Summary
Primary culture and long-term maintenance of primary retinal pigment epithelium (RPE) is a useful model system for the study of ocular pathologies such as age-related macular degeneration. Here, we detail the steps for the isolation and long-term culture of primary porcine RPE. We also describe steps for cryoprotecting, cryosectioning, and interrogating with immunofluorescence and histochemistry RPE cells grown on transwell membranes. These techniques can be used in histological studies to detect sub-RPE deposits.
For complete details on the use and execution of this protocol, please refer to Pilgrim et al., (2017).
Subject areas: Cell biology, Cell culture, Cell isolation
Graphical abstract
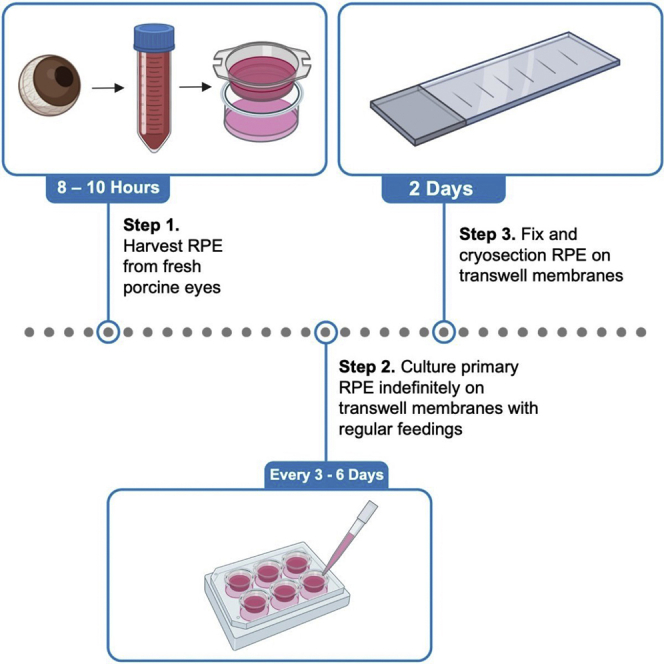
Highlights
-
•
Step-by-step protocol for isolation of primary RPE from porcine eyes
-
•
Long-term primary RPE culture on transwell cell culture inserts
-
•
Transwell membrane cryosectioning technique
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Primary culture and long-term maintenance of primary retinal pigment epithelium (RPE) is a useful model system for the study of ocular pathologies such as age-related macular degeneration. Here, we detail the steps for the isolation and long-term culture of primary porcine RPE. We also describe steps for cryoprotecting, cryosectioning, and interrogating with immunofluorescence and histochemistry RPE cells grown on transwell membranes. These techniques can be used in histological studies to detect sub-RPE deposits.
Before you begin
This protocol provides steps for isolation and long-term culture of primary retinal pigment epithelium (RPE) cells from 50 pig eyes, in addition to histology techniques that allow for reproducible sectioning of RPE attached to transwell membranes. This protocol can be modified for different eye quantities, has been used successfully to isolate primary RPE from bovine eyes with similar results, and is likely transferable to other species, including human donor tissue.
Obtain fresh eyes and prepare workspace
Timing: <6 h
-
1.
Obtain 50 fresh porcine eyes from a local abattoir. Eyes should ideally be recovered less than 6 h post-mortem and transported on ice for optimum RPE recovery and survival.
-
2.
Set out 2 bottles of L-15 media on workbench at least 1 h before beginning dissection to ensure media is 20°C–25°C in temperature.
-
3.Set up workspace in a biosafety cabinet. Useful items to be placed inside hood include:
-
a.Bucket lined with biohazard waste bag.
-
b.Autoclaved dissection tools: #10 blade scalpel, curved tip forceps, scissors.
-
c.Povidone-iodine prep (PVP) solution (10%) in a Petri dish.
-
a.
-
4.
Once biosafety cabinet is prepared and L-15 media is 20°C–25°C, place eyes in biosafety cabinet, and keep on ice until ready to use.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Fresh Pig Eyes | Johnsonville, LLC Sustainable Swine Resources |
https://www.ssr-solutions.com/ |
| Chemicals, peptides, and recombinant proteins | ||
| Leibovitz’s L-15 Medium | Gibco | 11415064 |
| DMEM, High Glucose, HEPES Medium | Gibco | 12430054 |
| Antibiotic-Antimycotic | Gibco | 15240062 |
| Fetal Bovine Serum (not heat inactivated) | Gibco | 26140079 |
| PVP Prep Solution | Medline | MDS093940 |
| Percoll® | Sigma-Aldrich | P4937 |
| Sodium phosphate dibasic (Na2HPO4) | Sigma-Aldrich | S5136 |
| Sodium Chloride (NaCl) | Sigma-Aldrich | S3014 |
| Deoxyribonuclease I | Sigma-Aldrich | DN25 |
| Trypsin powder | Gibco | 27250018 |
| Sakura Finetek™ Tissue-Tek™ O.C.T. Compound | Fisher Scientific | 12351753 |
| Other | ||
| Falcon® Permeable Support for 6-well Plate with 0.4 μm Transparent PET Membrane | Corning | 353090 |
| Falcon® 6-well TC-treated Polystyrene Permeable Support Companion Plate | Corning | 353502 |
| Falcon® Permeable Support for 12-well Plate with 0.4 μm Transparent PET Membrane | Corning | 353180 |
| Falcon® 12-well TC-treated Polystyrene Permeable Support Companion Plate | Corning | 353503 |
| Falcon® Permeable Support for 24-well Plate with 0.4 μm Transparent PET Membrane | Corning | 353095 |
| Falcon® 24-well TC-treated Cell Polystyrene Permeable Support Companion Plate | Corning | 353504 |
| Fisherbrand™ Petri Dishes | Fisher Scientific | FB0875712 |
| Autoclaved Dissection Tools | N/A | N/A |
| Costar® 6-Well Plates | Corning | 3516 |
| Falcon® 50mL Conical Tubes | Corning | 352070 |
| Falcon® 15mL Conical Tubes | Corning | 352096 |
| Epredia™ Disposable Base Molds | Fisher Scientific | 22-050-160 |
| Cryostat | N/A | N/A |
| Fisherbrand™ Superfrost™ Plus Microscope Slides | Fisher Scientific | 12-550-15 |
| Fine Tip Paint Brushes | N/A | N/A |
Materials and equipment
Trypsinization Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Leibovitz’s L-15 Medium | 99.75% | 400 mL |
| Trypsin | 0.25% | 1 g |
| Total | N/A | 400 mL |
Prepare fresh and use immediately.
DNaseI Stock Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Leibovitz’s L-15 Medium | 90% | 1 mL |
| DNase I | 10% | 0.1 g |
| Total | N/A | 1 mL |
Prepare fresh and use immediately.
Percoll Prep Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sterile H2O | N/A | Adjust to 50 mL |
| NaCl | 0.15 M | 0.44 g |
| Na+2P04 | 0.01 M | 0.07 g |
| Total | N/A | 50 mL |
Store at 4°C for up to 6 months.
40% Percoll Cushion
| Reagent | Final concentration | Amount |
|---|---|---|
| Percoll Prep Solution | 60% | 18 mL |
| Percoll | 40% | 12 mL |
| Total | N/A | 30 mL |
Prepare fresh and use immediately.
Primary RPE Culture Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM + HEPES | 89% | 445 mL |
| FBS | 10% | 50 mL |
| AA | 1% | 5 mL |
| Total | N/A | 500 mL |
Store at 4°C for up to 6 weeks.
Step-by-step method details
Isolation of primary porcine RPE
Timing: 8–10 h
This protocol provides effective preparation, harvesting, and purification steps for the isolation and culture of primary porcine RPE on transwell membranes. All steps should be carried out in a biosafety cabinet. Dissection tools should be autoclaved before use and should be routinely disinfected throughout the protocol using 70% ethanol to minimize the risk of contaminating the freshly isolated RPE.
-
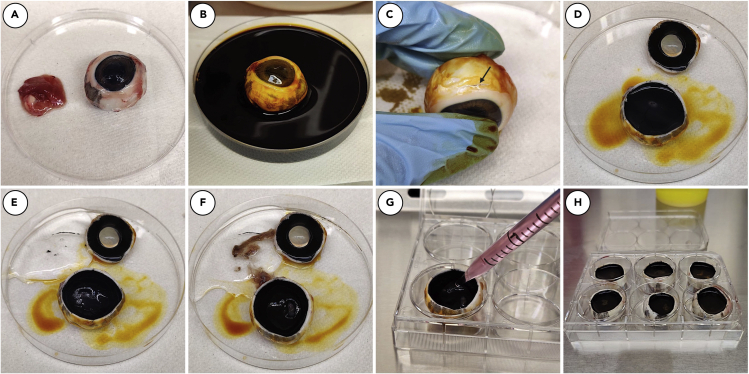
1.Prepare eyes for dissection.
-
a.Using scissors, carefully remove excess tissue surrounding the eye globe and trim the optic nerve back to the sclera without puncturing eye globe. (Figure 1A).
-
b.Thoroughly disinfect outside of eye with PVP solution. Place globe in a Petri dish. (Figure 1B).
-
c.Repeat steps 1a – 1b with desired number of eyes.
-
a.
Note: We recommend working through steps 1–3 in groups of 6 while leaving the remaining eyes on ice to maintain tissue integrity. Following removal of the extra-ocular tissues and optic nerves from the first batch of 6 eyes, proceed to step 2.
-
2.Working in batches of six globes, remove anterior chamber (cornea and iris), lens, vitreous humor, and retina.
-
a.Carefully pierce the globe approximately 0.5 cm posterior to the limbus using a #10 blade scalpel (Figure 1C).
-
b.Using sharp scissors carefully cut around the circumference of the globe, maintaining 0.5 cm distance from limbus.
-
c.Remove the anterior chamber. (Figure 1D).
-
d.From the remaining posterior chamber, carefully remove and discard the vitreous humor, using curved tip forceps (Figure 1E).Note: If the vitreous does not easily separate use sharp scissors to carefully cut around the opening in the globe to separate the vitreous body and anterior hyaloid membrane from the eye cup. Following this step, the vitreous should be easily dislodged using forceps. Any remaining vitreous will detach with the removal of the neural retina in step 2e.
-
e.Using curved tip forceps remove the neural retina, being careful not to disrupt the RPE below. (Figure 1F).
-
i.Starting near the optic nerve head gently puncture the neural retina.
-
ii.Slide the tips of the forceps underneath the retina and slowly lift the retina away from the underlying RPE.
-
iii.The retina will detach from the RPE and can be gently peeled away with minimal damage to the RPE below.Note: Due to the heavy pigmentation of the RPE, the neural retina is sometimes difficult to visualize; however, the retinal vasculature and optic nerve head can usually be clearly seen and can be helpful in locating and removing the neural retina.
-
i.
-
a.
-
3.Prepare to harvest RPE.
-
a.Once RPE is exposed, place each globe into a well of a sterile 6-well plate.
-
b.Fill each eye cup with L-15 media (20°C–25°C, 4–5 mL per globe; Figure 1G).
-
c.Place the 6-well plate containing globes filled with L-15 media (Figure 1H) into an incubator (37°C, 5% CO2).
-
d.Repeat steps a-c for remaining eyes (nine 6-well plates in total).
-
a.
-
4.Harvest and purify RPE.
-
a.After all eyes have been prepared for RPE harvesting (steps 1–3), prepare fresh 0.25% trypsin in L-15 media (20°C–25°C) and sterilize using a 0.22 μm bottle top filter.Note: We recommend making 8 ml of trypsinization media per eye (400 ml for 50 eyes).
-
b.First Trypsinization Step.
-
i.Working with one 6-well plate at a time in a biosafety cabinet, remove L-15 media from eye cups with a pipette and replace with 0.25% trypsin in L-15 media (about 4 mL per eye).Note: In this step, aspiration of the L-15 media will help to remove any remaining retinal tissue. (Figure 2).
-
ii.After filling each globe with trypsinization media, replace the 6-well plate into the incubator (37°C, 5% CO2) and repeat steps 4b with the remaining 6-well plates.
-
iii.Incubate each plate for 45 min (37°C, 5% CO2).
-
i.
-
c.Following the 45-min incubation period, retrieve each 6-well plate and place in a biosafety cabinet working one six-well plate at a time.
-
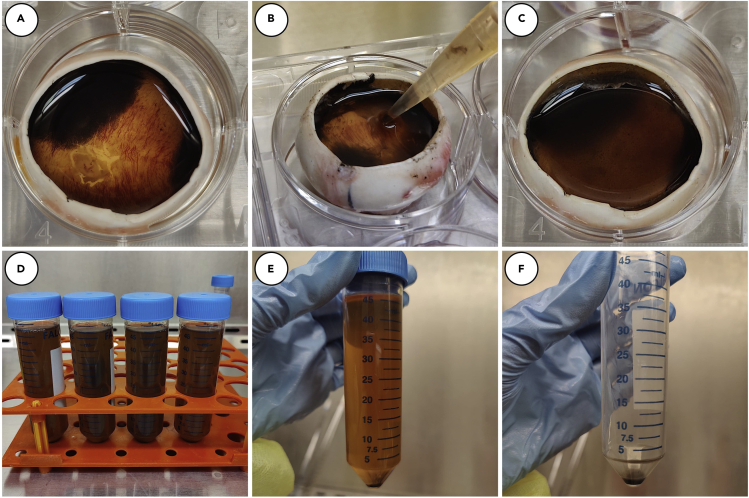
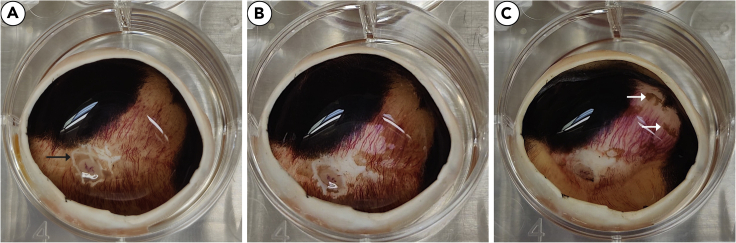
d.Use a P1000 pipette to gently triturate to loosen the RPE cells from the walls of each eye cup. When any loose RPE cells have been dislodged, transfer the trypsinization media from each globe into a 50 mL conical tube (Figures 3A–3D and 4B).Note: The trypsinization media containing RPE cells recovered from all 50 eyes should be pooled together and distributed evenly across four 50 mL conical tubes.
-
e.To maximize the harvesting efficiency, add 4 mL fresh trypsinization media (0.25% trypsin in L-15 media) to each globe. Return the 6-well plate to incubator.Note: Do not trypsinize the tissue for longer than necessary. Some eyes may release all RPE in first trypsinization incubation. In this case, skip the second trypsinization step (step 4f) and discard eye cup in biohazardous waste.
-
f.Second Trypsinization Step.
-
i.Repeat steps 4c – 4e with remaining 6-well plates.
-
ii.Incubate each plate for an additional 45 min (37°C, 5% CO2). During the incubation step:
-
iii.During the incubation step, centrifuge recovered media from step 4d (approximately 200 mL) at 400–500×g for 20 min. Resuspend all 4 pellets in 2 mL total of fresh L-15 media and set aside at 20°C–25°C. (Figures 3E and 3F).
-
iv.Place Primary RPE Culture Medium in 37°C water bath to warm.
-
i.
-
g.Following the 45-min incubation period, retrieve each 6-well plate and place in a biosafety cabinet working one six-well plate at a time.
- h.
-
i.Once the trypsinization media has been removed and RPE has been retrieved from the eye cup, discard eye cups and 6-well plate into biohazardous waste.
-
j.Repeat steps 4g – 4i with remaining 6 well plates.
-
k.Centrifuge recovered media (approximately 200 mL) at 400–500×g for 20 min. While centrifuging:
-
i.Prepare 1 mL of DNaseI Stock Solution in L-15 media in a 15-mL conical tube or Eppendorf tube. Sterile filter (0.22 μm) and set aside at 20°C–25°C for use in step 4n.
-
ii.In two 50 mL conical tubes, prepare two 30 mL 40% Percoll cushions (prepared with Percoll Prep Solution listed above in Materials and Equipment tables, pH 7.4).
-
i.
-
l.Resuspend all 4 pellets from Step 4k in 2 mL total of fresh L-15 media and add to recovered RPE from previous centrifugation step (4f-ii) in a 50mL conical tube (Figures 3E and 3F).
-
m.Dilute RPE suspension to 9 mL total using L-15 media (20°C–25°C).
-
n.Add 1 mL of DNaseI Stock Solution to RPE suspension. This should yield a working concentration of 1% DNaseI. Mix well by gentle inversion of the tube. Incubate for 2 min at 20°C–25°C.
-
o.Dilute RPE suspension to 40 mL using L-15 media (20°C–25°C).
-
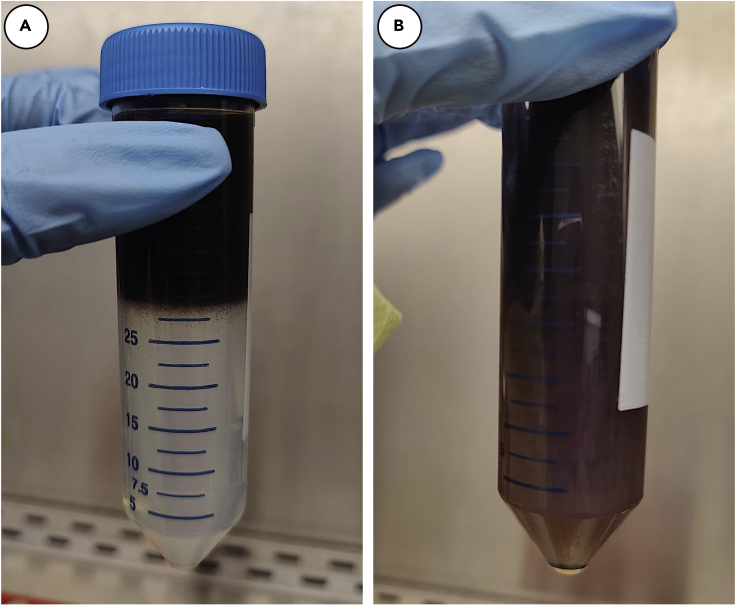
p.Carefully layer 20 mL of RPE suspension on top of each of the 30 mL Percoll cushions. (Figure 5A).
-
q.Centrifuge at 500×g for 3 min. After centrifugation, the Percoll cushion should be disrupted and a pellet of pigmented cells should be visible (Figure 5B).
-
a.
-
5.Plate RPE on transwell inserts.
-
a.Pour off Percoll and resuspend pellets at a density of 100,000 cells per mL in prewarmed Primary RPE Culture Medium (step 4f). Plate into the upper chamber of a transwell insert at the following densities and volumes:
-
i.For 24-well plates add 0.25 mL of resuspended cells to the upper chamber of each well. Add 0.8 mL of prewarmed Primary RPE Culture Medium to lower chamber.
-
ii.For 12-well plates add 0.5 mL of resuspended cells to the upper chamber of each well. Add 1 mL of prewarmed Primary RPE Culture Medium to lower chamber.
-
iii.For 6-well plates add 1.5 mL of resuspended cells to the upper chamber of each well. Add 2 mL of prewarmed Primary RPE Culture Medium to lower chamber.
-
i.
-
b.Place freshly plated RPE in incubator (37°C, 5% CO2).
-
c.Carefully change 100% of media in top and bottom wells 36–48 h after isolation in a biosafety cabinet, using fresh, prewarmed Primary RPE Culture Medium. For this media change, use the same media volumes as in Step 5a.
 CRITICAL: Do not move fresh plates until this time, except to place them in the biosafety cabinet. Moving them before their first media change can cause cells to gather in center of the well and disrupts even distribution.
CRITICAL: Do not move fresh plates until this time, except to place them in the biosafety cabinet. Moving them before their first media change can cause cells to gather in center of the well and disrupts even distribution. -
d.After first change, primary RPE can be maintained for up to 8 months with complete media changes every 3–5 days. (Figure 6, troubleshooting 1 and 2).Note: Newly isolated RPE may take several days to anchor on membrane and spread out (Figure 7). For this reason, media should be changed with care for first 10 days to prevent loss of cells.Note: Signs of unsuccessful RPE isolation should present within one month of isolation. RPE cells should be monitored for loss of typical RPE morphology, cell layering, and vessel formation during this time (Figure 8, troubleshooting 3).Note: Some loss of pigmentation may occur over extended periods of culture, but this does not negative affect cell viability.
-
a.
Figure 1.
Dissection and preparation of eye cups for RPE isolation
After extraocular tissue is removed from eye (A) and eye is disinfected with PVP prep solution (B), a scalpel is used to make an incision in the eye (C). Clean scissors are used to cut around scleral-corneal interface and anterior chamber (D), vitreous (E), and retina (F) is be removed. Eye cup is then placed in a 6-well plate and filled with L-15 medium (20°C–25°C) (G and H).
Figure 2.
Retinal fragments in eye cup
Any retinal fragments left in eye cup (white arrows) should be removed using a pipette prior to first trypsinization step.
Figure 3.
Dissection and preparation of eye cups for RPE isolation
Eye cups are filled with trypsinization media (A) and incubated for 30 min at 37oC. A pipette is used to gently dislodge any loose RPE (B and C) and trypsinization media is collected in 50mL conical tubes (D). Collected media is centrifuged at ∼450×g for 20 min and pellet is collected (E and F).
Figure 4.
RPE in eye cup before and after first and second trypsinization steps
Before trypsinization steps, RPE coverage is uniform (A). Some damage to RPE may occur with with removal of the retinal (black arrow). RPE will begin to lift after first trypsinization step, however large regions of RPE may remain (B). After second trypsinization step, most RPE should be harvested, with small regions of RPE remaining (white arrows) (C).
Figure 5.
Centrifugation of isolated RPE on Percoll cushion
Isolated RPE is layered on a cushion of 40% Percoll (A) and centrifuged for 3 min at 500×g. After centrifugation, pellet is recovered (B).
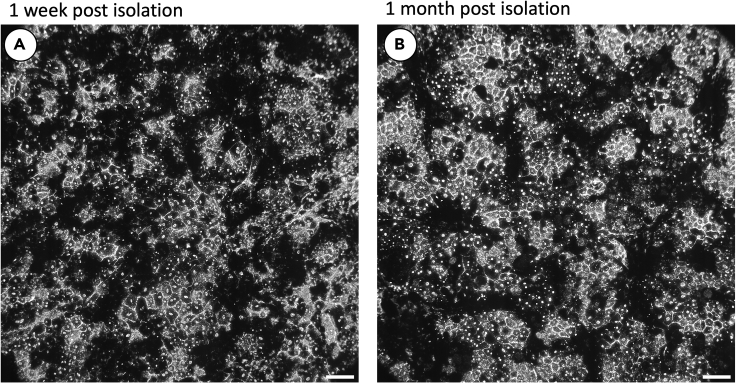
Figure 6.
Isolated primary porcine RPE
The primary RPE shown was isolated using the above protocol and was imaged at 1 week (A) and 1 month (B) post isolation. After 1 week (approximately 2 feedings), cell shedding should cease and surviving RPE should be well anchored to the membrane. These can be maintained indefinitely with regular feeding. Scale bars approximately 50um.
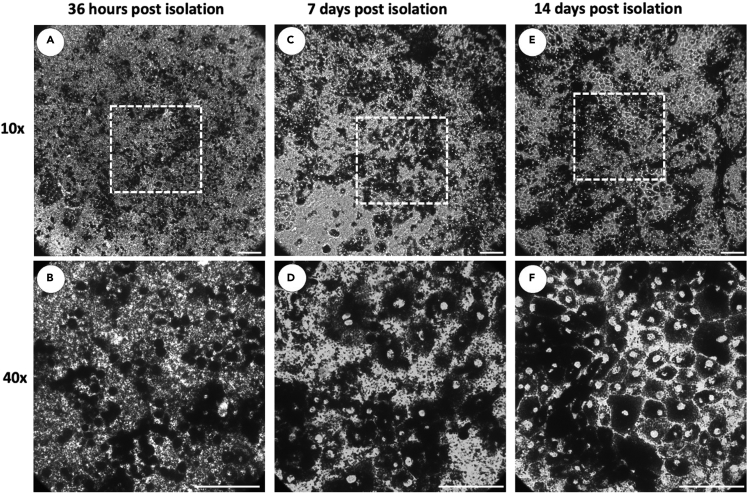
Figure 7.
Isolated primary porcine RPE. Isolated primary RPE take several days to anchor to the membrane and recover organization
After the first feeding, 36–48 h post isolation, cells are tightly contracted (A and B). At 1 week post isolation, cells have begun to release and anchor to membrane (C and D) with cell organization largely recovered 2 weeks after isolation (E and F). Scale bars approximately 50um.
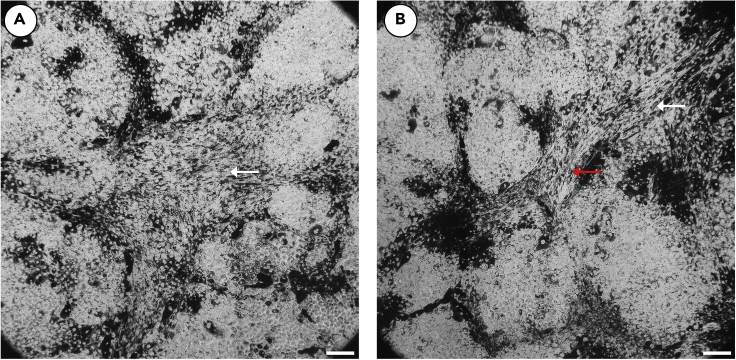
Figure 8.
Loss of typical RPE morphology and vessel formation in unsuccessful RPE isolation
Unsuccessful RPE isolation will typically present in the first month as a loss of typical RPE morphology. Most notably, RPE cultures may demonstrate a lengthening of RPE cells (A, white arrows) and vessel formation (B, red arrow). If this occurs refer to troubleshooting Problem 3. Scale bars approximately 50um.
Cryosectioing of transwell inserts
Timing: 2 days
This step describes a technique that reliably yields satisfactory histological sections from transwell membranes.
-
6.Fix and cryoprotect cells on transwell membrane.
-
a.Remove cell culture media and gently wash upper and lower chambers of the transwell membrane with PBS to remove excess cell culture media. Apply and remove PBS gently using the walls of the transwell, being careful not to dislodge any cells from the membrane.
-
b.Fix cells on the transwell membrane for 30 min at 20°C–25°C by adding 4% paraformaldehyde (PFA) to both the upper and lower chambers.
-
c.Remove the PFA and wash twice with PBS (20°C–25°C).
-
d.Using a #10 blade scalpel, gently cut the transwell membrane from the well around the edge to remove the membrane from the plastic.
-
e.Using curved tip forceps and a small pair of scissors, cut membrane in half to provide a flat edge for cryosectioning (Figure 9A).
-
f.Cryoprotect tissue using a series of sucrose solutions, switching the membrane between wells containing each sucrose solution.
-
i.10% sucrose: 30 min at 20°C–25°C.
-
ii.20% sucrose: 30 min at 20°C–25°C.
-
iii.30% sucrose: Overnight at 4°C.
-
i.
-
a.
-
7.Embed membrane and prepare block.
-
a.Embed membrane in Optimal Cutting Temperature (OCT) media in an appropriately sized embedding mold and allow to freeze on dry ice. (Figure 9A).
-
i.Place a layer of OCT into empty mold, minimizing bubbles.
-
ii.Place transwell membrane cell side down onto OCT layer, taking care to align the flat edge of membrane with edge of mold.
-
iii.Apply more OCT on top of membrane so that the membrane is located in the middle of the block when frozen.
-
iv.It may be helpful to mark the location of the membrane on the mold (shown in black marks in Figure 9A).
-
i.
-
b.Following freezing, remove the embedded membrane from mold and mount into cryostat so the membrane is orientated vertically and perpendicular to blade. (Figures 9B and 9C).
-
a.
-
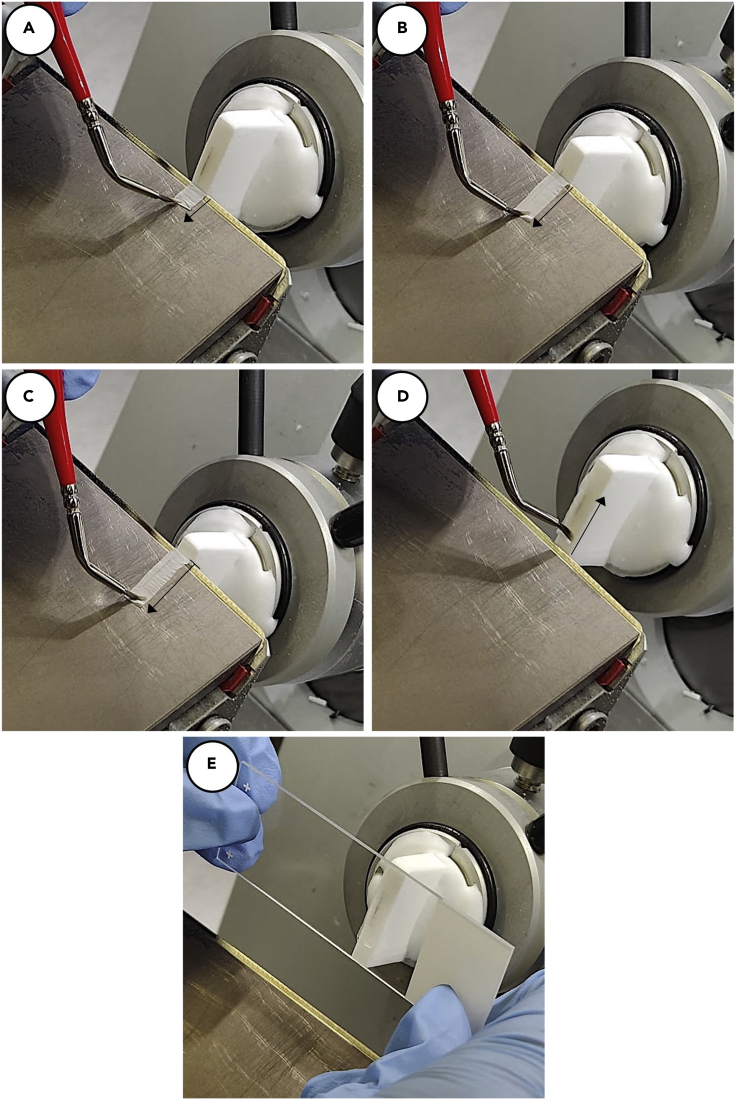
8.Hanging cryosection of membrane (Figure 10).
-
a.At a thickness of 5–10 μm, slowly begin sectioning through block.
-
b.Using a very fine tipped paintbrush, pull down section while slowly continuing to move block down on the blade. (Figures 10A and 10B).
-
c.Stop the section after the blade has traversed the membrane, but before the edge of the OCT block (Figure 10C, troubleshooting 4).
-
d.Reverse the direction of the block, removing the block from the blade and using the paintbrush press the section back onto the block surface (Figure 10D).
-
e.Retrieve section by gently pressing Superfrost Plus Microscope Slide directly to section on block (Figure 10E, troubleshooting 5).
-
f.After section is retrieved, finish advancing the blade through the block before starting a new section.
-
g.Repeat through block as necessary.
-
a.
Note: This cryosectioning technique should be performed without the use of an anti-roll cover glass. The cover glass will cause the separation of the membrane from the OCT block and will result in loss of orientation of the membrane.
- 9.
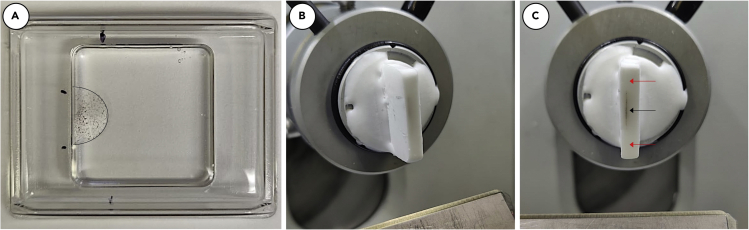
Figure 9.
Embedding of membrane and mounting of block
Once fixed, removed from transwell support, and cryoprotected, the membrane should be embedded in OCT in an appropriate size mold. It may be helpful to mark the location of the membrane, shown as black marks on mold. Note: The membrane has been outlined for easier visibility (A). The OCT block should be mounted into cryostat so membrane is perpendicular to the blade (black arrow) with excess OCT both above and below membrane (red arrows) (B and C).
Figure 10.
Stepwise images of hanging cryosection technique
Table 1.
Tested antibodies and suggested antibody dilutions
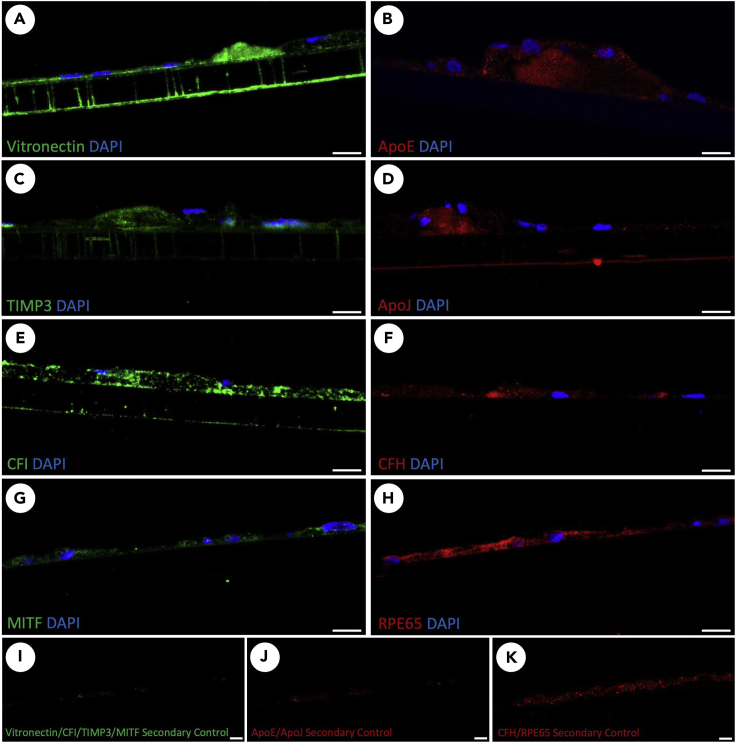
| Antigen | Dilution | Supplier | Identifier |
|---|---|---|---|
| ZO-1 (Figure 11B) | 1:250 | Thermo Fisher Scientific | Invitrogen 40-2200 |
| Nile Red (1mg/mL acetone) (Figure 11C) | 1:1000 | Thermo Fisher Scientific | Invitrogen N1142 |
| Collagen 5 (Figure 11E) | 1:200 | Thermo Fisher Scientific | Rockland 600-401107-01 |
| Vitronectin (Figure 12A) | 1:100 | Thermo Fisher Scientific | Proteintech 15833-1-AP |
| ApoE (Figure 12B) | 1:100 | Thermo Fisher Scientific | Invitrogen PA5-18361 |
| TIMP3 (Figure 12C) | 1:100 | Abcam | ab39184 |
| Clusterin/ApoJ (Figure 12D) | 1:100 | Thermo Fisher Scientific | Invitrogen PA5-46931 |
| Complement factor I (CFI) (Figure 12E) | 1:100 | Thermo Fisher Scientific | Invitrogen PA5-96371 |
| Complement factor H (CFH) (Figure 12F) | 1:100 | Thermo Fisher Scientific | Invitrogen GAU 018-03-03 |
| MITF (Figure 12G) | 1:100 | Thermo Fisher Scientific | Invitrogen PA5-38294 |
| RPE65 (Figure 12H) | 1:100 | Santa Cruz Biotechnology | sc-73616 |
Figure 12.
Panel of tested antibodies stained with suggested antibody dilutions from Table 1
Transwell membranes with primary porcine RPE, aged 20 weeks, were fixed and cryosectioned using the outlined protocol and standard staining procedures. Immunofluorescence staining for vitronectin (A), ApoE (B), TIMP3 (C), ApoJ (D), CFI (E), CFH (F), MITF (G), and RPE65 (H) are shown as well as appropriate secondary controls (I–K). Scale bars approximately 10um.
Expected outcomes
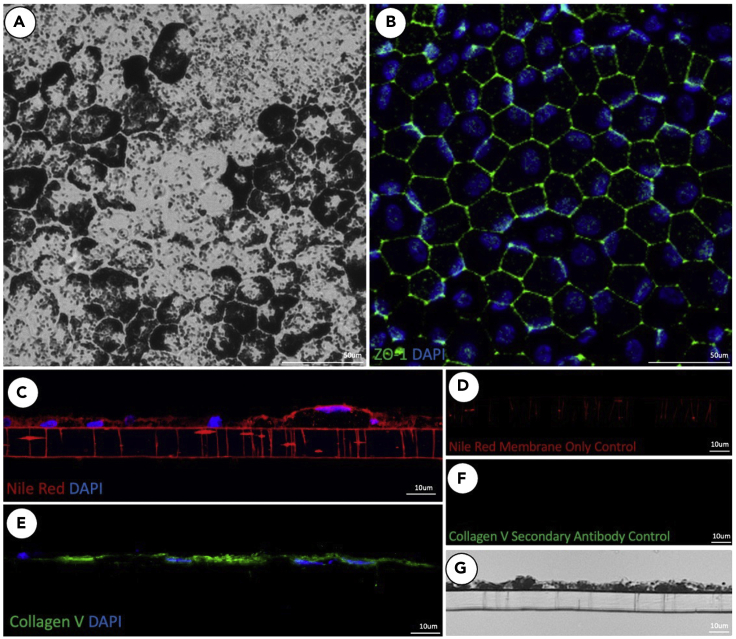
This protocol provides a reproducible and reliable method for the isolation of RPE cells from porcine eyes. Upon completion of this protocol, RPE maintain organization and pigment and can be cultured indefinitely with regular media changes (Figure 6). Cells maintain pigmentation as well as organization after 20 weeks in culture (Figures 11A and 11B) and show deposition of collagen 5, indicating the development of a basement membrane (Figure 11E). RPE cells also demonstrate lipid deposition through the pores of the transwell membrane, indicating nutrient and metabolite trafficking (Figure 11C).
Figure 11.
Isolated primary porcine RPE maintain organization and pigmentation through 20 weeks of aging while also showing evidence of lipid trafficking through membrane pores and basement membrane development
Transwell membranes were fixed and flatmounted or cryosectioned using the outlined protocol. Brightfield images of flatmounted RPE reveal persistence of pigment throughout 20-week aging period (A), while ZO-1 staining revealed presence of tight junctions and polygonal morphology (B). Cryosections demonstrated evidence of lipid deposition through membrane pores, shown by nile red lipid stain (C) and evidence of a collagenous basement membrane via collagen V staining (E). Nile red stained membrane only and secondary antibody only controls (D and F). Brightfield images of cryosections show maintenance of pigment (G).
Limitations
As this protocol isolates RPE from fresh pig eyes, results are subject to natural variation in eye size and RPE density of pig tissue. This may cause some variation in the amount of RPE isolated. Cell viability after isolation may also vary based on time between eye harvest and RPE isolation. For best results, we suggest using eyes that are less than 6 h post-mortem. In addition, as this is primary cell culture, RPE harvested in this protocol are more susceptible to infection.
Troubleshooting
Problem 1
High infection rate of isolated RPE (Step 5d).
Potential solution
-
•
Be sure to thoroughly disinfect the eyes before using PVP prep solution.
-
•
Remember to utilize autoclaved dissection tools and to regularly disinfect them with 70% ethanol throughout the dissection process.
Problem 2
Low RPE survival rate after isolation or low yield (step 5d).
Potential solution
-
•
Work with your supplier to ensure eyes are as fresh as possible (<6 h post mortem). Extended amounts of time on ice can decrease cell viability.
-
•
Utilize an additional trypsinization step to release any remaining RPE from eye cup.
Problem 3
Loss of RPE morphology or vessel formation (step 5d, Figure 11).
Potential solution
-
•
Decrease length of both trypsinization incubations.
-
•
Do not leave eye cup in trypsin for longer than necessary (step 4d). Once the majority of RPE has been released from the eyecup, cease collect the media and discard eyecup. Keep in mind that some eyes only require a single trypsinization step to release all RPE.
Problem 4
Curving, rolling, or breakage of tissue while pulling down cryosections (step 8c).
Potential solution
-
•
Pull down section at the same speed as the section, pulling too fast can cause it to rip and too slow can cause it to bunch.
-
•
Replace microtome blade.
Problem 5
Membrane not sticking to microscope slide or membrane section losing proper orientation upon retrieval (step 8f).
Potential solution
-
•
Remember to pull straight down with paintbrush at same speed as section. Any bunching, curving, or rolling of section can cause the membrane to knock loose.
-
•
Press microscope slide straight onto awaiting section on the block. Allowing the section to passively transfer to slide can disrupt the position of the membrane.
-
•
Remember to provide excess OCT block both above and below the membrane to provide plenty of room to manipulate section without disturbing the membrane.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Daniel Lipinski (dlipinski@mcw.edu).
Materials availability
This study did not generate unique reagents.
Acknowledgments
Research reported in this publication was supported in part by the National Eye Institute under award numbers R01EY027767, R01EY032478, and T32EY014537 and the National Institute of General Medical Sciences under award number T32GM080202. This investigation was conducted in part in a facility constructed with support from a Research Facilities Improvement Program, grant number C06RR016511 from the National Center for Research Resources of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Thank you to Christine Duris at the MCW/CRI Histology Core for helping with transwell membrane sectioning. The graphical abstract was created with Biorender.com.
Author contributions
E.H. optimized protocols, performed experiments, collected images, and wrote original draft of manuscript. D.L. and C.C. reviewed and edited the manuscript. D.L. and C.C. provided mentorship and expert advice.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Erika M.S. Hood, Email: eshaw@mcw.edu.
Daniel Lipinski, Email: dlipinski@mcw.edu.
Data and code availability
This study did not generate or analyze datasets or code.
References
- Pilgrim M.G., Lengyel I., Lanzirotti A., Newville M., Fearn S., Emri E., Knowles J.C., Messinger J.D., Read R.W., Guidry C., Curcio C.A. Subretinal pigment epithelial deposition of drusen components including hydroxyapatite in a primary cell culture model. Invest. Ophthalmol. Vis. Sci. 2017;58:708–719. doi: 10.1167/iovs.16-21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate or analyze datasets or code.