Summary
Immunoengineering technologies harness the power of immune system modulators such as monoclonal antibodies, cytokines, and vaccines to treat myriad diseases. Immunoengineering innovations have showed great promise in various practices including oncology, infectious disease, autoimmune diseases, and transplantation. Despite the countless successes, the majority of immunoengineering products contain active moieties that are prone to instability. The current review aims to feature freeze-drying as a robust and scalable solution to the inherent stability challenges in immunoengineering products by preventing the active moiety from degradation. Furthermore, this review describes the stability issues related to immunoengineering products and the utility of the lyophilization process to preserve the integrity and efficacy of immunoengineering tools ranging from biologics to nanoparticle-based vaccines. The concept of the freeze-drying process is described highlighting the quality by design (QbD) for robust process optimization. Case studies of lyophilized immunoengineering technologies and relevant clinical studies using immunoengineering products are discussed.
Subject areas: Biological sciences, Immunology, Biotechnology
Biological sciences; Immunology; Biotechnology
Introduction
Over the past few decades, immunoengineering has increasingly gained interests from the scientific community and have been advanced by healthcare industries of various practices including oncology, infectious disease, autoimmune diseases, and transplantation (Green, 2021). For instance, chimeric antigen receptor T (CAR-T) cells, genetically engineered T-cells that attack cancerous cells, are among the latest immunoengineering innovations. The US Food and Drug Administration (FDA) has approved the CAR T technology for the treatment of blood cancer (Jhunjhunwala, 2018). Such technological breakthrough is opening up new avenues for generating novel pathogen vaccines, moving our focus to biological-derived materials. The therapeutic translation of cytokines has been realized in the treatment of cancer and autoimmune illnesses. Immunoengineered vaccines against malaria (Fotoran et al., 2017) and biodegradable polymeric nanoparticles as nicotine vaccines are also being developed to treat infectious diseases (Hu et al., 2016). Most recently, immunoengineering technologies enabled the development of mRNA-based vaccines against SARS and CoV-2 in response to COVID-19 pandemic (Juncker et al., 2021).
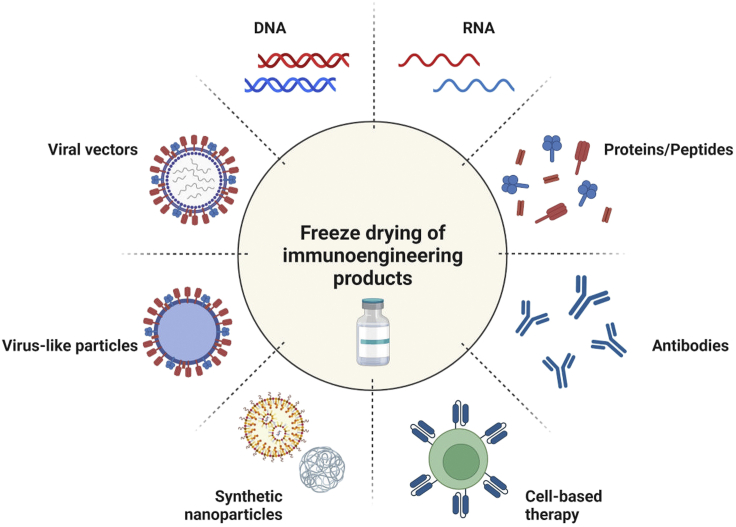
Despite the myriad successes of immunoengineering technologies, most products are susceptible to instability because of oxidation, hydrolysis, aggregation, and photolytic degradation which ultimately lead to reduced efficacy on shipping and long-term storage. This necessitates the utilization of a robust and scalable approach to preserve the integrity and efficacy of the immunoengineering products. Freeze-drying is a promising strategy which has been widely explored to stabilize small molecular therapeutics, proteins, peptides, and cells. Figure 1 illustrates immunoengineering technologies manufactured into lyophilized products.
Figure 1.
Thematic representation of application of freeze-drying for immunoengineering technologies and products
Created with BioRender.com.
Herein, the present review portrayed various immunoengineering technologies and products, stability issues, and the role of freeze-drying in improving the stability. The concept of the freeze-drying process is described highlighting the quality by design (QbD) for a robust process optimization. More importantly, the review discussed the case studies that utilize lyophilization technique to preserve immunomodulatory materials under development and in clinical trials, and FDA approved products.
Immunoengineering technologies
Cytokines
Cytokines is the foremost engineering tool approved by the US FDA as immunotherapy. Cytokines are primarily secreted by immune cells which are of low molecular weight proteins and regulate the immune response by mediating communication among the cells in the body (Duque and Descoteaux, 2014). Hence cytokines are well explored as immunomodulators, and they are classified as pro-inflammatory cytokines and anti-inflammatory cytokines. Pro-inflammatory cytokines are chiefly generated by the activated immune cells and are implicated in the elevation of immune response. Although anti-inflammatory cytokines produced by immune cells exhibit immunomodulation by suppressing the inflammation. This suppression of inflammation is achieved by either inhibiting or controlling the cellular activities of pro-inflammatory cytokines (Kany et al., 2019). Interleukin-2 (IL-2) and interferon-alpha (IFN-α) are two pro-inflammatory cytokines widely explored for the management of various malignancies. Apart from that interferon-gamma (IFN-γ), granulocyte-macrophage colony- stimulating factor, IL-7, IL-12 and IL-21 are being explored for the immunoengineering (Conlon et al., 2019). However, IL-10 is the only anti-inflammatory cytokine being studied in the treatment of rheumatoid arthritis, inflammatory bowel disease and systemic lupus erythematosus (Georgescu et al., 1997).
A crucial consideration regarding the administration of cytokines is its short half-life and to tackle this issue, cytokines are being pegylated to improve the circulation half-life. Polyethylene glycol (PEG) is conjugated to the cytokine which increases the molecular weight thereby reducing the renal clearance. IFNα-2a conjugated with a 5kD PEG in a molar ratio of 1:1 resulted in a half-life of 51 h where the unconjugated cytokine showed a half-life of 0.7 h (Monkarsh et al., 1997).
Antibodies
Antibodies are generated by B cells to target specific antigens. They are extremely efficient in binding to target antigens and interfere with molecular pathways that contribute to disease progression (Wang et al., 2020). Monoclonal antibodies are produced in laboratories by molecular engineering that are used to mimic natural antibodies and manipulate the immune system. Monoclonal antibodies are widely explored for the immunotherapy of cancer and other autoimmune diseases (Bayer, 2019). In spite of their advantage in immunotherapy, they are prone to denaturation, oxidation, and hydrolysis when subjected to extreme temperature, pH and solvents.
Vaccines
Vaccines are one of the prominent groups of immunoengineering tool to manage infectious diseases and cancer. Conventional vaccines are developed using a live attenuated or killed organism (Epstein et al., 2011). However, subunit vaccines, DNA and RNA-based vaccines are also being explored as new vaccine modality (Luke et al., 2011). Although, these subunit and nucleic acid-based vaccines are safer than conventional vaccines, they endure with poor immunogenicity. The immunogenicity can be efficiently enhanced by incorporating the nanotechnology in vaccine delivery. The nanocarrier systems ensure efficient delivery of vaccine antigens and also adjuvants to the targeted site owing to their size, composition and surface properties. They can co-deliver both the antigen and the adjuvant (Thoryk et al., 2016). Targeted delivery of nanocarrier systems can be accomplished by either passive targeting achieved by their size, which is actively taken up by macrophages or by active targeting by surface modification with ligands (Oyewumi et al., 2010). Thus, the antigens which trigger the immune response are delivered directly to the antigen-presenting cells via nanotechnology.
Nucleic acids
Nucleic acids such as plasmid DNA (pDNA), messenger RNA (mRNA), therapeutic RNA (refers to antisense oligonucleotides (ASOs)), micro RNA (miRNA), and small interfering RNA (siRNA) can be utilized for gene therapy and immunotherapy. They have shown promise in treating a wide range of disorders including infectious diseases, cancers, immune diseases in both preclinical and clinical stages (Kulkarni et al., 2021). For nucleic acid-based therapy to work, they must function inside target cells without evoking undesired immune responses. In many circumstances, a vector is required to transport nucleic acids into target cells. Viral and non-viral vector based delivery systems have been developed to protect the nucleic acid from degradation, minimize exposure to off-target cells, and maximize the delivery to target cells, and overcome biological barriers to facilitate the safe and effective delivery (Paunovska et al., 2022).
Nanoparticle carriers for immunoengineering products
Viral vectors have been widely implemented for the delivery of gene to the host cells which include adenovirus, retrovirus and adeno-associated virus (Rajagopal et al., 2018). Nonetheless, concerned with the safety issues of viral vectors, non-viral carriers are widely adopted for the gene delivery. Lipoplexes and lipid-based nanoparticles have gained interest for the nucleic acid delivery especially in the delivery of nucleotides and mRNA. Lipids with positive charge are easily associated with the nucleic acids which are negatively charged and form nanostructures. Polymers are also great alternatives to the lipid-based drug delivery where polymers can be fabricated as per the requirement for the delivery of nucleic acids. Cationic polymers self-assemble with the nucleic acids and are often used for gene delivery (Olden et al., 2018).
Cells
Cell-based therapy is one of the fastest growing fields of immuno-oncology where it leads to advances in immunotherapy strategies. Cells are engineered to avoid host immune rejection cells which may hamper their use as long-term effective therapies (Musiał-Wysocka et al., 2019). CAR-T cells are one of the advanced engineered T cells developed to treat the cancer. The T cells are isolated from the patient and engineered by replacing the T cells receptor with CAR which enables the targeting of cancerous cells. These engineered T cells are injected back to the patient where T cells fights against the tumor cells with effective targeting (Larson and Maus, 2021). Apart from CAR-T cells, CAR-NK cells, dendritic cells and cells for payload are being widely used immunotherapies. CAR-NK cells are similar to CAR-T cells where NK cells are engineered the receptors on their surface to target the cancerous cells (Xie et al., 2020). Dendritic cells, which are antigen-presenting cells, are engineered to express the cancer antigens ex vivo and are infused into the patient. Dendritic cells are also implemented in the treatment of autoimmune diseases (Hasegawa and Matsumoto, 2018) thus offering a wide range of opportunities in immunotherapy.
Instability of immunoengineering materials
Chemical instability of proteins
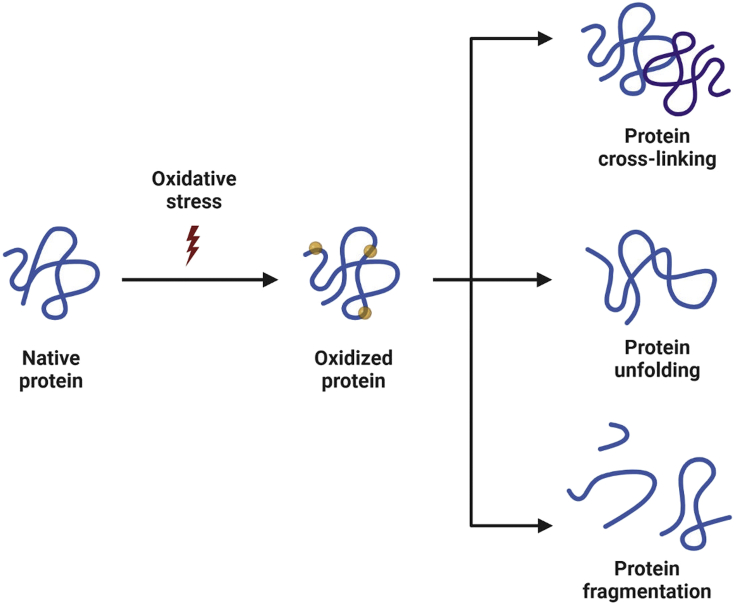
Oxidation causes changes in protein structure that affect the conformational stability, safety, and efficacy of immunogenic materials (Junping et al., 2000). Oxidation pathways and factors affecting the oxidation of immunological and protein formulations are listed in Figure 2. Amino acids such as lysine, histidine, and cysteine are susceptible to metal catalyzed oxidation (Stadtman and Levine, 2003). Hydrogen peroxide is one primary source of the oxidation reaction. Hydrogen peroxide can directly react with amino acids such as methionine and cysteine induce oxidation of other amino acids (tryptophan) through radicals generated via Fenton or Photo-Fenton reaction (Katritzky et al., 2003). Photo-oxidation is mediated by chromophores in proteins, as the light absorbed by chromophores leading to susceptibility of proteins to photo-oxidation. The details of type (site specific versus non-site specific), source, and oxidation induced protein instability discussed elsewhere (Gupta et al., 2021).
Figure 2.
Schematic of protein oxidation and most common outcomes (Modified from (Lund et al., 2011))
Created with BioRender.com.
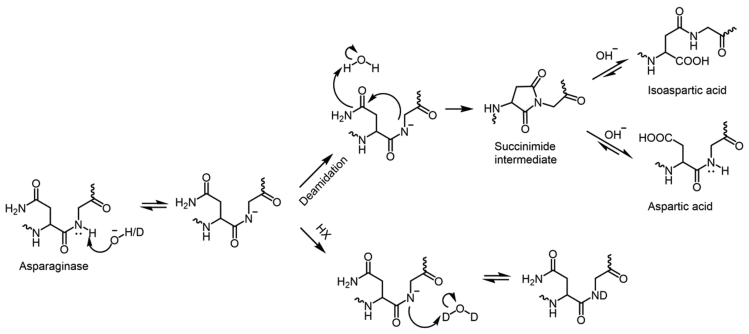
Deamidation is a non-enzymatic post translational modification, where asparagine converts to aspartate or isoaspartate and glutamine coverts to glutamate (Gervais, 2016). Deamidation of protein leads to the formation of a negatively charged side chain that increases the molecular weight of protein by 0.984 Da (Gamage et al., 2019). Furthermore, conversion to isoaspartate modifies the protein back bone by introducing a methylene group. These post translational modification conformational changes alter the charge, size, structural heterogeneity in proteins, thereby affecting protein function, stability, efficacy, purity, and immunogenicity (Noguchi, 2010). Deamidation mainly occurs via an acid-base catalyzed pathway. The backbone amide nitrogen on the C-terminal side of asparagine is deprotonated by OH in the solvent in the base catalyzed pathway, as shown Figure 3. The nucleophilic attack of the resulting amidate anion on the carbonyl carbon of the asparagine side chain forms the succinimide cyclic intermediate, which is the rate determining step of the deamidation reaction. Furthermore, the hydrolysis of succinimide intermediate leads to the formation of a mixture of isoaspartic acid and aspartatic acid (Catak et al., 2006). The rate of deamidation is dependent on the solution properties such as pH, temperature, ionic strength, protein sequence and high order structure of the protein. The detailed mechanism of deamidation is described elsewhere (Gamage et al., 2019).
Figure 3.
Base-catalyzed deamination and HX mechanism at an NG motif
Physical instability of proteins
Protein aggregation is a phenomena that occurs through the self-assembly of improper folded/misfolded proteins to form soluble oligomers and insoluble aggregates (Wang and Roberts, 2018). Aggregates become irreversible when the protein aggregates beyond the solubility limit, resulting in precipitation out of the solution. Irreversible aggregates can be removed from preparatory separation techniques such as filtration. The self-assembly of the protein molecules could cause reversible aggregate formation because of changes in pH or ionic strength of the protein formulation (Pham and Meng, 2020). Protein can aggregate by multiple pathways, including physical aggregation via unfolding intermediates and unfolded states, aggregation because of protein self-association (protein-protein interaction), chemical linkages, and aggregation due to chemical degradation (Wang and Roberts, 2018). The factors responsible for the aggregation of proteins include intrinsic (primary, secondary, tertiary or quaternary structure) and extrinsic (environmental and solution or processing conditions) factors (Wang et al., 2010). General environmental factors include temperature, light, and container/closure system. Solution conditions include protein concentration, pH, buffer type, salt conditions, and processing factors (fermentation/expression, unfolding/refolding, purification, freeze–thaw, shaking and shearing, pressurization, formulation/filling, drying), preparation of modified protein or delivery systems, analytical methodologies, etc., play a critical role in protein aggregation (Krebs et al., 2009).
Denaturation refers to protein unfolding because of loss of secondary and tertiary structure of the protein. Protein denaturation may result from environmental conditions such as extreme temperature and pH, leading to loss of protein high order structure through unfolding. The denaturation changes the protein physical state while maintaining its chemical composition (Butreddy et al., 2021a).
Surface adsorption or surface induced aggregation is a physical degradation process, which involves accumulation and adhesion of protein molecules to surface without penetration, leading to change of physical state and loss of protein concentration in solution (Zapadka et al., 2017).
Proteins are amphiphilic in nature and have surface activity, which allows them to adsorb on the liquid surface, thereby decreasing the solution interfacial and surface tension (Begum and Amin, 2019). The interfacial adsorption of proteins causes exposure of hydrophobic regions in proteins, interactions between hydrophobic regions, leading to protein structural changes and aggregation (Lajnaf et al., 2017). The hydrophobicity of the proteins increased with decreasing surface tension of the proteins, which promotes the interfacial instability of proteins (Xiao et al., 2020). Factors responsible for surface adsorption of proteins include; intramolecular interactions, hydrophobicity, ionic or electrostatic interactions (Zapadka et al., 2017). The surface adsorption of proteins occurs at various interfaces such as air-liquid, solid-liquid interfaces. During manufacturing, different surfaces are responsible for the adsorption of proteins include; processing components manufacturing tanks, glass vials, rubber stoppers, tubes, and filters (Sacha et al., 2010).
Nanoparticle instability
Recent immunoengineering products may include nanoparticles which are manufactured in an aqueous solution suffer from both chemical and physical instabilities. Chemical instability mainly related to oxidation, deamidation, hydrolysis, disulfide bond formation/exchange, whereas physical instability may include particle aggregation. Most nanoparticle formulations must be stored frozen, otherwise they can only be used for short time. Hence, the long-term stability of nanoparticles at ambient condition is a key developmental goal. Furthermore, nanoparticles that differ in morphological structure, size, charge, and chemical instabilities require different considerations related to formulation, process, and storage. In addition, formulation design of freeze-dried nanoparticles should consider liquid state colloidal stabilization mechanism as nanoparticles are also predisposed to aggregation during freeze drying processes (Trenkenschuh and Friess, 2021).
Freeze-drying for preservation of immunoengineering products
Freeze-drying or lyophilization is a well-known method for improving the stability of labile pharmaceuticals. The freeze-drying process consist of sublimation of ice from its frozen form, followed by desorption of moisture under the vacuum. The lyophilization process allows the creation of dry or solid-state presentations with improved long-term storage, helping to avoid the costly and time-consuming cold-chain supply that many biologics require.
Freeze-drying process
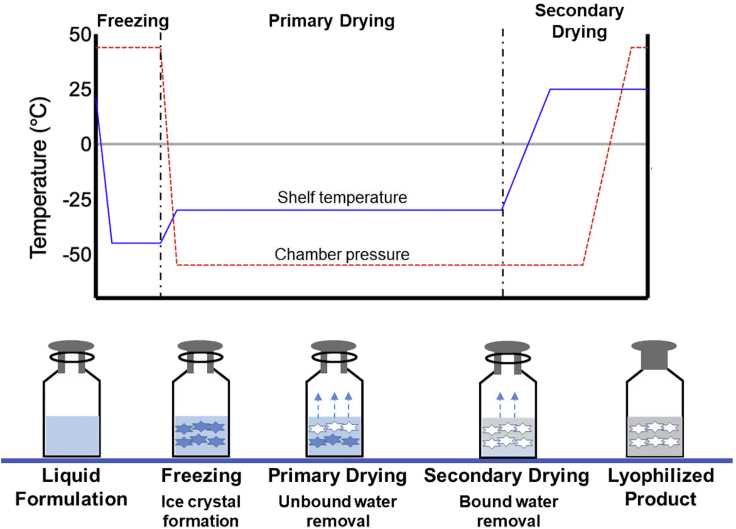
Protein molecules exhibit instability because of physical and chemical degradation. To obtain an acceptable shelf life, protein products are often freeze-dried to remove water in the drug product. The freeze-drying process consists of three steps: Freezing, primary drying and secondary drying.
Freezing step
The freezing process involves cooling the solution until ice nucleation starts and ice crystals to grow at a certain rate that can result in freeze concentration of the solution (Ward and Matejtschuk, 2021). The rate of ice crystal growth and size of ice crystals can be expressed as a function of the degree of supercooling. The main parameters during the freezing step are the minimum freezing temperature, rate of freezing and hold time at the freezing temperature. Typically, freezing temperature is selected below the formulations glass transition temperature and the formulations freezing rate is chosen depending on the ice crystal size and protein degradation. The hold time at freezing temperature should be sufficient to ensure all vials are equilibrated with the shelf temperature (Butreddy et al., 2020).
The annealing step often added after the freezing step is completed to increase the ice crystal size and allow the crystallization of excipients such as glycine and mannitol. This annealing step can increase the primary drying rate and improve the appearance and homogeneity of the freeze-dried cake. Annealing temperature and time are key parameters in the annealing step. Annealing temperature is selected 10–20°C above the Tg but well below the eutectic melting temperature (Kasper and Friess, 2011).
Primary drying step
The primary drying step consumes a large portion of the freeze-drying process. In primary drying, frozen water is removed under optimal shelf temperature and chamber pressure. The shelf temperature is chosen to be a few degrees below the formulation collapse temperature to obtain a dried cake with an acceptable appearance. The chamber pressure selected should be 10–30% of ice vapor pressure at the target product temperature (Butreddy et al., 2020). Knowledge of sublimation rates and product temperature profiles associated with the combination of chamber pressure and shelf temperature is important for selecting primary drying process parameters (Bjelošević et al., 2018). Furthermore, determination of the end of primary drying is critical because the transition to secondary drying without completion of primary drying may lead to a partial collapse of the cake.
Secondary drying step
After primary drying, depending on the formulation, a fair amount of residual water (5–20%) is present in the amorphous product (Butreddy et al., 2020). The purpose of secondary drying is to remove the water from the solute phase by desorption. In secondary drying, the desorption rate is depending on the product temperature and is not sensitive to chamber pressure. Generally, a slow ramp rate (0.1 °C/min) is chosen during the transition from the end of primary drying to the start of secondary drying to avoid collapse of the amorphous product (Tang and Pikal, 2004). The final product temperature and duration of drying are critical factors in secondary drying because these parameters determine the product final moisture content (Schneid et al., 2011a).
Once secondary drying is complete, when the moisture content of the freeze-dried cake reaches the target level, the vials are stoppered under a partial vacuum inside the chamber (Figure 4). Then the shelf temperature is maintained at 2–8°C, following the aeration of the chamber until the vials are unloaded.
Figure 4.
Schematic representation of lyophilization process
Excipients used in freeze-dried formulations
The selection of excipients for protein formulation depends on the nature of the proteins, and its degradation pathways. Excipients are often used to optimize the physical and chemical instabilities, and to slow down or prevent the destabilization stresses, induced during manufacturing. The commonly employed excipients in protein formulation are stabilizers, surfactants, buffers (Table 1).
Table 1.
Typically used excipients in protein formulations with their mechanism of stabilization
| Stabilizing excipient | Examples | Mechanism of stabilization |
|---|---|---|
| Amino acids | Arginine, Glycine, Methionine, Histidine | Preferential hydration Preferential exclusion Decrease protein-protein interactions Increase solubility (reduce viscosity) |
| Sugars | Sucrose, Trehalose | Preferential hydration Preferential exclusion Reduction of mobility resulting from increased viscosity |
| Surfactants | Polysorbate 80 Polysorbate 20 Poloxamer |
Competitive adsorption |
| Buffers/salts | Histidine Succinate Phosphate Citrate Acetate |
Preferential binding Interaction with protein bound water |
Stabilizers
Cryoprotectants, mainly sugars, and polyols are added to the freeze-dried protein formulation to protect the proteins from freezing induced stresses. Sucrose and trehalose are the most popular cryoprotectants or stabilizers. The mechanisms by which cryoprotectants exhibit the stabilizing effects include; preferential exclusion/hydration, vitrification, and water replacement hypothesis. Cryoprotectants stabilize the native state of proteins because cryoprotectants are selectively excluded from the vicinity of the protein surface, increasing the free energy of unfolding during the initial stage of the freeze process (Thakral et al., 2021).
Freeze concentration or ice-crystallization makes the system approach glassy state (vitrification), which reduces the molecular mobility and increases freeze-concentrate viscosity, thereby slowing down the denaturation and degradation reactions (Bhatnagar et al., 2007).
During freeze-concentration, the water molecules will not be available to form a hydrogen bond with the polar surface of the proteins. The water replacement/substitution or preferential solute interaction allows the selective interaction (hydrogen bonding) of solutes (hydroxyl group of sugar) with the proteins, thereby maintaining the proteins native conformation (Thakral et al., 2021). Furthermore, amino acids are used in freeze dried protein formulation to improve stability/solubility or suppress aggregation. Excipients such as arginine, histidine, glycine and methionine are deemed to be commonly used amino acid stabilizers (Falconer, 2019).
Surfactants
Proteins are vulnerable to aggregation because of their surface-active nature, which causes surface-induced denaturation (Lee et al., 2011). Proteins adsorb at various interfaces such as air-liquid interface because of mixing and ice-liquid or ice-air interface due to freeze-drying.
Surfactants are added to protein formulation to stabilize the proteins, possibly by two mechanisms (Thakral et al., 2021); i) surfactants form a surfactant-protein complex by interacting with the protein molecules, preventing the proteins from further interactions, ii) preferential binding of surfactant molecules at the interface leading to binding of surfactants at hydrophobic surfaces. The non-ionic surfactants Polysorbate (20 and 80), Poloxamer 188 are widely used in liquid and freeze-dried protein formulations to inhibit the surface induced aggregation (Ohtake et al., 2011).
Buffers
The selection of buffers for freeze-dried protein formulations depends on the target pH range, buffer capacity, and potential of buffer specific catalysis (Zbacnik et al., 2017). The most widely used buffers in protein formulations include histidine, sodium phosphate, potassium phosphate, citrate, tris and succinate buffers (Gervasi et al., 2018). In freeze-drying of proteins, it is important to use buffer levels at the lowest effective concentration owing to the potential of buffer crystallization. During freezing, the recrystallization propensity of buffer components affects the protein stability because of pH shift. For example, crystallization of disodium phosphate dodecahydrate, the component of sodium phosphate buffer during freezing may affect the protein stability because of lowering the pH of the freeze concentrate. Therefore, buffer components need to be remained amorphous during freezing. Furthermore, crystallization and the pH shift of the buffer components depend on the factors such as type and concentration of buffers, initial solution pH, the concentration of other components in a formulation. The use of non-crystallizing formulation components such as sucrose is known to inhibit buffer crystallization, thereby avoiding any pH shift (Sundaramurthi and Suryanarayanan, 2011).
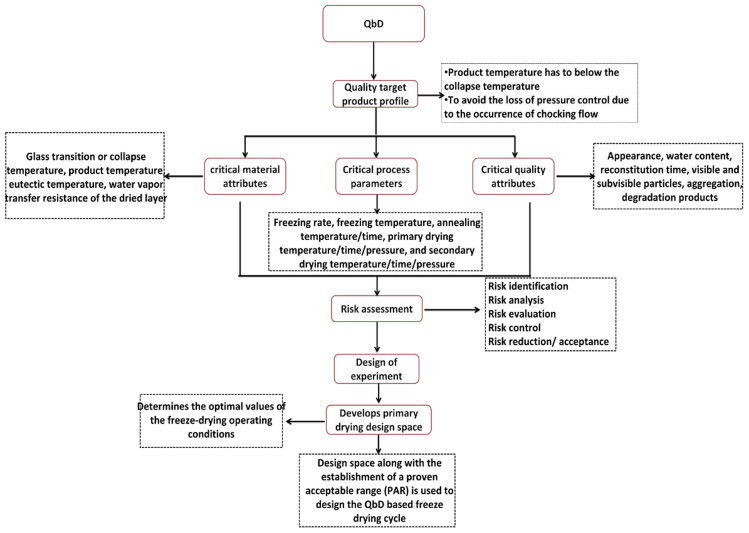
Freeze-drying quality by design
QbD is a systematic approach that emphasizes process understanding and control to guarantee process to meet the specific product critical quality attributes (CQAs) as represented in Figure 5. (Arsiccio and Pisano, 2018). QbD approach involves a formalized risk assessment to identify the critical formulation and processing factors that could affect product quality, a systematic experimental approach intended to place specifications on key formulation and process variables, and the identification of design spaces for formulation and process (Mockus et al., 2011).
Figure 5.
Schematic flow of QbD involved in freeze-drying of immunoengineering products
The key elements of QbD include critical quality attributes (CQAs), critical process parameters (CPPs), and critical material attributes (CMAs). The CMAs of the lyophilized formulations include glass transition or collapse temperature, product temperature, eutectic temperature, and water vapor transfer resistance of the dried layer. CPAs of the lyophilization process include freezing rate, freezing temperature, annealing temperature/time, primary drying temperature/time/pressure, and secondary drying temperature/time/pressure. CQAs of lyophilized proteins include; appearance, water content, reconstitution time, visible and subvisible particles, aggregation, degradation products (Kawasaki et al., 2019).
Identifying proper shelf temperature and chamber pressure to minimize the drying time is critical for optimizing the primary drying. For primary drying optimization, two constraints must be satisfied: (1) the product temperature has to remain below the collapse temperature to preserve the cake structure and to avoid product degradation, (2) when the sublimation flux is too high in the drying chamber, it is necessary to avoid the loss of pressure control because of the occurrence of chocking flow (Patel et al., 2010). QbD approach is designed to develop a primary drying design space to help determine the optimal values of the freeze-drying operating conditions. Typically, the design space approach together with the establishment of a proven acceptable range (PAR) is used to design the QbD based freeze-drying cycle. The QbD based freeze-drying cycle development begins with: (1) experimental determination of formulation critical temperature (collapse or glass transition temperature) and mathematical model parameters, (2) calculation of design space and designing of PAR around the process parameters such as chamber pressure, shelf temperature and time, (3) testing of selected process parameters profiles to verify the model and analyzing of CQA of freeze-dried product (Scutellà and Bourlès, 2020).
Applications of freeze-drying and case studies of freeze-dried immunoengineering technologies
PEGylated molecules
The covalent chemical conjugation of biomolecules to hydrophilic polymers (PEG) by PEGylation is a widely used technology to provide a slow release of biotherapeutic drugs over time. In PEGylation, the linker-functionalized PEG polymer conjugated with the reactive side chains of proteins or peptides (Pak and Finn, 2013). PEGylated proteins have gained increased interest owing to the decreased immunogenicity, low dose frequency and longer circulation half-life compared to the non-pegylated proteins. PEGylation can alter the protein physicochemical properties such as isoelectric point, hydrophobicity, aqueous solubility, binding properties, and molecular confirmation. PEG conjugation masks the surface of the protein and increases the molecular size of the polypeptide, thereby preventing the approach of antibodies or antigen processing cells and reduces the enzymes proteolytic degradation (Tattini et al., 2005). Mosharraf et al., studied the effect of formulation (sucrose, PEG, Pegylated rhGH analog alone or in combination) and process parameters (slow and fast freezing rate) on the properties such as amorphicity, protein/PEG/excipient interactions, and dissolution of lyophilized pegylated recombinant human growth hormone (rhGH). Results showed that the interaction between sucrose and pegylated protein in co-lyophilized pegylated protein/sucrose systems. As an increase in the concentration of sucrose in a formulation, the degree of amorphicity increased and the solid dissolved faster. The cooling rate of freeze-drying has a less pronounced effect on the solid-state structure when the concentration of sucrose is high (50 mg/mL). PEGylation affected the solid-state properties of the freeze-dried cake, causing a faster dissolution and lower moisture content and increased freeze-dried cake amorphicity, and more rapid moisture-induced crystallization of sucrose compared to the freeze-dried cake consist of sucrose alone (Mosharraf et al., 2007). Furthermore, the presence of PEG molecules in the PEGylated rhGH analogue resulted in a lyophilized cake with high degree of crystallinity. The coupling of PEG to protein causes alteration in physicochemical characteristics of both protein and PEG, and the resulting molecule shows characteristics that are halfway between the two.
Monoclonal antibodies (mAbs)
mAbs are widely used in the treatment and disease diagnosis owing to their high homogeneity and antigen specificity, therapeutic efficiency, and relatively rare side effects (Cui et al., 2017). Freeze-drying can be explored as a mitigation strategy by the biotechnology industry to inhibit proteinaceous particles from monoclonal antibodies and certain therapeutic proteins. The absence of water in lyophilized mAbs limits molecular mobility, which ultimately retards degradation mechanisms and improves protein stability.
The formulation development of mAbs aims to choose the excipients that can stabilize the mAbs throughout the manufacturing process, storage, shipment, dilution, and administration to the patient. Although sucrose is commonly used as a cryoprotectant and lyoprotectant in freeze-dried proteins, sucrose-based protein formulations require lower primary drying temperature to maintain an elegant cake appearance and to avoid cake collapse.
Haeuser et al. investigated different excipients to enable aggressive, shorter lyophilization cycle and storage at room temperature for mAb formulations. The formulations with 2-hydroxypropyl-beta-cyclodextrin/sucrose and 2-hydroxypropyl-beta-cyclodextrin/polyvinylpyrrldione/sucrose showed good product quality attributes such as cake appearance, reconstitution time, residual moisture, and enable storage for at least 9months at room temperature or above compared to formulations with pure sucrose. Thus, the addition of 2-hydroxypropyl-beta-cyclodextrin in mAb formulations could allow shorten lyophilization cycle, greatly reduce the cost of goods (Haeuser et al., 2020).
Parkt al., investigated the effect of pH (3–7) and stabilizers (sucrose and mannitol) on long term stability of freeze-dried anti-streptavidin IgG1 monoclonal antibody. The results show that structural changes were observed in the solid state at low pH. Furthermore, freeze-drying improved the physical stability by retaining the native conformation (secondary and tertiary structure) of IgG1 in the solid state across the pH range examined (Park et al., 2013).
Otto et al., investigate the impact of nucleation behavior (controlled and uncontrolled nucleation) on freeze-drying process performance and quality attributes of mAb formulation. Uniform nucleation was observed at temperature between −2.3 and 3.2°C under controlled ice nucleation, whereas random nucleation was observed at temperature between −10 and −16.4°C under uncontrolled nucleation. Controlled nucleation cycle resulted in the formation of large ice crystals, lower product resistance, higher sublimation rate (reduction in primary drying). Furthermore, freeze-dried mAb showed no visible collapse or shrinkage and low reconstitution time than for the uncontrolled nucleation freeze-dried mAb. Thus, controlled ice nucleation helps the development of efficient freeze-dried mAb formulation with improved quality attributes (Awotwe-Otoo et al., 2013). The lyophilized cake of mAb shows a more rigid and compact structure under uncontrolled ice nucleation cycle with slight shrinkage at the sides and bottom of the vial, and their respective SEM images shows sponge-like matrix with smaller pores. Whereas the lyophilized cakes from the controlled ice nucleation cycle exhibited more porous with no shrinkage at the vial sides and bottom, and their SEM images showed plate-like structures with bigger pores.
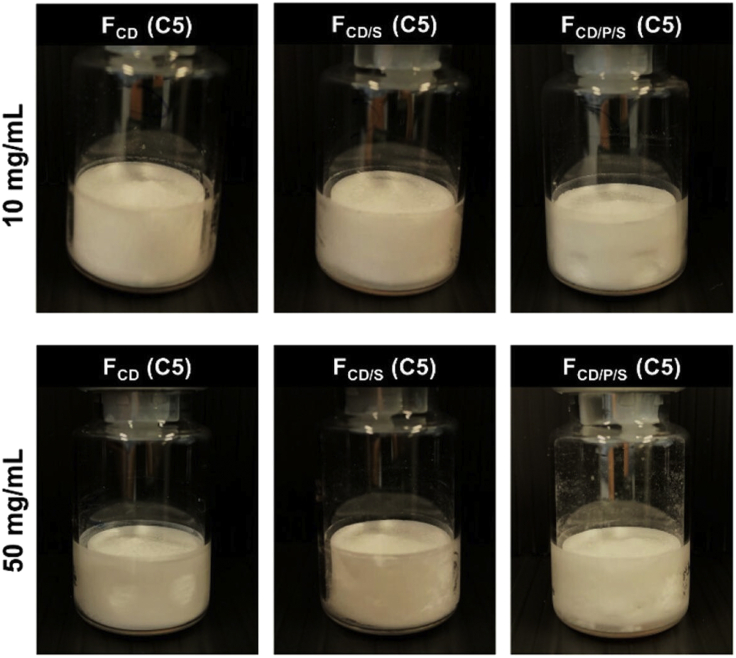
Christina Haeuser et at., reported the usage of amorphous excipients (Hydroxypropyl betacyclodextrin (HPBCD), sucrose and polyvinylpyrrolidone (PVP)) enabled single step to freeze-drying process for monoclonal antibody (mAb) (IgG1, pI ∼8.2, 149 kDa) formulation at different concentrations (10 mg/mL and 50 mg/mL) by shortening the commercial lyophilization cycle time by 50%. The results demonstrated the acceptability of single step freeze-drying and provided elegant lyophilizates for HPBCD and HPBCD/sucrose formulations. HPBCD/PVP/sucrose showed minor dents with good mAb stability even at accelerated stability condition for 3 months (Haeuser et al., 2019) as represented in Figure 6.
Figure 6.
Lyophilized mAb formulation cake appearance
Representative lyophilizates for 10 mg/mL formulations (upper row) and 50 mg/mL (lower row) formulations. FCD only HPBCD mAb formulation, FCD/S: mAb containing HPBCD in combination with sucrose, FCD/P/S: mAb formulation with HPBCD, PVP and sucrose (Haeuser et al., 2019) this image was taken from open access journal Pharmaceutics MDPI: https://doi.org/10.3390/pharmaceutics11110616.
Vaccines
For infectious diseases and cancer, there are different type of vaccine platform, including live-attenuated, whole-inactivated, subunit, virus-like particles, viral-vector, mRNA and DNA vaccines (Jain et al., 2020). An ideal vaccine platform should comprise of a simple, rapid, reproducible, thermostable, and developed with less cost and low risk (Gary and Weiner, 2020).
During freeze-drying, the freezing step can damage the individual components of the virus, such as viral lipid membrane, viral-coated proteins. The stresses that viruses can face during freezing can be tackled by use of cryoprotectants and appropriate freezing rate. The lipid membrane and the coated viral proteins are the main attributes prone to degradation, the use of stabilizer can protect these two attributes during freeze-drying (Hansen et al., 2015).
Influenza hemagglutinin is the most studied virus glycoprotein, found at the surface of the live, attenuated virus vaccines and can be used as antigen in influenza virosome and influenza subunit vaccine. The ionic strength, pH change and freezing stresses can cause the secondary and tertiary structural changes of influenza hemagglutinin viral protein (Hansen et al., 2015). Lipid membrane is one important consideration in freeze-drying because the loss of the viral lipid membrane results in viral inactivation. The lipid layer must be protected during freezing step against ice crystal damage, fusion, and thermotropic phase transitions (Chen et al., 2010). Several factors influencing the stability of vaccines includes temperature, pH, suspension medium, inactivating agents, exposure to light, freezing, thawing, and anti-microbials. However, the antigen itself is the most important factor as its antigenicity and infectivity depend on the antigen’s intrinsic stability (Hansen et al., 2015).
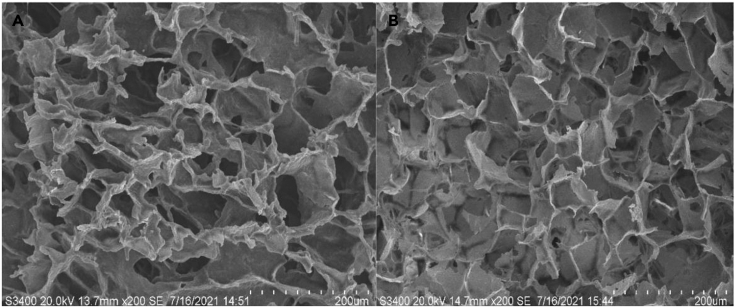
Schneid et al., evaluated robustness testing of freeze-drying cycle for a vaccine formulation (a suspension of live bacteria) by freeze-drying different primary drying conditions. The impact of conservative (below onset of collapse temperature), intermediate (at the onset of collapse temperature) and aggressive (above onset of collapse temperature) primary drying conditions on the key product quality attributes were investigated. An acceptable cake appearance was observed using conservative primary drying conditions, whereas, intermediate drying conditions led to formation of cracks, increased moisture content. The aggressive primary drying conditions greatly reduced the overall cycle time. However, product quality attributes were not met under aggressive primary conditions. Thus, for freeze-drying of bacterial vaccine solution, product temperature during primary drying should remain below critical formulation (collapse) temperature (Schneid et al., 2011b). In a study, Anamur et al., performed electron microscopy to confirm the structure of the collapse lyophilizate of influenza vaccine. In the electron micrographs of lyophilizates, characteristic large pores with a rough surface and sharp edges were clearly detectable for collapse lyophilizates. After cryo-milling, the lyophilizates displayed different surface morphology with the powder size between 20 μm and 80 μm (Anamur et al., 2015). Similarly, Zhao et al., reported the optimization of heat resistant technology for a Duck Hepatitis Lyophilized Live Vaccine. The optimized heat protectors were made of a mixture of 10% sucrose, 1.2% pullulan, 0.5% PVP, and 1% arginine to develop safe and cost-effective vaccine formulation with short time lyophilization cycle. The lyophilized cake formulation with SEM image has shown similar porous honey comb like structure which is similar to commercial formulation (Figure 7) (Zhao et al., 2022).
Figure 7.
SEM image for hepatitis lyophilized live vaccine A
Commercial formulation B. in house formulation both are with porous honeycomb like morphology (Zhao et al., 2022), this image was obtained from open access journal Vaccines MDPI: https://doi.org/10.3390/vaccines10020269.
Nucleic acid delivery using lipid particles
Lipid nanoparticles (LNPs) are widely investigated for the delivery of nucleic acid (mRNA, siRNA) drugs. As an alternative to plasmid DNA, mRNA shows its function in the cell cytosol, thereby circumventing the potential risk associated with genome mutagenesis (Granot-Matok et al., 2019). LNPs of mRNA delivery systems formulated by ionizable lipids, cholesterol, helper lipids, and polyethylene glycol (PEG) can deliver mRNAs in vitro and in vivo (Cheng and Lee, 2016). Although, the transfection rates of the frozen DNA formulations are maintained at frozen conditions, the requirement of strict storage and shipping temperatures are one main constraint (Hinrichs et al., 2006). The lyophilization technique has gained increasing interest to generate a dried or dehydrated nucleic acid-based formulation.
del Pozo-Rodríguez et al., evaluated the stability of freeze-dried solid lipid nanoparticles (SLNs) containing the pCMS-EGFP plasmid. The lyophilized SLNs and lyophilized SLN-DNA vectors have been evaluated for transfection capacity and morphological characteristics. The characterization parameters evaluated in stability studies include; size, zeta potential, conformation, DNA protection, transfection capacity, and cell viability. Results showed that the particle size of lipid nanoparticles increased after freeze-drying, without reducing the transfection capacity. Furthermore, the storage of trehalose stabilized lyophilized lipid nanoparticles did not affect cell viability (del Pozo-Rodríguez et al., 2009).
Zhao et al., investigated different conditions (aqueous, freezing, and lyophilization) for long-term storage of mRNA encapsulated LNPs. The stability of LNPs evaluated with various cryoprotectants, namely, sucrose, trehalose and mannitol under freezing or lyophilization process conditions. The results showed that the stability of mRNA-LNPs cannot be maintained over long-term storage under aqueous conditions. The lyophilized mRNA-LNPs with 20% (w/v) sucrose or trehalose showed mRNA delivery efficiency in-vitro, stabilization of nanoparticle size. However, it failed to show efficiency in vivo delivery. The lyophilization and reconstitution process could change the nanostructure of LNPs-mRNA, which can affect the serum protein interaction in-vivo, resulting in different mRNA delivery efficiency in-vivo. The authors concluded that freezing in liquid nitrogen with 5% sucrose or trehalose may be suitable for the long-term storage of mRNA-LNPs (Zhao et al., 2020).
Hiromi Muramatsu et al., studied about long term stability of lyophilized influenza virus hemagglutinin-encoding mRNA-LNP vaccine for 12 weeks on storage at room temperature and 24 weeks at 4°C. The mRNA-LNPs maintained their high expression without decrease in the immunogenicity (Muramatsu et al., 2022).
Cell based therapy
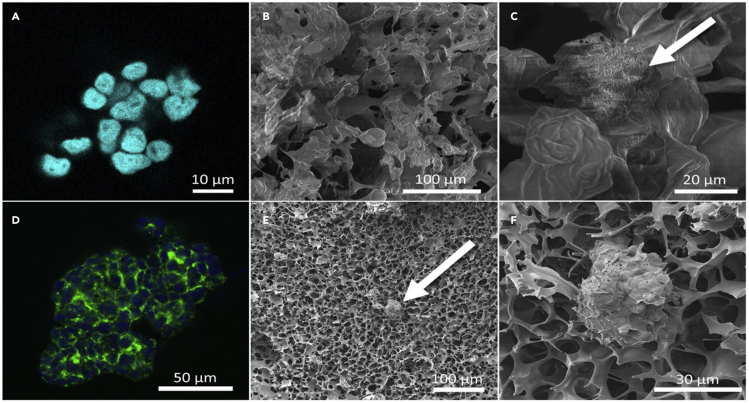
Cell-based therapy as a regenerative medicine modality is regarded as one of the most promising disciplines in modern science and medicine. Such powerful technology opens the door to a plethora of revolutionary and potentially curative remedies for some of humanity’s deadliest diseases. Regenerative medicine is quickly emerging as the next big thing in health care, with the goal of repairing and perhaps replacing sick cells, tissues, or organs in order to restore normal function (Lanza and Seghatchian, 2020). Typically, cells are cryopreserved at −80°C in a liquid nitrogen tank and ultra-freezers for long-term storage. However, cryopreservation requires constant maintenance, large space within freezers, continuous cost (electricity and liquid nitrogen), and risk of cross contamination. Freeze-drying approach counter these challenges (Merivaara et al., 2021). Exosomes are the type of cell-based product secreted from various cells such as mesenchymal stem cells, adipose tissue cells (Butreddy et al., 2021b). Exosomes are an important mediator in intercellular communications, transfer their cargo (proteins, RNA) to the recipient cells, play an important role in cancer, infectious and neurodegenerative diseases (Gebeyehu et al., 2021). Storage of exosomes at −80°C for 4 days altered the exosomes morphology compared to the freshly prepared exosomes (Maroto et al., 2017). Furthermore, structural changes or degradation was observed when exosomes stored at 4°C or 37°C, suggesting that the storage of exosomes is a critical issue (Sokolova et al., 2011). Merivaara et al., reported freeze-dried encapsulated human retinal pigment epithelial cells ARPE 19 cells and 3D cultured human hepatocellular carcinoma (HepG2) cells. They successfully preserved the 3D structure of HepG2 spheroids and partly enzymatic activity of the cell spheroids. The cytoskeleton of freeze-dried and reconstituted cell spheroids was preserved, and the dry aerogel scaffold appeared regular (Figure 8) (Merivaara et al., 2021).
Figure 8.
Freeze-drying of cells and 3D cell spheroids: freeze-dried encapsulated human retinal pigment epithelial cells ARPE 19 cells (A, B and C) and 3D cultured human hepatocellular carcinoma (HepG2) cells (D, E and F) A and D are fluorescent labeled cells and spheroids, B, C, E and F are SEM images of cells and spheroids in dry aerogel scaffold (Merivaara et al., 2021). This image was taken from open access article from Journal of Controlled Release, Elsevier: license and disclaimer available from: https://doi.org/10.1016/j.jconrel.2021.06.042
Charoenviriyakul et al., reported the lyophilization method for exosomes and investigated the effect of lyophilization on the exosomes physical properties. Results showed that lyophilization of B16BL6 melanoma-derived exosomes with trehalose as a cryoprotectant prevented the exosomal damage (aggregation) during lyophilization. Furthermore, room temperature storage of lyophilized exosomes did not affect the exosome protein and RNA content, and physicochemical, pharmacokinetic behavior of the protein, DNA loaded exosomes. Thus, lyophilization in the presence of trehalose is an effective approach for the preserving the exosomes for various applications (Charoenviriyakul et al., 2018). Despite the use of appropriate excipients, several process parameters should be optimized to obtain best drying conditions. Parameters such as drying method, freezing rate, cell density, residual moisture level, rehydration procedure can affect the cell survival during and after lyophilization. Therefore, to achieve maximum cell survival rates it is important to load trehalose intracellularly (Rockinger et al., 2021).
Zhang et al., studied the intactness of DNA in freeze-dried fibroblasts. The freeze-dried formulations containing trehalose alone or a mixture of albumin and trehalose, characterized for overall protein secondary structure and membrane lipid phase behavior, thereby DNA damage in freeze-dried cells. Regardless of the presence or absence of trehalose, membrane phase separation was observed one day after rehydration. The addition of trehalose reduces the DNA damage during storage. Despite increase in formulation glass transition temperature, presence of albumin did not have a protective effect on DNA integrity. Overall, the DNA integrity in freeze-dried somatic cells was preserved using trehalose as protectant when stored at or below 4°C (Zhang et al., 2017).
Comparison of freeze-drying and other drying techniques for the preservation of immunoengineering products
The outstanding feature of the freeze-drying technique as compared to spray drying is that it avoids the heating process and aids in drying of heat liable products. In a study reported the drying of Infliximab by both freeze-drying and spray-drying. Inulin and dextran were incorporated in the process as stabilizing excipients along with sucrose. Integrity of the sample and the biological activity were not disturbed by both the drying techniques. However, after subjecting the dried Infliximab to the accelerated stability conditions for 1monthat 60°C, the biological activity of Infliximab (binding ability to TNF-α) was conserved in freeze-dried samples. However, spray dried samples showed reduced activity. Also, the thermostability of sample was compromised in case of spray dried technique as compared to the freeze-drying which was concluded from the aggregate formation (Kanojia et al., 2016). Thus, the study revealed the potential of freeze-drying in preserving the biological activity and thermostability of samples as compared to spray drying. Furthermore, proteins also subjected to shear stress during the spraying process where the solution was passed through fine nozzle and comes into contact with the drying air. This exposure to the stress in spray-drying and spray-freeze-drying process leads to the denaturation of biological molecules as compared to the freeze-drying process (Adler and Lee, 1999). Protein isolate from rice dreg protein was both freeze-dried and spray-dried where the freeze-drying technique showed higher thermal stability as compared to the spray dried product (Zhao et al., 2013). In a study, the spray drying and freeze-drying techniques were compared in the case of protein hydrolysates from sesame bran. The study revealed that freeze-dried product exhibited better wettability, low bulk density and tapped density as compared to the spray dried sample which was useful in processing and transportation (Özdemir et al., 2022). However, as compared to freeze-drying, spray drying is simple, fast, cost-effective and scalable (Feng et al., 2019) and the limitation of scalability of freeze-drying is the dependence of parameters on the drying efficiency. For instance, pressure within the freezer varies with the geometry of the freeze dryer, heat transfer in the sample varies with the equipment used and the nucleation temperature varies with the environmental conditions in the manufacturing unit (Pisano et al., 2013).Therefore, based on the requirement and conditions, spray drying can be preferred over freeze-drying subjecting to no compromise in the stability of the sample (Feng et al., 2019). The vacuum condition used in the freeze-drying technique protects the product susceptible to oxidation as in the case of spray freeze-drying and spray-drying, products are exposed to atomized air leading to oxidation and denaturation of proteins.
The intactness of the product is protected in freeze-drying with the aid of cryoprotectants especially in the case of proteins and peptides whereas in supercritical drying, the product is subjected to stress conditions. Jovanović N et al., studied the effect of freeze-drying and supercritical fluid drying on stability and physicochemical characteristics of protein sample. Lysozyme and myoglobin proteins were subjected to freeze-drying and supercritical fluid drying process with trehalose and sucrose as stabilizers. The freeze-dried samples were found to be stable with intact protein structure. However, the supercritical fluid dried sample with sucrose showed agglomeration. The myoglobin sample dried with supercritical fluid drying was not reconstitutable (Jovanović et al., 2006). These studies showed the superiority of freeze-drying over other drying techniques.
Despite the many benefits associated with the freeze-drying technique, it suffers from limitations that include longer processing times and batch variability (Walters et al., 2014). These issues challenged the scientific fraternity and addressed the limitations by translating the practice from batch to continuous process. The translation is achieved by transferring the vials continuously from the freezing to secondary drying modules where the modules are separated by load-lock system and operated at different conditions. This enabled in improvement of drying process reducing the duration of process by 2–4 times and minimized the vial-to-vial variability and established homogeneity in the products manufactured (Capozzi et al., 2019). The other alternative to the conventional freeze-drying is active freeze-drying where the product is frozen dynamically and mixed with the aid of a mixing screw. This mixing step results in breaking of frozen blocks and enhanced the efficiency of the process shortening the drying time (Touzet et al., 2018). However, the amalgamation of these advances in the freeze-drying process with the quality by design enables the optimization of the process improving the suitability of this technique for further use in pharmaceutical product development (Arsiccio and Pisano, 2018).
FDA approved products and products under clinical trials
Numerous immunomodulators are approved by the US FDA which falls under the category of cytokines, monoclonal antibodies, vaccines and cell-based therapies. The US FDA approved 20 different cytokines (Table 2) where Interferon alpha (IFN-α) is the first FDA approved in 1986 as cancer immunotherapy cytokine which is indicated in the treatment of hairy cell leukemia, malignant melanoma, and follicular lymphoma (Waldmann, 2018). There are 3 pegylated cytokines in the market approved by FDA which includes pegademase bovine, Pegfilgrastim and Peginterferon alpha-2b. Pegademase bovine is the first pegylated cytokine approved in 1990 for the treatment of severe adenosine deaminase (ADA) deficiency with immunodeficiency disease (SCID) (Moncalvo et al., 2020). There are more than 90 monoclonal antibodies are approved for various diseases. The first monoclonal antibody approved was muromonab-CD3 (Orthoclone OKT3) which was approved in 1986 as an immunosuppressant drug given to reduce acute rejection in patients with organ transplants (Pollinger et al., 2007). Transtuzumab (Herceptin®) is the first monoclonal antibody to be approved for HER2-positive metastatic breast cancer in 1998 (Jeyakumar and Younis, 2012). Rituximab (Rituxan®) was approved in 1997 which is the first monoclonal antibody for the lymphoma therapy (Grillo-López et al., 1999). Daratumumab is the first monoclonal antibody for patients with multiple myeloma and Fingolimod (GilenyaTM) was the first monoclonal antibody to be approved for multiple sclerosis as an oral therapy and it is the first-line therapy for the relapsing forms (McKeage, 2016).
Table 2.
Cytokine based immunomodulators approved by US FDA
| Name | Approval year | Category | Therapeutic use | Route of administration | Trade name |
|---|---|---|---|---|---|
| Aldesleukin (rhIL-2) | 1992 | Human recombinant interleukin-2 product | Metastatic renal cell carcinoma | Intravenous infusion | PROLEUKIN |
| Oprelvekin (rhIL-11) | 1997 | Recombinant interleukin eleven (IL-11), a thrombopoietic growth factor | Prevention of severe thrombocytopenia and the reduction of the need for platelet transfusions following myelosuppressive chemotherapy | NEUMEGA® | |
| Filgrastim | 1991 | Leukocyte growth factor | To decrease the incidence of infection as manifested by febrile neutropenia in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs | Injection, for subcutaneous or intravenous use | NEUPOGEN® |
| Tbo-filgrastim (rhG-CSF) | 2012 | Leukocyte growth factor | Reduction in the duration of severe neutropenia in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia | Injection, for subcutaneous use | GRANIX® |
| Sargramostim(rhGM-CSF) | 1991 | Leukocyte growth factor | To accelerate bone marrow recovery in diverse settings of bone marrow failure | Injection, for subcutaneous or intravenous use | LEUKINE® |
| Metreleptin (rh-leptin) | 2014 | Leptin analog | As adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy | Injection for subcutaneous use | MYALEPTTM |
| Epoetin alfa | 1989 | Erythropoiesis-stimulating agent | Treatment of anemia due to- chronic kidney disease (CKD) and Zidovudine treated HIV infected patients | For intravenous or subcutaneous use | EPOGEN® |
| Darbepoietin alfa | 2001 | Erythropoiesis-stimulating agent | Treatment of anemia due to: chronic kidney disease (CKD) and concomitant myelosuppressive chemotherapy | For intravenous or subcutaneous use | ARANESP® |
| Interferon alfa-2b | 1986 | Alpha interferon | Hairy Cell Leukemia, Malignant Melanoma, Follicular Lymphoma, Condylomata Acuminata, AIDS-Related Kaposi's Sarcoma and Chronic Hepatitis C and B | Intramuscular, subcutaneous, intralesional, or intravenous Injection | INTRON® A |
| Interferon beta-1 | 1996 | Glycoprotein | Treatment of patients with relapsing forms of multiple sclerosis to slow the accumulation of physical disability and decrease the frequency of clinical exacerbations. | Intramuscular injection | AVONEX , Betaseron® |
| Interferon gamma-1 | 1990 | Interferon gamma | Reducing the frequency and severity of serious infections associated with Chronic Granulomatous Disease (CGD) and Delaying time to disease progression in patients with severe, malignant osteopetrosis (SMO) | Subcutaneous use | ACTIMMUNE® |
| Palifermin(rhKGF) | 2004 | Mucocutaneous epithelial human growth factor | To decrease the incidence and duration of severe oral mucositis inpatients with hematologic malignancies receiving myelotoxic therapy | Intravenous use | KEPIVANCE® |
| Becaplermin (rhPDGF) | 2005 | Recombinant human platelet-derived growth factor (rhPDGF-BB) | Treatment of lower extremity diabetic neuropathic ulcers that extend into the subcutaneous tissue or beyond and have an adequate blood supply | Gel | REGRANEX® |
| RhBMP-2 | 2002 | Recombinant human Bone Morphogenetic Protein-2 (rhBMP-2, known as dibotermin alfa) | Treating acute, open tibial shaft fractures that have been stabilized with IM nail fixation after appropriate wound management. | Bone graft | INFUSE® |
| Pegademase bovine | 1990 | (monomethoxypolyethylene glycol succinimidyl) 11-17adenosine deaminase. It is a conjugate of numerous strands of monomethoxypolyethylene glycol (PEG) | Severe adenosine deaminase (ADA) deficiency with immunodeficiency disease (SCID). | Intramuscular injection | ADAGEN® |
| Pegfilgrastim | 2002 | Covalent conjugate of recombinant methionyl human G-CSF (filgrastim) and monomethoxypolyethylene glycol | To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia | Subcutaneous use | NEULASTA® |
| Peginterferon alfa-2b | 2011 | Covalent conjugate of recombinant alfa-2b interferon with monomethoxy polyethylene glycol (PEG) | Adjuvant treatment of melanoma with microscopic or gross nodal involvement within 84 days of definitive surgical resection including complete lymphadenectomy | For injection, for subcutaneous use | SYLATRON™ |
Numerous vaccines are being approved by the US FDA where approximately 103 vaccines are approved and licensed for use in United States (FDA, 2022b). Sipuleucel-T (Provenge) is the first cancer vaccine which was approved in 2010 for the treatment of prostate cancer (Cheever and Higano, 2011). Recently 2 mRNA-based coronavirus disease 2019 (COVID-19) vaccines were approved for the active immunization against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for the prevention of COVID-19. SPIKEVAX is a nucleoside modified messenger RNA (mRNA) encoding the pre-fusion stabilized spike glycoprotein of SARS-CoV-2 virus is approved in 2022 while COMIRNATY nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2 approved in 2021 (FDA, 2022a).
Cellular and gene-based therapies are growing at a fast rate inculcating the advances leading to immunomodulation and cancer vaccines. Human gene therapy leads to the manipulation of gene expression thereby affecting the biological properties manifesting the therapeutic benefit. Four CAR-T cell-based therapies are approved till now and about 15 cellular and gene-based products are approved by US FDA (FDA, 2021) (Table 3).
Table 3.
US FDA approved cellular and gene therapy products
| Name | Approval year | Category | Therapeutic use | Formulation | Trade name |
|---|---|---|---|---|---|
| Idecabtagene vicleucel | 2021 | B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy | Relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody | Suspension for intravenous infusion | ABECMA® |
| Ciltacabtagene autoleucel | 2022 | B-cell maturation antigen (BCMA)-directed chimeric antigen receptor t-cell therapy | Relapsed or refractory multiple myeloma (RRMM) after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. | Infusion | CARVYKTI™ |
| HPC, Cord Blood | 2013 | HPC (Hematopoietic Progenitor Cell), Cord Blood, is an allogeneic cord blood hematopoietic progenitor cell therapy | Unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment | Suspension for Intravenous Use | ALLOCORD |
| 2016 | CLEVECORD™ | ||||
| 2011 | HEMACORD | ||||
| 2012 | DUCORD | ||||
| Allogeneic Cultured Keratinocytes and Fibroblasts in Bovine Collagen |
2012 | Allogeneic cellularized scaffold produc | Topical (non-submerged) application to a surgically created vascular wound bed in the treatment of mucogingival conditions in adults. | Cellular Sheet for Topical Oral Application | GINTUIT |
| Talimogene laherparepvec | 2015 | A genetically modified oncolytic viral therapy | Local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery | Suspension for intralesional injection | IMLYGIC |
| Azficel-T | 2011 | Autologous cellular product | For improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults. | Suspension for Intradermal Injection | LAVIV® |
| Voretigene neparvovec-rzyl | 2017 | Adeno-associated virus vector-based gene therapy | Confirmed biallelic RPE65 mutation-associated retinal dystrophy. Patients must have viable retinal cells as determined by the treating physician | Intraocular suspension for subretinal injection | LUXTURNA |
| Autologous Cultured Chondrocytes on a Porcine Collagen Membrane | 2016 | Autologous cellularized scaffold product | Repair of symptomatic, single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults. | Cellular sheet for autologous implantation | MACI |
| Sipuleucel-T | 2010 | Autologous cellular immunotherapy | Treatment of asymptomatic or minimally symptomatic metastatic castrate resistant (hormone refractory) prostate cancer | Suspension for Intravenous Infusion | PROVENGE® |
| Allogeneic processed thymus tissue–agdc | 2021 | Allogeneic processed thymus tissue | Immune reconstitution in pediatric patients with congenital athymia | For surgical implantation | RETHYMIC |
| Allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen-dsa | 2021 | Allogeneic cellularized scaffold product (allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen-dsat) | Treatment of adults with thermal burns containing intact dermal elements for which surgical intervention is clinically indicated (deep partial-thickness burns) | Topical us | StrataGraft® |
| Onasemnogene abeparvovec-xioi | 2019 | Adeno-associated virus vector-based gene therapy | Treatment of pediatric patients less than 2 years of age with spinal muscular atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene | Suspension, for intravenous infusion | ZOLGENSMA |
| Tisagenlecleucel | 2017 | CD19-directed genetically modified autologous T-cell immunotherapy | B-cell precursor acute lymphoblastic leukemia (ALL) | Suspension for intravenous infusion | Kymriah |
| Axicabtagene ciloleucel | 2017 | CD19-directed genetically modified autologous T cell immunotherapy | Relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy | Suspension for intravenous infusion | Yescarta |
| Brexucabtagene autoleucel | 2020 | CD19-directed genetically modified autologous T cell immunotherapy | Adult patients with relapsed or refractory mantle cell lymphoma (MCL) | Suspension for intravenous infusion | Tecartus |
| Lisocabtagene maraleucel | 2021 | CD19-directed genetically modified autologous T cell immunotherapy | Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) | Suspension for intravenous infusion | Breyanzi |
Apart from the approved marketed products there are numerous products under clinical pipeline which shows the evaluation and scope of immunotherapy in addressing the unmet medical need. IL-7, 12, 21, interferon gamma and granulocyte-macrophage colony-stimulating factor are under clinical investigations (Conlon et al., 2019).
Among the diverse immunoengineering products studies under clinical trials, lyophilized monoclonal antibodies and vaccines are widely reported (Tables 4 and 5) (trials, 2022). A number of clinical trials compared the immunogenicity, reactogenicity, equivalence and safety of liquid formulation and the lyophilized formulation where no variation was found between both the formulations. In the case of live-attenuated dengue tetravalent vaccine, given that WHO recommended the prequalification requirement storage conditions of vaccines at +2° to +8°C, a clinical trial was performed to compare its immunologic equivalence with that of liquid formulation (Vaccines and WHO, 2000). The study concluded that there was no variation of immunogenicity between both the formulations and reported no serious adverse events and showed similar safety profiles (Turner et al., 2020). These results suggested the application of lyophilization on biologics preservation with no compromise in efficacy and safety.
Table 4.
Monoclonal antibodies availing lyophilization under various phases of clinical trials
| Category | Aim of the study | Condition | Intervention | Phase | Lyophilized details |
|---|---|---|---|---|---|
| Monoclonal antibody | Efficacy and Safety in Participants with Multiple Myeloma | Drug: Belantamab mafodotin frozen liquid|Drug: Belantamab mafodotin lyophilized powder | Phase 2 | Belantamab mafodotin lyophilized powder | |
| Monoclonal antibody (novel humanized anti-CD37 protein therapeutic) | Multiple-dose escalation study to determine the maximum-tolerated dose (MTD) of TRU-016 given in combination with rituximab and bendamustine and to determine a safe dosing regimen for the combination | B-cell Small Lymphocytic Lymphoma Recurrent | Drug: TRU-016 (Otlertuzumab)|Drug: Bendamustine|Drug: Rituximab | Phase 1 | Otlertuzumab (TRU-016) lyophilized solution for infusion |
| Monoclonal antibody covalently linked to the cytotoxic agent drug | safety, feasibility, and efficacy of trastuzumab emtansine (T-DM1) after the completion of anthracycline-based adjuvant/neoadjuvant chemotherapy in patients with early HER2-positive breast cancer | Breast Cancer | Drug: Trastuzumab emtansine | Phase 2 | Trastuzumab emtansine as a single-use lyophilized formulation in a glass vial |
| Monoclonal antibody against human immunoglobulin E | To investigate the effect of omalizumab on the number of tissue eosinophils and other markers of airway inflammation. | Allergic Asthma | Drug: omalizumab at a dose of 0.016mg/kg/IU/mL|Drug: Placebo | Phase 4 | Omalizumab as lyophilized, sterile powder |
| Hydrolytic lysosomal glucocerebroside-specific enzyme | safety and effectiveness of velaglucerase alfa in patients with type 3 Gaucher disease. | Gaucher Disease, Type 3 | Biological: velaglucerase alfa | Phase 1|Phase 2 | lyophilized powder, intravenous infusion |
| Anti-interleukin-5 (IL-5) monoclonal antibody | compare the pharmacokinetics and safety of mepolizumab administered as a liquid drug product in two different devices with the reconstituted lyophilized drug product | Asthma | Biological: Lyophilized mepolizumab|Biological: Liquid mepolizumab|Device: Prefilled autoinjector|Device: Prefilled Safety Syringe | Phase 3 | Lyophilized Drug Product |
| Anti-interleukin-5 (IL-5) monoclonal antibody | Efficacy and Safety of Mepolizumab in the Treatment of Adolescent and Adult Subjects with Severe Hypereosinophilic Syndrome | Hypereosinophilic Syndrome | Drug: Mepolizumab 300 mg|Drug: Placebo matching mepolizumab|Drug: Active OCS capsules (5 mg prednisolone or prednisone) |Drug: Placebo matching OCS capsules | Phase 3 | Lyophilized powder for injection |
| Humanised, agonistic IgG1 anti-OX40 monoclonal antibody | safety, tolerability, pharmacokinetics (PK), pharmacodynamics, and preliminary clinical activity of GSK3174998 administered intravenously to participants with selected advanced or recurrent solid tumors. | Neoplasms | Drug: GSK3174998|Drug: Pembrolizumab | Phase 1 | Lyophilized powder |
| Human IgGκ monoclonal antibody | safety and efficacy of canakinumab pre-filled syringes in comparison to triamcinolone acetonide 40 mg and canakinumab lyophilizate in patients that have frequent flares of acute gouty arthritis. | Acute Gouty Arthritis | Drug: Canakinumab pre-filled syringe|Drug: Canakinumab lyophilized powder|Drug: Triamcinolone Acetonide|Drug: Placebo | Phase 3 | Lyophilized powder |
| Fully human immunoglobulin G1 lambda (IgG1λ) monoclonal agonistic antibody (mAb) targeting TRAIL-R1 | efficacy and safety of mapatumumab in combination with sorafenib in subjects with advanced hepatocellular carcinoma. | Carcinoma, Hepatocellular | Drug: Mapatumumab|Drug: Placebo|Drug: Sorafenib | Phase 1|Phase 2 | lyophilized formulation |
| PEGylated anti-TNFα monoclonal antibody | safety of long-term therapy with Certolizumab Pegol | Crohn Disease | Biological: Cimzia | Phase 3 | lyophilized powder formulation |
| Monoclonal antibody against human immunoglobulin E | to Evaluate the Efficacy and Safety of Lyophilized and Aged Liquid Omalizumab in the Prevention of Allergen-Induced Airway Obstruction in Adults with Mild Allergic Asthma | Allergic Asthma | Drug: omalizumab|Drug: placebo | Phase 2 | lyophilized formulation of omalizumab |
Table 5.
List of vaccines (lyophilized) under clinical trials
| Aim of the study | Condition | Intervention | Phase | Lyophilized details |
|---|---|---|---|---|
| To Assess the Reactogenicity and Safety of the Porcine Circovirus (PCV) Free Liquid Formulation of GSK's Oral Live Attenuated Human Rotavirus (HRV) Vaccine as Compared to the Lyophilized Formulation of the GSK's HRV Vaccine | Infections, Rotavirus | Biological: PCV-free liquid formulation of GSK's oral live attenuated HRV vaccine|Biological: Lyophilized formulation of GSK's oral live attenuated HRV vaccine | Phase 3 | Lyophilized formulation of GSK's oral live attenuated HRV vaccine |
| Immunogenicity and Safety Study of GSK's Investigational Vaccine (GSK3277511A) | Respiratory Disorders | Biological: GSK's investigational non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) multi-antigen vaccine (GSK3277511A) adjuvanted with AS01E|Biological: Shingrix GSK's lyophilized formulation of the herpes zoster (HZ) vaccine (GSK1437173A) | Phase 2 | Shingrix GSK's lyophilized formulation of the herpes zoster (HZ) vaccine |
| Safety, Tolerability and Long-term Immunogenicity of Different Dose Regimens and Formulations of MV-CHIK in Healthy Volunteers | Chikungunya Virus Infection | Biological: MV-CHIK lyophilized formulation, low dose|Biological: MV-CHIK liquid frozen formulation, low dose|Biological: MV-CHIK SPS® formulation, low dose|Biological: MV-CHIK liquid frozen formulation, high dose|Other: Placebo | Phase 2 | MV-CHIK lyophilized formulation |
| Immunogenicity and Safety of Meningococcal MenABCWY Vaccine, and of rMenB+OMV NZ and MenACWY Administered Concomitantly | Meningitis, Meningococcal | Biological: MenABCWY vaccine|Biological: rMenB+OMV NZ (Bexsero) vaccine|Biological: MenACWY (Menveo) vaccine | Phase 2 | MenACWY lyophilized component |
| Safety and Immunogenicity of Clade C ALVAC-HIV (vCP2438) and Bivalent Subtype C gp120 Alone, With MF59 Adjuvant, and With Alum Adjuvant | HIV Infections | Biological: ALVAC-HIV (vCP2438) Biological: Bivalent Subtype C gp120/MF59|Biological: Bivalent Subtype C gp120 admixed with Al(OH)3 Suspension|Biological: Bivalent Subtype C gp120 | Phase 1|Phase 2 | lyophilized vaccine |
| Safety of MV-CHIK in a previously epidemic area | Chikungunya | Biological: MV-CHIK|Biological: MMR-vaccine | Phase 2 | Lyophilized, life attenuated, measles vectored Chikungunya vaccine |
| Dose-Finding Study of Lyophilized Shigella Sonnei 53G Challenge Strain | Healthy | Biological: Shigella sonnei 53G | Phase 1 | Lyophilized Shigella Sonnei 53G |
| Safety and immunogenicity of the second dose of GBS Trivalent Vaccine | Bacterial Infection Due to Streptococcus, Group B | Biological: GBS Trivalent Vaccine | Phase 2 | lyophilized formulation of a GBS trivalent vaccine |
| Immune Response to Pentavalent Rotavirus Vaccine After a Supplemental Dose | Diarrhea Rotavirus | Biological: pentavalent rotavirus vaccine (PRV)|Biological: measles vaccine (MV)|Biological: yellow fever vaccine (YFV)|Biological: meningitis conjugate vaccine (PsA-TT-5μg) | Phase 4 | lyophilized measles vaccine |
| Safety and immunogenicity of a liquid formulation of Group B Streptococcus (GBS) Trivalent Vaccine (not requiring reconstitution prior to administration), and of the lyophilized formulation of GBS Trivalent Vaccine | Infections, Streptococcal|Streptococcal Infections | Biological: GBS Vaccine | Phase 2 | lyophilized formulation of GBS Trivalent Vaccine |
| To evaluate the equivalence of the lyophilized formulation of Takeda's Tetravalent Dengue Vaccine Candidate (TDV) compared with the liquid formulation of TDV | Dengue Fever | Drug: TDV Liquid Formulation 1|Drug: TDV Liquid Formulation 2|Drug: TDV IDT Lyophilized|Drug: Placebo | Phase 2 | lyophilized formulation of Takeda's Tetravalent Dengue Vaccine Candidate |
| Safety and Immunogenicity Evaluation of an Intramuscular Capsule-Conjugate Campylobacter Vaccine (CJCV1) | Campylobacter Infection | Biological: Capsule-Conjugate Campylobacter Vaccine (CJCV1)|Drug: Alhydrogel®, aluminum hydroxide adjuvant (alum) | Phase 1 | lyophilized CPS-CRM197 conjugate |
| Immunogenicity and safety of a booster dose of a MenABCWY vaccine | Meningococcal Disease | Biological: MenABCWY+OMV|Biological: MenABCWY+¼OMV|Biological: Placebo | Phase 2 | lyophilized MenACWY vaccine |
| Safety, Tolerability and Immunogenicity of a Third Dose of One of Four Different Formulations of rMenB + MenACWY | Meningococcal Disease|Meningococcal Meningitis | Biological: Meningococcal (groups A, C, W, and Y) oligosaccharide diphtheria CRM-197 conjugate combined with meningococcal (group B) multicomponent recombinant vaccine|Biological: Meningococcal (group B) multicomponent recombinant adsorbed vaccine|Biological: Tdap | Phase 2 | lyophilized Meningococcal (groups A, C, W, and Y) oligosaccharide diphtheria CRM-197 conjugate vaccine |
| Safety and Immunogenicity of 4 Doses of MenACWY Conjugate Vaccine | Meningococcal Disease | Biological: MenACWY-CRM|Biological: DTaP-IPV/Hib|Biological: HBV|Biological: PCV|Biological: MMR | Phase 3 | lyophilized component (MenA) |
| To assess dHER2+AS15 Cancer Immunotherapeutic Given in Combination with Lapatinib to Patients With ErbB2 Overexpressing Metastatic Breast Cancer Refractory to Trastuzumab | Metastatic Breast Cancer | Biological: dHER2 + AS15 ASCI|Drug: Lapatinib | Phase 1|Phase 2 | lyophilized preparation |
| Safety and Immunogenicity Study of V710 Lyophilized Formulation (V710-004) | Healthy | Biological: Comparator: V710|Biological: Comparator: placebo | Phase 1 | Lyophilized Formulation (V710-004) |
| Safety and effectiveness of two different formulations of an investigational dengue vaccine (T-DEN) | Dengue Fever|Dengue Hemorrhagic Fever|Dengue Shock Syndrome | Other: Placebo|Biological: T-DEN-Post-Transfection F17|Biological: T-DEN-Post-Transfection F19 | Phase 2 | Lyophilized T-DEN-Post-Transfection F17 |
| Safety, Tolerability and Immunogenicity of Lyophilized ChimeriVax-WN02 West Nile Vaccine | West Nile Fever | Biological: ChimeriVax-WN02 Low Dose|Biological: ChimeriVax-WN02 Medium Dose|Biological: ChimeriVax-WN02 High Dose|Biological: 0.9% Saline solution|Biological: 0.9 % NaCl solution | Phase 2 | Lyophilized ChimeriVax-WN02 West Nile Vaccine |
| Safety and Immunogenicity of WRAIR's MSP1 Candidate Malaria Vaccine (FMP1) Adjuvant in GSK Bio's AS02A vs. Rabies Vaccine | Malaria | Biological: FMP1/AS02A|Biological: Imovax Rabies Vaccine | Phase 1 | Lyophilized FMP1/AS02A |
| Non-Inferiority of the Concurrent Administration of Japanese Encephalitis Live Attenuated SA 14-14-2 Vaccine and Measles Vaccine Given Alone | Encephalitis, Japanese B | Biological: Live Japanese encephalitis vaccine SA 14-14-2 (LJEV)|Biological: Measles Vaccine (MV) | Phase 3 | Lyophilized live Japanese encephalitis vaccine SA 14-14-2 (LJEV) |
Conclusion and future prospective
Upon several biomedical breakthroughs and successes, immunoengineering technologies are witnessing an exponential growth. New generations of cytokines, monoclonal antibodies, and vaccines are being developed and investigated in clinical trials. Undoubtedly, global market of immunoengineering products is expected to continuously rise in years to come. However, instability remains one of the major limiting factors that hinder immunoengineering products from reaching the world’s population especially in rural areas and underdeveloped countries which may not be able to support complex cold chain.
Lyophilization is being widely implemented for enhancing the stability and shelf life of immunoengineering technologies including vaccines and various biologicals. To achieve the best preservation outcomes, vigilant optimization of formulation and freeze-drying process are required. Excipients such as cryprotectants, surfactants, and buffers are formulated with the active moiety to prevent freezing induced stress, aggregation and stabilize pH of the product during freeze-drying. Monoclonal antibodies, vaccines, PEGylated molecules, nanoparticles loaded with biologics, cellular and gene-based therapies are formulated as lyophilized products, and various marketed products and products under clinical trials are also reported as lyophilized products. This highlights the potential role of lyophilization in preserving, stabilizing, and enhancing the shelf life of various biologics employed for immunotherapy.
Acknowledgments
We thank funding supported by NIH grant R01AI150324 to PA; and the Sheldon J. Segal Post-Doctoral Fellowship to NK.
Author contributions
Conceptualization, P.A. and N.K.; writing – original draft preparation, N.K., A.B., V. S.N. J.; writing–review and editing, N.K., A.B., V.S.N. J., and P.A.; supervision, P.A. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no conflict of interest that could have appeared to influence the work reported in this review paper.
References
- Adler M., Lee G. Stability and surface activity of lactate dehydrogenase in spray-dried trehalose. J. Pharm. Sci. 1999;88:199–208. doi: 10.1021/js980321x. [DOI] [PubMed] [Google Scholar]
- Anamur C., Winter G., Engert J. Stability of collapse lyophilized influenza vaccine formulations. Int. J. Pharm. 2015;483:131–141. doi: 10.1016/j.ijpharm.2015.01.053. [DOI] [PubMed] [Google Scholar]
- Arsiccio A., Pisano R. Application of the quality by design approach to the freezing step of freeze-drying: building the design space. J. Pharm. Sci. 2018;107:1586–1596. doi: 10.1016/j.xphs.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Awotwe-Otoo D., Agarabi C., Read E.K., Lute S., Brorson K.A., Khan M.A., Shah R.B. Impact of controlled ice nucleation on process performance and quality attributes of a lyophilized monoclonal antibody. Int. J. Pharm. 2013;450:70–78. doi: 10.1016/j.ijpharm.2013.04.041. [DOI] [PubMed] [Google Scholar]
- Bayer V. Vol. 5. Elsevier; 2019. An Overview of Monoclonal Antibodies; p. 150927. [DOI] [PubMed] [Google Scholar]
- Begum F., Amin S. Investigating the influence of polysorbate 20/80 and polaxomer P188 on the surface & interfacial properties of bovine serum albumin and lysozyme. Pharm. Res. (N. Y.) 2019;36:107. doi: 10.1007/s11095-019-2631-6. [DOI] [PubMed] [Google Scholar]
- Bhatnagar B.S., Bogner R.H., Pikal M.J. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm. Dev. Technol. 2007;12:505–523. doi: 10.1080/10837450701481157. [DOI] [PubMed] [Google Scholar]
- Bjelošević M., Seljak K.B., Trstenjak U., Logar M., Brus B., Ahlin Grabnar P. Aggressive conditions during primary drying as a contemporary approach to optimise freeze-drying cycles of biopharmaceuticals. Eur. J. Pharm. Sci. 2018;122:292–302. doi: 10.1016/j.ejps.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Butreddy A., Dudhipala N., Janga K.Y., Gaddam R.P. Lyophilization of small-molecule injectables: an industry perspective on formulation development, process optimization, scale-up challenges, and drug product quality attributes. AAPS PharmSciTech. 2020;21:252. doi: 10.1208/s12249-020-01787-w. [DOI] [PubMed] [Google Scholar]
- Butreddy A., Janga K.Y., Ajjarapu S., Sarabu S., Dudhipala N. Instability of therapeutic proteins - an overview of stresses, stabilization mechanisms and analytical techniques involved in lyophilized proteins. Int. J. Biol. Macromol. 2021;167:309–325. doi: 10.1016/j.ijbiomac.2020.11.188. [DOI] [PubMed] [Google Scholar]
- Butreddy A., Kommineni N., Dudhipala N. Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomaterials. 2021;11:1481. doi: 10.3390/nano11061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi L.C., Trout B.L., Pisano R. From batch to continuous: freeze-drying of suspended vials for pharmaceuticals in unit-doses. Ind. Eng. Chem. Res. 2019;58:1635–1649. doi: 10.1021/acs.iecr.8b02886. [DOI] [Google Scholar]
- Catak S., Monard G., Aviyente V., Ruiz-López M.F. Reaction mechanism of deamidation of asparaginyl residues in peptides: effect of solvent molecules. J. Phys. Chem. A. 2006;110:8354–8365. doi: 10.1021/jp056991q. [DOI] [PubMed] [Google Scholar]
- Charoenviriyakul C., Takahashi Y., Nishikawa M., Takakura Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018;553:1–7. doi: 10.1016/j.ijpharm.2018.10.032. [DOI] [PubMed] [Google Scholar]
- Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- Chen C., Han D., Cai C., Tang X. An overview of liposome lyophilization and its future potential. J. Control. Release. 2010;142:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Cheng X., Lee R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- Conlon K.C., Miljkovic M.D., Waldmann T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Cui P., Chen B., Li S., Guan H. Monoclonal antibodies: formulations of marketed products and recent advances in novel delivery system. Drug Dev. Ind. Pharm. 2017;43:519–530. doi: 10.1080/03639045.2017.1278768. [DOI] [PubMed] [Google Scholar]
- del Pozo-Rodríguez A., Solinís M.A., Gascón A.R., Pedraz J.L. Short-and long-term stability study of lyophilized solid lipid nanoparticles for gene therapy. Eur. J. Pharm. Biopharm. 2009;71:181–189. doi: 10.1016/j.ejpb.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Duque G.A., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491–512. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J.E., Tewari K., Lyke K.E., Sim B.K.L., Billingsley P.F., Laurens M.B., Gunasekera A., Chakravarty S., James E.R., Sedegah M., et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science. 2011;334:475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- Falconer R.J. Advances in liquid formulations of parenteral therapeutic proteins. Biotechnol.Adv. 2019;37:107412. doi: 10.1016/j.biotechadv.2019.06.011. [DOI] [PubMed] [Google Scholar]
- FDA . U.S. FDA; 2021. Approved Cellular and Gene Therapy Products. [Google Scholar]
- FDA . U.S. FDA; 2022. Active Immunization to Prevent Coronavirus Disease 2019 (COVID-19) Caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Individuals 18 Years of Age and Older. [Google Scholar]
- FDA . U.S. FDA; 2022. Vaccines Licensed for Use in the United States. [Google Scholar]
- Feng J., Zhang Y., McManus S.A., Qian R., Ristroph K.D., Ramachandruni H., Gong K., White C.E., Rawal A., Prud'homme R.K. Amorphous nanoparticles by self-assembly: processing for controlled release of hydrophobic molecules. Soft Matter. 2019;15:2400–2410. doi: 10.1039/C8SM02418A. [DOI] [PubMed] [Google Scholar]
- Fotoran W.L., Santangelo R., de Miranda B.N.M., Irvine D.J., Wunderlich G. DNA-loaded cationic liposomes efficiently function as a vaccine against malarial proteins. Mol. Ther. Methods Clin. Dev. 2017;7:1–10. doi: 10.1016/j.omtm.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage C.L.D., Hageman T.S., Weis D.D. Rapid prediction of deamidation rates of proteins to assess their long-term stability using hydrogen exchange–mass spectrometry. J. Pharm. Sci. 2019;108:1964–1972. doi: 10.1016/j.xphs.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Gary E.N., Weiner D.B. DNA vaccines: prime time is now. Curr.Opin.Immunol. 2020;65:21–27. doi: 10.1016/j.coi.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebeyehu A., Kommineni N., Meckes D.G., Jr., Sachdeva M.S. Role of exosomes for delivery of chemotherapeutic drugs. Crit. Rev. Ther. Drug Carrier Syst. 2021;38:53–97. doi: 10.1615/CritRevTherDrugCarrierSyst.2021036301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu L., Vakkalanka R.K., Elkon K.B., Crow M.K. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J. Clin. Invest. 1997;100:2622–2633. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais D. Protein deamidation in biopharmaceutical manufacture: understanding, control and impact. J. Chem. Technol. Biotechnol. 2016;91:569–575. [Google Scholar]
- Gervasi V., Dall Agnol R., Cullen S., McCoy T., Vucen S., Crean A. Parenteral protein formulations: an overview of approved products within the European Union. Eur. J. Pharm. Biopharm. 2018;131:8–24. doi: 10.1016/j.ejpb.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Granot-Matok Y., Kon E., Dammes N., Mechtinger G., Peer D. Therapeutic mRNA delivery to leukocytes. J. Control. Release. 2019;305:165–175. doi: 10.1016/j.jconrel.2019.05.032. [DOI] [PubMed] [Google Scholar]
- Green J.J. Immunoengineering has arrived. J. Biomed. Mater. Res. 2021;109:397–403. doi: 10.1002/jbm.a.37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo-López A.J., White C.A., Varns C., Shen D., Wei A., McClure A., Dallaire B.K. Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin. Oncol. 1999;26:66–73. [PubMed] [Google Scholar]
- Gupta S., Jiskoot W., Schöneich C., Rathore A.S. Oxidation and deamidation of monoclonal antibody products: potential impact on stability, biological activity, and efficacy. J. Pharmaceut. Sci. 2021;111:903–918. doi: 10.1016/j.xphs.2021.11.024. [DOI] [PubMed] [Google Scholar]
- Haeuser C., Goldbach P., Huwyler J., Friess W., Allmendinger A. Be aggressive! Amorphous excipients enabling single-step freeze-drying of monoclonal antibody formulations. Pharmaceutics. 2019;11:616. doi: 10.3390/pharmaceutics11110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeuser C., Goldbach P., Huwyler J., Friess W., Allmendinger A. Excipients for room temperature stable freeze-dried monoclonal antibody formulations. J. Pharm. Sci. 2020;109:807–817. doi: 10.1016/j.xphs.2019.10.016. [DOI] [PubMed] [Google Scholar]
- Hansen L.J.J., Daoussi R., Vervaet C., Remon J.P., De Beer T.R.M. Freeze-drying of live virus vaccines: a review. Vaccine. 2015;33:5507–5519. doi: 10.1016/j.vaccine.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Matsumoto T. Mechanisms of tolerance induction by dendritic cells in vivo. Front. Immunol. 2018;9:350. doi: 10.3389/fimmu.2018.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs W.L.J., Manceñido F.A., Sanders N.N., Braeckmans K., De Smedt S.C., Demeester J., Frijlink H.W. The choice of a suitable oligosaccharide to prevent aggregation of PEGylated nanoparticles during freeze thawing and freeze drying. Int. J. Pharm. 2006;311:237–244. doi: 10.1016/j.ijpharm.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Hu Y., Smith D., Frazier E., Hoerle R., Ehrich M., Zhang C. The next-generation nicotine vaccine: a novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials. 2016;106:228–239. doi: 10.1016/j.biomaterials.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Batra H., Yadav P., Chand S. COVID-19 vaccines currently under preclinical and clinical studies, and associated antiviral immune response. Vaccines. 2020;8:649. doi: 10.3390/vaccines8040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar A., Younis T. Trastuzumab for HER2-positive metastatic breast cancer: clinical and economic considerations. Clin.Med. Insights Oncol. 2012;6:179–187. doi: 10.4137/CMO.S6460. CMO. S6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhunjhunwala S. Springer; 2018. Immunoengineering—From Biologics to Biomaterials. [Google Scholar]
- Jovanović N., Bouchard A., Hofland G.W., Witkamp G.-J., Crommelin D.J.A., Jiskoot W. Distinct effects of sucrose and trehalose on protein stability during supercritical fluid drying and freeze-drying. Eur. J. Pharm. Sci. 2006;27:336–345. doi: 10.1016/j.ejps.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Juncker H.G., Mulleners S.J., van Gils M.J., Bijl T.P.L., de Groot C.J.M., Pajkrt D., Korosi A., van Goudoever J.B., van Keulen B.J. Comparison of SARS-CoV-2-specific antibodies in human milk after mRNA-based COVID-19 vaccination and infection. Vaccines. 2021;9:1475. doi: 10.3390/vaccines9121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junping W., Maitani Y., Takayama K., Nagai T. In vivo evaluation of doxorubicin carried with long circulating and remote loading proliposome. Int. J. Pharm. 2000;203:61–69. doi: 10.1016/S0378-5173(00)00410-5. [DOI] [PubMed] [Google Scholar]
- Kanojia G., Have R.t., Bakker A., Wagner K., Frijlink H.W., Kersten G.F.A., Amorij J.-P. The production of a stable infliximab powder: the evaluation of spray and freeze-drying for production. PLoS One. 2016;11:e0163109. doi: 10.1371/journal.pone.0163109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper J.C., Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011;78:248–263. doi: 10.1016/j.ejpb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Katritzky A.R., Akhmedov N.G., Denisko O.V. 1H and 13C NMR spectroscopic study of oxidation of d, l-cystine and 3, 3′-dithiobis (propionic acid) with hydrogen peroxide in aqueous solution. Magn.Reson. Chem. 2003;41:37–41. doi: 10.1002/mrc.1110. [DOI] [Google Scholar]
- Kawasaki H., Shimanouchi T., Kimura Y. Recent development of optimization of lyophilization process. J. Chem. 2019;2019:1–14. doi: 10.1155/2019/9502856. [DOI] [Google Scholar]
- Krebs M.R.H., Domike K.R., Donald A.M. Protein aggregation: more than just fibrils. Biochem. Soc. Trans. 2009;37:682–686. doi: 10.1042/bst0370682. [DOI] [PubMed] [Google Scholar]
- Kulkarni J.A., Witzigmann D., Thomson S.B., Chen S., Leavitt B.R., Cullis P.R., van der Meel R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021;16:630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- Lajnaf R., Picart-Palmade L., Attia H., Marchesseau S., Ayadi M.A. Foaming and adsorption behavior of bovine and camel proteins mixed layers at the air/water interface. Colloids Surf. B Biointerfaces. 2017;151:287–294. doi: 10.1016/j.colsurfb.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Lanza F., Seghatchian J. An overview of current position on cell therapy in transfusion science and medicine: from fictional promises to factual and perspectives from red cell substitution to stem cell therapy. Transfus.Apher.Sci. 2020;59:102940. doi: 10.1016/j.transci.2020.102940. [DOI] [PubMed] [Google Scholar]
- Larson R.C., Maus M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer. 2021;21:145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., McAuley A., Schilke K.F., McGuire J. Molecular origins of surfactant-mediated stabilization of protein drugs. Adv. Drug Deliv. Rev. 2011;63:1160–1171. doi: 10.1016/j.addr.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Luke J.M., Simon G.G., Söderholm J., Errett J.S., August J.T., Gale M., Jr., Hodgson C.P., Williams J.A. Coexpressed RIG-I agonist enhances humoral immune response to influenza virus DNA vaccine. J. Virol. 2011;85:1370–1383. doi: 10.1128/jvi.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund M.N., Heinonen M., Baron C.P., Estévez M. Protein oxidation in muscle foods: a review. Mol. Nutr. Food Res. 2011;55:83–95. doi: 10.1002/mnfr.201000453. [DOI] [PubMed] [Google Scholar]
- Maroto R., Zhao Y., Jamaluddin M., Popov V.L., Wang H., Kalubowilage M., Zhang Y., Luisi J., Sun H., Culbertson C.T., et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles. 2017;6:1359478. doi: 10.1080/20013078.2017.1359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeage K. Daratumumab: first global approval. Drugs. 2016;76:275–281. doi: 10.1007/s40265-015-0536-1. [DOI] [PubMed] [Google Scholar]
- Merivaara A., Zini J., Koivunotko E., Valkonen S., Korhonen O., Fernandes F.M., Yliperttula M. Preservation of biomaterials and cells by freeze-drying: change of paradigm. J. Control. Release. 2021;336:480–498. doi: 10.1016/j.jconrel.2021.06.042. [DOI] [PubMed] [Google Scholar]
- Mockus L.N., Paul T.W., Pease N.A., Harper N.J., Basu P.K., Oslos E.A., Sacha G.A., Kuu W.Y., Hardwick L.M., Karty J.J., et al. Quality by design in formulation and process development for a freeze-dried, small molecule parenteral product: a case study. Pharm. Dev. Technol. 2011;16:549–576. doi: 10.3109/10837450.2011.611138. [DOI] [PubMed] [Google Scholar]
- Moncalvo F., Martinez Espinoza M.I., Cellesi F. Nanosized delivery systems for therapeutic proteins: clinically validated technologies and advanced development strategies. Front. Bioeng.Biotechnol. 2020;8:89. doi: 10.3389/fbioe.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkarsh S.P., Ma Y., Aglione A., Bailon P., Ciolek D., Debarbieri B., Graves M.C., Hollfelder K., Michel H., Palleroni A., et al. Positional isomers of monopegylated interferon α-2a: isolation, characterization, and biological activity. Anal.Biochem. 1997;247:434–440. doi: 10.1006/abio.1997.2128. [DOI] [PubMed] [Google Scholar]
- Mosharraf M., Malmberg M., Fransson J. Formulation, lyophilization and solid-state properties of a pegylated protein. Int. J. Pharm. 2007;336:215–232. doi: 10.1016/j.ijpharm.2006.11.064. [DOI] [PubMed] [Google Scholar]
- Muramatsu H., Lam K., Bajusz C., Laczkó D., Karikó K., Schreiner P., Martin A., Lutwyche P., Heyes J., Pardi N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated nucleoside-modified mRNA vaccine. Mol. Ther. 2022;30:1941–1951. doi: 10.1016/j.ymthe.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiał-Wysocka A., Kot M., Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28:801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S. Structural changes induced by the deamidation and isomerization of asparagine revealed by the crystal structure of Ustilago sphaerogena ribonuclease U2B. Biopolymers. 2010;93:1003–1010. doi: 10.1002/bip.21514. [DOI] [PubMed] [Google Scholar]
- Ohtake S., Kita Y., Arakawa T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv. Drug Deliv. Rev. 2011;63:1053–1073. doi: 10.1016/j.addr.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Olden B.R., Cheng Y., Yu J.L., Pun S.H. Cationic polymers for non-viral gene delivery to human T cells. J. Control. Release. 2018;282:140–147. doi: 10.1016/j.jconrel.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyewumi M.O., Kumar A., Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines. 2010;9:1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir E.E., Görgüç A., Gençdağ E., Yılmaz F.M. Physicochemical, functional and emulsifying properties of plant protein powder from industrial sesame processing waste as affected by spray and freeze drying. LWT. 2022;154:112646. doi: 10.1016/j.lwt.2021.112646. [DOI] [Google Scholar]
- Pak R.H., Finn R.F. Sterile Product Development. Springer; 2013. Formulation approaches and strategies for PEGylated biotherapeutics; pp. 61–97. [DOI] [Google Scholar]
- Park J., Nagapudi K., Vergara C., Ramachander R., Laurence J.S., Krishnan S. Effect of pH and excipients on structure, dynamics, and long-term stability of a model IgG1 monoclonal antibody upon freeze-drying. Pharm. Res. (N. Y.) 2013;30:968–984. doi: 10.1007/s11095-012-0933-z. [DOI] [PubMed] [Google Scholar]
- Patel S.M., Chaudhuri S., Pikal M.J. Choked flow and importance of Mach I in freeze-drying process design. Chem. Eng. Sci. 2010;65:5716–5727. doi: 10.1016/j.ces.2010.07.024. [DOI] [Google Scholar]
- Paunovska K., Loughrey D., Dahlman J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022;23:265–280. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham N.B., Meng W.S. Protein aggregation and immunogenicity of biotherapeutics. Int. J. Pharm. 2020;585:119523. doi: 10.1016/j.ijpharm.2020.119523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano R., Fissore D., Barresi A.A., Rastelli M. Quality by design: scale-up of freeze-drying cycles in pharmaceutical industry. AAPS PharmSciTech. 2013;14:1137–1149. doi: 10.1208/s12249-013-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollinger H.S., Burns J.M., Casingal V.P., Gores P.F. Cellular Transplantation. Elsevier; 2007. Current immunosuppressive drugs and clinical use; pp. 13–28. [Google Scholar]
- Rajagopal P., Duraiswamy S., Sethuraman S., Giridhara Rao J., Krishnan U.M. Polymer-coated viral vectors: hybrid nanosystems for gene therapy. J. Gene Med. 2018;20:e3011. doi: 10.1002/jgm.3011. [DOI] [PubMed] [Google Scholar]
- Rockinger U., Funk M., Winter G. Current approaches of preservation of cells during (freeze-) drying. J. Pharm. Sci. 2021;110:2873–2893. doi: 10.1016/j.xphs.2021.04.018. [DOI] [PubMed] [Google Scholar]
- Sacha G.A., Saffell-Clemmer W., Abram K., Akers M.J. Practical fundamentals of glass, rubber, and plastic sterile packaging systems. Pharm. Dev. Technol. 2010;15:6–34. doi: 10.3109/10837450903511178. [DOI] [PubMed] [Google Scholar]
- Schneid S.C., Gieseler H., Kessler W.J., Luthra S.A., Pikal M.J. Optimization of the secondary drying step in freeze drying using TDLAS technology. AAPS PharmSciTech. 2011;12:379–387. doi: 10.1208/s12249-011-9600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneid S.C., Stärtzel P.M., Lettner P., Gieseler H. Robustness testing in pharmaceutical freeze-drying: inter-relation of process conditions and product quality attributes studied for a vaccine formulation. Pharm. Dev. Technol. 2011;16:583–590. doi: 10.3109/10837450.2011.581287. [DOI] [PubMed] [Google Scholar]
- Scutellà B., Bourlès E. Development of freeze-drying cycle via design space approach: a case study on vaccines. Pharm. Dev. Technol. 2020;25:1302–1313. doi: 10.1080/10837450.2020.1806298. [DOI] [PubMed] [Google Scholar]
- Sokolova V., Ludwig A.K., Hornung S., Rotan O., Horn P.A., Epple M., Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Stadtman E.R., Levine R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins Amino Acids 25207218 2003. 139. Stadtman ER and Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Sundaramurthi P., Suryanarayanan R. The effect of crystallizing and non-crystallizing cosolutes on succinate buffer crystallization and the consequent pH shift in frozen solutions. Pharm. Res. (N. Y.) 2011;28:374–385. doi: 10.1007/s11095-010-0282-8. [DOI] [PubMed] [Google Scholar]
- Tang X., Pikal M.J. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm. Res. (N. Y.) 2004;21:191–200. doi: 10.1023/b:pham.0000016234.73023.75. [DOI] [PubMed] [Google Scholar]
- Tattini V., Jr., Parra D.F., Polakiewicz B., Pitombo R.N.M. Effect of lyophilization on the structure and phase changes of PEGylated-bovine serum albumin. Int. J. Pharm. 2005;304:124–134. doi: 10.1016/j.ijpharm.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Thakral S., Sonje J., Munjal B., Suryanarayanan R. Stabilizers and their interaction with formulation components in frozen and freeze-dried protein formulations. Adv. Drug Deliv. Rev. 2021;173:1–19. doi: 10.1016/j.addr.2021.03.003. [DOI] [PubMed] [Google Scholar]
- Thoryk E.A., Swaminathan G., Meschino S., Cox K.S., Gindy M., Casimiro D.R., Bett A.J. Co-administration of lipid nanoparticles and sub-unit vaccine antigens is required for increase in antigen-specific immune responses in mice. Vaccines (Basel) 2016;4 doi: 10.3390/vaccines4040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzet A., Pfefferlé F., der Wel P.v., Lamprecht A., Pellequer Y. Active freeze drying for production of nanocrystal-based powder: a pilot study. Int. J. Pharm. 2018;536:222–230. doi: 10.1016/j.ijpharm.2017.11.050. [DOI] [PubMed] [Google Scholar]
- Trenkenschuh E., Friess W. Freeze-drying of nanoparticles: how to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021;165:345–360. doi: 10.1016/j.ejpb.2021.05.024. [DOI] [PubMed] [Google Scholar]
- trials C. 2022. https://clinicaltrials.gov/
- Turner M., Papadimitriou A., Winkle P., Segall N., Levin M., Doust M., Johnson C., Lucksinger G., Fierro C., Pickrell P., et al. Immunogenicity and safety of lyophilized and liquid dengue tetravalent vaccine candidate formulations in healthy adults: a randomized, phase 2 clinical trial. Hum. Vaccin.Immunother. 2020;16:2456–2464. doi: 10.1080/21645515.2020.1727697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccines D.O., WHO A.B. Vaccines and Biologicals Update. 2000;34:1–4. [Google Scholar]
- Waldmann T.A. Cytokines in cancer immunotherapy. Cold Spring Harb.Perspect.Biol. 2018;10:a028472. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R.H., Bhatnagar B., Tchessalov S., Izutsu K.-I., Tsumoto K., Ohtake S. Next generation drying technologies for pharmaceutical applications. J. Pharm. Sci. 2014;103:2673–2695. doi: 10.1002/jps.23998. [DOI] [PubMed] [Google Scholar]
- Wang H., Zheng X., Jin J., Zheng L., Guan T., Huo Y., Xie S., Wu Y., Chen W. LncRNA MALAT1 silencing protects against cerebral ischemia-reperfusion injury through miR-145 to regulate AQP4. J. Biomed. Sci. 2020;27:40. doi: 10.1186/s12929-020-00635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Roberts C.J. Protein aggregation - mechanisms, detection, and control. Int. J. Pharm. 2018;550:251–268. doi: 10.1016/j.ijpharm.2018.08.043. [DOI] [PubMed] [Google Scholar]
- Wang W., Nema S., Teagarden D. Protein aggregation--pathways and influencing factors. Int. J. Pharm. 2010;390:89–99. doi: 10.1016/j.ijpharm.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Ward K.R., Matejtschuk P. The principles of freeze-drying and application of analytical technologies. Methods Mol. Biol. 2021;2180:99–127. doi: 10.1007/978-1-0716-0783-1_3. [DOI] [PubMed] [Google Scholar]
- Xiao H., Huang L., Zhang W., Yin Z. Damage of proteins at the air/water interface: surface tension characterizes globulin interface stability. Int. J. Pharm. 2020;584:119445. doi: 10.1016/j.ijpharm.2020.119445. [DOI] [PubMed] [Google Scholar]
- Xie G., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapadka K.L., Becher F.J., Gomes Dos Santos A.L., Jackson S.E. Factors affecting the physical stability (aggregation) of peptide therapeutics. Interface Focus. 2017;7:20170030. doi: 10.1098/rsfs.2017.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbacnik T.J., Holcomb R.E., Katayama D.S., Murphy B.M., Payne R.W., Coccaro R.C., Evans G.J., Matsuura J.E., Henry C.S., Manning M.C. Role of buffers in protein formulations. J. Pharm. Sci. 2017;106:713–733. doi: 10.1016/j.xphs.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang M., Li G., Wang Y., Wang Y., Zhao S., Haihong P., Zhao H., Wang Y. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci. Rep. 2017;7:10255. doi: 10.1038/s41598-017-10925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Hou X., Yan J., Du S., Xue Y., Li W., Xiang G., Dong Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020;5:358–363. doi: 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Xiong H., Selomulya C., Chen X.D., Huang S., Ruan X., Zhou Q., Sun W. Effects of spray drying and freeze drying on the properties of protein isolate from rice dreg protein. Food Bioproc. Tech. 2013;6:1759–1769. doi: 10.1007/s11947-012-0844-3. [DOI] [Google Scholar]
- Zhao Y., Deng B., Pan X., Zhang J., Zuo X., Wang J., Lv F., Lu Y., Hou J. Optimization of heat-resistance technology for a Duck hepatitis lyophilized live vaccine. Vaccines. 2022;10:269. doi: 10.3390/vaccines10020269. [DOI] [PMC free article] [PubMed] [Google Scholar]