Abstract
Although aroma is one of the most essential factors determining the quality of Fu brick tea (FBT), the aroma profiles of FBTs from different manufacturing areas are rarely investigated. The aroma profiles of FBTs manufactured in five typical provinces of China were comprehensively analyzed on the basis of headspace gas chromatography–ion mobility spectrometry (HS–GC–IMS), headspace solid-phase microextraction gas chromatography–mass spectrometry (HS–SPME–GC–MS), sensory evaluation, odor activity value (OAV), and relative odor activity value (ROAV). HS–GC–IMS and HS–SPME–GC–MS identified 63 and 93 volatile organic compounds (VOCs), respectively. Multivariate statistical analysis indicated that the FBTs from different production regions had remarkably varied aromas. HS–SPME–GC–MS revealed that 27 VOCs (OAV >1) contributed to the overall aroma of the samples, of which 15 key differential compounds can effectively distinguish the aroma profiles of different FBTs. FBT from Shaanxi manifested a strong floral and fruity aroma; that from Hunan had a floral, grassy, and pine-woody aroma; that from Guizhou presented a grassy and herbal aroma; that from Guangxi exhibited a sweet, floral, and minty aroma; and that from Zhejiang possessed various fruit flavors and floral fragrance. OAV analysis identified the biomarkers responsible for the variation in the aroma characteristics of diverse FBTs. These biomarkers included linalool, 6-methyl-5-hepten-2-one, α-ionone, hexanal, and ethyl hexanoate. Sensory evaluation demonstrated that the infusion color and aroma of FBT samples from different provinces also greatly varied. Network correlation analysis revealed that Aspergillus and Eurotium were the crucial microorganisms for the metabolism and formation of VOCs. These findings provide new insight into the VOCs and fragrance features of FBTs produced in different regions of China.
Keywords: Fu brick tea, Aroma characteristics, Multivariate statistical analysis, Sensory characteristics
Abbreviations: FBT, Fu brick tea; HS–GC–IMS, headspace gas chromatography–ion mobility spectrometry; HS–SPME–GC–MS, headspace solid-phase microextraction gas chromatography mass spectrometry; VOCs, volatile organic compounds; PCA, principal component analysis; PLS-DA, partial least squares–discriminant analysis; HCA, hierarchical cluster analysis; VIP, variable importance in the projection
Graphical abstract
Highlights
-
•
Volatiles in Fu brick tea from five provinces of China were comprehensively analyzed.
-
•
A total of 63 and 93 VOCs were identified by GC-IMS and GC-MS, respectively.
-
•
Aroma profiles of Fu brick tea from five regions were greatly different.
-
•
15 key volatiles were proposed to discriminate Fu brick tea from different regions.
-
•
The correlations between the key VOCs and fungal community were analyzed.
1. Introduction
Fu brick tea (FBT) is a type of Chinese dark tea that undergoes microbial fermentation during processing (Xu et al., 2011). It is gaining popularity worldwide due to its unique flavor and health effects. The main production areas of FBT in China are Hunan, Zhejiang, Guizhou, Guangxi, and Shaanxi Provinces (Chen et al., 2022). Piling, steaming, pressing, fermentation, and drying are the main steps in the manufacture of typical postfermented FBT and contribute to the formation of the distinct aroma features of FBT (Ling et al., 2010). Among these steps, microbial fermentation is thought to be the most important for developing the distinct flavors of FBT (Ling et al., 2010). Several previous works reported that this process involved a wide range of microorganisms, including fungi (e.g., Eurotium, Cyberlindnera, Debaryomyces, Aspergillus, and Candida) and bacteria (Xu et al., 2011; Li et al., 2019b; Xiao et al., 2022a). These microorganisms secrete a variety of extracellular enzymes (e.g., α-amylase, pectinase, polyphenol oxidase, cellulase, and protease) during the manufacturing of FBT (Ward et al., 2005). These enzymes decompose and convert protein, pectin, cellulose, and polyphenols for the formation of specific volatile organic compounds (VOCs). Alcohols, ketones, aldehydes, and heteroatomic compounds considerably accumulate in FBT during fermentation (Xu et al., 2007). Additionally, the aroma characteristics of FBT differ substantially in accordance with raw materials, processing conditions, and storage duration (Zheng et al., 2022), which may influence the microbial compositions of FBT that subsequently affect the flavor quality of the tea. For example, the production environment and processing conditions could jointly affect bacterial succession in dark tea (Yao et al., 2017). Tian et al. (2013) revealed that the bacterial community structure of Pu'er tea differed depending on storage conditions and that bacterial diversity was linked to aging time. Our previous study revealed that the fungal communities of FBT samples from different regions of China varied greatly and influenced the metabolites, antioxidant activity, and taste characteristics of the teas (Chen et al., 2022). However, only a few previous studies have focused on the dynamic changes in microbial composition and metabolites during the manufacturing of FBT. Therefore, the differences in the aroma profiles of FBT samples with different origins and their key differential VOCs remain poorly understood.
In recent years, many advanced techniques for food flavor analysis have been developed with the rapid evolution of science and technology. Headspace solid-phase microextraction gas chromatography–mass spectrometry (HS–SPME–GC–MS) combines the advantages of the high separation capacity of gas chromatography (GS) with the strong metabolite identification ability of mass spectrometry (MS) and has been broadly used on food samples for odor analysis and quality classification (Chen et al., 2021). However, GC–MS is not sensitive enough for low levels of VOCs, leading to the neglect of VOCs (Chen et al., 2021). Headspace–gas chromatography–ion mobility spectrometry (HS–GC–IMS) is a convenient scientific technique for food flavor analysis due to its rapid detection, lack of sample pretreatment requirement, excellent resolution, ease of operation, intuitive data visualization, ultrahigh sensitivity and analytical speed, and operation under atmospheric pressure. It combines the benefits of the high separation efficiency of GC with the fast response of IMS. In recent years, research on food flavor using HS–GC–IMS has increased (Xiao et al., 2022b). However, some VOCs have not been identified because of the lack of a complete database for HS–GC–IMS. Hence, a combination of HS–GC–IMS and HS–SPME–GC–MS could enable the comprehensive analysis of the VOCs in FBT samples.
Therefore, the objective of this study is to determine the variations in the VOCs of five FBTs from different regions of China through GC–IMS and GC–MS analyses and identify the key contributory VOCs to elucidate the characteristic aroma formation of FBT samples from different regions. This study could provide crucial information on the formation mechanism of the flavor characteristics of FBT samples from different regions of China.
2. Materials and methods
2.1. Reagents and tea samples
Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China) provided the C4–C9 n-ketones used in the GC–IMS study. Ethyl caprate was purchased from CATO Co., Ltd. (Guangzhou, China). All other reagents and compounds were of analytical grade. The FBT samples collected from five production areas in China were provided by Guangxi Jinhua Tea Co., Ltd. (GX); Hunan Anhua Yuntiange Tea Industry Co., Ltd. (HN); Guizhou Fanjin Tea Industry Co., Ltd. (GZ); Shaanxi Xianxi lamu Fu Tea Co., Ltd. (SX); and Zhejiang Wuyi luotuo Jiulong Brick Tea Co., Ltd. (ZJ). All of these FBT samples were produced by using traditional processing methods in the same year (2018). The obtained FBTs were ground, sealed, and frozen at −20 °C before VOC analysis.
2.2. HS–GC–IMS analysis
The VOCs of FBT were analyzed by using a GC–IMS FlavourSpec instrument (Dortmund, Germany) equipped with an Agilent Technologies 490 micro gas chromatograph and a MXT-5 capillary column (15 m × 0.53 mm). The temperature of the chromatographic column for separation was set as 60 °C. The GC–IMS instrument was equipped with an automatic headspace injector (CTC Analytics AG, Zwingen, Switzerland) with a headspace sampling unit. A total of 1.5 g of sample was placed into a headspace sample vial and incubated for 15 min at 80 °C. Then, 500 μL of headspace was injected by an injection syringe (splitless mode) heated to 85 °C. The carrier gas (nitrogen, purity ≥99.99%) was delivered in programmed flow starting at the rate of 2 mL/min for 2 min. The rate was gradually increased to 100 mL/min over 18 min. The analytes were ionized by using a 3H ionization source in the IMS ionization chamber in positive ion mode. Then, the resulting ions were driven into a 9.8 cm drift tube that was conducted at a constant voltage (5 kV) and temperature (45 °C). Nitrogen (purity ≥99.99%) was used as the drift gas (150 mL/min). The average of 12 scans was used to create each spectrum. The n-ketones C4–C9 were used to calculate the VOC retention index (RI). VOCs were identified by comparing the drift time and RI of the standard in the GC–IMS library (Dortmund, Germany), and the contents were estimated on the basis of the peak intensity in GC–IMS.
2.3. HS–SPME–GC–MS analysis
The GC–MS analysis of VOCs was performed by using an Agilent 7000D system (Agilent, Palo Alto, CA, USA). A HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm, Agilent, USA) was used to separate the VOCs. The VOCs were extracted through headspace solid-phase microextraction (HS–SPME) as follows: 0.5 g of finely powdered FBT samples were placed in a headspace vial with a volume of 20 mL. Subsequently, the headspace vial was added with 0.5 g of sodium chloride, 5 mL of boiled ultrapure water, and 10 μL of ethyl caprate internal standard solution. The headspace glass bottle was pre-equilibrated on a heated plate (80 °C) for 10 min, after which a 50/30 μm DVB/CAR/PDMS fiber (Supelco, Bellefonte PA, USA) was injected into the headspace vial for the extraction of VOCs for 50 min under the same conditions. Then, the fiber was desorbed in splitless injection mode at 250 °C for 5 min in the injection port of the GC–MS. The following chromatographic separations for the column oven were carried out: The initial temperature was set at 40 °C and maintained for 3 min, then increased to 80 °C at the rate of 2 °C/min, ramped to 90 °C at the rate of 2 °C/min, increased to 150 °C at the rate of 3 °C/min, and raised to 180 °C at the rate of 5 °C/min. The temperature was subsequently increased to 230 °C at 15 °C/min and held for 2 min. Helium (99.999%) was used as the carrier gas at the flow rate of 1 mL/min. Mass spectrometry was carried out in electron impact ionization mode with a 70 eV electron energy and a mass scan range of 35–400 m/z. The temperature of the MS ion source was 230 °C. The VOCs were identified by comparing the mass spectra of all detected metabolites with those in the NIST 17.0 library, Wiley MS library, and other public databases. Quantitative analysis was performed on the basis of the internal standard ethyl caprate.
2.4. Odor activity values and relative odor activity value analysis
Odor activity values (OAV) are widely used to assess the contributions of certain flavor compounds. Compounds with OAV >1 are generally regarded as aroma-active compounds that greatly contribute to the overall aroma characteristics of a sample. OAV was calculated by using the ratio of the concentration (C) of each VOC to the odor threshold (OT) of the VOC in water (Zhong et al., 2022). OTs were obtained from earlier reports (Guo et al., 2022; Yang et al., 2022) and used in the following formula: OAV = C/OT, where OAV is the odor activity value of the compound, C is the content of the compound, and OT is the odor threshold concentration of the compound in water.
In this study, the concentration of VOCs obtained by GC-IMS was expressed by the relative content (%), therefore, relative odor activity values (ROAV) were performed to evaluate the specific contribution of each compound to the overall aroma, which was well reported in previous studies (Zhu et al., 2020; Zhong et al., 2022). The ROAV of a volatile compound (i) was calculated as ROAVi = (OAVi/OAVmax) × 100 (Zhu et al., 2020). Given that OAVi = Ci/OTi, then ROAVi = (Ci/OTi) × (OTmax/Cmax) × 100, where ‘max’ is the volatile compound, which has the highest value calculated by dividing the relative content compound by odor threshold concentration among the detected volatile compounds. OAVmax is the odor activity value of the above compound (‘max’) and is regarded as 100, Cmax is the relative content of the compound (‘max’), and OTmax is the odor threshold of the compound (‘max’). OAVi is the odor activity value of a compound, Ci is the relative content of the compound, and OTi is the odor threshold of the compound.
A high ROAV is also indicative of the great contribution of a component to the overall flavor of the sample. Components with ROAV ≥1 are generally considered as the key aroma compounds of the analyzed samples, whereas components with 0.1 ≤ ROAV <1 have important modifying effects on the overall aroma of the samples (Zhang et al., 2020; Zhong et al., 2022).
2.5. Sensory evaluation
Ten well-trained panelists from Hunan Agricultural University (five females and five males, ages 22 years–50 years) evaluated the odor and taste qualities of FBT by using the Chinese Standard Methodology of Tea (GB/T 23776–2018) with minor modifications. All assessors were trained in the ability to identify, describe, and differentiate different aroma qualities. Each assessor completed 200 h of training on a specific tea infusion. After training, a discussion was held to determine accurate aroma characteristic terms to summarize the attributes of the five kinds of FBTs. Finally, five aroma attributes were selected to describe the overall aroma characteristics of the five FBTs: fungal floral, minty, floral, green, and woody. The color of the tea infusions was also evaluated. Briefly, 3.0 g of each FBT sample was placed in a teacup and brewed for 5 min with 150 mL of boiling water. Subsequently, the tea infusions were filtered into tea bowls and assessed by the panelists. Before the evaluation, all instructions were given to the panelists. Infusions of FBT from different regions were assigned three-digit numbers and presented in random order. During the examination of odor qualities, the panelists were given water to rinse their palates in between each sample. Panelists recorded scores for the sensory qualities of the FBT samples. High scores indicate strong intensities (10 = extremely high intensity, 5 = medium intensity, and 0 = none or unnoticeable intensity) in the sensory attribute descriptions of tea on a 10-point hedonic scale. The average sensory attribute scores were calculated on the basis of the scores recorded by the ten panelists.
2.6. Statistical analysis
All measurements were carried out at least three times, with the results reported as the mean ± SD. SPSS 17.0 software was used to evaluate statistical significance (p < 0.05) by using one-way analysis of variance and Duncan's multiple range test (Chicago, IL, USA). SIMCA-P+ 12.0 software (Umea, Sweden) was used to perform principal component analysis (PCA), partial least squares–discriminant analysis (PLS–DA), and hierarchical cluster analysis (HCA). Graphical presentations, heat maps, and network plots were generated with Origin v8.5 (OriginLab Corporation, MA, USA), TBtools version 0.655, and Cytoscape software (version 3.5.1) (http://www.cytoscape.org/). GC–IMS data were processed by using Reporter plug-ins, Laboratory Analytical Viewer software (Dortmund, Germany), dynamic PCA, and gallery plot analysis.
3. Results and discussion
3.1. Analysis of VOCs by HS–GC–IMS
3.1.1. Topographic plots of the HS–GC–IMS results of FBT samples from different regions
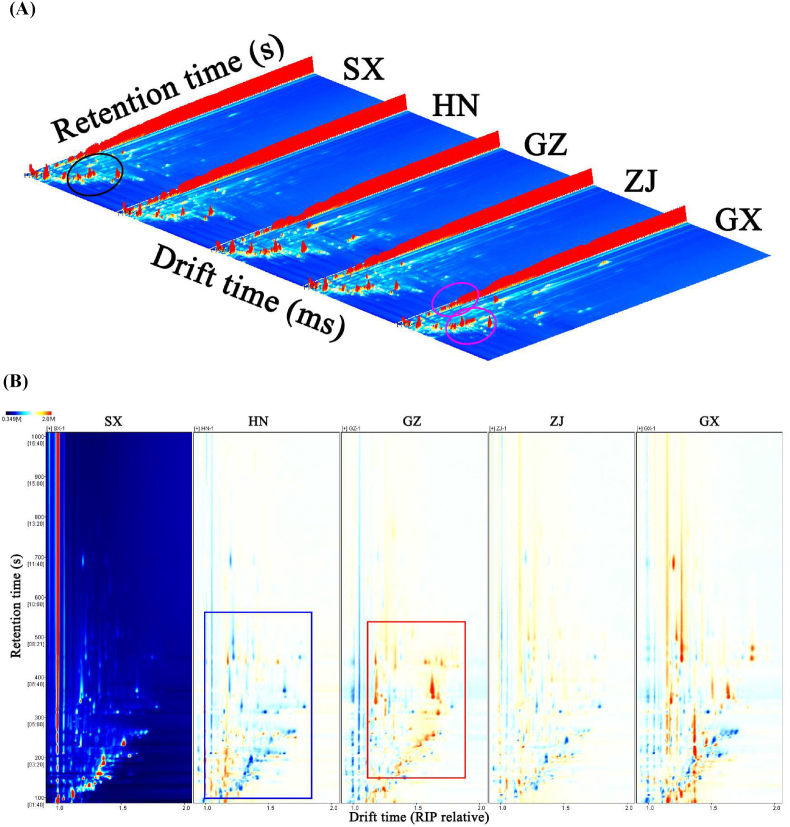
HS–GC–IMS was performed to elucidate the variations in the VOCs of different FBTs. Fig. 1A shows a three-dimensional (3D) topographic map of the VOCs of five samples. Although the VOCs of the five FBT samples were similar, several peaks were clearly different, indicating that the VOCs of the FBT samples from different regions differed drastically. An overhead view was conducted to observe the variations in VOCs with increased clarity, and the result is shown in Fig. 1B. SX was used as the reference, and the spectra of HN, GZ, ZJ, and GX were obtained by subtracting the reference. Blue and red indicate lower and higher VOCs in the sample than in the reference, respectively. The majority of signals had a retention time of 100–800 s and a drift time of 1.0–2.0 s (relative RIP). The presence of more red spots in GZ and GX than in the reference suggested that VOCs were present in larger concentrations in these samples than in the reference. By contrast, HN and ZJ had numerous blue specks in the backdrop, indicating that they had lower VOC concentrations than the reference.
Fig. 1.
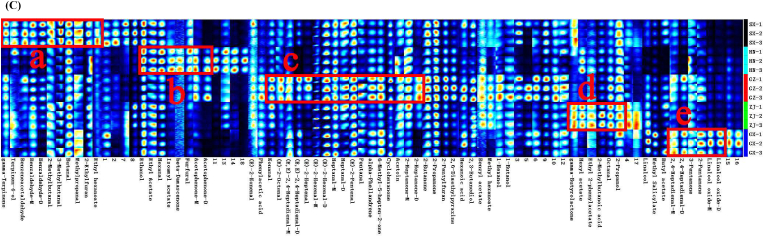
(A) 3D–topographic and (B) 2D–topographic subtraction plots of the VOCs of different Fu brick tea. (C) VOCs fingerprint comparisons of different Fu brick tea.
3.1.2. Differential VOCs of FBT samples
Supplementary Fig. 1 displays the qualitative analysis of VOCs, which were plotted in topographic plots (Fig. S1). A total of 63 VOCs were tentatively identified. They included nine esters, 24 aldehydes, nine alcohols, 12 ketones, three acids, two hydrocarbons, two furans, one pyrazine, and one lactone. The other 18 peaks were unidentifiable due to the limited data in the library databases. The detailed information on the 63 VOCs identified in the five FBT samples are listed in Table 1. The carbon chain of these identified VOCs were all within C2–C13, and several single compounds were seen to produce multiple spots or signals (dimer or monomer forms) owing to their varied levels and characteristics (Rodríguez–Maecker et al., 2017). These compounds included heptanal, (E)-2-hexenal, 2,4-heptadienal, linalool oxide, and 2-heptanone. The results of fingerprint analysis showed that the contents of VOCs in SX, HN, GZ, ZJ, and GX varied greatly in the gallery pot regions (a, b, c, d, and e). In Fig. 1C, each column represents a VOC, and each line represents a FBT sample. The SX sample had high concentrations of γ-terpinene, terpinen-4-ol, benzeneacetaldehyde, butanal, benzaldehyde-M, benzaldehyde-D, 2-methylbutanal, 3-methylbutanal, ethyl hexanoate, methylpropanal, and 2-ethylfuran as illustrated in box a. The HN sample showed high levels of ethanol, β-damascenone, hexanal, isoamyl acetate, ethyl acetate, furfural, acetophenone-M, and acetophenone-D as displayed in box b. The GZ sample showed high contents of phenylacetic acid, (E)-2-octenal, (E,E)-2,4-heptadienal-M, (E,E)-2,4-heptadienal-D, (E)-2-heptenal, (E)-2-hexenal-M, (E)-2-hexenal-D, pentanal, α-phellandrene, and 2-heptanone-D as depicted in box c. The ZJ sample had high concentrations of γ-butyrolactone, ethyl 2-phenylacetate 2-methylbutanoic acid, octanal, and 2-propanol as shown in box d. The GX sample displayed high concentrations of 2,4-heptadienal-M, 2-pentanone, 3-pentanone, 2,4-heptadienal-D, linalool oxide-M, and linalool oxide-D as illustrated in box e. The peak intensities of these substances in the five samples of FBT were different.
Table 1.
GC–IMS integration parameters and peak intensity for the analysis of volatile components in Fu brick tea from 5 different places by HS–GC–IMS.
| No | Compounds | Odor descriptiona | CAS | Formula | MW | RIb | Rtc [sec] | Dtd [RIPrel] | peak intensity |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SX | HN | GZ | ZJ | GX | |||||||||
| Esters | |||||||||||||
| a1 | Methyl Salicylate | Wintergreen, mint | 119-36-8 | C8H8O3 | 152.1 | 1232.8 | 684.21 | 1.20761 | 1175.06 ± 81.53b | 636.96 ± 134.72b | 1250.69 ± 242.36b | 936.80 ± 72.61b | 2324.23 ± 1132.39a |
| a2 | Butyl acetate | Ethereal, solvent, fruity, banana | 123-86-4 | C6H12O2 | 116.2 | 804 | 210.85 | 1.62989 | 186.99 ± 29.99a | 17.97 ± 1.76b | 113.62 ± 25.15 ab | 154.69 ± 82.83a | 193.14 ± 91.08a |
| a3 | Ethyl acetate | Ethereal, fruity, sweet, weedy, green | 141-78-6 | C4H8O2 | 88.1 | 605 | 139.544 | 1.34393 | 574.22 ± 76.32a | 527.03 ± 64.43a | 424.45 ± 23.48b | 563.09 ± 38.98a | 425.72 ± 7.03b |
| a4 | Isoamyl acetate | Sweet, fruity, banana, solvent | 123-92-2 | C7H14O2 | 130.20 | 880.4 | 250.575 | 1.3078 | 133.94 ± 11.39b | 287.66 ± 48.02a | 111.61 ± 11.29bc | 97.39 ± 23.21bc | 78.12 ± 15.19c |
| a5 | Ethyl hexanoate | Sweet, fruity, pineapple, waxy, green, banana | 123-66-0 | C8H16O2 | 144.20 | 1014.7 | 371.007 | 1.33039 | 669.26 ± 210.63a | 299.64 ± 63.34b | 359.33 ± 14.35b | 271.03 ± 39.05b | 328.42 ± 23.80b |
| a6 | Hexyl acetate | Fruity, green, apple, banana, sweet | 142-92-7 | C8H16O2 | 144.20 | 1031 | 394.412 | 1.40906 | 122.76 ± 8.21b | 370.00 ± 39.70a | 121.70 ± 8.92b | 367.00 ± 8.51a | 129.77 ± 8.94b |
| a7 | Benzyl acetate | Sweet, floral, fruity, jasmin, fresh | 140-11-4 | C9H10O2 | 150.20 | 1154.3 | 571.519 | 1.33512 | 139.64 ± 10.96b | 167.08 ± 34.78 ab | 193.30 ± 9.25a | 152.31 ± 9.51b | 95.64 ± 12.43c |
| a8 | Ethyl 2-phenylacetate | Sweet, floral, honey, rose, balsam, cocoa | 101-97-3 | C10H12O2 | 164.20 | 1241.3 | 696.42 | 1.28639 | 161.70 ± 8.48c | 243.43 ± 43.61 ab | 217.98 ± 26.04bc | 285.57 ± 9.65a | 180.72 ± 47.83bc |
| a9 | Methyl hexanoate | Ethereal, fruity, pineapple, apricot, strawberry, tropical, banana, bacon | 106-70-7 | C7H14O2 | 130.20 | 922.8 | 282.609 | 1.28394 | 60.71 ± 9.13b | 48.12 ± 3.53b | 106.67 ± 23.45a | 111.65 ± 5.23a | 43.28 ± 6.83b |
| Aldehydes | |||||||||||||
| a10 | (E)-2-Nonenal | Fatty, green, cucumber, aldehydic, citrus | 18829-56-6 | C9H16O | 140.20 | 1186 | 617.03 | 1.41242 | 260.39 ± 51.87a | 231.18 ± 10.23a | 252.30 ± 14.70a | 235.73 ± 13.46a | 263.37 ± 34.12a |
| a11 | Nonanal | Waxy, aldehydic, rose, fresh, orris, orange, peel, fatty, peely | 124-19-6 | C9H18O | 142.20 | 1109.2 | 506.74 | 1.47094 | 469.51 ± 32.15bc | 384.80 ± 46.77cd | 706.82 ± 94.96a | 604.57 ± 111.66 ab | 314.70 ± 56.94d |
| a12 | (E)-2-Octenal | Fresh, cucumber, fatty, green, herbal, banana, waxy, leaf | 2548-87-0 | C8H14O | 126.20 | 1057 | 431.77 | 1.33579 | 367.81 ± 9.41b | 325.30 ± 18.26bc | 648.49 ± 65.87a | 277.04 ± 56.19c | 270.53 ± 23.68c |
| a13 | Benzeneacetaldehyde | Green, sweet, floral, hyacinth, clover, honey, cocoa | 122-78-1 | C8H8O | 120.20 | 1041.8 | 409.92 | 1.25245 | 512.44 ± 129.86a | 152.47 ± 61.14b | 331.45 ± 149.15 ab | 184.32 ± 40.42b | 229.50 ± 50.78b |
| a14 | (E,E)-2,4-Heptadienal–M | Fatty, green, oily, aldehydic, vegetable, cake, cinnamon | 4313-03-5 | C7H10O | 110.20 | 1012.3 | 367.63 | 1.18994 | 1508.49 ± 239.20c | 1019.71 ± 41.82d | 2964.98 ± 43.84a | 1419.67 ± 177.04c | 2425.11 ± 247.65b |
| a15 | (E,E)-2,4-Heptadienal–D | Fatty, green, oily, aldehydic, vegetable, cake, cinnamon | 4313-03-5 | C7H10O | 110.20 | 1012.8 | 368.34 | 1.62748 | 1055.03 ± 108.37b | 155.47 ± 21.21b | 2503.27 ± 906.68a | 778.98 ± 72.29b | 2022.63 ± 572.62a |
| a16 | 2,4-Heptadienal-M | Green, pungent, fruity, spicy | 5910-85-0 | C7H10O | 110.20 | 1001.1 | 351.60 | 1.19574 | 313.20 ± 44.30b | 254.01 ± 11.66b | 611.20 ± 81.76a | 254.83 ± 68.50b | 727.58 ± 61.76a |
| a17 | 2,4-Heptadienal-D | Green, pungent, fruity, spicy | 5910-85-0 | C7H10O | 110.20 | 1001.1 | 351.60 | 1.63398 | 243.96 ± 21.11b | 71.00 ± 18.31b | 728.61 ± 400.33a | 183.71 ± 35.06b | 964.37 ± 232.53a |
| a18 | Benzaldehyde-M | Strong, sharp, sweet, bitter, almond, cherry | 100-52-7 | C7H6O | 106.10 | 960.5 | 314.48 | 1.15266 | 699.78 ± 108.97a | 393.58 ± 18.96c | 545.64 ± 41.05b | 624.60 ± 26.81 ab | 540.33 ± 65.70b |
| a19 | Benzaldehyde-D | Strong, sharp, sweet, bitter, almond, cherry | 100-52-7 | C7H6O | 106.10 | 960.5 | 314.48 | 1.47651 | 542.25 ± 103.35a | 91.15 ± 3.38c | 340.10 ± 60.05b | 269.35 ± 24.59b | 104.23 ± 12.93c |
| a20 | (E)-2-Heptenal | Pungent, green, vegetable, fresh, fatty | 18829-55-5 | C7H12O | 112.20 | 954.7 | 309.56 | 1.67558 | 103.33 ± 16.74b | 64.63 ± 9.48b | 562.20 ± 178.83a | 114.36 ± 25.33b | 141.61 ± 21.13b |
| a21 | Heptanal-M | Fresh, aldehydic, fatty, green, herbal, wine-lee, ozone | 111-71-7 | C7H14O | 114.20 | 905 | 267.52 | 1.33093 | 387.27 ± 87.39a | 413.61 ± 52.12a | 483.19 ± 23.58a | 341.92 ± 79.73a | 365.33 ± 74.94a |
| a22 | Heptanal-D | Fresh, aldehydic, fatty, green, herbal, wine-lee, ozone | 111-71-7 | C7H14O | 114.20 | 903.9 | 266.62 | 1.70083 | 730.83 ± 133.39a | 316.14 ± 26.71b | 758.55 ± 63.14a | 583.74 ± 142.17a | 236.01 ± 52.28b |
| a23 | (E)-2-Hexenal-M | Green, banana, aldehydic, fatty, cheesy | 6728-26-3 | C6H10O | 98.10 | 847.2 | 233.31 | 1.18472 | 1705.52 ± 19.21b | 2007.49 ± 171.73a | 1543.32 ± 44.42b | 2065.85 ± 93.43a | 1496.07 ± 125.87b |
| a24 | (E)-2-Hexenal-D | Green, banana, aldehydic, fatty, cheesy | 6728-26-3 | C6H10O | 98.10 | 847.2 | 233.31 | 1.52169 | 5268.13 ± 568.76b | 4389.53 ± 363.82bc | 6978.86 ± 485.37a | 3514.24 ± 542.61c | 4675.11 ± 682.86b |
| a25 | Hexanal | Fresh, green, fatty, aldehydic, grass, leafy, fruity, sweaty | 66-25-1 | C6H12O | 100.20 | 792.4 | 204.79 | 1.57116 | 1428.27 ± 142.16a | 1716.64 ± 106.13a | 1318.76 ± 51.31 ab | 940.73 ± 372.23b | 907.35 ± 246.07b |
| a26 | (E)-2-Pentenal | Pungent, green, fruity, apple, orange, tomato | 1576-87-0 | C5H8O | 84.10 | 748.2 | 185.89 | 1.36557 | 4555.79 ± 280.65b | 2725.16 ± 56.78d | 5146.09 ± 259.56a | 3529.27 ± 167.78c | 3544.20 ± 463.10c |
| a27 | Pentanal | Fermented, bready, fruity, nutty, berry | 110-62-3 | C5H10O | 86.10 | 696.5 | 164.86 | 1.43204 | 274.79 ± 17.32b | 144.26 ± 90.46c | 451.47 ± 64.38a | 289.48 ± 18.43b | 318.24 ± 49.81b |
| a28 | 2-Methylbutanal | Musty, cocoa, phenolic, coffee, nutty, malty, fermented, fatty, alcoholic | 96-17-3 | C5H10O | 86.10 | 659 | 153.81 | 1.40576 | 1032.91 ± 9.28a | 546.24 ± 50.11d | 586.02 ± 25.56d | 905.73 ± 30.39b | 714.89 ± 118.48c |
| a29 | 3-Methylbutanal | Ethereal, aldehydic, chocolate, peach, fatty | 590-86-3 | C5H10O | 86.10 | 637.4 | 148.10 | 1.41658 | 589.52 ± 14.64a | 223.29 ± 21.65c | 246.22 ± 13.97c | 409.71 ± 34.27b | 364.54 ± 112.84b |
| a30 | Butanal | Pungent, cocoa, musty, green, malty, bready | 123-72-8 | C4H8O | 72.10 | 590.1 | 135.622 | 1.29602 | 1268.41 ± 51.67a | 1035.27 ± 60.71b | 977.03 ± 11.15b | 1008.86 ± 86.66b | 963.78 ± 23.78b |
| a31 | Methylpropanal | Fresh, aldehydic, floral, green | 78-84-2 | C4H8O | 72.10 | 553.7 | 125.996 | 1.28365 | 1010.99 ± 21.48a | 280.94 ± 26.82c | 768.07 ± 63.53b | 333.07 ± 89.66c | 725.32 ± 94.53b |
| a32 | Octanal | Aldehydic, waxy, citrus, orange, peel, green, herbal, fresh, fatty | 124-13-0 | C8H16O | 128.20 | 1005.6 | 358.063 | 1.40123 | 164.99 ± 48.78b | 191.11 ± 20.24b | 281.01 ± 17.23a | 321.15 ± 5.53a | 148.73 ± 5.95b |
| a33 | 2-Furfural | Sweet, woody, almond, fragrant, baked, bread | 98-01-1 | C5H4O2 | 96.10 | 828.8 | 223.72 | 1.08249 | 144.24 ± 4.35a | 182.62 ± 18.26a | 89.84 ± 9.91b | 68.19 ± 33.91b | 55.41 ± 16.52b |
| Alcohols | |||||||||||||
| a34 | Terpinen-4-ol | Pepper, woody, earth, musty, sweet | 562-74-3 | C10H18O | 154.30 | 1159.5 | 578.93 | 1.2387 | 281.75 ± 33.70a | 169.87 ± 75.73bc | 105.09 ± 9.75c | 215.72 ± 66.69 ab | 168.07 ± 50.65bc |
| a35 | Linalool | Citrus, floral, sweet, rose, woody, green, blueberry | 78-70-6 | C10H18O | 154.30 | 1095.3 | 486.75 | 1.222 | 869.88 ± 108.43b | 236.52 ± 24.28c | 1126.92 ± 147.86b | 1733.67 ± 183.43a | 1711.42 ± 250.68a |
| a36 | 2,3-Butanediol | Fruity, creamy, buttery | 513-85-9 | C4H10O2 | 90.10 | 780.6 | 199.09 | 1.36248 | 2147.88 ± 502.00 ab | 1472.48 ± 391.36b | 3130.60 ± 287.90a | 2545.50 ± 970.29 ab | 2334.71 ± 766.84 ab |
| a37 | Ethanol | Strong, alcoholic, ethereal, medical | 64-17-5 | C2H6O | 46.10 | 451.1 | 98.9 | 1.05025 | 1655.17 ± 99.12a | 1664.72 ± 146.53a | 1080.75 ± 68.91c | 1379.07 ± 126.22b | 1523.98 ± 108.93 ab |
| a38 | 2-Propanol | Alcohol, musty, woody | 67-63-0 | C3H8O | 60.10 | 565.2 | 129.051 | 1.21957 | 732.26 ± 6.61a | 616.94 ± 18.23 ab | 655.61 ± 70.72 ab | 739.49 ± 30.38a | 534.50 ± 169.82b |
| a39 | Linalool oxide-M | Musty, camphor, fenchyl, alcohol | 60047-17-8 | C10H18O2 | 170.30 | 1066.4 | 445.364 | 1.26608 | 144.79 ± 27.49b | 451.95 ± 135.88b | 426.45 ± 63.36b | 362.11 ± 12.92b | 1824.81 ± 474.85a |
| a40 | Linalool oxide-D | Musty, camphor, fenchyl, alcohol | 60047-17-8 | C10H18O2 | 170.30 | 1067.8 | 447.275 | 1.82189 | 95.34 ± 15.74b | 133.13 ± 25.71b | 229.11 ± 29.74b | 134.29 ± 10.43b | 729.04 ± 272.41a |
| a41 | 1-Hexanol | Ethereal, fusel oil, fruity, alcoholic, sweet, green | 111-27-3 | C6H14O | 102.20 | 873.8 | 247.109 | 1.32584 | 198.94 ± 26.26bc | 159.56 ± 11.06c | 508.23 ± 40.91a | 256.74 ± 41.00b | 238.69 ± 52.96b |
| a42 | 1-Butanol | Fusel oil, sweet, balsam, whiskey | 71-36-3 | C4H10O | 74.10 | 657.1 | 153.301 | 1.38066 | 291.58 ± 39.45a | 310.22 ± 37.12a | 465.09 ± 163.00a | 376.08 ± 62.66a | 443.71 ± 38.99a |
| Ketones | |||||||||||||
| a43 | Acetophenone-M | Sweet, pungent, hawthorn, mimosa, almond, acacia, chemical | 98-86-2 | C8H8O | 120.20 | 1061.4 | 438.11 | 1.18834 | 318.69 ± 10.41b | 1228.10 ± 226.60a | 1052.03 ± 148.12a | 345.24 ± 13.67b | 369.01 ± 135.56b |
| a44 | Acetophenone-D | Sweet, pungent, hawthorn, mimosa, almond, acacia, chemical | 98-86-2 | C8H8O | 120.20 | 1062.5 | 439.63 | 1.57705 | 41.67 ± 7.01b | 605.84 ± 241.57a | 588.74 ± 253.34a | 45.21 ± 1.03b | 51.50 ± 21.28b |
| a45 | 6-Methyl-5-hepten-2-one | Citrus, green, musty, lemongrass, apple | 110-93-0 | C8H14O | 126.20 | 992.7 | 341.76 | 1.1794 | 1066.98 ± 26.36c | 1338.92 ± 104.58b | 2523.89 ± 210.16a | 1287.07 ± 66.59bc | 2394.50 ± 163.39a |
| a46 | Cyclohexanone | Mint, cool | 108-94-1 | C6H10O | 98.10 | 898.7 | 262.15 | 1.15266 | 912.42 ± 30.04 ab | 782.86 ± 28.52b | 1024.71 ± 182.43a | 746.01 ± 41.23b | 1007.87 ± 39.23a |
| a47 | 2-Heptanone-M | Fruity, spicy, sweet, herbal, coconut, woody | 110-43-0 | C7H14O | 114.20 | 894.4 | 258.57 | 1.26111 | 248.68 ± 66.75 ab | 338.97 ± 5.31a | 285.33 ± 10.57a | 267.86 ± 71.61a | 165.05 ± 38.93b |
| a48 | 2-Heptanone-D | Fruity, spicy, sweet, herbal, coconut, woody | 110-43-0 | C7H14O | 114.20 | 892.3 | 256.78 | 1.63695 | 121.01 ± 22.83c | 94.64 ± 27.79cd | 481.09 ± 55.70a | 212.25 ± 5.00b | 43.03 ± 6.79d |
| a49 | Acetoin | Sweet, buttery, creamy, dairy, milky, fatty | 513-86-0 | C4H8O2 | 88.10 | 731.6 | 179.12 | 1.3393 | 692.06 ± 55.12b | 269.84 ± 32.16c | 930.64 ± 111.33a | 880.67 ± 29.29a | 653.03 ± 34.25b |
| a50 | 2-Butanone | Fragrant, fruit, pleasant | 78-93-3 | C4H8O | 72.10 | 571.2 | 130.631 | 1.2481 | 2778.55 ± 159.22b | 2077.00 ± 115.76c | 3118.85 ± 67.77a | 2142.26 ± 187.92c | 2047.56 ± 159.61c |
| a51 | 2-Propanone | Solvent, ethereal, apple, pear | 67-64-1 | C3H6O | 58.10 | 487.5 | 108.526 | 1.1229 | 9132.59 ± 616.63a | 6907.91 ± 400.71bc | 9473.44 ± 380.97a | 7706.43 ± 461.39b | 6855.33 ± 14.45c |
| a52 | 3-Pentanone | Ethereal, acetone | 96-22-0 | C5H10O | 86.10 | 683 | 160.157 | 1.34757 | 1914.46 ± 42.33b | 1750.00 ± 283.11b | 2414.75 ± 101.02a | 1884.24 ± 44.98b | 2541.42 ± 160.98a |
| a53 | 2-Pentanone | Sweet, fruity, ethereal, wine, banana, woody | 107-87-9 | C5H10O | 86.10 | 694.1 | 163.847 | 1.36926 | 457.02 ± 17.56c | 516.71 ± 129.12c | 1087.87 ± 202.60b | 498.76 ± 40.93c | 1433.31 ± 135.39a |
| a54 | β-Damascenone | Apple, rose, honey, tobacco, sweet | 23726-93-4 | C13H18O | 190.30 | 1402.4 | 927.741 | 1.39395 | 153.92 ± 4.91b | 286.27 ± 35.11a | 175.75 ± 18.52b | 165.21 ± 36.86b | 152.08 ± 4.84b |
| Acids | |||||||||||||
| a55 | Phenylacetic acid | Sweet, honey, floral, honeysuckle, sour, waxy, civet | 103-82-2 | C8H8O2 | 136.10 | 1283.1 | 756.40 | 1.32282 | 270.22 ± 13.42c | 291.86 ± 21.38c | 648.28 ± 97.98a | 422.43 ± 9.12b | 488.43 ± 30.37b |
| a56 | Hexanoic acid | Sour, fatty, sweat, cheese | 142-62-1 | C6H12O2 | 116.20 | 976.9 | 328.34 | 1.28785 | 543.01 ± 234.77 ab | 288.09 ± 72.76b | 821.92 ± 276.86a | 387.34 ± 32.75b | 698.78 ± 253.75 ab |
| a57 | 2-Methylbutanoic acid | Pungent, acid, roquefort, cheese | 116-53-0 | C5H10O2 | 102.10 | 900.2 | 263.46 | 1.2072 | 219.82 ± 7.53b | 321.47 ± 33.98b | 251.36 ± 25.45b | 595.58 ± 172.20a | 176.94 ± 13.65b |
| Hydrocarbons | |||||||||||||
| a58 | γ-Terpinene | Oily, woody, terpene, lemon/lime, tropical, herbal | 99-85-4 | C10H16 | 136.20 | 1072.2 | 453.62 | 1.2236 | 856.25 ± 133.88a | 235.52 ± 3.59c | 771.72 ± 63.22a | 609.94 ± 22.74b | 494.37 ± 88.27b |
| a59 | α-Phellandrene | Citrus, herbal, terpene, green, woody, peppery | 99-83-2 | C10H16 | 136.20 | 1003.9 | 355.62 | 1.22694 | 273.03 ± 42.22d | 363.60 ± 18.61c | 598.04 ± 28.78a | 215.12 ± 13.79d | 430.51 ± 48.96b |
| Furans | |||||||||||||
| a60 | 2-Pentylfuran | Fruity, green, earthy, beany, vegetable, metallic | 3777-69-3 | C9H14O | 138.20 | 996.8 | 345.33 | 1.26111 | 579.36 ± 80.90c | 770.08 ± 38.47b | 1187.40 ± 20.42a | 601.57 ± 20.32c | 400.74 ± 18.12d |
| a61 | 2-Ethylfuran | Chemical, beany, ethereal, cocoa, bready, malty, coffee, nutty | 3208-16-0 | C6H8O | 96.10 | 683.3 | 160.22 | 1.32075 | 2419.96 ± 119.20a | 1329.25 ± 106.35b | 1662.85 ± 440.16b | 2295.62 ± 81.35a | 1529.12 ± 59.07b |
| Pyrazines | |||||||||||||
| a62 | 2,6-Dimethylpyrazine | Ethereal, cocoa, nutty, roasted, roasted, meaty, beefy, brown, coffee, buttermilk | 108-50-9 | C6H8N2 | 108.10 | 933 | 291.22 | 1.13186 | 173.41 ± 11.90b | 268.11 ± 20.52b | 415.94 ± 79.96a | 242.03 ± 58.98b | 200.84 ± 32.40b |
| Lactones | |||||||||||||
| a63 | γ-Butyrolactone | Creamy, oily, fatty, caramel | 96-48-0 | C4H6O2 | 86.10 | 919.3 | 279.59 | 1.08284 | 235.22 ± 34.38a | 127.43 ± 50.11b | 79.99 ± 8.86b | 224.18 ± 11.15a | 141.47 ± 41.48b |
Odor descriptions were from FEMA database. Different small letters in the same row indicated significant difference (p < 0.05). D: Dimer, M: Monomer.
Represents the retention index in the capillary GC column.
Represents the retention time in the capillary GC column.
Represents the drift time in the drift tube.
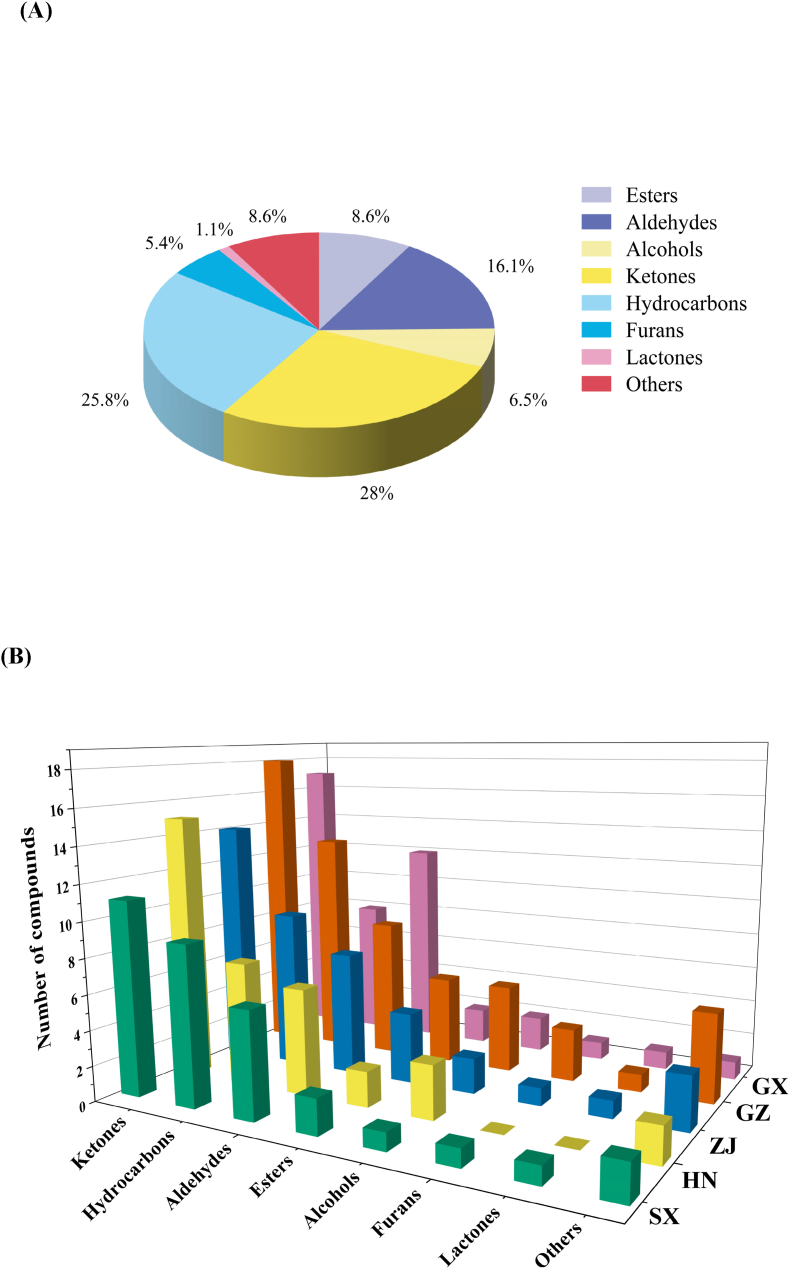
Analyzing the VOC distributions revealed that aldehydes and ketones were the predominant groups in all FBT samples. Specifically, in these five FBT samples, aldehydes and ketones accounted for more than 57% of the total VOCs (Fig. S2A). Previous research has shown that ketones and aldehydes may be the key contributors to the distinctive scent of FBT (Li et al., 2019a). This result was in agreement with our current findings. In the tea samples, alcohols and esters each accounted for 28% of the total VOCs, followed by acids (5%), hydrocarbons (3%), furans (3%), pyrazine (2%), and lactone (2%). Aldehydes were the largest groups of VOCs present in the five FBT samples (Fig. S2B) as follows: SX (42.70%), HN (38.26%), GZ (42.56%), ZJ (37.18%), and GX (38.78%). SX had a larger quantity of aldehydes than the teas from other regions. Aldehydes are produced during the postfermentation stage via the oxidative deamination and decarboxylation of amino acids and the hydrolysis and oxidation of fatty acids (Xu et al., 2007). Nonanal may contribute to the differentiation of the diverse FBTs because it was present at low levels (p < 0.05) in GX and at comparable levels in the SX, HN, GZ, and ZJ samples. The concentrations of (E, E)-2,4-heptadienals (dimers and monomers) in GZ (7.80%) were higher than those in GX (7.64%), SX (4.44%), ZJ (4.25%), and HN (2.59%). (E)-2-hexenals, which confer a green flavor, were the most abundant aldehydes in all FBT samples and could help discriminate the characteristic aromas of diverse FBTs due to the variation in their levels.
Ketones were the second largest group of VOCs and were present at comparable levels in the five FBTs (30.54%–35.70%). They were mainly composed of 2-butanone, 2-propanone, and 3-pentanone. Specifically, in the SX sample, 2-propanone and 2-butanone contents were the highest at 15.83% and 4.82%, respectively. The GX samples had the lowest 2-propanone and 2-butanone contents of 11.82% and 3.54%, respectively, and were remarkably different from other samples (p < 0.05). Therefore, 2-propanone and 2-butanone contribute to the identification of the geographical origin of FBTs. The amounts of 3-pentanone also differed in the different FBTs. The lowest 3-pentanone content was found in SX (3.33%), followed by GZ (3.45%), whereas the highest 3-pentanone level was detected in GX (4.39%).
The levels of alcohols in GZ (11.04%) were lower than those in SX (11.10%), HN (11.50%), ZJ (15.02), and GX (16.34%), and linalool, linalool oxide, and 2,3-butanediol, which provided a floral flavor, were the most abundant compounds. Linalool and linalool oxide accounted for 0.52% and 0.28% of the VOCs in HN, respectively, and were present at greater concentrations in SX (1.50, 0.41%), GZ (1.61%, 0.94%), ZJ (3.36, 0.96%), and GX (2.95%, 4.40%). The GX sample had the highest contents of the monomers and dimers of linalool oxide (p < 0.05), which could help to distinguish the different FBTs.
Nine esters were identified and measured in the five FBTs. These esters were dominated by methyl salicylate and ethyl acetate. Methyl salicylate imparts the aroma of wintergreen oil and mint and has been reported to be one of the key VOCs contributing to the characteristic flavor of FBT samples (Li et al., 2020a, 2020b). Notably, organic acids were present at low levels in all FBT samples. We detected three organic acids and found that the content of 2-methylbutanoic acid was the highest in the ZJ sample at 1.16% (p < 0.05). This compound could help distinguish FBTs from ZJ from those from other production regions.
3.1.3. Odor profiles of FBTs from five different regions
The ROAVs of the 63 identified VOCs were determined to assess the role of these compounds in the aroma scent of the teas. A high ROAV indicates the great contribution to the scent qualities of FBT. A compound with ROAV ≥1 was considered as a crucial volatile molecule, whereas a VOC with 0.1 ≤ ROAV <1 plays a major part in modifying the overall flavor of a sample. A total of 20 key VOCs with ROAV ≥0.1 were found among the 63 VOCs (Table S1), which separated into two classes. The first group (ROAV ≥1) included β-damascenone, isoamyl acetate (highest ROAV of 1.34 ± 0.07 in sample HN), (E)-2-nonenal (highest ROAV of 1.83 ± 0.29 in sample GX), 2-methylbutanal (highest ROAV of 1.34 ± 0.03 in sample SX), and linalool (highest ROAV of 10.27 ± 1.79 in sample GX). The second group (0.1 ≤ ROAV <1) included ethyl hexanoate, nonanal, (E)-2-octenal, benzeneacetaldehyde, (E,E)-2,4-heptadienal-M, (E,E)-2,4-heptadienal-D, heptanal-M, heptanal-D, hexanal, 3-methylbutanal, butanal, 2-pentylfuran, octanal, 1-hexanol, and methylpropanal. (E,E)-2,4-heptadienal, which provides a fatty, floral odor, is also detected in stale tea and in tea with a sun-baked flavor due to photic oxidation. Microbial metabolism could contribute to the production of (E,E)-2,4-heptadienal during the fermentation of FBT (Xu et al., 2007). An early study reported that nonanal and heptanal are generated by the oxidation of the precursors oleic acid and palmitoleic (Ho et al., 2015) and contribute to fresh and greenish odors in tea infusions (Takeo and Tsushida, 1980). β-Damascenone had the highest ROAV value (ROAV = 100) and provide a strong contribution to the flavor characteristic of FBT. Given that β-damascenone has an apple-like flavor and a very low water threshold (0.002 ppb), it could easily affect the aroma attributes of teas. It results from the oxidation of neoxanthin by enzymes (Ho et al., 2015).
3.2. VOCs identified via GC–MS
3.2.1. Analysis of VOCs in FBT from five different production regions
The VOCs were also analyzed through GC–MS with HS–SPME to illuminate the flavor differences of diverse FBTs comprehensively. The representative total ion chromatograms of VOCs are illustrated in Fig. S3A, and Table 2 shows the detailed results of all VOCs in five FBT samples. A total of 93 VOCs were characterized through GC–MS. They included aldehydes (15), esters (8), alcohols (6), ketones (26), hydrocarbons (24), furans (5), lactone (1), and others (8). Similar to the results of GC–IMS, the peak areas and numbers of the VOCs in the five FBT samples greatly differed, indicating that the VOCs were drastically distinct. Fig. 2A and B illustrate the proportion and category distributions of the VOCs in the five FBT samples. These figures indicated that the number of VOCs varied among the five samples given that 33, 35, 58, 41, and 44 VOCs were identified in SX, HN, GZ, ZJ, and GX, respectively. The release of VOCs through the Maillard reaction, glycoside hydrolysis, carotenoid degradation, or lipid breakdown during processing resulted in either the reduction or generation of VOCs in distinct categories (Ho et al., 2015).
Table 2.
Concentration of volatile compounds among five aroma types of FBT by GC–MS.
| No | Compounds | CAS | Formula | Rt | Odor description | Content (ng/g) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| SX | HN | GZ | ZJ | GX | ||||||
| Esters | ||||||||||
| b1 | Ethyl caprylate | 106-32-1 | C10H20O2 | 32.498 | Apricot, brandy, fat, floral, pineapple | ND | ND | 21.56 ± 6.18 | ND | ND |
| b2 | Methyl hexanoate | 106-70-7 | C7H14O2 | 13.332 | Ester, fresh, fruit, pineapple | ND | ND | 58.58 ± 0.72 | ND | ND |
| b3 | Methyl salicylate | 119-36-8 | C8H8O3 | 31.889 | Almond, caramel, peppermint, sharp | 568.55 ± 17.35 | 294.69 ± 30.48 | 606.82 ± 24.88 | 228.89 ± 3.47 | 622.63 ± 77.99 |
| b4 | Propionic anhydride | 123-62-6 | C6H10O3 | 4.025 | Like acetaldehyde | ND | ND | ND | 22.12 ± 12.87 | 49.50 ± 54.12 |
| b5 | Ethyl hexanoate | 123-66-0 | C8H16O2 | 18.196 | Apple peel, brandy, fruit gum, overripe fruit, pineapple | 91.78 ± 14.11 | ND | ND | 80.62 ± 2.65 | ND |
| b6 | Vinyl acrylate | 2177-18-6 | C5H6O2 | 6.163 | – | ND | ND | 4.40 ± 4.53 | ND | ND |
| b7 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 6846-50-0 | C16H30O4 | 52.276 | – | ND | ND | ND | 10.73 ± 3.58 | ND |
| b8 | Linalyl butyrate | 78-36-4 | C14H24O2 | 25.243 | Bergamot, fruity, banana, berry | ND | 72.23 ± 4.24 | 303.46 ± 19.66 | ND | ND |
| Aldehydes | ||||||||||
| b9 | Benzaldehyde | 100-52-7 | C7H6O | 15.317 | Bitter almond, burnt sugar, cherry, malt, roasted pepper | 508.83 ± 18.45 | 177.18 ± 16.88 | 555.84 ± 37.46 | 317.10 ± 27.60 | 536.52 ± 48.33 |
| b10 | 3,3-Diethoxy-1-propyne | 10160-87-9 | C7H12O2 | 20.413 | – | ND | ND | ND | 24.59 ± 31.99 | ND |
| b11 | Heptanal | 111-71-7 | C7H14O | 11.895 | Citrus, fat, green, nut | ND | 76.48 ± 4.94 | 217.91 ± 42.63 | ND | 432.04 ± 48.25 |
| b12 | Decanal | 112-31-2 | C10H20O | 32.948 | Floral, fried, orange peel, penetrating, tallow | ND | ND | 30.65 ± 7.28 | ND | 25.43 ± 2.06 |
| b13 | 2,3-Dihydro-2,2,6-trimethylbenzalhyde | 116-26-7 | C10H14O | 32.256 | Fresh, herbal, phenolic, metallic, rosemary, tobacco, spicy | 171.42 ± 12.55 | 175.16 ± 5.19 | 143.25 ± 10.34 | 177.84 ± 11.44 | 175.82 ± 12.32 |
| b14 | Benzeneacetaldehyde | 122-78-1 | C8H8O | 20.908 | Berry, geranium, honey, nut, pungent | 49.87 ± 11.41 | ND | ND | ND | ND |
| b15 | (E)-2-Nonenal | 18829-56-6 | C9H16O | 29.485 | Fatty, green, cucumber, aldehydic, citrus | ND | ND | ND | ND | 62.13 ± 5.79 |
| b16 | 5-Ethylcyclopent-1-enecarboxaldehyde | 36431-60-4 | C8H12O | 20.021 | – | ND | ND | 84.11 ± 27.11 | ND | ND |
| b17 | (E,E)-2,4-Heptadienal | 4313-03-5 | C7H10O | 18.759 | Fat, nut | ND | 101.16 ± 4.97 | 399.23 ± 32.27 | 174.69 ± 21.66 | 1170.43 ± 103.67 |
| b18 | 2,6,6-Trimethylcyclohex-2-ene-1-carbaldehyde | 432-24-6 | C10H16O | 26.092 | – | ND | ND | ND | ND | 84.00 ± 20.74 |
| b19 | 2,6,6-Trimethyl-1-cyclohexene-1-carboxaldehyde | 432-25-7 | C10H16O | 33.694 | Tropical, saffron, herbal, clean, rose, oxide, sweet, tobacco, damascone, fruity | 117.04 ± 6.72 | 190.27 ± 6.65 | 214.94 ± 7.04 | 132.46 ± 4.49 | 548.62 ± 27.45 |
| b20 | 2,5-Dimethylbenzaldehyde | 5779-94-2 | C9H10O | 30.449 | – | ND | ND | ND | ND | 39.27 ± 0.91 |
| b21 | Isophthalaldehyde | 626-19-7 | C8H6O2 | 30.405 | – | 22.79 ± 4.48 | ND | ND | 11.08 ± 5.98 | 34.43 ± 1.44 |
| b22 | Hexanal | 66-25-1 | C6H12O | 6.995 | Apple, fat, fresh, green, oil | 373.51 ± 52.14 | 481.86 ± 15.88 | 521.05 ± 246.03 | 402.30 ± 76.54 | 1610.57 ± 182.86 |
| b23 | (E)-2-Hexenal | 6728-26-3 | C6H10O | 9.285 | Green, banana, aldehydic, fatty, cheesy | ND | ND | ND | ND | 773.38 ± 66.13 |
| Alcohols | ||||||||||
| b24 | 2-Ethyl-1-hexanol | 104-76-7 | C8H18O | 20.435 | Citrus, fresh, floral, oily, sweet | ND | 128.85 ± 15.11 | 74.63 ± 18.80 | 172.70 ± 21.94 | ND |
| b25 | 1-Hexanol | 111-27-3 | C6H14O | 10.506 | Banana, flower, grass, herb | ND | 125.94 ± 8.19 | 139.84 ± 18.01 | ND | ND |
| b26 | (S)-(+)-3-Methyl-1-pentanol | 42072-39-9 | C6H14O | 10.409 | – | ND | ND | 159.30 ± 33.82 | ND | ND |
| b27 | Terpinen-4-ol | 562-74-3 | C10H18O | 30.729 | Pepper, woody, earth, musty, sweet | ND | ND | ND | ND | 31.53 ± 5.70 |
| b28 | 2,4,4-Trimethyl-2-cyclohexen-1-ol | 73741-61-4 | C9H16O | 21.816 | – | ND | 103.68 ± 9.06 | 107.60 ± 4.15 | ND | ND |
| b29 | Linalool | 78-70-6 | C10H18O | 25.154 | Coriander, floral, lavender, lemon, rose | 149.79 ± 45.98 | ND | 179.10 ± 19.21 | 360.10 ± 71.45 | 364.77 ± 124.02 |
| Ketones | ||||||||||
| b30 | 6-Methyl-5-hepten-2-one | 110-93-0 | C8H14O | 17.202 | Citrus, mushroom, pepper, rubber, strawberry | 294.92 ± 32.29 | 571.02 ± 29.81 | 620.00 ± 31.96 | 177.45 ± 13.23 | 1181.21 ± 19.30 |
| b31 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | 1125-21-9 | C9H12O2 | 28.162 | Floral | 30.29 ± 3.65 | 72.80 ± 6.53 | 49.25 ± 1.89 | 40.50 ± 5.36 | 20.65 ± 2.48 |
| b32 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | 1203-08-3 | C13H18O | 45.159 | – | 76.34 ± 33.10 | 85.14 ± 12.21 | ND | ND | 99.11 ± 4.20 |
| b33 | α-Ionone | 127-41-3 | C13H20O | 44.974 | Violet, wood | 100.26 ± 19.76 | 310.32 ± 162.35 | 386.17 ± 86.12 | 174.03 ± 11.52 | 813.81 ± 40.52 |
| b34 | 4,4,6-Trimethyl-2-cyclohexen-1-one | 13395-73-8 | C9H14O | 23.437 | – | ND | ND | ND | 41.56 ± 1.77 | ND |
| b35 | 3-Nonen-2-one | 14309-57-0 | C9H16O | 28.055 | Fruity, berry, fatty, oily, ketonic, weedy, spicy, licorice | ND | ND | 67.08 ± 5.30 | ND | ND |
| b36 | 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | 14901-07-6 | C13H20O | 47.584 | Floral, violet | 282.09 ± 108.33 | ND | ND | ND | 624.31 ± 75.96 |
| b37 | 6-Methyl-3,5-heptadiene-2-one | 1604-28-0 | C8H12O | 25.457 | Cinnamon, coconut, spice, woody, sweet, weedy | ND | ND | 361.74 ± 58.76 | ND | ND |
| b38 | 6,10-Dimethyl-2-undecanone | 1604-34-8 | C13H26O | 43.986 | – | ND | ND | 24.49 ± 2.15 | 12.13 ± 0.15 | ND |
| b39 | 1-Penten-3-one | 1629-58-9 | C5H8O | 5.558 | Fish, green, mustard, pungent | ND | ND | ND | 13.15 ± 8.15 | ND |
| b40 | 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-2-butanone | 17283-81-7 | C13H22O | 45.476 | Earthy, woody, mahogany, orris, dry amber | 39.76 ± 9.15 | 23.07 ± 5.25 | 46.31 ± 15.40 | 38.38 ± 10.38 | 30.60 ± 6.41 |
| b41 | 3,4,4-Trimethyl-2-cyclohexen-1-one | 17299-41-1 | C9H14O | 23.443 | – | ND | 95.07 ± 5.78 | 131.08 ± 6.40 | 52.44 ± 8.68 | 77.24 ± 49.59 |
| b42 | trans-3-Nonen-2-one | 18402-83-0 | C9H16O | 28.101 | – | ND | ND | 63.98 ± 8.17 | ND | 37.49 ± 6.93 |
| b43 | 4-Methyleneisophorone | 20548-00-9 | C10H14O | 33.543 | – | ND | 32.57 ± 0.64 | 46.97 ± 5.38 | ND | 12.27 ± 5.07 |
| b44 | 2,2,6-Trimethylcyclohexanone | 2408-37-9 | C9H16O | 20.206 | Floral | ND | ND | ND | ND | 108.42 ± 6.90 |
| b45 | 4-(2,6,6-Trimethyl-2-cyclohexen-1-yl)-2-butanone | 31499-72-6 | C13H22O | 44.430 | Fruit | ND | 55.45 ± 5.53 | ND | ND | ND |
| b46 | (E)-6,10-Dimethylundeca-5,9-dien-2-one | 3796-70-1 | C13H22O | 46.154 | Fresh, green, fruity, waxy, rose, woody, magnolia, tropical | ND | 32.19 ± 1.41 | 80.07 ± 78.21 | ND | 266.95 ± 82.34 |
| b47 | 3,5-Octadien-2-one | 38284-27-4 | C8H12O | 24.573 | – | ND | ND | 355.70 ± 159.60 | 114.12 ± 10.82 | 297.63 ± 28.96 |
| b48 | 3,3,6-Trimethyl-1,5-heptadien-4-one | 546-49-6 | C10H16O | 5.566 | Fruity, fatty, mushroom | ND | ND | 46.43 ± 20.19 | 30.57 ± 20.05 | 145.89 ± 48.37 |
| b49 | (R,S)-5-Ethyl-6-methyl-3E-hepten-2-one | 57283-79-1 | C10H18O | 28.501 | – | 31.32 ± 3.16 | 34.09 ± 6.94 | 105.26 ± 7.36 | 46.12 ± 2.09 | 47.64 ± 7.38 |
| b50 | 1-(2,3,6-Trimethylphenyl)-3-butanone | 58720-40-4 | C13H18O | 51.515 | – | 52.94 ± 35.56 | 62.98 ± 44.27 | 58.30 ± 22.38 | 49.59 ± 16.54 | ND |
| b51 | Acetone | 67-64-1 | C3H6O | 7.211 | Pungent | ND | ND | 56.28 ± 13.14 | ND | ND |
| b52 | Isophorone | 78-59-1 | C9H14O | 21.958 | Cedarwood, spice | 113.97 ± 10.58 | 164.20 ± 5.80 | ND | ND | ND |
| b53 | β-Ionone | 79-77-6 | C13H20O | 47.615 | Floral, violet | ND | 40.92 ± 7.22 | 181.62 ± 126.57 | 172.52 ± 129.06 | 700.34 ± 15.87 |
| b54 | 1-(4-tert-Butylphenyl)propan-2-one | 81561-77-5 | C13H18O | 42.791 | – | 26.05 ± 5.84 | 42.28 ± 5.62 | ND | ND | 28.18 ± 3.34 |
| b55 | Acetophenone | 98-86-2 | C8H8O | 22.423 | Almonds, flower, meat, must | 93.99 ± 9.91 | 665.33 ± 26.28 | 636.03 ± 14.15 | 71.38 ± 6.69 | 89.40 ± 5.92 |
| Hydrocarbons | ||||||||||
| b56 | Styrene | 100-42-5 | C8H8 | 11.154 | Sweet, balsam, floral, plastic | 721.64 ± 66.27 | ND | ND | ND | ND |
| b57 | 1,3-Bis(1,1-dimethylethyl)benzene | 1014-60-4 | C14H22 | 35.929 | – | 315.95 ± 30.34 | 96.40 ± 56.12 | 189.61 ± 22.43 | ND | 362.08 ± 62.90 |
| b58 | 1,4-Dimethyl-2,5-bis(1-methylethyl)-benzene | 10375-96-9 | C14H22 | 47.469 | – | ND | ND | ND | ND | 58.63 ± 3.35 |
| b59 | 1,3-Dimethyl-benzene | 108-38-3 | C8H10 | 10.010 | Plastic | 233.33 ± 33.05 | 36.79 ± 9.28 | ND | ND | ND |
| b60 | Toluene | 108-88-3 | C7H8 | 5.833 | Sweet | 86.48 ± 16.74 | ND | ND | ND | ND |
| b61 | β-Himachalene | 1461-03-6 | C15H24 | 43.118 | – | ND | ND | ND | ND | 55.19 ± 6.32 |
| b62 | Acenaphthylene | 208-96-8 | C12H8 | 45.586 | – | ND | ND | 35.62 ± 6.09 | ND | ND |
| b63 | 1,6,7-Trimethylnaphthalene | 2245-38-7 | C13H14 | 50.652 | Earthy | ND | 17.45 ± 1.58 | 28.53 ± 17.62 | 17.66 ± 11.46 | ND |
| b64 | Ethylpentamethylbenzene | 2388-04-7 | C13H20 | 46.768 | – | ND | ND | 39.66 ± 18.84 | ND | ND |
| b65 | Germacrene D | 23986-74-5 | C15H24 | 43.125 | Woody, spice | ND | ND | ND | ND | 58.34 ± 5.77 |
| b66 | 1,2,3,4-Tetrahydro-1,6,8-trimethylnaphthalene | 30316-36-0 | C13H18 | 41.426 | – | 81.07 ± 101.63 | ND | ND | ND | ND |
| b67 | 2,2′,5,5′-Tetramethyl-1,1′-biphenyl | 3075-84-1 | C16H18 | 54.526 | – | ND | ND | ND | 11.92 ± 7.73 | ND |
| b68 | 1,7,7-Trimethylbicyclo[2.2.1]hept-2-ene | 464-17-5 | C10H16 | 34.217 | – | ND | 42.57 ± 1.23 | 29.75 ± 3.59 | 22.80 ± 4.88 | 104.81 ± 8.76 |
| b69 | α-Cedrene | 469-61-4 | C15H24 | 44.155 | Woody, cedar, sweet, fresh | ND | 38.93 ± 3.49 | 32.97 ± 0.28 | 16.95 ± 3.51 | ND |
| b70 | 1,2,3,4-Tetrahydro-1,1,6-trimethylnaphthalene | 475-03-6 | C13H18 | 41.416 | Fruit | ND | ND | 125.62 ± 77.43 | ND | 41.25 ± 10.16 |
| b71 | Tridecane | 629-50-5 | C13H28 | 38.683 | – | 11.73 ± 2.11 | ND | ND | ND | ND |
| b72 | Tetradecane | 629-59-4 | C14H30 | 43.737 | Mild, waxy | 12.58 ± 1.00 | ND | ND | ND | ND |
| b73 | (Z)-3-Methyl-4-undecene | 74645-87-7 | C12H24 | 22.073 | – | ND | ND | 227.12 ± 24.23 | 84.81 ± 5.93 | ND |
| b74 | Acenaphthene | 83-32-9 | C12H10 | 47.169 | – | ND | ND | 9.87 ± 0.21 | ND | ND |
| b75 | Fluorene | 86-73-7 | C13H10 | 51.361 | – | ND | ND | 11.17 ± 2.00 | ND | ND |
| b76 | Naphthalene | 91-20-3 | C10H8 | 30.758 | Pungent, dry, tarry | 70.28 ± 3.28 | 168.29 ± 18.81 | 442.84 ± 23.54 | 66.07 ± 6.83 | 47.65 ± 8.00 |
| b77 | 2-Methylnaphthalene | 91-57-6 | C11H10 | 38.822 | Sweet, floral, woody | 7.50 ± 1.37 | 56.23 ± 3.42 | 68.42 ± 4.10 | 11.88 ± 1.92 | ND |
| b78 | Biphenyl | 92-52-4 | C12H10 | 42.464 | Pungent, rose, green, geranium | ND | ND | 51.71 ± 6.75 | 9.61 ± 3.58 | ND |
| b79 | α-Terpinene | 99-86-5 | C10H16 | 19.091 | Woody, terpene, lemon, herbal, medicinal, citrus | ND | ND | ND | 22.77 ± 0.50 | 67.61 ± 35.05 |
| Furans | ||||||||||
| b80 | Dibenzofuran | 132-64-9 | C12H8O | 48.551 | – | ND | ND | 27.35 ± 5.52 | ND | ND |
| b81 | 2-Acetyl-2-methyltetrahydrofuran | 32318-87-9 | C7H12O2 | 25.427 | – | ND | ND | ND | 22.05 ± 2.22 | ND |
| b82 | 4-[(S)-1-Methylpropyl]-2,3-dihydrofuran | 34379-54-9 | C8H14O | 16.253 | – | 100.45 ± 24.79 | ND | 56.49 ± 14.56 | ND | ND |
| b83 | 5-Methoxy-6,7-dimethyl-1-benzofuran | 35355-35-2 | C11H12O2 | 46.777 | – | ND | ND | 35.60 ± 3.74 | ND | ND |
| b84 | 2-Butylfuran | 4466-24-4 | C8H12O | 25.743 | Wet, hay | ND | ND | ND | ND | 37.11 ± 4.63 |
| Lactones | ||||||||||
| b85 | 5,6,7,7a-Tetrahydro-4,4,7a-trimethyl-2(4H)-benzofuranone | 17092-92-1 | C11H16O2 | 49.310 | Musk, coumarin | 66.49 ± 23.41 | ND | 60.82 ± 25.44 | 68.49 ± 7.73 | 100.04 ± 18.81 |
| Others | ||||||||||
| b86 | Butylated Hydroxytoluene | 128-37-0 | C15H24O | 48.736 | Mild, phenolic, camphor | ND | ND | ND | 11.85 ± 0.16 | ND |
| b87 | Dimethyl ether | 115-10-6 | C2H6O | 3.109 | Ethereal | ND | 56.00 ± 20.40 | ND | ND | ND |
| b88 | Hexanoic acid | 142-62-1 | C6H12O2 | 20.445 | Sour, fatty, sweat, cheese | ND | ND | 179.31 ± 10.77 | ND | ND |
| b89 | 3,4-Dimethoxytoluene | 494-99-5 | C9H12O2 | 35.191 | – | ND | ND | 21.56 ± 0.67 | ND | ND |
| b90 | 1,2,3-Trimethoxybenzene | 634-36-6 | C9H12O3 | 39.456 | – | 25.59 ± 7.07 | ND | ND | 33.60 ± 12.69 | ND |
| b91 | Dimethylphosphinic fluoride | 753-70-8 | C2H6FOP | 4.171 | – | ND | ND | 49.59 ± 22.75 | ND | 137.30 ± 92.04 |
| b92 | 1,2-Dimethoxybenzene | 91-16-7 | C8H10O2 | 28.612 | Earth, moss, wood | 42.29 ± 3.19 | 30.82 ± 2.89 | 70.55 ± 6.72 | 66.62 ± 5.90 | ND |
| b93 | Precocene I | 17598-02-6 | C12H14O2 | 46.523 | – | ND | ND | 135.22 ± 103.55 | ND | ND |
* Odor descriptions were from FEMA database. ND, not detected.
Fig. 2.
(A) Classification of all VOCs, (B) category distributions of volatile organic compounds by GC–MS of five FBT samples.
The FBT samples had 12 VOCs in common, and FBTs from different origins had unique VOCs. Among all the detected VOCs, six were detected only in SX, 10 only in GX, seven only in ZJ, 17 only in GZ, and two only in HN (Fig. S3B). Among these VOCs, benzeneacetaldehyde, styrene, toluene, 1,2,3,4-tetrahydro-1,6,8-trimethylnaphthalene, tridecane, and tetradecane were found only in SX samples. The VOCs unique to HN were 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-2-butanone and dimethyl ether. The VOCs unique to GZ were ethyl caprylate, methyl hexanoate, vinyl acrylate, 5-ethylcyclopent-1-enecarboxaldehyde, (S)-(+)-3-methyl-1-pentanol, 3-nonen-2-one, 6-methyl-3,5-heptadiene-2-one, acetone, acenaphthylene, ethylpentamethylbenzene, acenaphthene, fluorene, dibenzofuran, 5-methoxy-6,7-dimethyl-1-benzofuran, hexanoic acid, 3,4-dimethoxytoluene, and precocene I. The VOCs unique to ZJ were 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, 3,3-diethoxy-1-propyne, 4,4,6-trimethyl-2-cyclohexen-1-one, 2,2,5,5-tetramethyl-1,1-biphenyl, 2-acetyl-2-methyltetrahydrofuran, 1-penten-3-one, and butylated hydroxytoluene. The VOCs unique to GX were (E)-2-nonenal, 2,6,6-trimethylcyclohex-2-ene-1-carbaldehyde, 2,5-dimethylbenzaldehyde, (E)-2-hexenal, terpinen-4-ol, 2,2,6-trimethylcyclohexanone, 1,4-dimethyl-2,5-bis(1-methylethyl)-benzene, β-himachalene, germacrene D, and 2-butylfuran. These distinct VOCs could help discriminate FBTs from different regions.
GC–IMS and GC–MS displayed different identification capabilities for VOCs as previously demonstrated (Wang et al., 2018a). In the present study, GC–MS and GC–IMS measured aldehydes and ketones sensitively. Ketones could be generated by microbial enzymatic activity on lipids or amino acids, as well as Maillard reactions (Wang et al., 2018b). Although ketone molecules are often present, their aromatic contribution may be modest due to their high thresholds. Table 2 shows the distribution of VOCs in the five FBT samples. Ketones were the main VOCs in the five samples. A total of 11, 15, 18, 14, and 17 ketones were detected in SX, HN, GZ, ZJ, and GX, respectively, at the contents of 841.61 ± 265.25, 2152.07 ± 277.26, 2873.91 ± 360.04, 894.76 ± 173.06, and 3710.16 ± 410.27 ng/g, respectively. The GX sample had the highest content of 6-methyl-5-hepten-2-one (1181.21 ± 19.30 ng/g) among the five samples. This compound may originate from the degradation of carotenoids in FBT (Takeo and Tsushida, 1980). α-Ionone and β-ionone contribute to the floral aroma of tea. GX had the highest contents of these two compounds (813.81 ± 40.52 ng/g for α-ionone and 700.34 ± 15.87 ng/g for β-ionone). Among all the ketones detected, 3-nonen-2-one with a fruity aroma (67.08 ± 5.30 ng/g); 6-methyl-3,5-heptadiene-2-one with a woody, sweet aroma (361.74 ± 58.76 ng/g); and acetone with a pungent scent (56.28 ± 13.14 ng/g) were detected only in GZ samples. 4-(2,6,6-Trimethyl-2-cyclohexen-1-yl)-2-butanone (55.45 ± 5.53 ng/g) with fruity aroma and 2,2,6-trimethylcyclohexanone (108.42 ± 6.90 ng/g) with floral aroma were detected only in the samples of HN and GX, respectively. 4,4,6-Trimethyl-2-cyclohexen-1-one (41.56 ± 1.77 ng/g) and 1-penten-3-one (13.15 ± 8.15 ng/g) were detected only in ZJ. These compounds conferred fishy, green, mustardy, and pungent aromas.
Aldehydes constituted the second largest group of VOCs in the five FBT samples. Surprisingly, the variation in aldehyde content was similar to that in ketone content: the content of aldehydes was the largest in GX (4939.20 ± 565.45 ng/g), followed by that in GZ (2015.99 ± 349.23 ng/g). Benzaldehyde and benzeneacetaldehyde, which impart floral scents, were the crucial flavor compounds contributing to the characteristic aroma of tea (Ho et al., 2015; Wang et al., 2000). Benzaldehyde was found in all FBT samples, and its levels were highest in GZ (555.84 ± 37.46 ng/g), whereas benzeneacetaldehyde was observed only in SX (49.87 ± 11.41 ng/g). Interestingly, we identified diverse derivatives of benzaldehyde. For example, we tentatively characterized the methyl derivatives 2,3-dihydro-2,2,6-trimethylbenzalhyde and 2,5-dimethylbenzaldehyde. The content of the former was highest in ZJ (177.84 ± 11.44 ng/g), whereas the latter was detected only in GX (39.27 ± 0.91 ng/g).
The GZ sample showed greater ester contents than the other samples, e.g., methyl salicylate, linalyl butyrate, methyl hexanoate, and ethyl caprylate, which impart fruity odors to tea. Methyl salicylate was the only ester identified in each FBT sample and was present at the concentrations of 568.55 ± 17.35, 294.69 ± 30.48, 606.82 ± 24.88, 228.89 ± 3.47, and 622.63 ± 77.99 ng/g in SX, HN, GZ, ZJ, and GX, respectively. Hydrocarbons were present at the lowest levels in ZJ (181.15 ± 29.91 ng/g) and the highest levels in SX (1408.97 ± 124.52 ng/g). Compared with other compounds, hydrocarbons had less effect on the overall flavor of the teas due to their higher threshold values.
3.2.2. Key aroma active compounds forming the aroma characteristics of the five FBTs
Note that not all VOCs contribute to tea aroma. The sensory effects of VOCs are not only influenced by their level and odor attributes, they are also greatly associated with the odor threshold (Deng et al., 2021). In addition, a synergistic effect among different VOCs with diverse olfactory characteristics and ratios might have occurred and consequently influenced the types of aroma. OAV analysis is generally used to assess the contribution of VOCs to aroma, and high values represent great aroma contribution. Although many kinds of VOCs were detected in the five FBT samples, OAV analysis revealed that not all the VOCs contributed greatly to the flavor of FBT. The OAVs of 27 VOCs in five FBT samples were greater than 1. These VOCs consisted of three esters, eight aldehydes, two alcohols, eight ketones, five hydrocarbons, and a furan. These results are also shown in Table S2 β-Ionone, which had the highest OAV and provided a floral, violet odor, was found in HN, GZ, ZJ, and GX (58 46.08–100 048.11). Meanwhile, α-ionone had the maximum OAV value (215.29 ± 10.72) in the GX sample and the minimum OAV value (26.52 ± 5.23) in the SX sample. Previous literature (Wang et al., 2021) stated that the continuous postfermentation of dark tea caused the degradation of carotenoids, leading to the accumulation of α- and β-ionones as demonstrated by our findings. Ionones (including α- and β-forms) were reported to have a remarkable effect on the aroma of teas (Zhou et al., 2019), and the different ionone values of FBT samples could be used to discriminate teas from diverse production regions.
In the SX sample, only linalool had OAV >600. VOCs with 100 > OAV >10 included methyl salicylate, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, ethyl hexanoate, hexanal, α-ionone, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one, naphthalene, styrene, and 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone. VOCs with 10 > OAV >1 included benzeneacetaldehyde, 6-methyl-5-hepten-2-one, 2-methylnaphthalene, acetophenone, and 2,6,6-trimethyl-2-cyclohexene-1,4-dione. The unique active aroma components of SX were benzeneacetaldehyde and styrene, which imparted floral and berry-like aromas.
The OAV of β-ionone in the HN sample exceeded 5000. Meanwhile, the VOCs in the HN sample with 100 > OAV >10 included 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, hexanal, 1-hexanol, α-ionone, heptanal, acetophenone, naphthalene, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone, and 2-methylnaphthalene. The OAV scores of 2,6,6-trimethyl-2-cyclohexene-1,4-dione (floral) and acetophenone (almond, floral, meaty, and musty aromas) in HN were higher than in SX, GZ, ZJ, and GX. Furthermore, heptanal, (E,E)-2,4-heptadienal, 1-hexanol, and 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone, which were responsible for nutty, fatty, and green aromas, were lower in HN than in other samples. As a result, HN had a relatively weaker green odor characteristic than the other teas. Table S2 shows that 2,6,6-trimethyl-2-cyclohexene-1,4-dione and acetophenone (imparting floral and almond-like aromas) had the highest value in the samples and greatly contributed to discriminating the aroma of HN from that of teas from other regions.
The OAVs of β-ionone and linalool in the GZ sample were greater than 20 000 and 800, respectively. VOCs in the GZ sample with 500 > OAV >100 consisted of hexanal, α-ionone, and biphenyl; those with 100 > OAV >10 included methyl salicylate, heptanal, decanal, (E,E)-2,4-heptadienal, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, 2-methylnaphthalene, naphthalene, 1-hexanol, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone, and 1,2,3,4-tetrahydro-1,1,6-trimethylnaphthalene; and those with 10 > OAV >1 included 6-methyl-5-hepten-2-one, ethyl caprylate, dibenzofuran, acetophenone and 2,6,6-trimethyl-2-cyclohexene-1,4-dione. In this sample, β-ionone showed the highest OAV. Furthermore, several VOCs showed higher OAV scores in GZ than in other FBTs. These VOCs included floral–woody and floral–fruity compounds, such as decanal, 1-hexanol, and 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone, and fruity and sweet floral compounds, such as 2-methylnaphthalene and 1,2,3,4-tetrahydro-1,1,6-trimethylnaphthalene. The unique active aroma components in GZ included ethyl caprylate and dibenzofuran, which have oleaginous and fruity aromas.
In the ZJ sample, only β-ionone and linalool showed OAV >20 000 and OAV >1600; VOCs with 100 > OAV >10 included hexanal, (E,E)-2,4-heptadienal, ethyl hexanoate, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, naphthalene, biphenyl, α-ionone, and 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone; and those with 10 > OAV >1 included methyl salicylate, 6-methyl-5-hepten-2-one, 2-methylnaphthalene, acetophenone, and 2,6,6-trimethyl-2-cyclohexene-1,4-dione. All of these VOCs remarkably contributed to the formation of the aroma characteristics of ZJ.
In the GX sample, β-ionone and linalool also had the highest OAV values that exceeded 100 000 and 1500, respectively; VOCs with 500 > OAV >100 included heptanal, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one, (E)-2-nonenal, hexanal, α-ionone, and 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde; and VOCs with 100 > OAV >10 included methyl salicylate, (E,E)-2,4-heptadienal, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone, 6-methyl-5-hepten-2-one, and 1,2,3,4-tetrahydro-1,1,6-trimethylnaphthalene. Notably, the OAVs of most active aroma compounds in GX were larger than those of the compounds in other samples. These compounds included methyl salicylate, heptanal, (E,E)-2,4-hepadienal, hexanal, linalool, and β-ionone. The unique active aroma components in GX were (E)-2-nonenal, (E)-2-hexenal, and 2,2,6-trimethylcyclohexanone, which imparted a fatty and green odor.
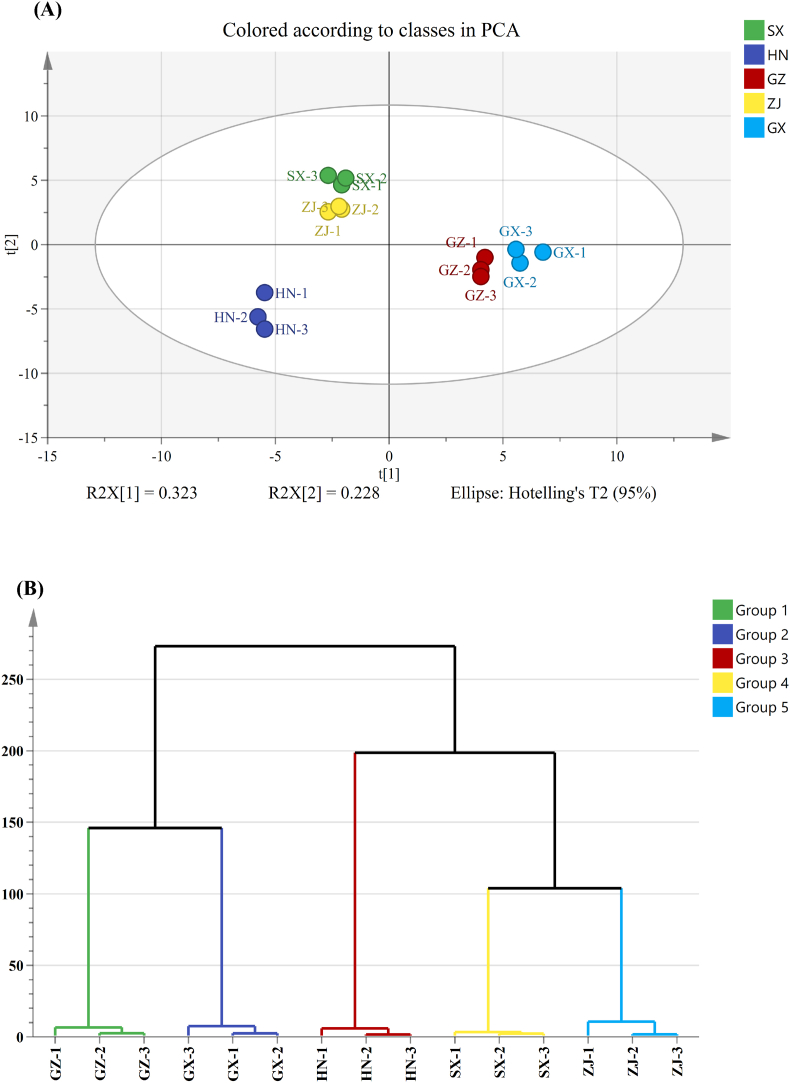
3.3. Differential VOCs in FBTs from different regions
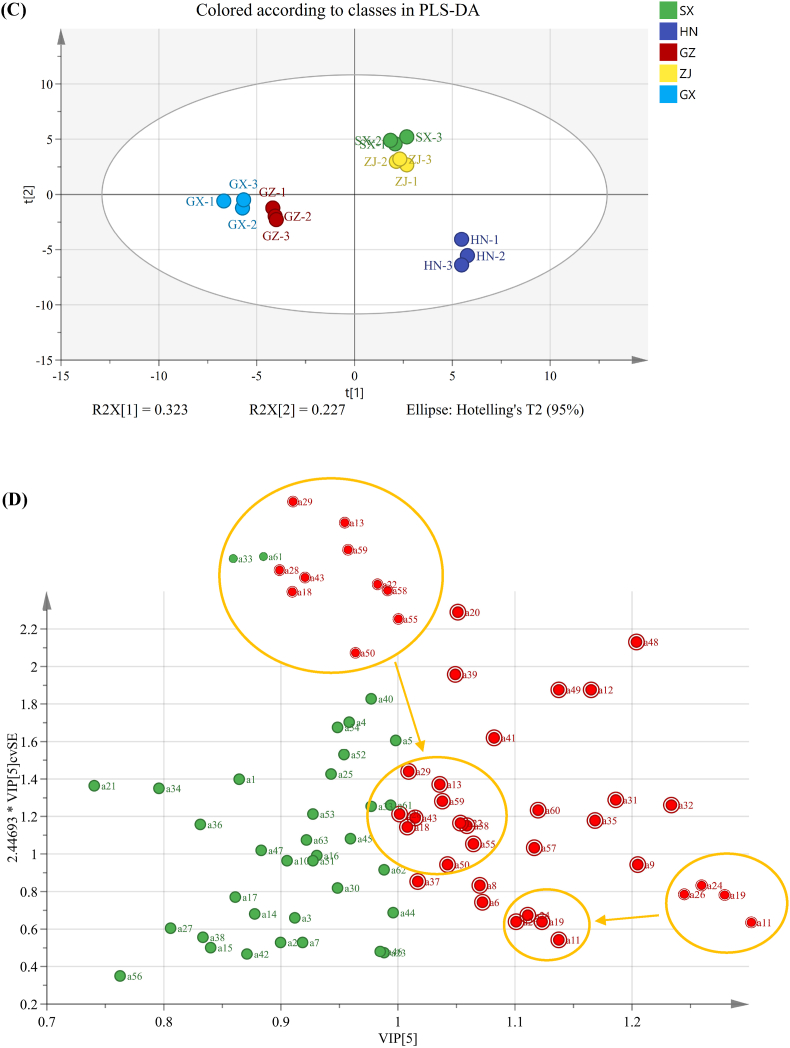
PCA and HCA were conducted on the VOCs identified by using GC–IMS to further reveal the similarities and differences in VOCs among the FBT samples. The results are shown in Fig. 3. The PCA plot constructed on the basis of GC–IMS results (Fig. 3A) showed clear separation among the FBTs from five different sources, implying that the VOCs of tea samples differed dramatically. The HN samples clustered in the bottom left corner, samples from ZJ and SX clustered in the upper left corner, and samples from GX and GZ were located in the right area. HCA also classified the FBT samples into three groups (HN, SX–ZJ, and GZ–GX) (Fig. 3B) in agreement with the PCA results. The PLS–DA model was further constructed to clarify the differential VOCs that were responsible for distinguishing FBTs with various origins, and the results are presented in Fig. 3C. A total of 29 VOCs were identified as differential metabolites on the basis of the common screening criteria of variable importance in the projection (VIP) > 1 and p < 0.05 (Fig. 3D). Notably, the levels of these differential VOCs varied drastically among the FBT samples. Among them, nine differential VOCs were found with the highest concentration in the SX sample; four kinds of VOCs were present at the highest concentration in the HN sample; and six, eight, and two discriminatory VOCs had the highest contents in the GZ, ZJ and GX samples, respectively (Table 1). In addition, the discriminatory aldehyde compounds, such as benzeneacetaldehyde, heptanal, and 3-methylbutanal, might greatly contribute to discriminating the aroma of SX from that of FBTs from other regions, and differential heteroatomic compounds and ketones, such as 2-pentylfuran and acetophenone, might differentiate HN from the other FBT samples. In addition, the discriminatory aldehyde compounds, including (E)-2-octenal, (E)-2-hexenal (dimer), and (E)-2-heptenal, might differentiate GZ from other teas. Discriminatory ester and alcohol compounds, including methyl hexanoate, ethyl 2-phenylacetate, and linalool, were might be responsible for distinguishing ZJ from the other samples. Discriminatory VOCs in the GX sample included phenylacetic acid and linalool oxide (monomer) might differentiate it with others samples.
Fig. 3.
Principal component analysis, hierarchical cluster analysis, partial least squares discriminant analysis, and variable importance in the projection in different manufacturing regions of FBT according to GC–IMS (A–D) and GC–MS (E–H).
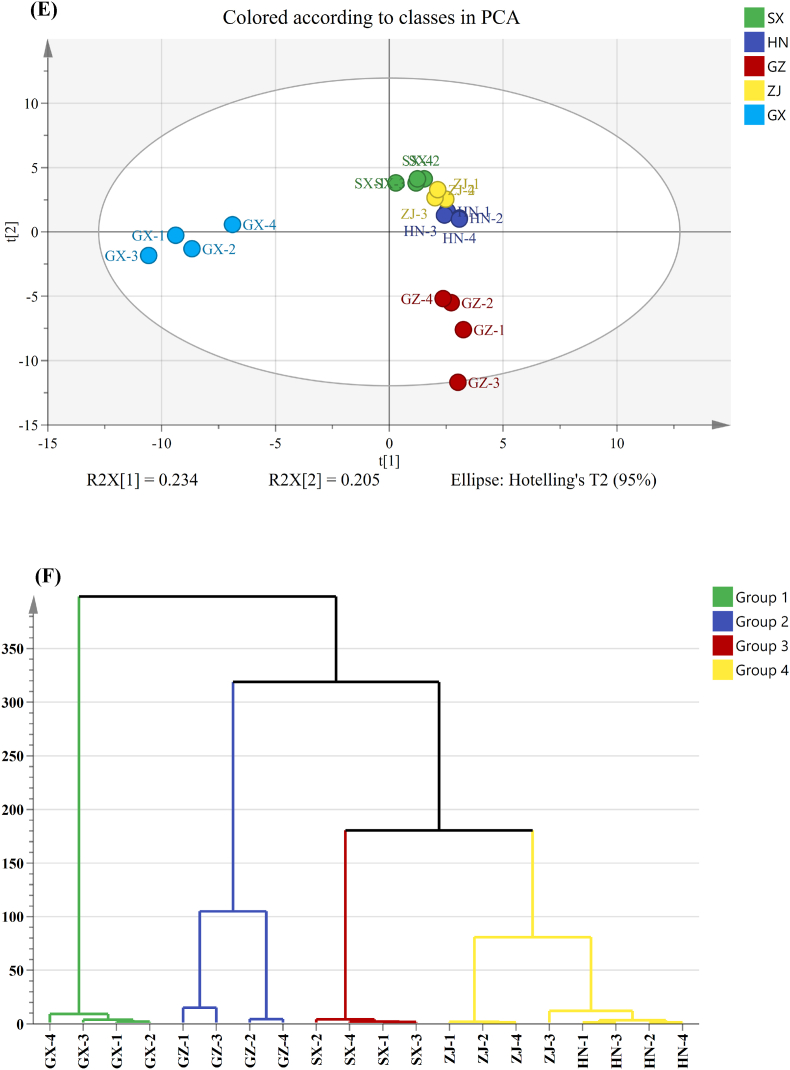
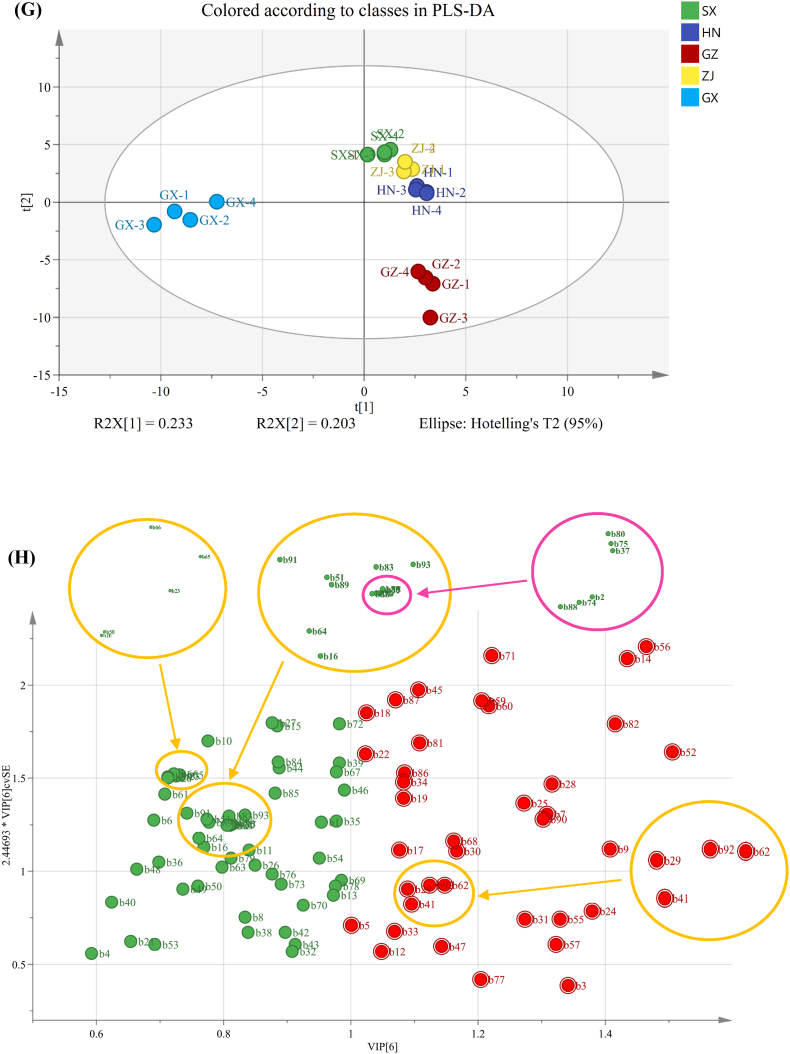
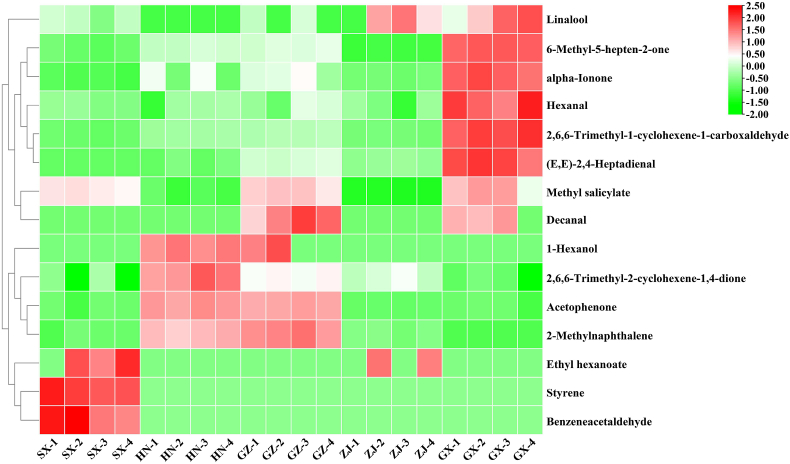
The PCA plot and HCA and PLS–DA analysis of VOCs were also obtained from the results of GC–MS and displayed in Fig. 3E–G. These results showed that the production area had a great influence on the flavor profiles of the FBT samples. In accordance with the screening criteria for VIP >1.0 and p < 0.05, 37 VOCs were screened as discriminating metabolites (Fig. 3H). A large value of VIP is indicative of the great difference between the groups of the aroma compounds and is important for distinguishing the aroma characteristics of diverse FBTs. Considering the lack of systematic comparative research on FBTs from different regions, we systematically compared the aroma attributes of FBTs from five different production regions. On the basis of the OAV results, 15 VOCs were considered as differential metabolites (OAV >1, p < 0.05, VIP >1) (Fig. 4). Three VOCs, namely, styrene, benzeneacetaldehyde, and ethyl hexanoate, were identified as discriminatory aroma components and regarded as the characteristic compounds in the SX sample. Among the five FBT samples, the SX sample had the greatest levels of these VOCs. Styrene, which imparts a sweet, balsamic, and almost floral aroma, has a crucial effect on improving the flavor of tea and forming the unique floral aroma characteristic of FBT. A previous study reported that styrene is a key odorant in Qingzhuan, Liubao, and Tibet teas (Takeo and Tsushida, 1980). In this study, styrene may have contributed to the differentiation of FBT samples from different sources because it was detected only in the SX sample. Benzeneacetaldehyde, a type of phenylalanine-derived volatile in tea, generally has floral, fruity, or sweet scents (Yang et al., 2013). Aromatic amino acid decarboxylases decarboxylate phenylalanine to produce phenylethylamine, which is then converted into benzeneacetaldehyde by amine oxidase, dehydrogenase, or transaminase (Tieman et al., 2006). Benzeneacetaldehyde has been reported to be an important fragrant compound of Keemun black tea (Su et al., 2022). The increase in its level might contribute to the floral and fruity aroma characteristics of black tea. In this study, benzeneacetaldehyde was detected only in the SX sample and could be considered as the key VOC for distinguishing SX from the teas from the other four regions. Therefore, the aroma characteristics of SX could be described as strongly floral and fruity. Two VOCs, including 2,6,6-trimethyl-2-cyclohexene-1,4-dione and acetophenone, could be regarded as discriminatory VOCs for the Hunan sample. These discriminatory aroma compounds had the highest content in the HN sample and could be used as biomarkers to distinguish this sample from other samples. In particular, acetophenone was considered to be the basic volatile of the aroma characteristics of FBT, and its production is related to microorganisms in the fermentation process (Lv et al., 2014). Li et al. (2020a) reported that Hunan FBT has high acetophone content and that acetophenone provides floral, woody, and green attributes, which could promote the formation of the fungal floral aroma characteristics of Hunan FBT. In this study, acetophenone might contribute to the discrimination of HN samples from the other FBT samples. Three volatiles were identified as differential VOCs for the FBT from Guizhou: 1-hexanol, 2-methylnaphthalene, and decanal. The three compounds displayed higher concentrations in the GZ sample than in the other samples. 1-Hexanol has been well-documented to be an important contributor to the flavor and aroma of various kinds of teas (Howard, 1979) and generally imparts strong grassy odors. In earlier investigations, decanal was identified as the primary fragrance molecule that forms the green characteristic of postfermented dark tea (Shi et al., 2019). 1-Hexanol and decanal are produced through the degradation of unsaturated fatty acids in tea and were important to forming the unique green aroma characteristics of the GZ sample. In addition, 2- methylnaphthalene, the main contributor to green, pungent, and herbal-like aromas, could be formed by microbes (Tanguler et al., 2017). Li et al. (2022) reported that 2-methylnaphthalene is a vital VOC contributing to the aroma characteristics of Liupao tea with a betel-nut type aroma. Therefore, the GZ sample was characterized by a grassy and herbal aroma, whereas its floral aroma was weak. For GX, seven VOCs were identified as differential metabolites, including (E,E)-2,4-heptadienal, α-ionone, methyl salicylate, linalool, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, hexanal, and 6-methyl-5-hepten-2-one. GX had the highest levels of these VOCs among all samples. Methyl salicylate, a vital contributor to the flavor quality of tea, confers the characteristics of fresh, faint gingery, grass, milky, and minty odors and could be generated through enzymatic hydrolysis (e.g., β-glucosidase and β-primeveroside) (Celik et al., 2016). This metabolite contributes to distinguishing semifermented tea from fully fermented tea (Wang et al., 2008) and is also considered as the most crucial contributor to the formation of the aroma characteristic profiles of FBT (Li et al., 2020a, 2020b). 6-Methyl-5-hepten-2-one, α-ionone, and 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde are important aromatic compounds that impart sweet, floral, or fruity aromas (Kang et al., 2019; Joshi and Gulati, 2015) and may be related to the degradation of carotenoids during the fermentation of FBT (Li et al., 2018). Linalool is generated by the hydrolysis of the geranyl pyrophosphate precursor by microbial glycosidase that is produced during FBT processing (Ho et al., 2015). In this study, these VOCs may contribute to discriminating the source of FBT samples and to forming the unique sweet, floral, minty aroma characteristic of the GX sample. However, no VOCs were found to clearly distinguish the ZJ sample from the other samples, and only ethyl caproate with a fruity aroma and linalool with a floral aroma were existed higher levels in this sample.
Fig. 4.
Heatmap analysis for the discriminatory volatile compounds among the five sources of FBT.
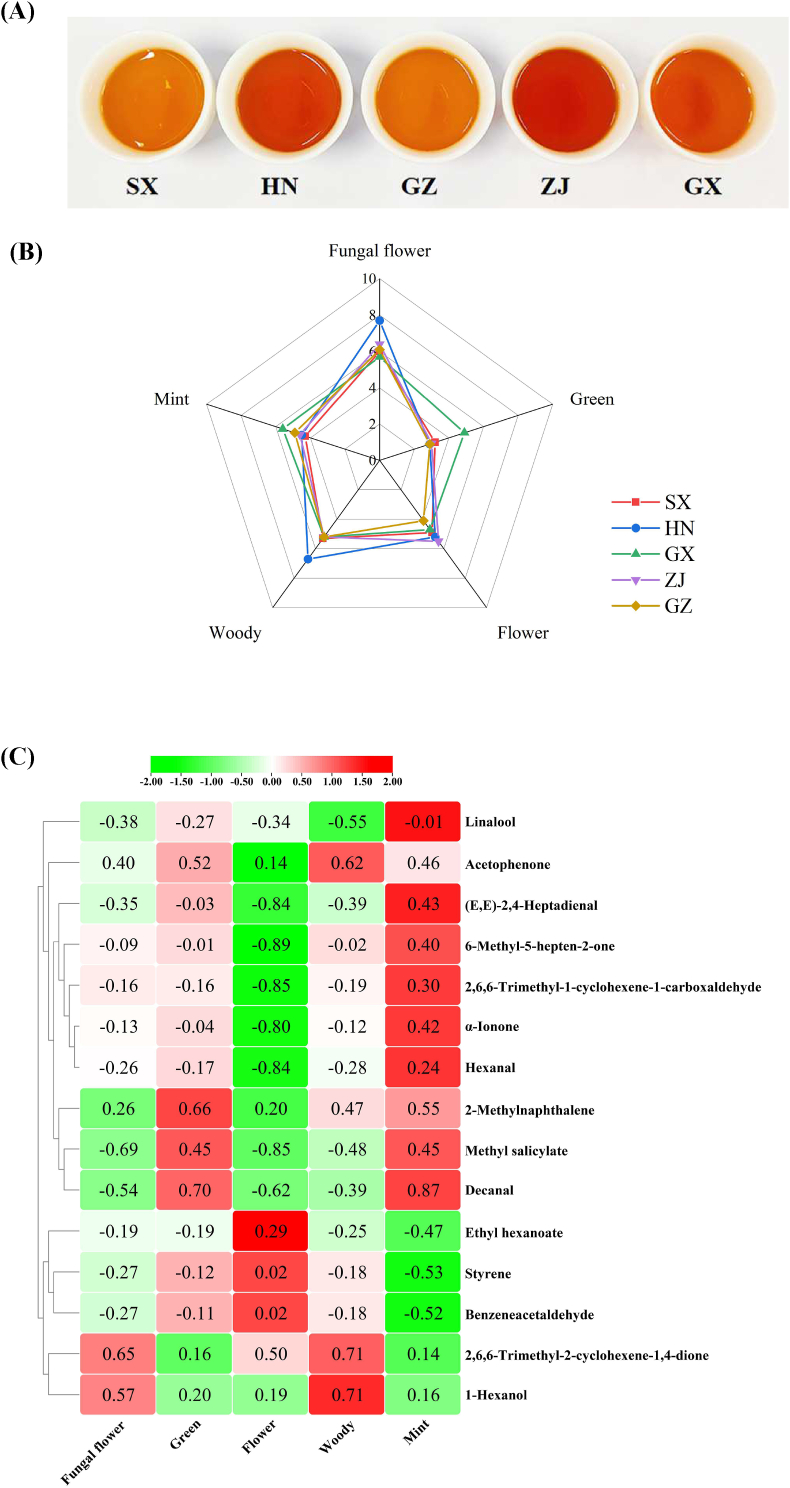
3.4. Sensory evaluation
Sensory evaluation was performed to identify the flavor differences in FBT samples from different sources. The results are presented in Fig. 5. The color of diverse FBT infusions varied. Specifically, the colors of the ZJ and HN samples were dark, whereas those of the GZ and SX samples were light. Five aroma characteristics (fungal floral, green, floral, woody, and minty) were used to describe the flavor attributes of FBT (Fig. 5B). The results indicated that among the samples, the HN sample showed the highest intensity of fungal floral and woody aromas; the GX sample showed the highest intensity of green and minty aromas; and the GZ sample presented lower intensities of floral aroma than the other samples. The results showed that the color and aroma characteristics of the FBT samples differed by production region likely due to the differences in climate, processing, and fermentation time, as well as the different microorganisms involved in their production. Correlation analysis was further conducted to investigate the relationships between sensory attributes and VOCs (OAV >1). The data are shown in Fig. 5C. The results revealed that methyl salicylate was closely associated with fungal floral and floral attributes (p < 0.05); 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, 6-methyl-5-hepten-2-one, (E,E)-2,4-heptadienal, and α-ionone were found to be greatly related to the floral attribute (p < 0.05); and 2-methylnaphthalene and decanal were highly related to the green attribute (p < 0.05). Consistent with a previous study (Lv et al., 2014), this work found that ethyl hexanoate and styrene were related to the mint attribute (p < 0.05).
Fig. 5.
(A) Color of tea infusions, (B) radar of sensory aroma and (C) the correlation results between the sensory aroma and the key volatile components.
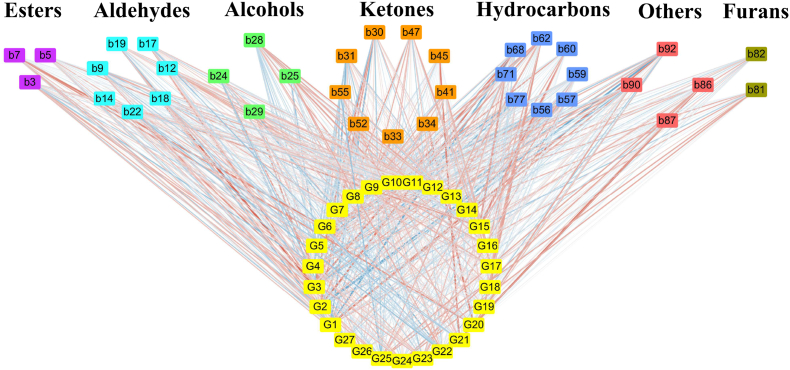
3.5. Association between microorganisms and volatile compounds in FBTs from different regions
Fungal communities have been reported to be a critical contributor to the drastic effects on the aroma compounds of postfermented FBT (Li et al., 2020a). In our previous study, we investigated the fungal communities of diverse FBT samples and found them to be considerably different (Chen et al., 2022). Spearman connection analysis was conducted to clarify the differences in fungal communities (at the genus level) leading to the variation in VOCs in different FBT samples, and the result is shown in Fig. 6. The important VOCs with VIPvalue ≥ 1.0 in GC–MS analysis were selected for correlation analysis. The results indicated that 19 fungal genera were significantly correlated with 30 VOCs (p < 0.05), including three esters, five aldehydes, four alcohols, six ketones, eight hydrocarbons, and four others. Specially, Aspergillus and Eurotium were the most important fungi in the fermentation of FBT (Xu et al., 2011). Consistent with our study, a previous work had also reported that these two fungi greatly contribute to aroma formation in FBT (Li et al., 2020a). It has also been reported that Aspergillus and Eurotium could produce extracellular enzymes, such as polyphenol oxidases, pectinases, and cellulases, to convert tea polyphenols into other active aroma compounds that are primarily responsible for the development of the unique flavor of tea (Chen et al., 2021; Xiao et al., 2022b). In this study, we noted that Aspergillus was closely positively correlated (p < 0.05) with 2,6,6-trimethylcyclohex-2-ene-1-carbaldehyde, 6-methyl-5-hepten-2-one, alpha-ionone, 1,3-bis(1,1-dimethylethyl)benzene, and 1,7,7-trimethylbicyclo[2.2.1]hept-2-ene. 6-Methyl-5-hepten-2-one and α-ionone might be formed through the oxidative decomposition of β-carotene and the oxidative condensation of carotenoids ( Waché et al., 2003) under the action of oxidases produced by Aspergillus (Chen et al., 2021). In this study, we discovered that two of the representative methoxyphenolic compounds, namely, 1,2,3-trimethoxybenzene and 1,2-dimethoxybenzene, were greatly positively correlated with Eurotium (p < 0.05). Methoxyphenolic compounds are thought to be the methylation products of gallic acid and tannins, which are metabolized by microorganisms (Du et al., 2013; Xu et al., 2016). Methoxyphenolic compounds have been reported as the main typical compounds in dark tea, and their accumulation during fermentation could effectively improve the coarse and old taste of raw dark tea and impart a mellow aroma to fermented dark tea (Cao et al., 2018). Notably, Thermoascus was significantly positively correlated with (E,E)-2,4-heptadienal, hexanal, linalool, 6-methyl-5-hepten-2-one, α-ionone, and 1,3-bis(1,1-dimethylethyl)benzene, implying that this fungus plays important role in the aroma characteristics of FBT. These results demonstrated that microorganisms provide great contributions to the formation of the characteristic aroma of FBT and that the variation in VOC profiles may be caused by the differences in the fungi present in FBT samples from different production regions.
Fig. 6.
Network correlation between fungal community and volatile flavor compounds. The abbreviations of fungi are shown in our previous study (Chen et al., 2022). G1: Eurotium, G2:Aspergillus, G3: Thermoascus, G4: Monascus, G5: Saccharomycopsis, G6: Debaryomyces, G7: Saccharomyces, G8: Udeniomyces, G9: Marasmius, G10: Alternaria, G11: Penicillium, G12: Chytridiomycota_unclassified, G13: Saccharomycetales_Incertae_sedis_unclassified, G14: Nectriaceae_unclassified, G15: Chaetothyriales_unclassified, G16: Mortierella, G17: Sporobolomyces, G18: Eurotiomycetes_unclassified, G19: Monosporascus, G20: Wallemia, G21: Agaricomycetes_unclassified, G22: Fungi_unclassified, G23: Thermomyces, G24: Quambalaria, G25: Spizellomycetaceae_unclassified, G26: Malassezia, G27: Ascomycota_unclassified.
4. Conclusion
In this study, we systematically analyzed the aroma characteristics of FBTs originating from five regions of China through GC–IMS, GC–MS, OAV, ROAV, and sensory analysis. A total of 63 and 93 VOCs were identified and quantified through GC–IMS and GC–MS, respectively. GC–MS and GC–IMS revealed that aldehydes and ketones were the major VOCs in the five samples. PCA, PLS-DA, and HCA results demonstrated that FBT samples from different production regions could be clearly separated, implying that their aroma characteristics greatly varied. GC–IMS found high levels of 2-propanone and (E)-2-pentenal in all FBT samples. The OAV and PCA based on GC–MS results showed that the key aroma components of FBT were β-ionone, linalool, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, heptanal, α-ionone, and hexanal. The OAV of these aroma substances in some samples exceeded 100. Therefore, these compounds provided a great contribution to the aroma characteristics of the FBT samples. Fifteen key differential VOCs (i.e., linalool, 6-methyl-5-hepten-2-one, α-ionone, hexanal, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, (E,E)-2,4-heptadienal, decanal, methyl salicylate, 1-hexanol, 2,6,6-trimethyl-2-cyclohexene-1,4-dione, acetophenone, 2-methylnaphthalene, ethyl hexanoate, styrene, and benzeneacetaldehyde) could discriminate the FBT samples from different regions. Sensory analysis also noted that the color and aroma of the infusions of the FBT samples from five regions differed and that methyl salicylate was significantly correlated with fungal floral and floral attributes (p < 0.01). In addition, the correlation analysis between VOC and fungal data showed that fungi played a crucial role in the formation of the characteristic aroma of FBT samples from different regions. Aspergillus and Eurotium were important beneficial fungi in FBT. The results of our work demonstrated that the VOCs and aroma characteristics of FBT samples from different regions of China differed greatly. These differences contributed to the classification of FBT samples from differing production regions and could also offer strong evidence for FBT quality identification.
CRediT authorship contribution statement
Yu Xiao: Project administration, Formal analysis, Methodology, Supervision, Funding acquisition, Conceptualization, Data curation, Validation, Writing – original draft, Writing – review & editing, Investigation, Resources. Yuxin Huang: Methodology, Data curation, Validation, Writing – original draft, Investigation. Yulian Chen: Writing – original draft, Investigation. Leike Xiao: Investigation. Xilu Zhang: Investigation. Chenghongwang Yang: Investigation. Zongjun Li: Resources. Mingzhi Zhu: Writing – review & editing. Zhonghua Liu: Project administration, Supervision, All authors have read and agreed to the published version of the manuscript. Yuanliang Wang: Writing – review & editing, Supervision, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate the financial support from the National Key R&D Project (2022YFE0111200), Natural Science Foundation of China (Nos. 32002095 & 32172217), Hunan Province Natural Science Foundation (No. 2020JJ5243), Hunan Province Science and Technology Innovation Program (No. 2020RC2055 & No. 2021NK4260). The authors also appreciate the Key R&D Program of Hunan Province (2020WK2017 & 2020NK2030) and the Undergraduate Students Innovation Training Project of Hunan Agricultural University (No. xcx202210537014). The authors thank Dr. Chi–Tang Ho for reviewing and editing of the manuscript.
Handling Editor: Dr. Quancai Sun
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.09.024.
Contributor Information
Yu Xiao, Email: yuxiao_89@163.com, xiaoyuhn@hotmail.com.
Zhonghua Liu, Email: zhonghua-liu@hunau.edu.cn.
Yuanliang Wang, Email: wangyuanliang@hunau.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Cao L., Guo X., Liu G., Song Y., Ho C.T., Hou R., et al. A comparative analysis for the volatile compounds of various Chinese dark teas using combinatory metabolomics and fungal solid–state fermentation. J. Food Drug Anal. 2018;26(1):112–123. doi: 10.1016/j.jfda.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik A., Dincer A., Aydemir T. Characterization of β–glucosidase immobilized on chitosan–multiwalled carbon nanotubes (MWCNTS) and their application on tea extracts for aroma enhancement. Int. J. Biol. Macromol. 2016;89:406–414. doi: 10.1016/j.ijbiomac.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen J., Chen R., Xiao L., Wu X., Hu L., et al. Comparison of the fungal community, chemical composition, antioxidant activity, and taste characteristics of Fu brick tea in different regions of China. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li P., Liao L., Qin Y., Jiang L., Liu Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS–GC–IMS and HS–SPME–GC–MS. Food Chem. 2021;361 doi: 10.1016/j.foodchem.2021.130055. [DOI] [PubMed] [Google Scholar]
- Deng X., Huang G., Tu Q., Zhou H., Li Y., Shi H., et al. Evolution analysis of flavor–active compounds during artificial fermentation of Pu–erh tea. Food Chem. 2021;357 doi: 10.1016/j.foodchem.2021.129783. [DOI] [PubMed] [Google Scholar]
- Du L., Wang C., Li J., Xiao D., Li C., Xu Y. Optimization of headspace solid–phase microextraction coupled with gas chromatography–mass spectrometry for detecting methoxyphenolic compounds in Pu–erh tea. J. Agric. Food Chem. 2013;61(3):561–568. doi: 10.1021/jf304470k. [DOI] [PubMed] [Google Scholar]
- Guo X., Schwab W., Ho C.T., Song C., Wan X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC–IMS. Food Chem. 2022;376 doi: 10.1016/j.foodchem.2021.131933. [DOI] [PubMed] [Google Scholar]
- Ho C.T., Zheng X., Li S. Tea aroma formation. Food Sci. Hum. Wellness. 2015;4(1):9–27. [Google Scholar]
- Howard G.E. The volatile constituents of tea. Food Chem. 1979;4(2):97–106. [Google Scholar]
- Joshi R., Gulati A. Fractionation and identification of minor and aroma–active constituents in Kangra orthodox black tea. Food Chem. 2015;167:290–298. doi: 10.1016/j.foodchem.2014.06.112. [DOI] [PubMed] [Google Scholar]
- Kang S., Yan H., Zhu Y., Liu X., Lv H.P., Zhang Y., et al. Identification and quantification of key odorants in the world's four most famous black teas. Food Res. Int. 2019;121:73–83. doi: 10.1016/j.foodres.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Li M.Y., Xiao Y., Zhong K., Bai J.R., Wu Y.P., Zhang J.Q., et al. Characteristics and chemical compositions of Pingwu Fuzhuan brick–tea, a distinctive post–fermentation tea in Sichuan province of China. Food Res. Int. 2019;22(1):878–889. [Google Scholar]
- Li Q., Chai S., Li Y., Huang J., Luo Y., Xiao L., et al. Biochemical components associated with microbial community shift during the pile–fermentation of primary dark tea. Front. Microbiol. 2018;9:1509. doi: 10.3389/fmicb.2018.01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Hong X., Zheng X., Xu Y., Lai X., Teng C., et al. Characterization of key aroma compounds and core functional microorganisms in different aroma types of Liupao tea. Food Res. Int. 2022;152 doi: 10.1016/j.foodres.2021.110925. [DOI] [PubMed] [Google Scholar]
- Li Q., Li Y., Luo Y., Zhang Y., Chen Y., Lin H., et al. Shifts in diversity and function of the bacterial community during the manufacture of Fu brick tea. Food Microbiol. 2019;80:70–76. doi: 10.1016/j.fm.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Li Q., Li Y., Luo Y., Xiao L., Wang K., Huang J., et al. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. LWT--Food Sci. Technol. 2020;127 [Google Scholar]
- Li Z., Huang L., Xia N., Teng J., Wei B., Peng D. Amount of Eurotium sp. in Chinese Liupao tea and its relationship with tea quality. J. Appl. Microbiol. 2020;128(6):1658–1668. doi: 10.1111/jam.14589. [DOI] [PubMed] [Google Scholar]
- Ling T.J., Wan X.C., Ling W.W., Zhang Z.Z., Xia T., Li D.X., et al. New triterpenoids and other constituents from a special microbial–fermented tea–fuzhuan brick tea. J. Agric. Food Chem. 2010;58(8):4945–4950. doi: 10.1021/jf9043524. [DOI] [PubMed] [Google Scholar]
- Lv S.D., Wu Y.S., Li C.W., Xu Y.Q., Liu L., Meng Q.X. Comparative analysis of Pu–erh and fuzhuan teas by fully automatic headspace solid–phase microextraction coupled with gas chromatography–mass spectrometry and chemometric methods. J. Agric. Food Chem. 2014;62(8):1810–1818. doi: 10.1021/jf405237u. [DOI] [PubMed] [Google Scholar]
- Rodríguez–Maecker R., Vyhmeister E., Meisen S., Rosales Martinez A., Kuklya A., Telgheder U. Identification of terpenes and essential oils by means of static headspace gas chromatography–ion mobility spectrometry. Anal. Bioanal. Chem. 2017;409(28):6595–6603. doi: 10.1007/s00216-017-0613-2. [DOI] [PubMed] [Google Scholar]
- Shi J., Zhu Y., Zhang Y., Lin Z., Lv H.P. Volatile composition of Fu–brick tea and Pu–erh tea analyzed by comprehensive two–dimensional gas chromatographytime–of–flight mass spectrometry. LWT-Food Sci. Technol. 2019;103:27–33. [Google Scholar]
- Su D., He J.J., Zhou Y.Z., Li Y.L., Zhou H.J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS–SPME–GC–MS, sensory evaluation, and chemometrics. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131587. [DOI] [PubMed] [Google Scholar]
- Takeo T., Tsushida T. Changes in lipoxygenase activity in relation to lipid degradation in plucked tea shoots. Phytochemistry (Oxf.) 1980;19(12):2521–2522. [Google Scholar]
- Tanguler H., Selli S., Sen K., Cabaroglu T., Erten H. Aroma composition of shalgam: a traditional Turkish lactic acid fermented beverage. J. Food Sci. Technol. 2017;54(7):2011–2019. doi: 10.1007/s13197-017-2637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Zhu Z., Wu B., Wang L., Liu X. Bacterial and fungal communities in Pu’er tea samples of different ages. J. Food Sci. 2013;78(8):M1249–M1256. doi: 10.1111/1750-3841.12218. [DOI] [PubMed] [Google Scholar]
- Tieman D., Taylor M., Schauer N., Fernie A.R., Hanson A.D., Klee H.J. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. U.S.A. 2006;103(21):8287–8292. doi: 10.1073/pnas.0602469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waché Y., Bosser–DeRatuld A., Lhuguenot J.C., Belin J.M. Effect of cis/trans isomerism of β–carotene on the ratios of volatile compounds produced during oxidative degradation. J. Agric. Food Chem. 2003;51(7):1984–1987. doi: 10.1021/jf021000g. [DOI] [PubMed] [Google Scholar]
- Wang D., Yoshimura T., Kubota K., Kobayashi A. Analysis of glycosidically bound aroma precursors in tea leaves. 1. Qualitative and quantitative analyses of glycosides with aglycons as aroma compounds. J. Agric. Food Chem. 2000;48(11):5411–5418. doi: 10.1021/jf000443m. [DOI] [PubMed] [Google Scholar]
- Wang L.F., Lee J.Y., Chung J.O., Baik J.H., So S., Park S.K. Discrimination of teas with different degrees of fermentation by SPME–GC analysis of the characteristic volatile flavour compounds. Food Chem. 2008;109(1):196–206. doi: 10.1016/j.foodchem.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Wang T., Li X., Yang H., Wang F., Kong J., Qiu D., et al. Mass spectrometry–based metabolomics and chemometric analysis of Pu–erh teas of various origins. Food Chem. 2018;268:271–278. doi: 10.1016/j.foodchem.2018.06.041. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kan Z., Thompson H.J., Ling T., Ho C.T., Li D., et al. Impact of six typical processing methods on the chemical composition of tea leaves using a single Camellia sinensis cultivar, Longjing 43. J. Agric. Food Chem. 2018;67(19):5423–5436. doi: 10.1021/acs.jafc.8b05140. [DOI] [PubMed] [Google Scholar]
- Wang Z., Ma B., Ma C., Zheng C., Zhou B., Guo G., et al. Region identification of Xinyang Maojian tea using UHPLC–Q–TOF/MS–based metabolomics coupled with multivariate statistical analyses. J. Food Sci. 2021;86(5):1681–1691. doi: 10.1111/1750-3841.15676. [DOI] [PubMed] [Google Scholar]
- Ward O.P., Qin W.M., Dhanjoon J., Ye J., Singh A.J. Physiology and biotechnology of Aspergillus. Adv. Appl. Microbiol. 2005;58:1–75. doi: 10.1016/S0065-2164(05)58001-8. [DOI] [PubMed] [Google Scholar]
- Xu A., Wang Y., Wen J., Liu P., Liu Z., Li Z. Fungal community associated with fermentation and storage of Fuzhuan brick–tea. Int. J. Food Microbiol. 2011;146:14–22. doi: 10.1016/j.ijfoodmicro.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Xiao Y., He C., Chen Y., Ho C.T., Wu X., Huang Y., et al. UPLC–QQQ–MS/MS–based widely targeted metabolomic analysis reveals the effect of solid–state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022;378 doi: 10.1016/j.foodchem.2021.131999. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Huang Y., Chen Y., Zhu M., He C., Li Z., et al. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT-Food Sci. Technol. 2022;169:113925. [Google Scholar]
- Xu X.Q., Mo H.Z., Yan M.C., Zhu Y. Analysis of characteristic aroma off ungal fermented Fuzhuan brick–tea by gas chromatography/mass spectro–photometry. J. Sci. Food Agric. 2007;87(8):1502–1504. [Google Scholar]
- Xu Y.Q., Wang C., Li C.W., Liu S.H., Zhang C.X., Li L.W., et al. Characterization of aroma–active compounds of Pu–erh tea by headspace solid–phase microextraction (HS–SPME) and simultaneous distillation–extraction (SDE) coupled with GC–olfactometry and GC–MS. Food Anal. Methods. 2016;9(5):1188–1198. [Google Scholar]
- Yang Y., Zhu H., Chen J., Xie J., Shen S., Deng Y., et al. Characterization of the key aroma compounds in black teas with different aroma types by using gas chromatography electronic nose, gas chromatography–ion mobility spectrometry, and odor activity value analysis. LWT-Food Sci. Technol. 2022;163 [Google Scholar]
- Yang Z., Baldermann S., Watanabe N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013;53(2):585–599. [Google Scholar]
- Yao Y., Wu M., Huang Y., Li C., Pan X., Zhu W., et al. Appropriately raising fermentation temperature beneficial to the increase of antioxidant activity and gallic acid content in Eurotium cristatum–fermented loose tea. LWT-Food Sci. Technol. 2017;82:248–254. [Google Scholar]
- Zhang H., Huang D., Pu D., Zhang Y., Chen H., Sun B., et al. Multivariate relationships among sensory attributes and volatile components in commercial dry porcini mushrooms (Boletus edulis) Food Res. Int. 2020;133 doi: 10.1016/j.foodres.2020.109112. [DOI] [PubMed] [Google Scholar]
- Zheng X.X., Hong X., Jin Y., Wang C., Liu Z.H., Huang J.N., et al. Characterization of key aroma compounds and relationship between aroma compounds and sensory attributes in different aroma types of Fu brick tea. Food Chem. X. 2022;13 doi: 10.1016/j.fochx.2022.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong A., Chen W., Hu L., Wu Z., Xiao Y., Li K., et al. Characterisation of key volatile compounds in fermented sour meat after fungi growth inhibition. LWT-Food Sci. Technol. 2022;165 [Google Scholar]
- Zhou P., Hu O., Fu H., Ouyang L., Gong X., Meng P., et al. UPLC–Q–TOF/MS–based untargeted metabolomics coupled with chemometrics approach for Tieguanyin tea with seasonal and year variations. Food Chem. 2019;283:73–82. doi: 10.1016/j.foodchem.2019.01.050. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Chen J., Chen X., Chen D., Deng S. Use of relative odor activity value (ROAV) to link aroma profiles to volatile compounds: application to fresh and dried eel (Muraenesox cinereus) Int. J. Food Prop. 2020;23(1):2257–2270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.