smoked or used illicit drugs. His family history was significant for CAD in both parents. He was started on statin after the cardiac event. He has had no further episodes of AMI and is now 63 years old. He is currently treated with the triple combination CFTR modulator therapy, elexacaftor–tezacaftor–ivacaftor (ETI), and has experienced improvement in lung function after starting ETI.
Case 2
A 54-year-old white male with severe lung disease (FEV1 26 %), hypertension, CFRD and PI presented with sudden onset chest pain and was diagnosed with NSTEMI requiring stent placement. CFRD with no known microvascular complications was diagnosed 7 year ago and was being managed with repaglinide with HbA1c of 6.9 %. Lipid panel showed LDL 53 mg/dL, total cholesterol 131 mg/dL, HDL 52 mg/dL, and TGL 132 mg/dL. He was started on statin and insulin therapy after the AMI. He has done well since with no further episodes of AMI and is now 66 years old. No history of smoking or use of illicit drugs. His family history is significant for a father with type 2 diabetes. He was not on CFTR modulator therapy at the time of the AMI.
Case 3
A 31-year-old black female with CF, 10 years post bilateral lung transplant with hypertension and PI presented with sudden onset chest pain and was found to have a NSTEMI requiring coronary stent placement. CFRD, diagnosed 3 years post-transplant and treated with insulin, was complicated by retinopathy and nephropathy. Nephropathy was likely also related to transplant medications. At the time of this admission, HbA1c was 5.5 % and BMI 35 kg/m2. Lipid profile showed LDL 55 mg/dL, HDL 56 mg/dL, and TGL 119 mg/dL. She has done well post-stent placement from a cardiac standpoint, with no further acute cardiac issues. She was a former smoker and quit tobacco use around seven years prior to her AMI. Family history was non-contributory.
Case 4
A 34-year-old White female with moderately severe lung disease (FEV1 45 %), poorly controlled CFRD, gastroesophageal reflux disease, PI, anxiety, depression, and chronic kidney disease presented with chest pain. No history of hypertension or smoking. She was diagnosed with an NSTEMI and ischemic cardiomyopathy with transthoracic echocardiogram showing EF 35 % with new wall motion abnormalities including hypokinetic mid to distal anterior, anteroseptal, distal inferoseptal and apical walls. Cardiac catheterization was notable for severe proximal stenosis in the left anterior descending (LAD) coronary artery and mild-moderate non-obstructive disease in the LCX and right coronary artery (RCA). A drug eluting stent was placed in the LAD at that time. This patient was diagnosed with CFRD at age 9 years and had poorly controlled diabetes for many years leading to complications including diabetic retinopathy, neuropathy, peripheral vascular disease, and severe gastroparesis. Her diabetes had been managed with an insulin pump for the previous 7 years, but she rarely gave herself insulin with meals or checked her glucose levels. She reported that her HbA1c was > 12 % for many years, and just prior to the cardiac event was 10.4 %. Her lipid profile showed total cholesterol 119 mg/dL, TGL 96 mg/dL, HDL 45 mg/dL, LDL 55 mg/dL. She is currently being treated with ETI (started after she was diagnosed with cardiac disease).
Case 5
A 68-year-old woman, with an CF diagnosed at age 59, and with moderate obstructive lung disease (FEV1 60 %), PI with malnutrition and BMI of 16 kg/m2. She had been on ETI for a year prior to presenting with acute shortness of breath and hypoxia. She was found to have an NSTEMI. A transthoracic echocardiogram showed an apical wall motion abnormality. She underwent coronary catheterization demonstrating a 95 % stenosis of the LAD resulting in the placement of a single drug eluting stent. Her initial hypoxia and dyspnea slowly improved after the stent placement. Her history is significant for CFRD without significant complications and not on insulin therapy, with an HbA1c of 6.2 %. No history of smoking, hypertension, or illicit drug use, and no family history of premature atherosclerotic cardiovascular disease (CVD). Lipid panel demonstrated total cholesterol 106 mg/dL, TGL 43, HDL 45, LDL 52.
Case 6
A 57-year-old male with mild CF (FEV1 93 %), morbid obesity, CFRD (A1C 7.1 %), HFpEF presented with acute left arm and neck pain and hypertensive emergency. He was found to have an NSTEMI. Cardiac catheterization revealed significant occlusion of the circumflex artery which was stented with a drug-eluting stent. His right coronary artery had an occlusion of the right to mid proximal artery which appeared chronic and had notable bridging collaterals that was medically managed. Ventricular ejection fraction was 55 to 65 %. The patient had a 15 pack-year smoking history but no notable family history of CVD. Lipid panel demonstrated total cholesterol 211 mg/dL, TG 450, HDL 41, LDL 116. He was already taking aspirin and a statin, and the statin was intensified. He was started on ETI prior to being diagnosed with cardiac disease.
Discussion
Atherosclerotic heart disease is believed to be rare in patients with CF since it is so seldom reported, even as patients are living longer. One possible reasoning is that people with CF do not develop symptomatic CAD is because their LDL levels are low, presumably related to fat malabsorption and perhaps to the basic CF defect. In this manuscript, we report six cases in which patients with CF presented with typical symptoms of CAD and were diagnosed with AMI requiring coronary stenting. Five out of the six patients in this series had low LDL levels.
In the CF literature, atherosclerosis appears to be rare in adults with CF, and actual CAD requiring intervention even less common. One study found no evidence of narrowing of coronary vessels or atherosclerotic plaques in 14 patients with severe CF lung disease (median age 47, 64 % with CFRD) who had undergone screening angiogram prior to lung transplant [5]. A cross sectional study assessing the prevalence of pulmonary hypertension and left ventricle (LV) function in 18 patients with severe stable lung disease in CF noted normal LV function in all study participants except for one, a 40-year-old patient with diabetes who underwent further evaluation with thallium perfusion scan confirming CAD [6]. CAD was incidentally found on autopsy in a 41-year-old woman with CFRD, dyslipidemia and severe hypertension with nephrosclerosis. She died of respiratory failure in the setting of pulmonary hypertension [7].
There have been a handful of isolated case reports of CAD requiring treatment. A 48-year-old man with CF with a BMI of 26 kg/m2, good lung function and a non-diabetic OGTT presented with throat pain radiating to his jaw and left arm. His cardiovascular risk factors included family history of a cardiovascular accident in his father and premature CAD in his cousin at the age of 30. Coronary angiography revealed complete occlusion of the proximal right coronary artery requiring stenting [8]. A 52-year-old male with CF presented with exertional dyspnea and productive cough. He had no documented history of hypertension or CFRD. Labs included HbA1c 5.1 %, total cholesterol 139 mg/dl, LDL 80 mg/dl, HDL 30 mg/dl and TGL 76 mg/dl. Cardiac catheterization revealed multi-vessel disease. He was not a candidate for coronary artery bypass due to his severe lung disease, and underwent PCI with stenting of his LAD [9]. Two cases of young women with CFRD and CF were recently reported to have STEMI requiring stenting. Both women had risk factors for CAD; in fact, one had elevated LDL levels and the other had history of organ transplant with hypertension [10].
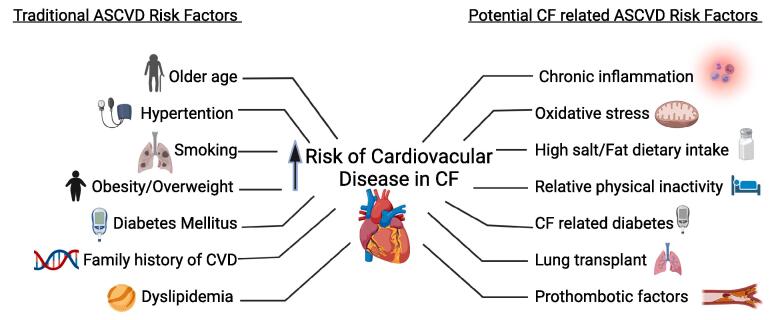
Age, sex, dyslipidemia, hypertension, obesity, cigarette smoking, family history, and diabetes are some of the traditional risk factors for CAD in the general population (Fig. 1). Reverri EJ et al discussed specific risk factors that potentially could promote the development of CVD in patients with CF, including chronic inflammatory respiratory tract disease, systemic inflammation, high oxidative stress, high dietary fat intake, a prothrombotic state, endothelial dysfunction, lung transplant, relative physical inactivity and low/dysfunctional HDL (Fig. 1). They also highlighted that other traditional CVD risk factors such as elevated LDL, obesity and tobacco use tend to be absent or minimally present in patients with CF [10].
Fig. 1.
Atherosclerotic cardiovascular disease (ASCVD) risk factors -traditional and potentially related to CF.
CAD has an estimated lifetime risk of 48.6 % for men and 31.7 % for women at the age of 40 in the general population [11]. Data from the Framingham cohort shows that the prevalence of CAD increases with age and is higher in men versus women. It is estimated to be 6.3 % between ages 40–59 years, and the trend is similar for myocardial infarctions, with a prevalence of 3.3 % [12]. These data are not available for patients with CF. It has been argued that patients with CF might not live long enough to experience CVD. CF lifespan continues to rapidly increase with newer and more effective therapies so this may become more apparent as time goes on. Four out of the six patients in this case series were in 50 s and 60 s when their myocardial event occurred. Case 3 and 4 were both women in their 30 s with risk factors for CAD such as lung transplant and poorly controlled diabetes. The question arises as to what made them more prone to having a myocardial event compared to their counterpart with similar conditions. Both type 1 and type 2 diabetes are associated with increased risk of CVD. Five out of the six patients in this series had CFRD. Case 1 had A1c of 6.2 % which is in the pre-diabetes range. Oral glucose tolerance test (OGTT) is the recommended screening test for diabetes in CF and low A1c does not rule out diabetes in CF. OGTT data was not available for case 1.
Cholesterol and LDL levels tend to be low in CF compared to the general population, and thus do not reflect the atherogenic profile that is typically associated with CVD. A study of 192 pediatric and adult CF patients with normal fasting glucose with age 5–75 years, demonstrated only 4 % of subjects had total cholesterol levels > 200 mg/dL; average total cholesterol level in those 35–44 years of age was 175 ± 35 mg/dl and in those age 45–75 was 166 ± 44. Only one patient had a total cholesterol level > 240 mg/dL. Average LDL in the cohort was 68 ± 29 mg/dL. These levels are lower than the ones reported by the American Heart Association; Heart Disease and Stroke statistics – 2022 update for the general population. Prevalence of adults with total cholesterol > 200 mg/dL was 38 % with a mean of 191 mg/dL [13], [14]. In a CF study (14), 16 % of CF patients had triglyceride levels > 200 mg/dL; this was hypothesized to be a marker of systemic inflammation. Most patients in this case series had low LDL levels, except for one. The composition of LDL, even if normal by measurement, is altered in patients with diabetes mellitus and is more atherogenic as compared to healthy individuals [15]. LDL composition has not been assessed in CF. Only one patient in this series (case 6) had elevated LDL. This patient was had a mild CF genotype, with BMI in overweight range and elevated TGL. He was treated with metformin and GLP-1 receptor agonist due to clinical picture of type 2 diabetes. As people with CF are considered to be at low risk of CVD, it is not clear when and if elevated LDL level should be treated in this population [4] Future research is needed to better understand which patients with CFRD may benefit from lipid lowering medications like statin.
Hypertension, a major risk factor for CVD, is not a usual feature of CF unless there has been an organ transplant, although all but one of the patients in our cohort had hypertension. Salt intake of higher than 1500 mg per day is associated with increased risk of CVD in the general population, and patients with CF tend to consume large amounts of salt. This is not felt to be an issue in patients with CF because of excessive loss of sodium chloride in their sweat [15]. However, with the recent increased use of new CFTR modulator drugs that partially correct the basic CF defect, we are seeing increase in the prevalence of hypertension in patients with CF [16] which could modify the future risk of CVD in this population. Similarly, obesity is becoming more prevalent in the CF population and is expected to rise further with the advent of modulator drugs. Obesity is a risk factor for CVD in the general population and is associated with hypertension and higher cholesterol levels in patients with CF [17]. Only one patient in this case series had a BMI in the overweight or obese range.
Patients with CF experience chronic inflammatory respiratory tract disease, chronic systemic inflammation, and oxidative stress, all of which can contribute to endothelial dysfunction. Poore et al examined endothelial function in CF patients without diabetes by utilizing brachial artery flow-mediated dilation, and found reduced flow mediated dilation compared to healthy controls. Diabetes is also associated with chronic inflammation, oxidative stress and endothelial dysfunction, and thus it would not be surprising if CF and CFRD acted synergistically to promote the development of atherosclerotic CVD [18].
Only 2 of the 6 patients in this case series was treated with a CFTR modulator at the time of their cardiac event. It is unknown if new highly effective CFTR modulator therapies will have an impact on CVD risk. Treatment with highly effective CFTR modulator therapy has been shown to improve lung function, leads to reduction in pulmonary exacerbations, weight gain, and decreased sweat chloride [19], [20], [21], [22], [23]. There are limited data on the cardiovascular safety of CFTR modulators. A systematic review by Degenais et al noted cases of decreased heart rate in patients receiving lumacaftor/ivacaftor. Additionally, one case report described the development of asymptomatic first degree heart block in a 21-year-old patient with use of tezacaftor/ivacaftor and azithromycin [24]. Elevation in systolic and diastolic blood pressure has been reported in clinical trials of CFTR modulators compared to placebo [20], [25].
This case series highlights that systematic studies are needed to establish the incidence of CAD among people with CF. Studies are also needed to investigate if there is a connection between CF and CAD and whether this not been observed previously due to survivor bias or competing risk between development of CAD and death.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ratjen F., Bell S.C., Rowe S.M., Goss C.H., Quittner A.L., Bush A. Cystic fibrosis. Cystic fibrosis Nat Rev Dis Primers. 2015;1(1) doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis C., Blackman S.M., Nelson A., Oberdorfer E., Wells D., Dunitz J., et al. Diabetes-related Mortality in Adults with Cystic Fibrosis. Role of Genotype and Sex. Am J Respir Crit Care Med. 2015;191(2):194–200. doi: 10.1164/rccm.201403-0576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarzenberg S.J., Thomas W., Olsen T.W., Grover T., Walk D., Milla C., et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care. 2007 May;30(5):1056–1061. doi: 10.2337/dc06-1576. Epub 2007 Feb 23 PMID: 17322485. [DOI] [PubMed] [Google Scholar]
- 4.Moran A, Brunzell C, et al. CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010 Dec;33(12):2697-708. doi: 10.2337/dc10-1768. PMID: 21115772; PMCID: PMC2992215. [DOI] [PMC free article] [PubMed]
- 5.Bright-Thomas RJ, Webb AK. The heart in cystic fibrosis. J R Soc Med. 2002; 95 Suppl 41:2-10. [PMC free article] [PubMed]
- 6.Schlesinger DM, Holsclaw DS, Fyfe B. Generalized atherosclerosis in an adult with CF and diabetes mellitus [abstract] Eleventh Annual North American Cystic Fibrosis Conference, Nashville, Tennessee, October 23–26, 1997.
- 7.Skolnik K., Levy R.D., Wilcox P.G., Quon B.S. Coronary artery disease in cystic fibrosis: An emerging concern? J Cyst Fibros. 2016 Nov;15(6):e70–e71. doi: 10.1016/j.jcf.2016.09.010. Epub 2016 Oct 14. [DOI] [PubMed] [Google Scholar]
- 8.Perrin F.M., Serino W. Ischaemic heart disease - a new issue in cystic fibrosis? J R Soc Med. 2010;103(1_suppl):44–48. doi: 10.1258/jrsm.2010.s11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onady G.M., Farinet C.L. An adult cystic fibrosis patient presenting with persistent dyspnea: case report. BMC Pulm Med. 2006;6:9. doi: 10.1186/1471-2466-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thambuluru S.R., Kyazimzade S., Despotes K.A., Kirk D., Goralski J.L. Acute ST-elevation myocardial infarction in two young women with cystic fibrosis and cystic fibrosis-related diabetes. J Cyst Fibros. 2022;21(1):e44–e47. doi: 10.1016/j.jcf.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Reverri E.J., Morrissey B.M., Cross C.E., Steinberg F.M. Inflammation, oxidative stress, and cardiovascular disease risk factors in adults with cystic fibrosis. Free Radic Biol Med. 2014 Nov;76:261–277. doi: 10.1016/j.freeradbiomed.2014.08.005. Epub 2014 Aug 27 PMID: 25172163. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones D.M., Larson M.G., Beiser A., Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999 Jan 9;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. PMID: 10023892. [DOI] [PubMed] [Google Scholar]
- 13.Mozafarrian et al. Heart Disease and Stroke Statistics—2022 Update A Report From the American Heart Association. Circulation. 2022;145:e153–e639. https://doi.org/10.1161/CIR.0000000000001052. [DOI] [PubMed]
- 14.Figueroa V., Milla C., Parks E.J., Schwarzenberg S.J., Moran A. Abnormal lipid concentrations in cystic fibrosis. Am J Clin Nutr. 2002 Jun;75(6):1005–1011. doi: 10.1093/ajcn/75.6.1005. [DOI] [PubMed] [Google Scholar]
- 15.Scheffer P.G., Teerlink T., Heine R.J. Clinical significance of the physicochemical properties of LDL in type 2 diabetes. Diabetologia. 2005 May;48(5):808–816. doi: 10.1007/s00125-005-1736-0. Epub 2005 Apr 14 PMID: 15830178. [DOI] [PubMed] [Google Scholar]
- 16.Li L., Somerset S. Dietary intake and nutritional status of micronutrients in adults with cystic fibrosis in relation to current recommendations. Clin Nutr. 2016 Aug;35(4):775–782. doi: 10.1016/j.clnu.2015.06.004. Epub 2015 Jun 23. [DOI] [PubMed] [Google Scholar]
- 17.Sergeev V., Desai S., Flores E., Kerr J., Victoria S.u., Wilcox P., et al. Safety and effectiveness of lumacaftor-ivacaftor in adults with cystic fibrosis: A single-center Canadian experience. Can J Respiratory Critical Care Sleep Med. 2020;4(3):174–179. doi: 10.1080/24745332.2019.1649608. [DOI] [Google Scholar]
- 18.Harindhanavudhi T., Wang Q., Dunitz J., Moran A., Moheet A. Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: A single-center analysis. J Cyst Fibros. 2020 Jan;19(1):139–145. doi: 10.1016/j.jcf.2019.10.004. Epub 2019 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross et al Reverri EJ, Cross, Rhodes B.
- 20.Middleton P.G., Mall M.A., Dřevínek P., Lands L.C., McKone E.F., Polineni D., et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E., et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. The Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skilton M, Krishan A, Patel S, et al.. Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Cochrane Database Syst Rev. 2019 Jan 7;1(1):CD009841. doi: 10.1002/14651858.CD009841.pub3. PMID: 30616300; PMCID: PMC6353056. [DOI] [PMC free article] [PubMed]
- 23.Wainwright CE, Elborn JS, et al. TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 2015 Jul 16;373(3):220-31. doi: 10.1056/NEJMoa1409547. Epub 2015 May 17. PMID: 25981758; PMCID: PMC4764353. [DOI] [PMC free article] [PubMed]
- 24.Song Y., Palacios A.C., Thiagalingam A., Middleton P.G. Azithromycin and tezacaftor/ivacaftor is associated with first-degree heart block in an adult with cystic fibrosis. J Cyst Fibros. 2021;20(2):e19–e21. doi: 10.1016/j.jcf.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Konstan M.W., McKone E.F., Moss R.B., Marigowda G., Tian S., Waltz D., et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5(2):107–118. doi: 10.1016/S2213-2600(16)30427-1. [DOI] [PubMed] [Google Scholar]