Abstract
Helicobacter hepaticus is a bacterial pathogen that causes chronic active hepatitis and inflammatory bowel disease in mice. The purpose of this study was to develop a recombinant antigen-based enzyme-linked immunosorbent assay (ELISA) to detect H. hepaticus-infected mice. A genomic library of H. hepaticus was constructed and was screened with sera from H. hepaticus-infected mice. A 459-bp open reading frame that coded for an 18-kDa immunoreactive protein, MAP18, was identified. The gene had high identity with genes coding for outer membrane proteins of other bacteria, and the predicted amino acid sequence of MAP18 had a putative membrane-trafficking signal sequence and a putative signal peptidase II cleavage site. The recombinant protein was expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein, GST-MAP18, and purified by affinity chromatography. The 44-kDa fusion protein was detected on Western blots probed with sera from H. hepaticus-infected mice but was not detected on blots probed with sera from mice infected with Helicobacter muridarum or Helicobacter bilis or with sera from mice free of Helicobacter infection. The GST-MAP18 fusion protein was used as an antigen in an ELISA to detect anti-H. hepaticus antibodies in sera from infected mice. This ELISA was compared to an H. hepaticus-specific ELISA that uses a detergent extract of H. hepaticus as the antigen. Sera from mice naturally and experimentally infected with H. hepaticus, H. bilis, or H. muridarum and sera from mice free of Helicobacter infection were evaluated. Both ELISAs performed with a high specificity (98%); however, the detergent extract-based ELISA performed with a higher sensitivity (89%) than the recombinant protein-based ELISA (sensitivity, 66%). These data indicate that H. hepaticus carries a gene that encodes an immunogenic 18-kDa membrane-associated protein; however, antibodies to this protein are not detected in all infected mice.
Helicobacter hepaticus is a helical, gram-negative, motile, microaerobic, bacterial pathogen of mice. It naturally colonizes the lower intestinal tract and can cause chronic active hepatitis or inflammatory bowel disease in a variety of immunocompetent mice (9–11, 13, 23, 24, 31, 39, 41) and immunodeficient mice (6, 8, 23, 40, 42). This bacterium is of concern to the scientific community because of its potential to invalidate studies that use infected mice (13). The hepatic and intestinal pathology induced by H. hepaticus can directly affect research involving these organ systems. Infected mice have abnormal liver function tests, as demonstrated by altered serum bile acid concentrations (39) and elevated levels of serum alanine aminotransferase (10, 11). Rectal prolapse, as a sequela to inflammatory bowel disease, may cause mice to be prematurely removed from an experiment. There is also evidence that H. hepaticus infections can disrupt host immune homeostasis. For example, infected mice mount a prompt and vigorous type 1 immune response against the bacterium (24, 42).
The potential for H. hepaticus to adversely affect scientific studies of mice is considerable, because infections in mouse colonies in the United States and Japan are widespread (30, 35). Since infected mice rarely display clinical signs of disease, accurate and inexpensive diagnostic tests to detect H. hepaticus infections are needed to effectively design and manage plans to eradicate this bacterium from mouse colonies. Diagnostic methods currently used to detect H. hepaticus-infected mice include histopathologic examination of silver-stained liver sections (39), culture of feces or livers (10), PCR analysis of liver, feces, or cecal tissue (3, 4, 27, 32), and enzyme-linked immunosorbent assay (ELISA) of serum (25) or feces (29) to detect anti-H. hepaticus specific antibodies.
Diagnostic ELISAs that use recombinant proteins as antigens to detect antibodies to bacterial pathogens have been described. Several of these recombinant protein-based ELISAs perform with high levels of sensitivity and specificity or function better than immunoassays that use alternative antigen sources (12, 14, 16, 17, 26). Recombinant protein antigens can be advantageous in bacterial ELISAs because they contain defined antigens that potentially have fewer cross-reactive epitopes than other antigen preparations. Also, large amounts of affinity-tagged recombinant proteins produced in prokaryotic or eukaryotic expression systems can be harvested and purified by affinity chromatography. In addition, the availability of recombinant antigens produced from fastidious organisms, such as H. hepaticus, eliminates the need to cultivate the bacteria.
In this report, we describe the identification and cloning of an immunogenic membrane-associated protein of H. hepaticus. This protein was expressed in and purified from Escherichia coli as a glutathione S-transferase (GST) fusion protein and used as an antigen to create an H. hepaticus-specific ELISA. The performance of this ELISA was compared to that of an H. hepaticus-specific ELISA that uses a detergent extract of H. hepaticus as the antigen. The recombinant protein-based ELISA was highly specific for detecting H. hepaticus-infected mice; however, this assay was less sensitive than the detergent extract-based H. hepaticus ELISA.
MATERIALS AND METHODS
Bacteria, plasmids, and sera.
An isolate of H. hepaticus was obtained from an adult male A/JCr mouse from an endemically infected colony (39, 41) as previously described (25). The isolate was cultured under microaerobic conditions (25) and was identified as H. hepaticus based on its ultrastructural morphology and urease activity and the results of DNA sequence analysis of the 16S rRNA gene (32). E. coli DH5αMCR (Gibco BRL, Gaithersburg, Md.) was used in all cloning and subcloning experiments. E. coli BL21(DE3)pLysS (Novagen, Madison, Wis.) was used to express recombinant proteins. The BamHI-digested plasmid Ready-To-Go pUC18 (Pharmacia Biotech, Piscataway, N.J.) was used to construct the genomic library, and the plasmid pUC18 (New England Biolabs, Beverly, Mass.) was used for subcloning procedures. The plasmid pGEX-5-X-3 (Pharmacia Biotech), which codes for an N-terminal GST moiety, was used to express fusion proteins in E. coli.
The H. hepaticus genomic library was screened with serum that was produced in adult C57BL/6 mice that were inoculated intraperitoneally with 108 viable H. hepaticus organisms. Six weeks after inoculation, mice were euthanized, blood was collected by cardiocentesis, and serum was separated, pooled, and preabsorbed against E. coli by pseudoscreening (33). Sera used to evaluate the H. hepaticus ELISAs (25) were collected from multiple strains of immunocompetent mice naturally infected with H. hepaticus (n = 44) or free of Helicobacter infection (n = 41) as determined by a fecal PCR assay (4, 32). To further evaluate the specificities of the ELISAs, groups of seven C57BL/6 mice were orally gavaged with 107 H. hepaticus, Helicobacter bilis, or Helicobacter muridarum organisms. Sixteen weeks following inoculation, mice were euthanized, blood was collected by cardiocentesis, and serum was separated and stored at −20°C.
Construction of genomic library.
H. hepaticus genomic DNA was isolated from cultured bacteria by using Genomic-tips (Qiagen, Inc., Valencia, Calif.) according to the manufacturer’s protocol. Genomic DNA was partially digested with Sau3AI (New England Biolabs), and DNA was separated on NuSieve GTG low-melting-temperature agarose (FMC, Rockland, Maine). DNA fragments in the 4- to 12-kb size range were recovered with the Qiaex II gel extraction kit (Qiagen). Partially digested genomic DNA was ligated into BamHI-digested Ready-To-Go pUC 18 (Pharmacia Biotech), and E. coli DH5αMCR (Gibco BRL) was transformed, plated onto Luria-Bertani agar plates (33) containing 50 μg of ampicillin per ml and 50 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml, and incubated for 14 to 16 h at 37°C.
Screening of genomic library.
Transformed bacteria were lifted onto nitrocellulose membranes (Nitropure; Micron Separations Inc., Westborough, Mass.), and bacterial colonies were incubated on filter paper saturated with lysis buffer (0.1 M NaHCO3, 1% Triton-X, and 2 mg of lysozyme per ml) in an atmosphere saturated with CHCl3 (18). The membranes were incubated with H. hepaticus-positive serum diluted to 1:2,000 in phosphate-buffered saline (PBS) (14.5 mM NaH2PO4, 108 mM Na2HPO4, 1.37 M NaCl [pH 7.4]) containing 5% nonfat dry milk (PBS-milk) and then incubated with an alkaline phosphatase-labeled antibody [F(ab′)2 fragment goat anti-mouse immunoglobulin G (heavy and light chains)] (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) diluted 1:2,000 in PBS-milk. The membranes were developed with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP–NBT) substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). Immunoreactive clones were identified and rechecked for immunoreactivity as described above. Three immunoreactive clones designated 1A, 1B, and B2, that contained plasmids p1A, p1B, and pB2, respectively, were identified.
Subcloning and sequence analysis.
Plasmids from positive clones were isolated by using the QIAprep Miniprep kit (Qiagen). To generate an immunoreactive clone that contained a small insert of H. hepaticus genomic DNA, plasmids p1A, p1B, and pB2 were subjected to restriction endonuclease digestion with HindIII, EcoRI, Sau3AI, PstI, or KpnI and DNA fragments were separated on 1% SeaPlaque GTG low-melting-temperature agarose (FMC). The DNA fragments were excised from the gel and, with an in-gel ligation procedure (33), ligated into pUC18 (New England Biolabs). DH5αMCR bacteria were transformed with the subcloned plasmids and tested for immunoreactivity as described above. Bacteria transformed with the plasmid p1A2, which contains a 3.2-kb HindIII fragment of the plasmid p1A, were immunoreactive. This plasmid, p1A2, was chosen for further study and was sequenced by the dideoxy chain termination method (34) at the University of Missouri DNA Core Facility. The DNA sequences were assembled and analyzed for open reading frames (ORFs) by using GCG software (Genetics Computer Group, Inc., Madison, Wis.) and by inspection. Gene sequences and the predicted translated gene products of ORFs were compared to existing sequences deposited in the GenBank database by using software from GCG, the National Center for Biotechnology Information (2), and the Human Genome Center, Baylor College of Medicine (1, 43).
PCR and subcloning into E. coli expression vectors.
The 3.2-kb insert of p1A2 was amplified by PCR in a thermal cycler (2400; Perkin-Elmer Cetus, Norwalk, Conn.) by using the Expand High Fidelity PCR System (Boehringer Mannheim, Indianapolis, Ind.). Each reaction mixture contained 40 ng of p1A2 as the template, a 0.4 μM concentration of the universal sequencing primer M13, and a 0.4 μM concentration of the reverse sequencing primer M13 (Pharmacia Biotech).
ORF 1 and ORF 2 were amplified by PCR in a thermal cycler (2400; Perkin-Elmer Cetus) by using the Expand High Fidelity PCR System (Boehringer Mannheim). Fifty nanograms of the 3.2-kb amplified insert of p1A2 was used as the template, and the annealing temperatures used to generate ORF 1 and ORF 2 were 49 and 53°C, respectively. The oligonucleotide primers used to generate ORF 1 were ORF1Forward (gcggatccggtaccTCTTAAAGGAGATGATGATGATG), which corresponds to nucleotides 276 to 295, and ORF1Reverse (cggaattcccatggCAACTTTATTCCCCATATTG), which corresponds to nucleotides 780 to 761. The primers used to generate ORF 2 were ORF2Forward (gcggatccggtaccAAGGATTCATTATGGAAGCTC), which corresponds to nucleotides 2347 to 2367, and ORF2Reverse (cggaattcccatggTAATGTGAGTTTGCACAGGC), which corresponds to nucleotides 2643 to 2624. The nucleotides of forward primers represented by lowercase letters were added to create restriction enzyme sites for BamHI and KpnI, and the nucleotides of reverse primers represented by lowercase letters were added to create restriction enzyme sites for EcoRI and NcoI. The amplified ORF 1 and ORF 2 genes were digested with BamHI and EcoRI and directionally cloned, in frame, into a GST gene fusion vector, pGEX-5X-3 (Pharmacia Biotech), to create plasmids pGEX-5X-3-MAP18 and PGEX-5X-3-P8, respectively. E. coli DH5αMCR was transformed with the recombinant plasmids and was tested for immunoreactivity by using the immunoscreening procedure described above.
Recombinant protein expression and purification.
E. coli BL21(DE3)pLysS transformed with the plasmid pGEX-5X-3-MAP18 produced an H. hepaticus-specific immunoreactive fusion protein, GST-MAP18, that was chosen for further study. E. coli BL21(DE3)pLysS transformed with the plasmid pGEX-5X-3, which contained no foreign DNA insert, was used to express GST for use as a control protein. Proteins were expressed at 30°C, and proteins were purified with glutathione-linked agarose beads by the pGEX vector protocol (Pharmacia Biotech). The recovered proteins were dialyzed overnight at 4°C against PBS through a 12,000- to 14,000-Da cutoff membrane (Spectrum Medical Industries, Inc., Houston, Tex.), and the protein concentrations of the eluted fractions were determined by a bicinchoninic acid procedure (36).
Western blot analysis.
Purified GST-MAP18 and GST or sonicated preparations of immunoreactive E. coli clones that harbored plasmids from an H. hepaticus genomic library were subjected to electrophoresis on a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel under nonreducing conditions (22). Proteins were electrophoretically transferred to an Immobilon-P membrane (Millipore, Bedford, Mass.) and blocked with PBS-milk. Western blots were incubated with sera from Helicobacter-free mice or mice infected with H. hepaticus, H. bilis, or H. muridarum diluted 1:400 in PBS-milk. Next, blots were sequentially incubated with biotinylated goat anti-mouse antibody diluted 1:1,000 in PBS-milk and Vectastain (Vector Laboratories, Burlingame, Calif.). Membranes were developed with 3,3′,5,5′-tetramethylbenzidine membrane peroxidase substrate (Kirkegaard and Perry Laboratories).
H. hepaticus-specific ELISAs.
The GST-MAP18 fusion protein and the control GST protein were diluted to 1 μg of protein/ml in coating buffer (0.1 M NaHCO3 [pH 9.6]), and 200 μl of each solution was added to separate wells of Immulon 2 96-well plates (Dynex, Chantilly, Va.). Proteins were allowed to bind to plates for 48 h at 4°C. The wells were emptied; 300 μl of blocking buffer (PBS with 0.5% nonfat dry milk and 0.05% Tween 20) was added to each well, incubated for 30 min at room temperature, and discarded; and 200 μl of serum diluted 1:100 in blocking buffer was added to duplicate wells coated with the antigen and to duplicate wells coated with the control protein. After incubation at 37°C for 2 h, plates were washed three times in PBS with 0.05% Tween 20 (PBS-Tween). Two hundred microliters of alkaline phosphatase-labeled secondary antibody [F(ab′)2 fragment goat anti-mouse immunoglobulin G (heavy and light chains)] (Jackson Immunoresearch Laboratories, Inc.) diluted 1:7,000 in PBS-Tween was added to each well and incubated at 37°C for 2 h. Plates were washed five times in PBS-Tween. One hundred microliters of phosphatase substrate solution (1 mg of Sigma 104 phosphatase substrate [Sigma] per 1 ml of substrate buffer [2 mM MgCl2, 27.5 mM Na2CO3, 22.5 mM NaHCO3]) was added to each well and incubated in the dark at room temperature for 30 min. The optical density (OD) of each well was measured (Kinetic Reader EL312E; Bio-Tek Instruments, Inc., Winooski, Vt.) and automatically calculated as the OD at 405 nm (OD405) minus the OD490 to correct for optical interference. The final OD405 for each serum sample was calculated as the mean OD405 of the paired recombinant-antigen-coated wells minus the mean OD405 of the paired control protein-coated wells. The cutoff OD405 was set at two standard deviations above the mean for 41 serum samples from mice negative for Helicobacter infection by PCR (4, 25, 32). To evaluate the performance of the recombinant-protein-based ELISA, this ELISA was directly compared to an H. hepaticus-specific ELISA that uses a detergent extract of H. hepaticus as the antigen (25).
Nucleotide sequence accession number.
The GenBank accession number of the sequence for the 3.2-kb HindIII fragment and the MAP18 gene is AF134212.
RESULTS
Identification of immunoreactive protein.
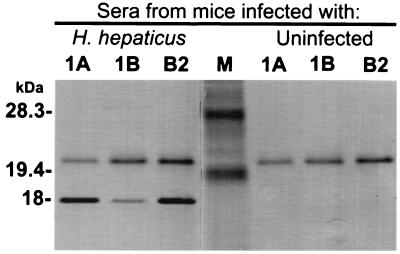
Immunoscreening of 8,000 white clones from the H. hepaticus genomic library with sera from H. hepaticus-infected mice revealed the three immunoreactive clones designated 1A, 1B, and B2, which contained the plasmids p1A, p1B, and pB2, respectively. Western blot analysis of all three positive clones demonstrated an 18-kDa immunoreactive protein when blots were probed with sera from H. hepaticus-infected mice (Fig. 1). The 18-kDa band was not visualized when clones were probed with sera from mice free of H. hepaticus infection (Fig. 1). Thus, subsequent experiments were focused toward identifying an ORF in the H. hepaticus genome contained in the plasmid p1A, p1B, or pB2 that coded for an H. hepaticus-specific 18-kDa immunoreactive protein. Restriction endonuclease digestion of the plasmids p1A, p1B, and pB2 revealed DNA inserts of approximately 8.5, 9.0, and 6.5 kb, respectively. Subcloning and immunoscreening of these plasmids revealed that a gene coding for an 18-kDa immunoreactive protein was located on a 3.2-kb piece of DNA generated by HindIII digestion of p1A. This subcloned plasmid, p1A2, was chosen for further study.
FIG. 1.
Western blot of sonicated preparations from cultures of three immunoreactive E. coli clones (1A, 1B, and B2) harboring plasmids from an H. hepaticus genomic library. Blots were probed with pooled sera from H. hepaticus-infected mice or with pooled sera from uninfected mice. Notice that the 18-kDa protein is detected only with the serum from H. hepaticus-infected mice. The 20-kDa band present in all test lanes likely represents an E. coli protein that is detected by anti-E. coli antibodies present in the sera of H. hepaticus-infected and uninfected mice. Each lane contains 10 μg of protein. M, prestained low-range SDS-polyacrylamide gel electrophoresis protein standards (Bio-Rad Laboratories, Hercules, Calif.).
Identification of an ORF encoding an immunogenic protein.
The DNA sequence of p1A2 was determined, and four ORFs that had putative ribosome binding sites and coded for predicted proteins of >7,000 Da were identified. The ORF 1 gene, which coded for a predicted 18,836-Da membrane-associated protein (MAP18), and the ORF 2 gene, which coded for a predicted 7,959-Da protein (P8), were generated by PCR, and the amplicons were directionally cloned in frame into the expression vector pGEX-5X-3 (Pharmacia Biotech) to create the plasmids pGEX-5X-3-MAP18 and pGEX-5X-3-P8, respectively. Colony lifts of E. coli harboring the plasmid pGEX-5X-3-MAP18 were immunoreactive when probed with sera from H. hepaticus-infected mice. Colony lifts of E. coli harboring pGEX-5X-3-P8 demonstrated no immunoreactivity when probed with sera from mice infected with H. hepaticus. Because ORF 1 was identified as the gene that coded for the 18-kDa immunoreactive H. hepaticus protein that was being sought, ORF 3 and ORF 4 were not evaluated for the production of immunoreactive proteins.
Gene sequence and predicted translated protein of ORF 1.
The 459-nucleotide sequence of the ORF that coded for the 18-kDa immunogenic protein of H. hepaticus, ORF 1, is presented in Fig. 2. The gene had a putative ribosome binding site at positions −6 through −11, a TAA stop codon, and a G+C content of 40.3%. The ORF had a 59.8% identity in a 316-bp overlap with an ORF of the H. pylori genome that codes for a putative 18-kDa peptidoglycan-associated lipoprotein (GenBank nucleotide sequence accession no. AE000619) (37). The gene also had a 61.8% identity in a 338-bp overlap with a gene coding for a peptidoglycan-associated outer membrane lipoprotein of Campylobacter jejuni (GenBank nucleotide sequence accession no. U47617) (20) and had a high sequence similarity with genes coding for outer membrane proteins of other gram-negative bacteria. The predicted translated protein of ORF 1, MAP18, contained 153 amino acids and had a high identity with predicted outer membrane lipoproteins of other gram-negative bacteria. For example, MAP18 had a 48% identity over 104 amino acids with a predicted 18-kDa peptidoglycan-associated lipoprotein of H. pylori (GenBank accession no. AE000619) (37) and had a 49% identity over 107 amino acids with a predicted 18-kDa peptidoglycan-associated lipoprotein of C. jejuni (GenBank accession no. X83374). The N-terminal 19-amino-acid peptide fragment of the predicted translated protein of MAP18 (Fig. 2) was a predicted prokaryotic membrane lipoprotein signal sequence (19, 28, 38) that had a 52% identity with the leader sequence of a 20-kDa membrane-associated lipoprotein of H. pylori (GenBank accession no. AE000645) (7, 21). In addition, the Ser-Val-Gly-Cys sequence at amino acid positions 15 through 18 is a putative cleavage site for a specific lipoprotein signal peptidase, signal peptidase II (15), with the serine at position 19 serving as a probable outer membrane trafficking signal (44) and the cysteine at position 18 serving as a possible site for the protein to be attached to a membrane lipid (15). Thus, it is likely that MAP18 is a membrane-associated protein of H. hepaticus.
FIG. 2.
Nucleotide sequence of ORF 1 and the predicted translated protein, MAP18. The DNA sequences shown are nucleotides 281 to 784 of the 3.2-kb HindIII fragment of the plasmid p1A2. The putative ribosome binding site (rbs) is double underlined. The possible leader signal peptide sequence is underlined, and the predicted signal peptidase II cleavage site is indicated (↓).
Western blotting of recombinant GST-MAP18.
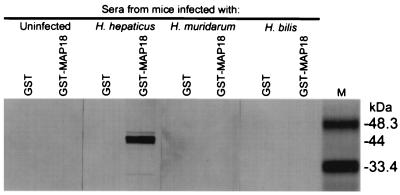
Western blots of affinity-purified GST-MAP18 demonstrated a 44-kDa fusion protein that reacted with sera from H. hepaticus-infected mice but did not react with sera from mice infected with H. bilis or H. muridarum or with sera from uninfected mice (Fig. 3). This 44-kDa recombinant protein, GST-MAP18, was composed of the 18-kDa immunoreactive protein of H. hepaticus (MAP18) coded for by ORF 1 fused with the 26-kDa GST protein affinity tag coded for by the expression vector.
FIG. 3.
Western blot of affinity-purified H. hepaticus recombinant protein (GST-MAP18) and control protein (GST) probed with pooled sera from mice infected with H. hepaticus, H. muridarum, or H. bilis or with pooled sera from uninfected mice. Note that antibodies to GST-MAP18 are detected only for mice infected with H. hepaticus. Each lane contains 10 μg of protein. M, prestained low-range SDS-polyacrylamide gel electrophoresis protein standards (Bio-Rad Laboratories).
Comparison of recombinant-antigen-based ELISA to detergent extract-based ELISA.
The GST-MAP18 recombinant protein was expressed in and purified from E. coli and used as the antigen in an ELISA to detect anti-H. hepaticus specific antibodies in the sera of infected mice. This recombinant-antigen-based ELISA was directly compared to an established H. hepaticus-specific ELISA that uses a detergent extract of H. hepaticus as the antigen (25) (Table 1). The sensitivity and specificity of each ELISA were determined by evaluating sera from multiple strains of immunocompetent mice that were documented by fecal PCR assay to be infected with H. hepaticus or free of Helicobacter infections. Both ELISAs were highly specific (98%); however, the recombinant-protein-based ELISA was 66% sensitive in identifying mice naturally infected with H. hepaticus while the detergent extract-based ELISA was 89% sensitive in identifying these mice. To further compare the sensitivities and specificities of the two H. hepaticus-specific ELISAs, sera from mice that were orally gavaged with H. hepaticus, H. bilis, or H. muridarum were tested. When these sera were evaluated, the detergent extract-based ELISA and the recombinant protein-based ELISA were both 100% specific and 100% sensitive in identifying the H. hepaticus-infected mice. Thus, the recombinant-antigen-based ELISA was less sensitive than the detergent extract-based ELISA in detecting mice naturally infected with H. hepaticus while both assays were equally highly specific.
TABLE 1.
Results for the performance of the two H. hepaticus-specific ELISAs
| ELISAa | ELISA result | No. (%) of mice with the indicated
Helicobacter infection status
|
||||
|---|---|---|---|---|---|---|
| Helicobacter free (n = 41) | H. hepaticusb(n = 44) | H. hepaticusc(n = 7) | H. bilisc(n = 7) | H. muridarumc(n = 7) | ||
| Recombinant | Positive | 1 (2) | 29 (66) | 7 (100) | 0 (0) | 0 (0) |
| Negative | 40 (98) | 15 (34) | 0 (0) | 7 (100) | 7 (100) | |
| Detergent extract | Positive | 1 (2) | 39 (89) | 7 (100) | 0 (0) | 0 (0) |
| Negative | 40 (98) | 5 (11) | 0 (0) | 7 (100) | 7 (100) | |
The recombinant-antigen-based ELISA used the fusion protein, GST-MAP18, as the antigen, and the detergent extract-based ELISA used a detergent extract of H. hepaticus as the antigen. Sera from mice free of Helicobacter infection, naturally infected with H. hepaticus, or experimentally inoculated with H. hepaticus, H. bilis, or H. muridarum were tested.
Mice naturally infected with H. hepaticus.
Mice experimentally inoculated with H. hepaticus, H. bilis, or H. muridarum.
DISCUSSION
In this report we describe the identification and cloning of a gene that codes for an 18-kDa immunogenic membrane-associated protein (MAP18) of H. hepaticus. The protein was expressed in and purified from E. coli as a GST fusion protein, GST-MAP18, and was used as the antigen in an ELISA to detect anti-H. hepaticus specific antibodies in the sera of infected mice. This ELISA was directly compared to an H. hepaticus-specific ELISA that uses a detergent extract of H. hepaticus as the antigen. Both ELISAs performed with high specificity; however, the recombinant protein-based ELISA performed with a lower sensitivity than did the detergent extract-based ELISA in identifying mice naturally infected with H. hepaticus. The decreased sensitivity of the recombinant protein-based ELISA may have resulted from the presence of only a limited number of antigenic epitopes on the single protein antigen and the failure of all infected mice to generate a vigorous antibody response against this protein. Several immunoreactive bands were detected when Western blots of the H. hepaticus detergent extract antigen were probed with sera from H. hepaticus-infected mice (data not shown), suggesting that this antigen contains more immunogenic epitopes than the recombinant-protein antigen. Thus, the increased sensitivity of the detergent extract-based ELISA may result from its ability to identify several different H. hepaticus-specific antibodies that are produced in response to infection. The decreased sensitivity of the recombinant-protein-based ELISA may also have resulted from antigenic variation of MAP18 so that antibodies directed against the MAP18 proteins of other H. hepaticus isolates may not recognize the antigenic epitopes of the recombinant MAP18 protein used in our ELISA. Therefore, potential ways to increase the sensitivity of the ELISA are to clone other antigenic variants of MAP18 or to clone other immunoreactive proteins of H. hepaticus and to combine these proteins with GST-MAP18 to create a multivalent antigen preparation for use in an H. hepaticus-specific ELISA.
Our results suggest that MAP18 is a membrane-associated lipoprotein of H. hepaticus. The gene coding for MAP18, ORF 1, had a high sequence identity with genes coding for outer membrane lipoproteins of other gram-negative bacteria, including a putative 18-kDa outer membrane lipoprotein of H. pylori (37) and an 18-kDa outer membrane lipoprotein of C. jejuni (5), a bacterium from a closely related genus. Also, the predicted amino acid sequence of MAP18 had high identities with the amino acid sequences of these same proteins. In addition, the N-terminal 19-amino-acid peptide fragment of MAP18 contained a prokaryotic signal peptide that predicted that the protein would be trafficked to the bacterial membrane (19, 28, 38). The C terminus of the signal peptide had a putative signal peptidase II cleavage site; signal peptidase II is an enzyme that is involved in cleavage of signal sequences of prokaryotic membrane-transported proteins (15). In addition, the cysteine residue following this cleavage site is a likely point of attachment of membrane proteins to a lipid moiety (15) and the serine at position two of the cleaved sequence has been shown to be an outer membrane trafficking signal in E. coli (44).
In summary we cloned a gene that encodes an immunogenic protein of H. hepaticus. The protein was expressed as a fusion protein and was used as an antigen in an ELISA to detect antibodies to H. hepaticus in sera from infected mice. This ELISA was compared to an H. hepaticus-specific ELISA that uses a detergent extract of H. hepaticus as the antigen. Both assays performed with an equally high specificity; however, the recombinant-protein-based ELISA was not as sensitive in detecting infected mice. This report provides a foundation for the further development of recombinant-protein-based immunoassays for the detection of H. hepaticus infections in mice.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battles J K, Williamson J C, Pike K M, Gorelick P L, Ward J M, Gonda M A. Diagnostic assay for Helicobacter hepaticus based on nucleotide sequence of its 16S rRNA gene. J Clin Microbiol. 1995;33:1344–1347. doi: 10.1128/jcm.33.5.1344-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckwith C S, Franklin C L, Hook R R, Jr, Besch-Williford C L, Riley L K. Fecal PCR assay for diagnosis of Helicobacter infection in laboratory rodents. J Clin Microbiol. 1997;35:1620–1623. doi: 10.1128/jcm.35.6.1620-1623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnens A, Stucki U, Nicolet J, Frey J. Identification and characterization of an immunogenic outer membrane protein of Campylobacter jejuni. J Clin Microbiol. 1995;33:2826–2832. doi: 10.1128/jcm.33.11.2826-2832.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao P, McClain M S, Forsyth M H, Cover T L. Extracellular release of antigenic proteins by Helicobacter pylori. Infect Immun. 1998;66:2984–2986. doi: 10.1128/iai.66.6.2984-2986.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foltz C J, Fox J G, Cahill R, Murphy J C, Yan L, Shames B, Schauer D B. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;3:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 9.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber A, Krell S, Morenz J. Recombinant Treponema pallidum antigens in syphilis serology. Immunobiology. 1996;196:535–549. doi: 10.1016/s0171-2985(97)80070-8. [DOI] [PubMed] [Google Scholar]
- 13.Hailey J R, Haseman J K, Bucher J R, Radovsky A E, Malarkey D E, Miller R T, Nyska A, Maronpot R R. Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve National Toxicology Program two-year carcinogenesis studies. Toxicol Pathol. 1998;26:602–611. doi: 10.1177/019262339802600503. [DOI] [PubMed] [Google Scholar]
- 14.Hauser U, Wilske B. Enzyme-linked immunosorbent assays with recombinant internal flagellin fragments derived from different species of Borrelia burgdorferi sensu lato for the serodiagnosis of Lyme neuroborreliosis. Med Microbiol Immunol (Berlin) 1997;186:145–151. doi: 10.1007/s004300050057. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 16.Ijsselmuiden O E, Schouls L M, Stolz E, Aelbers G N, Agterberg C M, Top J, van Embden J D. Sensitivity and specificity of an enzyme-linked immunosorbent assay using the recombinant DNA-derived Treponema pallidum protein TmpA for serodiagnosis of syphilis and the potential use of TmpA for assessing the effect of antibiotic therapy. J Clin Microbiol. 1989;27:152–157. doi: 10.1128/jcm.27.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaltenboeck B, Heard D, DeGraves F J, Schmeer N. Use of synthetic antigens improves detection by enzyme-linked immunosorbent assay of antibodies against abortigenic Chlamydia psittaci in ruminants. J Clin Microbiol. 1997;35:2293–2298. doi: 10.1128/jcm.35.9.2293-2298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp D J, Cowman A F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci USA. 1981;78:4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein P, Somorjai R L, Lau P C. Distinctive properties of signal sequences from bacterial lipoproteins. Protein Eng. 1988;2:15–20. doi: 10.1093/protein/2.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Konkel M E, Mead D J, Cieplak W., Jr Cloning, sequencing, and expression of a gene from Campylobacter jejuni encoding a protein (Omp18) with similarity to peptidoglycan-associated lipoproteins. Infect Immun. 1996;64:1850–1853. doi: 10.1128/iai.64.5.1850-1853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostrzynska M, O’Toole P W, Taylor D E, Trust T J. Molecular characterization of a conserved 20-kilodalton membrane-associated lipoprotein antigen of Helicobacter pylori. J Bacteriol. 1994;176:5938–5948. doi: 10.1128/jb.176.19.5938-5948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Fox J G, Whary M T, Yan L, Shames B, Zhao Z. SCID/NCr mice naturally infected with Helicobacter hepaticus develop progressive hepatitis, proliferative typhlitis, and colitis. Infect Immun. 1998;66:5477–5484. doi: 10.1128/iai.66.11.5477-5484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livingston, R. S., L. K. Riley, C. L. Besch-Williford, R. R. Hook, Jr., and C. L. Franklin. Elevated interferon-gamma mRNA levels and inflammatory bowel disease in the ceca of Helicobacter hepaticus infected female A/JCr mice. Submitted.
- 25.Livingston R S, Riley L K, Steffen E K, Besch-Williford C L, Hook R R, Jr, Franklin C L. Serodiagnosis of Helicobacter hepaticus infection in mice by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1997;35:1236–1238. doi: 10.1128/jcm.35.5.1236-1238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnarelli L A, Fikrig E, Berland R, Anderson J F, Flavell R A. Comparison of whole-cell antibodies and an antigenic flagellar epitope of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J Clin Microbiol. 1992;30:3158–3162. doi: 10.1128/jcm.30.12.3158-3162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malarkey D E, Ton T V, Hailey J R, Devereux T R. A PCR-RFLP method for the detection of Helicobacter hepaticus in frozen or fixed liver from B6C3F1 mice. Toxicol Pathol. 1997;25:606–612. doi: 10.1177/019262339702500611. [DOI] [PubMed] [Google Scholar]
- 28.Mattar S, Scharf B, Kent S B, Rodewald K, Oesterhelt D, Engelhard M. The primary structure of halocyanin, an archaeal blue copper protein, predicts a lipid anchor for membrane fixation. J Biol Chem. 1994;269:14939–14945. [PubMed] [Google Scholar]
- 29.Morgan T J, Whary M T, Foltz C A, Saunders K E, Fox J G. Enzyme-linked immunosorbent assay (ELISA) for fecal IgA to Helicobacter hepaticus: a rapid, noninvasive screening test for infection. Contemp Top Lab Anim Sci. 1997;36:45. [Google Scholar]
- 30.Ohashi H, Goto K, Takakura A. Identification of widespread Helicobacter hepaticus infection in mouse colonies in Japan, abstr. PS11. Contemp Top Lab Anim Sci. 1997;36:44–45. [Google Scholar]
- 31.Rice J M. Helicobacter hepaticus, a recently recognized bacterial pathogen, associated with chronic hepatitis and hepatocellular neoplasia in laboratory mice. Emerg Infect Dis. 1995;1:129–131. doi: 10.3201/eid0104.950404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley L K, Franklin C L, Hook R R, Jr, Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol. 1996;34:942–946. doi: 10.1128/jcm.34.4.942-946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shames B, Fox J G, Dewhirst F, Yan L, Shen Z, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. . (Erratum, 163:279, 1987.) [DOI] [PubMed] [Google Scholar]
- 37.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. . (Erratum, 389:412, 1997.) [DOI] [PubMed] [Google Scholar]
- 38.von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989;2:531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]
- 39.Ward J M, Anver M R, Haines D C, Benveniste R E. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 40.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 41.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 42.Whary M T, Morgan T J, Dangler C A, Gaudes K J, Taylor N S, Fox J G. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worley K C, Wiese B A, Smith R F. BEAUTY: an enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity search results. Genome Res. 1995;5:173–184. doi: 10.1101/gr.5.2.173. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi K, Yu F, Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]