Abstract
Five hundred and forty Cheery Valley ducks were used to investigate the effects of dietary supplementation of acidifier and compound probiotics, individually or in combination, on production performance, egg quality, immune and oxidative status, expression of reproductive, and calcium binding related genes from 42 wk to 48 wk of age. Ducks were randomly allocated to 9 treatment groups with 6 replicates and 10 ducks per replicate for each group. A 3 × 3 factorial arrangement, with 3 dietary inclusion levels of acidifier and probiotics (0, 2, and 3 g/kg acidifier; 0, 1, and 2 g/kg probiotics) were used. The acidifier used was mainly consisted of Benzoic acid, Fumaric acid, phosphoric acid, and formic acid. The main components of the probiotics were Bacillus subtilis and Clostridium butyricum. Dietary supplementation of probiotics improved the daily feed intake, egg production rate, and body weight of ducks (P < 0.05), and diet acidifier also increased the daily feed intake compared to the control (P < 0.01). Egg quality was improved by diet inclusion of probiotics, including Haugh unit, albumen height, egg shape index (P < 0.01), and eggshell hardness (P = 0.05). A significant increase in Haught unit and yolk weight was observed in ducks fed diet added with acidifier (P < 0.05). Acidifier supplementation reduced the total antioxidant capacity (T-AOC), immunoglobulin A (IgA), and IgG content and the catalase (CAT) activity in the serum (P < 0.05), in accompanied with an increased malondialdehyde (MDA) concentration (P < 0.05). Serum total superoxide dismutase (T-SOD) activities were improved by dietary inclusion of probiotics (P < 0.05). There was an interaction effects on serum IgA and IgG contents between acidifier and probiotics (P < 0.05). Diet supplementation of probiotics improved the ovary follicle-stimulating hormone receptor (FSHR) and estrogen receptor (ER) gene expressions (P < 0.01), while dietary acidifier reduced the transcription levels of FSHR and luteinizing hormone receptor (LHR) (P < 0.01) in ovary. In the uterus of the oviduct, expressions of FSHR, and carbonic anhydrase 2 (CA2) were also increased by diet probiotics (P < 0.01), and diet acidifier reduced the gene expressions of calbindin-D28k (CaBP-D28k) and CA2 (P < 0.05). Significant interaction effects between diet acidifier and probiotics were obtained on gene expressions of FSHR, LHR, and ovalbumin (OVAL) in the ovary (P < 0.05), and LHR, CaBP-D28k, and CA2 (P < 0.05) in the uterus. It can be concluded that production performance and egg quality of laying ducks can be improved in the late phase of reproduction by dietary inclusion of probiotics, while the organic acid mixture caused a decline in serum antioxidant and immune capacity of the ducks.
Key words: laying ducks, organic acids, probiotics, egg quality, antioxidant capacity
INTRODUCTION
As the European Union and China banning the use of certain antibiotics as growth promoter in poultry industry, exploitation of alternatives to dietary antibiotics is attracting more and more attention. Dietary supplementation of antibiotics at the subtherapeutic level could reduce the incidence of disease and improve the growth performance of birds, especially for birds that grow in overcrowded and unsanitary conditions (Islam et al., 2014). Nutritional additives that reduce or limit pathogen load and improve laying performance were widely investigated and used to replace the dietary antibiotics. Among such additives, acidifiers and probiotics are more favourable and potential alternatives of dietary antibiotics, as they compensate for gastric acidification and inhibition of pathogenic bacteria in the gastrointestinal tract of animals (Yang et al., 2009; Eftekhari et al., 2015).
Organic acids, generated during the metabolism in animals, enable lowering of pH in feed and digestive tract of animals, which help for defense against pathogens that are pH sensitive, and improving the nutrient digestibility and performance in poultry (Dittoe et al., 2018; Scicutella et al., 2021). Low gastric pH accelerates the conversion of pepsinogen to pepsin, which improves the absorption of amino acids, proteins, and minerals (Youn et al., 2005; Liem et al., 2008). The antimicrobial effect of organic acids and their salts is believed to be attributed to the undissociated part (Cherrington et al., 1991). There is a decline in eggshell quality as the hen ages, which is attributed to the increased egg weight without an increase in the amount of calcium carbonate deposited in the shell (Wistedt et al., 2014). The incidence of cracked eggs could exceed 20% at the end of the laying period, which causes great economic losses (Zhang et al., 2017). By lowering the pH of digestive tract, organic acids facilitates the P and Ca solubility and digestibility, which contributes to more deposition of calcium carbonate in the eggshell and better shell quality of laying hens (Swiatkiewicz and Arczewska-Wlosek, 2012). Experiments with layers and old broiler breeder hens have demonstrated that organic acids improved laying performance and eggshell quality (Sengor et al., 2007; Soltan, 2008). In modern poultry production, chickens appear to have compromised immune status as the fast growth, efficient feed conversion, and high stocking density for broilers and layers (Khan et al., 2012). In this tend, employment of organic acids in poultry diet plays a critical role in improving the immune system (Abbas et al., 2013; Khan et al., 2022). Dietary supplementation of commercial product (lactacid) in older laying hens (from 75 to 80 wk of age) reduced the soft-shell plus broken egg production and increased the IgY concentration in yolk (Park et al., 2009). Organic acid mixture, including formic, lactic and orthophosphoric acids, significantly increased egg weight of laying hens at 32 to 42 wk of age (Shalaei et al., 2014).
Probiotics are defined as live and harmless microorganisms that beneficially affects the host (Fuller, 1989). As a Gram-positive obligate anaerobic probiotic, both Bacillus subtilis (B. subtilis) and Clostridium butyricum (C. butyricum) are among the widely used beneficial bacteria that have been recognized as safe for animal dietary use (European, Food, Safety, and Authority 2007). In poultry, a variety of advantages of B. subtilis rang from anti-inflammation, modifying gut microflora balance, adjusting the immunological function and gut morphology (Rajput et al., 2013; Ar’Quette et al., 2018). Dietary inclusion of B. subtilis in laying hens from 64 to 75 wk of age significantly increased the egg production and egg weight (Rajput et al., 2013). Additionally, B. subtilis was reported to decrease the pH of intestinal digesta and increase the intestinal absorption surface of aged laying hens for improving Ca availability, and better eggshell quality was obtained in aged laying hens when B. subtilis was added at 2 × 109 CFU/kg (Abdelqader et al., 2013). As a butyrate-producing and normal flora in the intestines of health chicken, C. butyricum has been documented for promoting growth performance and meat quality, alleviating oxidative stress, strengthening immune function of broilers and ducks (Zhang et al., 2011a; Liao et al., 2015; Liu et al., 2018). Previous studies regarding the effect of dietary C. butyricum on the poultry mainly focused on broilers, and it is rare concerning the application of C. butyricum in laying ducks.
Both organic acids and probiotics were identified as potential alternatives to dietary antibiotics with a promoted productive performance in poultry. However, few studies demonstrated the synergistic effect between them, especially in laying ducks. The present study was therefore conducted to investigate the effects of a commercial probiotics, constituted of B. subtilis and C. butyricum, along with a commercial acidifier on the laying performance, egg quality, antioxidant capacity, and expression of reproductive genes of Cherry Valley ducks in the late period of production.
MATERIALS AND METHODS
Animals and Experimental Design
A total of 540 Cherry Valley ducks at 42 wk of age were randomly selected from a commercial flock to be used in this study. Ducks were raised in cages (2.00 m × 2.00 m × 0.52 m, 10 ducks per cage) and had free access to feed and water throughout the experiment. Room temperature was maintained at 22 ± 3°C, with 16 h of light/day. Ducks care and experiment protocols were in compliance with the regulations of the Animal Ethical Committee of Jiangsu Agri-animal Husbandry Vocational College (20210026).
A 6-wk feeding trial was conducted to evaluate the effects of acidifier and probiotics on laying performance of ducks from 42 wk to 48 wk of age. Ducks were randomly allocated to 9 treatment groups with 6 replicates and 10 ducks per replicate for each groups. The 9 groups received the following diets: 1) basal diet (control), 2) basal diet + 2 g/kg acidifier, 3) basal diet +3g/kg acidifier, 4) basal diet + 1 g/kg compound probiotics, 5) basal diet + 1 g/kg compound probiotics + 2 g/kg acidifier, 6) basal diet + 1g/kg compound probiotics + 3 g/kg acidifier, 7) basal diet + 2 g/kg compound probiotics, 8) basal diet + 2 g/kg compound probiotics + 2 g/kg acidifier, 6) basal diet + 2 g/kg compound probiotics + 3 g/kg acidifier. Basal diet was purchased from Chaohu COFCO group, Anhui, China and the formula was shown in Table 1. The acidifier used was a commercial product (provided by Hanove Animal Health Co., Ltd, Wuxi, China), which was mainly consisted of Benzoic acid (≥25.0%), Fumaric acid (≥20.0%), phosphoric acid (≥12.75%), formic acid (≥12.0%), and silica as the carrier. The compound probiotics was manufactured by Suzhou Co-Pullulation Bio-technology Co., Ltd, China with a main components being B. subtilis (≥1 × 108 CFU/g) and C. butyricum (CB, ≥3 × 107 CFU/g), supported by stone powder and fumed silica. Cages from each treatment groups were separated from each other to avoid mixing the experiment diets.
Table 1.
Ingredients and nutrient composition of the basal diet.
| Ingredients (%) | Composition |
|---|---|
| Maize | 34.65 |
| Wheat | 8 |
| Rice bran | 17 |
| Soybean meal | 23.75 |
| Corn gluten meal | 5 |
| Soybean oil | 1.36 |
| Limestone | 8.08 |
| Dicalcium phosphate | 0.28 |
| Sodium chloride | 0.30 |
| DL-methionine | 0.14 |
| Lysine sulfate | 0.14 |
| Colorant | 0.16 |
| Adhesive | 0.10 |
| 75% Choline chloride | 0.04 |
| Vitamin-mineral-premixa | 1 |
| Total | 100 |
| Calculated nutrient compositionb (%) | |
| Metabolizable energy (MJ/kg) | 11.31 |
| Crude protein | 19.02 |
| Crude fiber | 3.45 |
| Calcium | 3.10 |
| Total phosphorus | 0.60 |
| Lysine | 1 |
| Methionine and cystine | 0.76 |
The premix provided the following per kg of diet:vitamin A 9,800 IU; vitamin D3 3,850 IU; vitamin E 22 IU; vitamin K3 1.68 mg; vitamin B1 1 mg; vitamin B2 4.25 mg; vitamin B6 2 mg; vitamin B12 0.01 mg; nicotinic acid 52 mg; pantothenic acid 10.8 mg; folic acid 0.78 mg; iron 0.08 g; manganese 0.14 g; zinc 0.1 g; iodine 1.1 g; copper 0.01 g; selenium 0.3 g.
Based on ingredients composition provided by Chinese Feeding Standard of Chicken (Ministry of Agriculture of China, 2004) and National Research Council (1994).
Feed Mixing Procedures and Egg Collection
Acidifier and compound probiotics were fully incorporated with the complete formula feed weekly and the total amount was 100 kg for each group, exceeding the 1-wk-consumption. To ensure a well-mixed diets for each group, the ingredients were accurately weighted and thoroughly hand-mixed with 10 kg feed, then the homogenized feed were divided into 4 portions and blended in a small mixer with the remaining basal diet. Ducks were fed twice daily at 7:00 and 16:30, and eggs (intact, malformed, broken, and shell-less) from each replicates were collected, weighted, and recorded on a daily basis. Feed consumption was recorded weekly by replicates and used to calculate the daily feed intake (gram per day, g/day). Average feed conversion ratio (kg/kg) was calculated by the division of feed consumption to egg production during the experiment. At the last day of experiment, 6 ducks (1 ducks per replication) were randomly selected from each treatment after deprivation of feed for 12 h, weighted and euthanized by cervical dislocation. Individual blood samples were collected and centrifugation at 3,000 rpm for 15 min at 4°C was applied to separate the serum. Serum samples were frozen at −20°C until the analysis of antioxidant and immune parameters. Ovary and uterus of uterine tube were taken and stored at −80°C for mRNA extraction.
Egg Quality
One hundred sixty-two eggs (3 per replication, 18 per treatment) were randomly selected and examined to evaluate the egg quality once per week, with 6 times in total throughout the whole experiment. Haugh unit, albumen height (millimeter, mm), egg weight (g) and yolk color were measured by an egg-multi tester (Robotmation, Japan). Egg shape index was calculated by the formula index = egg length / egg width, where length and width were measured by vernier caliper. Albumen and yolk were separated and yolk was weighted. Eggshell hardness (Newton, N) was measured using a texture analyzer (Robotmation) and mean shell thickness (mm) was the average of the thickness from 3 sites at the blunt, sharp, and equator of the egg measured by vernier caliper.
Serum Antioxidant Parameters
Serum samples were analyzed for activities of total superoxide dismutase (T-SOD) and catalase (CAT), concentrations of total antioxidant capacity (T-AOC) and malondialdehyde (MDA) using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the manufacturer's protocol as follows. Briefly, T-SOD activity was measured based on SOD-mediated inhibition of nitrite formation from hydroxyammonium in the presence of O2− generators (xanthine/xanthine oxidase; Elstner and Heupel, 1976). CAT activity was estimated from absorbance at 405 nm according to the consumption of H2O2. In the process of T-AOC evaluation, ferric ion was reduced by antioxidant reducing agents and blue complex Fe2+-TPTZ (2,4,6-tri (2-pyridyl)-s-triazine) was produced, which then reacted with phenanthroline to generate a stable complex that could be tested by the absorbance at 520 nm. One unit of T-AOC was defined as 0.01 increase in absorbance value at 37°C per min. The MDA concentration was determined by a previous method (Placer et al., 1966), which utilized the thiobarbituric acid method to monitor MDA-reactive products spectrophotometrically. The absorbance of the organic layer was measured at 532 nm. The results were presented as nanomoles (nmol) per milliliter (mL) serum for MDA and T-AOC, and units of enzyme activities per ml of serum for T-SOD and CAT.
Serum Immunoglobulin
Serum immunoglobulin A (IgA) and immunoglobulin G (IgG) were tested with appropriately diluted serum samples by an enzyme-linked immune-sorbent assay (ELISA), and the results were presented as milligrams (mg) of immunoglobulin per mL of serum. The commercial kits were chicken-special IgA and IgG ELISA quantitation kits (Nanjing Jiancheng Bioengineering Institute).
RNA Extraction and Real-Time PCR
Total RNA from ovary and uterus of uterine tube were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNase І (RNase-free) (TaKaRa, Dalian, China) to remove genomic DNA. The total RNA concentration and purity were determined spectrophotometrically at 260 and 280 nm with a Nanodrop 8000 (Thermo Fisher Scientific, Wilmington, DE). For each sample, 1 μg of total RNA was reverse transcribed to cDNA with M-MLV reverse transcriptase (TaKaRa) and oligonucleotide primers. The housekeeping gene beta actin (β-actin) and target genes including the ovary follicle-stimulating hormone receptor (FSHR), estrogen receptor (ER), luteinizing hormone receptor (LHR), ovalbumin (OVAL), calbindin-D28k (CaBP-D28k), and carbonic anhydrase 2 (CA2) were quantified by real-time PCR on a QuantStudio 3 system using a commercial kit (SYBR Premix Ex Taq, TaKaRa). The gene-specific primers were designed based on the corresponding mRNA sequences with Primer Version 5.0 (Table 2). All samples were measured in duplicate. For quantification of real-time PCR results, the threshold cycle Ct was determined for each reaction. Ct values for each gene of interest were normalized to the housekeeping gene. The relative mRNA concentration was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). Normalized values were used to calculate the degree of induction or inhibition expressed as a “fold difference” compared to normalized control values.
Table 2.
Primers used for real-time PCR.
| Gene (abbreviation) | GenBank accession no. | Sequence (5′→3′) | Length of DNA product (bp) |
|---|---|---|---|
| β-actin | NM_001310421.1 | F:GGTATCGGCAGCAGTCTTA | 128 |
| R: TTCACAGAGGCGAGTAACTT | |||
| FSHR | XM_021267214.3 | F: GCGGCAAACTGCATAAGGAGA | 194 |
| R: TACACGAGGTTGTTGGCCTT | |||
| ER | NM_001346787.1 | F: GTACTGTGCTGTGTGCAACG | 182 |
| R: TTCTTAGTCGGCAGGCTTGG | |||
| LHR | NM_001243048.1 | F: ACTGGAGTCCCTGCCTAGTT | 177 |
| R: TCTCTGTAGTTCTCTGTCCTCA | |||
| OVAL | NM_053069.5 | F: CAGATGGACAGCTGCACACAC | 126 |
| R: GGGTTTCCAGCATTGGCTCTA | |||
| CaBP-D28k | XM_027452451.2 | F: TGTGCCTCCTTAGATTACATTGGA | 200 |
| R: TGGGAGACAGAAGAAGAGCTG | |||
| CA2 | XM_027452432.2 | F: GGCGGGAGCCTATAAAAGCC | 108 |
| R: TATCCCCAGTGGTGGGACAT |
Statistical Analysis
All the data were subjected to statistical analysis using a completely randomized design, in accordance with the GLM procedure by GraphPad Prism Version 8.0 soft-ware program (GraphPad Software, San Diego, CA). Data were analyzed using two-way ANOVA test and when significant difference in interaction effect was detected, one-way ANOVA was applied to perform the data variance between given groups. Statistical difference was accepted when P < 0.05 and P < 0.01, and data were presented as mean ± standard error (SEM).
RESULTS
Productive Performance
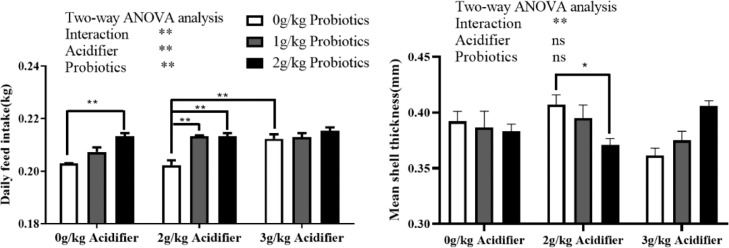
Two-way ANOVA test showed that body weight, egg production, and daily feed intake were improved (P < 0.05, P < 0.01) with dietary supplementation of compound probiotics, with no difference in FCR between all supplemented groups and the control (Table 3). Diet inclusion of acidifier improved the daily feed intake (P < 0.01) and significant interaction effects (P < 0.05) on feed intake and egg weight between acidifier and probiotics were observed (Table 3). One-way ANOVA test indicated an increased feed intake in 0 g/kg acidifier plus 2 g/kg probiotics group compared to the control (Figure 1, P < 0.01). Feed intakes were also improved in 2 g/kg acidifier plus 1 or 2 g/kg probiotics groups and 3 g/kg acidifier plus 0 g/kg probiotics group compared to the 2 g/kg acidifier plus 0 g/kg probiotics group (Figure 1, P < 0.01).
Table 3.
Effects of dietary acidifier and probiotics on the productive performance of cherry valley ducks at the late laying stage.
| Acidifier (g/kg) |
SEMa |
P value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | Acidi-fier | Probiotics | Interaction | ||||||||
| Probiotics (g/kg) | |||||||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | |||||
| Body weight (kg) | 4.1 | 4.3 | 4.2 | 4.0 | 4.3 | 4.7 | 3.9 | 4.8 | 4.4 | 0.2 | NS | 0.01 | NS |
| Egg production (%) | 7.5 | 3.4 | 8.7 | 8.7 | 4.8 | 10.2 | 4.8 | 6.7 | 12.5 | 2.4 | NS | 0.049 | NS |
| Egg weight (g) | 79.8 | 85.8 | 86.1 | 84.7 | 83.2 | 80.4 | 80.4 | 86.9 | 85.5 | 1.9 | NS | NS | 0.049 |
| Daily feed intake (g/day) | 203 | 207 | 213 | 202 | 213 | 213 | 212 | 213 | 215 | 1.1 | 0.01 | 0.01 | 0.01 |
| Feed conversion (kg/kg) | 34.6 | 100.8 | 35.6 | 44.1 | 138.2 | 28.3 | 65.9 | 96.2 | 22.3 | 29.1 | NS | NS | NS |
SEM, pooled standard error of the means.
Figure 1.
Effects of dietary acidifier and probiotics on the daily feed intake and egg shell thickness of cherry valley ducks at the late laying stage. Note: For two-way ANOVA test, * and ** indicate significant difference (P < 0.05, P < 0.01), and one-way ANOVA was performed as interaction effect between acidifier and probiotics was observed. * and ** indicate a significant difference between two groups for one-way ANOVA test.
Egg Quality
By two-way ANOVA test, we found that Haugh unit, albumen height, and egg shape index were improved for ducks fed probiotics inclusion diet (P < 0.05, Table 4). Additionally, dietary supplementation of probiotics tended to produce hard-to-break eggs by increasing the eggshell hardness (P = 0.053). Eggs laid by ducks fed acidifier exhibited improved Haugh unit and yolk weight (P < 0.01, P < 0.05) and lighter yolk color (P < 0.01). An interaction effect on shell thickness (P < 0.01) between diet acidifier and probiotics was observed, and among supplemented groups shell was thicker in 2 g/kg acidifier plus 2 g/kg probiotics group than that in 2 g/kg acidifier plus 0 g/kg probiotics group (P < 0.05) by ordinary one-way ANOVA (Figure 1).
Table 4.
Effects of dietary acidifier and probiotics on the egg quality of cherry valley ducks at the late laying stage.
| Parameters | Acidifier (g/kg) |
SEMa |
P value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | Acidifier | Probiotics | Interaction | ||||||||
| Probiotics (g/kg) | |||||||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | |||||
| Haugh unit | 87.2 | 79.3 | 91.4 | 86.0 | 90.6 | 97.3 | 89.9 | 97.9 | 97.1 | 2.7 | 0.01 | 0.01 | NS |
| Albumen height(mm) | 6.96 | 6.67 | 7.34 | 6.22 | 7.95 | 8.56 | 6.93 | 8.51 | 8.43 | 0.51 | NS | 0.01 | NS |
| Egg shape index | 1.39 | 1.38 | 1.37 | 1.40 | 1.39 | 1.35 | 1.38 | 1.45 | 1.34 | 0.02 | NS | 0.01 | NS |
| Yolk weight(g) | 23.6 | 25.6 | 24.8 | 24.2 | 21.8 | 23.2 | 25.9 | 24.1 | 24.8 | 0.9 | 0.048 | NS | NS |
| Yolk color | 13.0 | 13.7 | 13.8 | 13.0 | 12.7 | 12.9 | 13.8 | 12.9 | 13.4 | 0.2 | 0.01 | NS | NS |
| Eggshell strength(N) | 2.99 | 4.96 | 3.92 | 3.36 | 4.36 | 3.99 | 3.26 | 3.43 | 3.46 | 0.48 | NS | 0.053 | NS |
| Mean shell thickness(mm) | 0.39 | 0.39 | 0.38 | 0.41 | 0.40 | 0.37 | 0.36 | 0.38 | 0.41 | 0.01 | NS | NS | 0.01 |
SEM, pooled standard error of the means.
Serum Antioxidant Capacity and Immunoglobulin Content
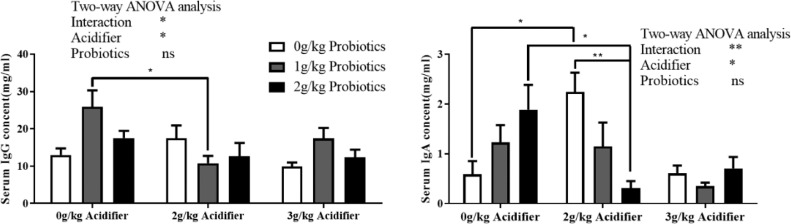
Two-way ANOVA test indicated higher concentration of MDA in the serum of ducks fed diet containing acidifier than these in acidifier-free groups (P < 0.05), especially in the 3 groups of 2 g/kg acidifier inclusion (Table 5). Both serum T-AOC content and CAT activities were reduced in groups supplemented with acidifier (P < 0.05), while ducks fed probiotics reversely improved the serum T-SOD activity (P < 0.05, Table 5). Dietary inclusion of acidifier lowered the serum IgA and IgG concentrations (P < 0.05), with no effects of probiotics supplementation on serum immunoglobulin contents (Table 5). There was an interaction effect on both IgA and IgG contents between acidifier and probiotics (P < 0.05), and application of ordinary one-way ANOVA observed an increase of IgA content in 2 g/kg acidifier plus 0 g/kg probiotics group compare to the control (Figure 2, P < 0.05), a decline in 2 g/kg acidifier plus 2 g/kg probiotics group compare to the 0 g/kg acidifier plus 2 g/kg probiotics group (P < 0.05) and the 2 g/kg acidifier plus 0 g/kg probiotics group (Figure 2, P < 0.01). Ducks in the 2 g/kg acidifier plus 1 g/kg probiotics group exhibited lower serum IgG content than the 0 g/kg acidifier plus 1 g/kg probiotics group (P < 0.05, Table 5).
Table 5.
Effects of dietary acidifier and probiotics on the serum antioxidants activity and immunoglobulin content of cherry valley ducks at the late laying stage.
| Parameters | Acidifier (g/kg) |
SEMa |
P value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | Acidifier | Probiotics | Interaction | ||||||||
| Probiotics (g/kg) | |||||||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | |||||
| MDA (nmol/ml) | 5.80 | 3.28 | 4.22 | 7.65 | 7.99 | 5.45 | 5.08 | 4.46 | 1.15 | 0.02 | NS | NS | |
| T-AOC (nmol/mL) | 0.34 | 0.30 | 0.34 | 0.32 | 0.20 | 0.12 | 0.25 | 0.20 | 0.22 | 0.04 | 0.01 | NS | NS |
| T-SOD (U/mL) | 24.5 | 27.1 | 29.9 | 22.9 | 21.4 | 30.3 | 25.2 | 27.9 | 29.8 | 2.63 | NS | 0.04 | NS |
| CAT (U/mL) | 3.31 | 3.23 | 5.38 | 3.34 | 1.93 | 3.02 | 1.61 | 2.75 | 2.66 | 0.64 | 0.04 | NS | NS |
| IgG (mg/mL) | 12.99 | 25.89 | 17.56 | 17.51 | 10.78 | 12.65 | 9.92 | 17.46 | 12.43 | 2.49 | 0.03 | NS | 0.02 |
| IgA (mg/mL) | 0.59 | 1.23 | 1.89 | 2.24 | 1.15 | 0.31 | 0.61 | 0.35 | 0.71 | 0.29 | 0.02 | NS | 0.01 |
SEM, pooled standard error of the means.
Figure 2.
Effects of dietary acidifier and probiotics on the concentrations of IgG and IgA in serum of cherry valley ducks at the late laying stage. Note: For two-way ANOVA test, * and ** indicate significant difference (P < 0.05, P < 0.01), and one-way ANOVA was performed as interaction effect between acidifier and probiotics was observed. * and ** indicate a significant difference between two groups for one-way ANOVA test.
Expression of Reproductive-Related Genes in the Ovary
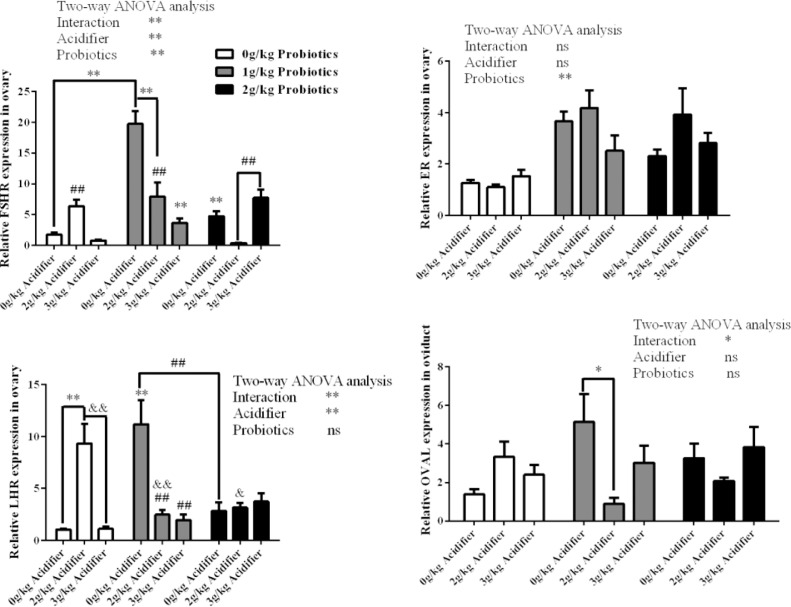
By two-way ANOVA test, a decline in transcription of FSHR and LHR gene in the ovary was observed by diet inclusion of acidifier (P < 0.01, Figure 3). Diet supplementation of probiotics improved the ovary FSHR and ER gene expressions (P < 0.01). Interaction effects on FSHR, LHR, and OVAL gene expression between acidifier and probiotics inclusion were detected (P < 0.01, P < 0.01, and P < 0.05). By one-way ANOVA test, 0 g/kg acidifier plus 1 g/kg probiotics group exhibited the highest FSHR expression compared with the control and other corresponding-supplemented groups (P < 0.01). The lowest FSHR expression was obtained in 2 g/kg acidifier plus 2 g/kg probiotics group compared with other 2 g/kg acidifier groups and the 3 g/kg acidifier plus 2 g/kg probiotics group (P < 0.01). Similarly, 0 g/kg acidifier plus 1 g/kg probiotics group also showed the highest transcription level of LHR in ovary compared with the control and other corresponding-supplemented groups (P < 0.01). Among the three 2 g/kg acidifier group, 0 g/kg group exhibited higher expression of LHR gene than the other two (P < 0.05). A higher OVAL gene expression was observed in 0 g/kg acidifier plus 1 g/kg probiotics group than the 2/kg acidifier plus 1 g/kg probiotics group (P < 0.05, Figure 3).
Figure 3.
Effects of dietary acidifier and probiotics on the expression of reproductive-related genes in the ovary of cherry valley ducks at the late laying stage. Note: For two-way ANOVA test, * and ** indicate significant difference (P < 0.05, P < 0.01); For FSHR expression, ** represent a significant difference compared to the 0 g/kg acidifier plus 1 g/kg probiotics group (P < 0.01), ## represent a significant difference compared to the 2 g/kg acidifier plus 2 g/kg probiotics group (P < 0.01); For LHR expression, ** represent a significant difference compared to the control (P < 0.01), & and && represent a significant difference compared to 2 g/kg acidifier plus 0 g/kg probiotics group (P < 0.05, P < 0.01), and ## represent a significant difference compared to 0 g/kg acidifier plus 1 g/kg probiotics group (P < 0.01).
Expression of Reproductive and Eggshell Formatting Related Genes in the Uterus of Uterine Tube
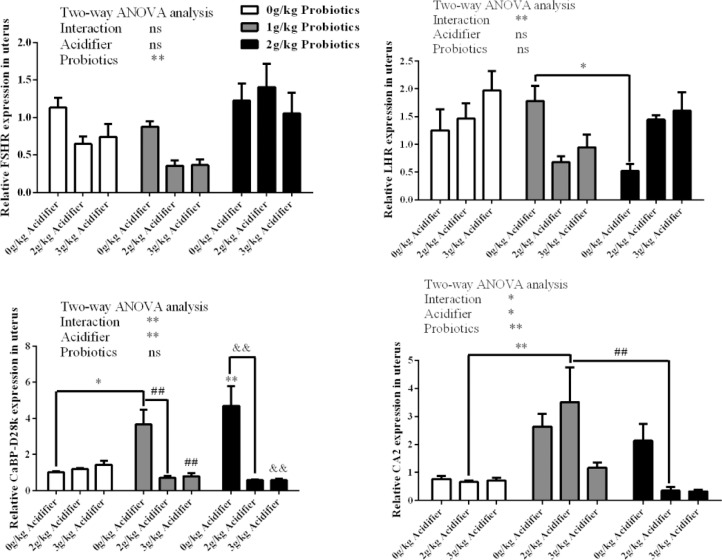
Two-way ANOVA test observed that dietary inclusion of probiotics improved the gene expressions of FSHR and CA2 in the uterus of uterine tube (P < 0.01), and diet acidifier reduced the gene expressions of CaBP-D28k and CA2 (P < 0.01, P < 0.05, Figure 4). Significant interaction effects between diet acidifier and probiotics were obtained on gene expressions of LHR, CaBP-D28k (P < 0.01) and CA2 (P < 0.05) in the uterus. Then one-way ANOVA was applied and higher LHR expression was detected in 0 g/kg acidifier plus 1 g/kg probiotics group than the 0 g/kg acidifier plus 2 g/kg probiotics group (P < 0.05). For CaBP-D28k gene expression, a similar variation in 2 probiotics alone supplemented groups was observed by a higher expression compared to the control (P < 0.05) and the other 2 corresponding acidifier-supplemented groups (P < 0.01), respectively. Two g/kg acidifier plus 1 g/kg probiotics group exhibited the highest CA2 expression in uterus among the three 2 g/kg acidifier groups (P < 0.01, Figure 4).
Figure 4.
Effects of dietary acidifier and probiotics on the expression of reproductive-related genes in the uterus of oviduct of cherry valley ducks. Note: For two-way ANOVA test, * and ** indicate significant difference (P < 0.05, P < 0.01); For CaBP-D28k expression, * and ** represent a significant difference compared to the control (P < 0.05, P < 0.01), ## represent a significant difference compared to the 0 g/kg acidifier plus 1 g/kg probiotics group (P < 0.01) and && represent a significant difference compared to the 0 g/kg acidifier plus 2 g/kg probiotics group (P < 0.01).
DISCUSSION
Productivity
Organic acid and probiotics were widely used in poultry industry to improve hens performance and healthy due to the ban on antimicrobial growth promoters in different production systems. Among these acids, fumaric, formic, lactic, butyrate, propionic and citric acids, and their salts were extensively studied and exploited (Zhang et al., 2011b; Yang et al., 2018). Usually, acidifier is a mixture composed of various organic acids. Laying hens at 26 wk of age fed a diet inclusion of organic acid mixture (60% formic acid, 20% propionic acid, and 20% soft acid) resulted in no beneficial influence on feed consumption, egg production, feed conversion ratio, and body weight (Kaya et al., 2015). Another research in layers at 44 wk of age also reported no effect of dietary acidifiers (70% propionic acid, 5% citric acid, and 25% soft acid) on the productive performance (Kaya et al., 2013). Our present study demonstrated similar result that organic acid mixture (benzoic acid, fumaric acid, phosphoric acid, and formic acid) had no effect on body weight, egg production, egg weight, and feed conversion ratio except for the daily feed intake, which showed an improvement. The increased feed intake agreed with Haque et al. (2010) and Fascina et al. (2012), who reported that broilers fed with 0.5% citric acid or organic acid mixture (30.0% lactic acid, 25.5% benzoic acid, 7% formic acid, 8% citric acid, and 6.5% acetic acid) has shown progress in feed consumption. While in other reports of layer hens, employment of organic acids markedly increased the egg reproduction (Youssef et al., 1997; Yesilbag and Çolpan, 2006). These contrasts may be attributed to variations in the addition amount and content of organic acids, chick strains, and housing conditions.
Probiotics, especially B. subtilis and C. butyricum, are believed to promote nutrient absorption and maintain intestinal health through colonization in the gut of the host and regulating intestinal flora balance, secreting exogenous enzymes to improve nutrinent digestibility, activating mitotic cell davision and improving the proliferation of gut epithelial cells, which will increase the villus height and enhance nutrient absorption, and adjusting the immune function (Hill et al., 2014; Shalaei et al., 2014; Abdel-Moneim et al., 2020). The increased absorption of nutrients by diet probiotic yields more energy to be potentially available for the net energy of production. Dietary supplementation of B. subtilis and C. butyricum in laying hens improved egg production (Abdelqader et al., 2013; Zhan et al., 2018), and broiler chickens fed diet inclusion of B. subtilis and C. butyricum showed remarkable increase in body weight and decline in feed conversion ratio (Rhayat et al., 2017; Svejstil et al., 2019; Wang et al., 2022). The present study of laying ducks in the late period of production confirmed the beneficial effects of dietary addition of these two probiotics on laying performance by increased egg production, feed intake, and body weight. The improvement of production performance may be related to extracellular digestive enzymes secreted by B. subtilis (Guo et al., 2020) and nutrients such as short-chain fatty acids by C. butyricum (Cao et al., 2012). Additionally, the positive effects of probiotics on egg production, observed in this study, may also be attributed to the increased expression of reproduction-related genes, such as FSHR in ovary and uterus of uterine tube, and ER in ovary.
Egg Quality
Egg quality parameters are one of the most crucial issues in the laying hen industry, since it influence both the economic profitability of egg production and hatchability (Swiatkiewicz and Arczewska-Wlosek, 2012). For example, egg shape index directly determines the choosing of a breeding egg, and is positively correlated with the hatchability (Narushin and Romanov, 2002). Damaged eggs due to a poor eggshell quality accounts for 6 to 10% of all eggs produced worldwide, which causes great economic losses (Roland, 1988). Organic acids may serve as a meaningful tool to improve the absorption of minerals, as the acidic anion has been shown to be complex with calcium and enhance calcium availability and absorption in the gut (Li et al., 1998). Increasing calcium absorption contributes to increased eggshell strength (Chen and Chen, 2004). Even researchers observed an increase in serum calcium content and eggshell thickness of laying hens fed a diet supplemented with organic acids (Soltan, 2008; Kaya et al., 2013), our present study found no beneficial influence of acidifier on eggshell hardness and shell thickness, which was in consistent with the results of Park et al. (2009) and Kaya et al. (2015). Haugh unit is one of the most common indicators of egg freshness and an obvious increase in Haugh unit and yolk weight were observed by dietary acidifier in this study. The increased Haugh unit was also observed by Sandi et al. (2022) who reported that organic acid derived from grass silage improved the Haugh unit of duck eggs.
Except for laying performance, inclusion of probiotics in the diets of laying hens can also result in improved egg quality: higher values for albumen height and yolk color (Upadhaya et al., 2019); increased eggshell thickness and strength (Xiang et al., 2019; Souza et al., 2021). Similarly, the present study confirmed that the combination of B. subtilis and C. butyricum inclusion in diets of laying ducks contributed to an improvement in Haugh unit, albumen height, and egg shape index. Meanwhile, the tendency toward an increase in eggshell strength was observed in compound probiotic groups. Eggs usually exhibit a decline in eggshell quality as the hen ages, due to the increased egg weight without an increase in the amount of calcium carbonate deposited in the shell (Etches, 1998). The greater eggshell quality produced by ducks fed diets inclusion of probiotics was directly associated with the rising ratio of marketable eggs. Carbonic anhydrase (CA) and calcium-bing protein (calbindin, CaBP) of chicken play an important role in the eggshell formation: CA catalyzes the reversible hydration of CO2 to form HCO3− and protons, and HCO3− provides CO32− to bind with Ca2+, which is ultimately deposited as a CaCO3 shell on the egg (Simkiss, 2010); CaBP exists as a high-molecular weight protein of 28kDa (CaBP-D28k) in avian intestine and eggshell grand, which are characterized by their massive transport of Ca2+ (Arie, 2009), and the concentration of CaBP in the eggshell grand is positively correlated with rate of shell Ca2+ deposition (Yosefi et al., 2003). As shown in Figure 4, diet added with probiotics enhanced the CA2 expression in uterus and CaBP-D28k gene also showed higher expression in two 0g/kg acidifier plus probiotics groups, and these elevation of gene expressions may result from the enhanced Ca2+ uptake in the intestine of the corresponding probiotics groups, which contributed to the increased eggshell strength. Probiotics can stimulate the quantitative or qualitative composition of the intestinal microflora to improve calcium bioavailability (Raveschot et al., 2020; Wawrzyniak and Suliburska, 2021). Rats fed dietary multispecies probiotics exhibited higher calcium deposition in the hair (Suliburska, et al., 2021).
Serum Antioxidant Capacity and Immune Status
Oxidative stress refers to the imbalance between oxidation and anti-oxidation in the body, and it could result in varieties of reactive oxygen species (ROS), which can damage the proteins, nucleic acids and lipids, contributing to tissue damage and the development of diseases. Antioxidant enzymes (SOD, CAT, GSH, and GSH-Px) serve as the first-line to eliminate the ROS and MDA exists as the final product of lipid peroxidation, which is frequently used as biomarker to estimate oxidative stress (Geret et al., 2003). Due to the capacity of penetrating through the cell well of bacterial and cellular pH-reduction, acidifiers were proved to inhibit the growth of pathogenic bacteria in intestine, especially the gram negative bacteria, such as E.coli and Salmonella (Hassan et al., 2010; Nguyen et al., 2020). The reduced pathogen colonization by acidifiers contributed to a lower cases of intestinal disease, such as necrotic enteritis, a common problem in the poultry industry caused by Clostridium perfringens (Jerzsele et al., 2012). In addition, dietary organic acids were reported to have positive effects on intestinal villi morphology and content of beneficial bactetia in heat-stressed broliers (Abdelqader and Al-Fataftah, 2016). The inhibition of pathogenic bacteria and improvement of intestinal epithelium functions help to maintain a health and sound condition of animals. Broiler chickens fed with organic acids showed lower MDA concentration in the serum (Hashemi et al., 2012). In contrast, in our present study ducks fed with the organic acid mixture showed reductions of CAT activity and T-AOC concentrations, accompanied by a marketable higher MDA content in the serum. Differ from the acidifier, we observed that diet supplementation of compound probiotics improved the serum SOD activity, and the positive impact was in agreement with the previous studies. For instance, laying hens fed with C. butyricum exhibited increased serum CAT and T-SOD concentrations (Zhan et al., 2018) and serum GSH-Px activity increased linearly in breeding hens as dietary inclusion of B. subtilis increased (Liu et al., 2019). The improvement in SOD activity may due to that C. butyricum can produce butyrate and hydrogen, and stimulate the intraepithelial lymphocytes in the small intestinal, which will enhance the activity of antioxidative enzymes and reduce ROS production (Franziska et al., 2014; Bai et al., 2018).
Serum immunoglobulin play a critical role in immune function, among which IgA, IgG, and IgM are usually used to evaluate immune status in hens. Organic acids were demonstrated to improve the health of young broilers by enhance the immune system (Dittoe et al., 2018). Broiler chicks fed with dietary organic acids showed higher serum IgG concentration (Mustafa et al., 2021). Contrastingly, here we observed a decline in serum IgA and IgG contents of laying ducks fed diet with organic acid mixture. The inconsistent results of the serum antioxidant capacity and immunoglobulin concentration with the previous studies may be attributed to the type of composition and dosage of the organic acids, nutrient composition of feed, age, and health status of the ducks (Nguyen et al., 2020). The reduced immunoglobulin content and antioxidant capacity, coupled with the elevation of MDA concentration allowed to conclude that ducks fed with dietary acidifier were vulnerable to infections or disease in the present study. Several studies have reported that B. subtilis alone or combined with other probiotic can serve as immune-modulatory factors to increase performance in chickens, such as higher index of humoral and cell-mediated immunities (Molnár et al., 2011; Biswas et al., 2022). The immunostimulatory impact of probiotics might be attributed to their ability to activete T lymphocytes in the intestinal immune system via augmenting Toll-like receptor (TLR) signalling (Abd El-Hack et al., 2020). Diet supplementation of B. subtilis improved IgM concentration both in laying hens and broilers (Fathi et al., 2017; Liu et al., 2019). Studies on diet inclusion of C. butyricum indicated promoted concentrations of serum IgG and IgM in broilers and laying hens (Yang et al., 2012; Zhan et al., 2018), and serum IgM content in Cherry Valley ducks (Zhuang et al., 2015). Our present study found that there were interaction effects beween the compound probiotics and the acidifiers on serum IgA and IgG contents of ducks, and dietary probiotics mitigated the significant decline in serum IgA and IgG caused by diet inclusion of acidifiers.
CONCLUSIONS
Egg reproductive performance and quality of aged laying ducks can be improved by diet inclusion with compound probiotics composed of B. subtilis and C. butyricum, accompanied by increased expressions of reproductive genes and eggshell formation related genes. Compound probiotics also increased the serum antioxidant enzyme activity. Organic acid mixture supplementation beneficially affected the egg quality, while the serum antioxidant capacity and immunoglobulin content were reduced by acidifier. The negative impact of the acidifier in this study suggests that further studies with different combinations of organic acids on laying ducks deserve to be carried out to distinguish the beneficial acids from the harmful ones.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support provided by the Agricultural Science and Technology Independent Innovation Fund of Jiangsu Province (grant number. CX (18) 1004) and Innovation and Entrepreneurship Training Program for College Students of Jiangsu Province (grant number. 202112806072H).
DISCLOSURES
The authors declare that they have no conflict of interest.
References
- Abbas G., Khan S.H., Rehman H.U. Effects of formic acid administration in the drinking water on production performance, egg quality and immune system in layers during hot season. Avian. Biol. Res. 2013;6:227–232. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y.A., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. An. N. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A., Selim D.A., Basuony H.A., Sabic E.M., Saleh A.A., Ebeid T.A. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health. Pro. 2020;52:671–680. doi: 10.1007/s11250-019-02055-1. [DOI] [PubMed] [Google Scholar]
- Abdelqader A., Al-Fataftah A.R. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livest. Sci. 2016;183:78–83. [Google Scholar]
- Abdelqader A., Al-Fataftah A.R., Das G. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim. Feed. Sci. Tech. 2013;179:103–111. [Google Scholar]
- Arie B. Differential regulation of calbindin in the calcium-transporting organs of birds with high calcium requirements. J. Poult. Sci. 2009;46:265–297. [Google Scholar]
- Ar’Quette G., Gay G.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity and gut health in poultry. Avian. Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Bai K.W., Feng C.C., Jiang L.Y., Zhang L.G., Zhang J.F., Zhang L.L., Wang T. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 2018;97:2312–2321. doi: 10.3382/ps/pey116. [DOI] [PubMed] [Google Scholar]
- Biswas A., Dev K., Tyagi P.K., Mandal A. The effect of multi-strain probiotics as feed additives on performance, immunity, expression of nutrient transporter genes and gut morphometry in broiler chickens. Anim. Biosci. 2022;35:64–74. doi: 10.5713/ab.20.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G.T., Xiao Y.P., Yang C.M., Chen A.G., Liu T.T., Zhou L., Zhang L., Ferket P.R. Effects of clostridium butyricum on growth performance, nitrogen metabolism, intestinal morphology and cecal microflora in broiler chickens. J. Anim. Vet. Adv. 2012;11:2665–2671. [Google Scholar]
- Chen Y.C., Chen T.C. Mineral utilization in layers as influenced by dietary oligofructose and inulin. Inter. J. Poult. Sci. 2004;3:442–445. [Google Scholar]
- Cherrington C.A., Hinton M., Mead G.C., Chopra I. Organic acids: chemistry, antibacterial activity and practical applications. Adv. Microb. Physiol. 1991;32:87–108. doi: 10.1016/s0065-2911(08)60006-5. [DOI] [PubMed] [Google Scholar]
- Dittoe D.K., Ricke S.C., Kiess A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018;5:216. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A., Rezaeipour V., Abdullahpour R. Effects of acidified drinking water on performance, carcass, immune response, jejunum morphology, and microbiota activity of broiler chickens fed diets containing graded levels of threonine. Livest. Sci. 2015;180:158–163. [Google Scholar]
- Elstner E.F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 1976;70:616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Etches R.J. Reproduction in poultry. Livest. Prod. Sci. 1998;48:83–84. [Google Scholar]
- European, Food, Safety, and Authority Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA-Opinion of the Scientific Committee. EFSA J. 2007;587:1–16. [Google Scholar]
- Fascina V.B., Sartori J.R., Gonzales E., Carvalho F.B., Souza I.M.G.P., Polycarpo G.D., Stradiotti A.C., Pelícia V.C. Phytogenic additives and organic acids in broiler chicken diets. Rev. Bras. Zootecn. 2012;41:2189–2197. [Google Scholar]
- Fathi M.M., Ebeid T.A., Al-Homidan I., Soliman N.K., Abou-Emera O.K. Influence of probiotic supplementation on immune response in broilers raised under hot climate. Br. Poult. Sci. 2017;58:512–516. doi: 10.1080/00071668.2017.1332405. [DOI] [PubMed] [Google Scholar]
- Franziska J., Wilhelm A., Jablonowski N., Mothes H., Greulich K.O., Glei M. Butyrate modulates antioxidant enzyme expression in malignant and non-malignant human colon tissues. Mol. Carcinogene. 2014;54:249–260. doi: 10.1002/mc.22102. [DOI] [PubMed] [Google Scholar]
- Fuller R. Probiotics in man and animals. J. Appl. Microbiol. 1989;66:365–378. [PubMed] [Google Scholar]
- Geret F., Serafim A., Bebianno M.J. Antioxidant enzyme activities, metallothioneins and lipid peroxidation as biomarkers in Ruditapes decussatus? Ecotoxicology. 2003;12:417–426. doi: 10.1023/a:1026108306755. [DOI] [PubMed] [Google Scholar]
- Guo M.J., Li M.T., Zhang C.C., Zhang X.R., Wu Y.T. Dietary administration of the Bacillus subtilis enhances immune responses and disease resistance in chickens. Front. Microbiol. 2020;11:1768. doi: 10.3389/fmicb.2020.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M.N., Islam K.M.S., Akbar M.A., Chowdhury R., Khatun M., Karim M.R., Kemppainen B.W. Effect of dietary citric acid, flavomycin and their combination on the performance, tibia ash and immune status of broiler. Can. J. Anim. Sci. 2010;90:57–63. [Google Scholar]
- Hashemi S.R., Zulkifli I., Davoodi H., Zunita Z., Ebrahimi M. Growth performance, intestinal microflora, plasma fatty acid profile in broiler chickens fed herbal plant (Euphorbia hirta) and mix of acidifiers. Anim. Feed. Sci. Tech. 2012;178:167–174. [Google Scholar]
- Hassan H.M.A., Mohamed M.A., Youssef A.W., Hassan E.R. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers. Asian. Austral. J. Anim. 2010;23:1348–1353. [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastro. Hepat. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Ahmed S.T., Kim Y.J., Mun H.S., Kim Y.J., Yang C.J. Effect of sea tangle (laminaria japonica) and charcoal supplementation as alternatives to antibiotics on growth performance and meat quality of ducks. Asian. Austral. J. Anim. 2014;27:217–224. doi: 10.5713/ajas.2013.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J.J., Galfi P. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Kaya A., Kaya H., Gül M., Yildirim B.A., Timurkaan S. Effect of different levels of organic acids in the diets of hens on laying performance, egg quality criteria, blood parameters, and intestinal histomorphology. Indian J. Anim. Res. 2015;49:919–932. [Google Scholar]
- Kaya H., Kaya A., Gul M., Celebi S. The effect of zeolite and organic acid mixture supplementation in the layer diet on performance, egg quality traits and some blood parameters. J. Anim. Vet. Adv. 2013;12:782–787. [Google Scholar]
- Khan R.U., Naz S., Javdani M., Nikousefat Z., Selvaggi M., Tufarelli V., Laudadio V. The use of Turmeric (Curcuma longa) in poultry feed. World. Poult. Sci. J. 2012;68:97–103. [Google Scholar]
- Khan R.U., Naz S., Raziq F., Qudratullah Q., Khan N.A., Laudadio V., Tufarelli V., Ragni M. Prospects of organic acids as safe alternative to antibiotics in broiler chickens diet. Environ. Sci. Pollut. R. 2022;29:32594–32604. doi: 10.1007/s11356-022-19241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.F., Che X.R., Wang Y.Q., Hong C., Thacker P.A. Effect of microbial phytase, vitamin D3, and citric acid on growth performance and phosphorus, nitrogen and calcium digestibility in growing swine. Anim. Feed. Sci. Tech. 1998;73:173–186. [Google Scholar]
- Liao X.D., Wu R.J., Ma G., Zhao L.M., Zheng Z.J., Zhang R.J. Effects of Clostridium butyricum on antioxidant properties, meat quality and fatty acid composition of broiler birds. Lipids. Health. Dis. 2015;14:36. doi: 10.1186/s12944-015-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem A., Pesti G.M., Edwards H.M. The effect of several organic acids on phytate phosphorus hydrolysis in broiler chicks. Poult. Sci. 2008;87:689–693. doi: 10.3382/ps.2007-00256. [DOI] [PubMed] [Google Scholar]
- Liu X., Peng C.Y., Qu X.Y., Guo S.C., Chen J.F., He C.Q., Zhou X.B., Zhu S.W. Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. J. Anim. Physiol. An. N. 2019;103:182–190. doi: 10.1111/jpn.13022. [DOI] [PubMed] [Google Scholar]
- Liu Y.H., Li Y.Y., Feng X.C., Wang Z., Xia Z.F. Dietary supplementation with Clostridium butyricum modulates serum lipid metabolism, meat quality, and the amino acid and fatty acid composition of Peking ducks. Poult. Sci. 2018;97:3218–3229. doi: 10.3382/ps/pey162. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Molnár A.K., Podmaniczky B., Kürti P., Glávits R., Virág G., Szabó Z., Farkas Z. Effect of different concentrations of Bacillus subtilis on immune response of broiler chickens. Probiotics. Antimicro. 2011;3:8–14. doi: 10.1007/s12602-011-9063-x. [DOI] [PubMed] [Google Scholar]
- Mustafa A., Bai S.P., Zeng Q.F., Ding X.M., Wang J.P., Xuan Y., Su Z.W., Zhang K.Y. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express. 2021;11:140. doi: 10.1186/s13568-021-01299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushin V.G., Romanov M.N. Egg physical characteristics and hatchability. World. Poult. Sci. J. 2002;58:297–303. [Google Scholar]
- Nguyen D.H., Seok W.J., Kim I.H. Organic acids mixture as a dietary additive for pigs–a review. Animals. 2020;10:952. doi: 10.3390/ani10060952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.W., Rhee A.R., Um J.S., Paik I.K. Effect of dietary available phosphorus and organic acids on the performance and egg quality of laying hens. J. Appl. Poult. Res. 2009;18:598–604. [Google Scholar]
- Placer Z.A., Cushman L.L., Johnson B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Rajput I.R., Li L.Y., Xin X., Wu B.B., Juan Z.L., Cui Z.W., Yu D.Y., Li W.F. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013;92:956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- Raveschot C., Coutte F., Fremont M., Vaeremans M., Dugersuren J., Demberel S., Drider D., Dhulster P., Flahaut C., Cudennec B. Probiotic Lactobacillus strains from Mongolia improve calcium transport and uptake by intestinal cells in vitro. Food. Res. Int. 2020;133 doi: 10.1016/j.foodres.2020.109201. [DOI] [PubMed] [Google Scholar]
- Rhayat L., Jacquier V., Brinch K.S., Nielsen P., Nelson A., Geraert P.A., Devillard E. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult. Sci. 2017;96:2274–2280. doi: 10.3382/ps/pex018. [DOI] [PubMed] [Google Scholar]
- Roland A.D. Research note: egg shell problems: estimates of incidence and economic impact. Poult. Sci. 1988;67:1801–1803. [Google Scholar]
- Sandi S., Sari M.L., Yosi F., Sahara E., Maharani B.P., Asmak A., Rofip M.N., Ali A.I.M. Organic acid and probiotic derived from grass silage improved egg quality in Pegagan laying duck: a research note. Trop. Anim. Health. Pro. 2022;54:1–5. doi: 10.1007/s11250-022-03060-7. [DOI] [PubMed] [Google Scholar]
- Scicutella F., Mannelli F., Daghio M., Viti C., Buccioni A. Polyphenols and organic acids as alternatives to antimicrobials in poultry rearing: a review. Antibiotics-Basel. 2021;10:1010. doi: 10.3390/antibiotics10081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengor E., Sahin H., Bayram I., Dogan I., Cetingul S., Yardimci M. Effects of short chain fatty acid (SCFA) supplementation on performance and egg characteristics of old breeder hens: short communication. S. Afr. J. Anim. Sci. 2007;37:158–163. [Google Scholar]
- Shalaei M., Hosseini S.M., Zergani E. Effect of different supplements on eggshell quality, some characteristics of gastrointestinal tract and performance of laying hens. Vet. Res. Forum. 2014;5:277–286. [PMC free article] [PubMed] [Google Scholar]
- Simkiss K. Calcium metabolism and avian reproduction. Biol. Rev. 2010;36:321–359. [Google Scholar]
- Soltan M.A. Effect of dietary organic acid supplementation on egg production, egg quality and some blood serum parameters in laying hens. Inter. J. Poult. Sci. 2008;7:613–621. [Google Scholar]
- Souza O., Adams C., Rodrigues B., Krause A., Bonamigo R., Zavarize K., Stefanello C. The impact of Bacillus subtilis PB6 and chromium propionate on the performance, egg quality and nutrient metabolizability of layer breeders. Animals. 2021;11:3084. doi: 10.3390/ani11113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliburska J., Harahap I.A., Skrypnik K., Bogdanski P. The impact of multispecies probiotics on calcium and magnesium status in healthy male rats. Nutrients. 2021;13:3513. doi: 10.3390/nu13103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstil R., Plachy V., Joch M., Salmonova H., Duskova D., Hautekiet V., Vlkova E. Effect of probiotic Clostridium butyricum CBM 588 on microbiota and growth performance of broiler chickens. Czech. J. Anim. Sci. 2019;64:387–394. [Google Scholar]
- Swiatkiewicz S., Arczewska-Wlosek A. Prebiotic fructans and organic acids as feed additives improving mineral availability. World. Poult. Sci. J. 2012;68:269–279. [Google Scholar]
- Upadhaya S.D., Florence R., Kim I.H. Efficacy of dietary Bacillus subtilis and Bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poult. Sci. 2019;98:4722–4728. doi: 10.3382/ps/pez184. [DOI] [PubMed] [Google Scholar]
- Wang J., Ishfaq M., Miao Y.S., Liu Z.Y., Hao M.T., Wang C.Y., Wang J.Q., Chen X.P. Dietary administration of Bacillus subtilis KC1 improves growth performance, immune response, heat stress tolerance, and disease resistance of broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzyniak N., Suliburska J. Nutritional and health factors affecting the bioavailability of calcium: a narrative review. Nutr. Rev. 2021;79:1307–1320. doi: 10.1093/nutrit/nuaa138. [DOI] [PubMed] [Google Scholar]
- Wistedt A., Ridderstrale Y., Wall H., Holm L. Exogenous estradiol improves shell strength in laying hens at the end of the laying period. Acta. Vet. Scand. 2014;56:34. doi: 10.1186/1751-0147-56-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Q.H., Wang C., Zhang H., Lai W., Wei H.K., Peng J. Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals. 2019;9:1110. doi: 10.3390/ani9121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Xin H.L., Yang C.B., Yang X.J. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018;4:388–393. doi: 10.1016/j.aninu.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.M., Cao G.T., Ferket P.R., Liu T.T., Zhou L., Zhang L., Xiao Y.P., Chen A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012;91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- Yang Y., Iji P.A., Choct M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World. Poultry. Sci. J. 2009;65:97–114. [Google Scholar]
- Yesilbag D., Çolpan I. Effects of organic acid supplemented diets on growth performance, egg production and quality and on serum parameters in laying hens. Rev. Med. Vet-toulouse. 2006;157:280–284. [Google Scholar]
- Yosefi S., Braw-Tal R., Bar A. Intestinal and eggshell calbindin, and bone ash of laying hens as influenced by age and molting. Comp. Biochem. Phys. A. 2003;136:673–682. doi: 10.1016/s1095-6433(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Youn B.S., Nam K.T., Chang K.M., Hwang S.G., Choe I.S. Effects of wood vinegar addition for meat quality improvement of old layer. Korean. J. Poult. Sci. 2005;32:101–106. [Google Scholar]
- Youssef A.W., Hassan H., Ali H.M., Mohamed M.A. Effect of probiotics, prebiotics and organic acids on layer performance and egg quality. Asian J. Poult. Sci. 1997;7:65–74. [Google Scholar]
- Zhan H.Q., Dong X.Y., Li L.L., Zheng Y.X., Gong Y.J., Zou X.T. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2018;98:896–903. doi: 10.3382/ps/pey436. [DOI] [PubMed] [Google Scholar]
- Zhang B.K., Yang X., Guo Y.M., Long F.Y. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr. 2011;65:329–339. doi: 10.1080/1745039x.2011.568274. [DOI] [PubMed] [Google Scholar]
- Zhang W.H., Jiang Y., Zhu Q.F., Gao F., Dai S.F., Chen J., Zhou G.H. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011;52:292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]
- Zhang Y.N., Zhang H.J., Wang J., Yue H.Y., Qi X.L., Wu S.G., Qi G.H. Effect of dietary supplementation of organic or inorganic zinc on carbonic anhydrase activity in eggshell formation and quality of aged laying hens. Poult. Sci. 2017;96:2176–2183. doi: 10.3382/ps/pew490. [DOI] [PubMed] [Google Scholar]
- Zhuang S., Jiang F.B., Jia Z.X., Yan R. Clostridium butyricum can be used as a potential alternative for the antibiotic in Cherry Valley ducks. J. Anim. Plant. Sci. 2015;25:1227–1232. [Google Scholar]