Abstract
Background
Numerous systematic reviews and meta-analyses have examined the effects of probiotics used perinatally on prevention or treatment of atopic disease in infants and children. However, to date, no review has examined randomized controlled trials of Lactobacillus rhamnosus, specifically, administered both prenatally and postnatally and its effect over a long period of time.
Objective
The objective was to determine if L. rhamnosus either used solely or in conjunction with other probiotics demonstrates a long-term preventive effect on atopic disease in pediatric patients when used perinatally.
Methods
A systematic review was undertaken to identify those studies where L. rhamnosus was used (either solely or in conjunction with other probiotics). The following databases were searched from the year 2000 through December 8, 2021: PubMed, Cochrane Reviews and Cochrane Central Database of Controlled Trials; systematic reviews were hand searched to identify randomized controlled trials (RCTs). Meta-analytic statistical techniques were then employed. Evaluation of the incidence of atopic eczema was also examined longitudinally based on timeframe. Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessments were employed to determine the quality of the evidence.
Results
Eleven randomized controlled trials were identified which examined L. rhamnosus in its effect on atopy. Risk of bias was low on the majority of the domains assessed. Meta-analysis of the timeframes ≤ 2 years (RR 0.60, 95% CI 0.47–0.75; p < 0.00001) and 6–7 years (RR 0.62, 95% CI 0.50–0.75; p < 0.00001) demonstrated statistically significant reductions in atopic eczema with use of L. rhamnosus. For the 4 to 5-year (RR 0.74, 95% CI 0.55–1.00; p = 0.05) and 10–11-year (RR 0.68, 95% CI 0.37–1.27; p = 0.23) timeframes there was no statistically significant reduction. GRADE assessment for each timeframe was considered moderate in two, owing to high attrition rates in all of the studies, and low in two due to imprecision.
Conclusion
Based on the meta-analysis and GRADE assessments, the use of L. rhamnosus with or without other probiotics appears to have a positive effect in reducing the incidence of atopic eczema in pediatric patients at least out to 7 years. Attrition rates temper these findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-022-00723-x.
Key Points
| The use of Lactobacillus rhamnosus with or without other probiotics when administered to infants pre- and postnatally has a positive effect in reducing the incidence of eczema/atopic dermatitis in children when evaluated at 2 years out and 6–7 years out. |
Introduction
Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin disease that has recently been recognized as a leading cause of, or precursor to, other atopic conditions such as food allergy and asthma [1, 2]. The age of onset of eczema and the severity of the symptoms has been directly correlated to the risk of future atopic conditions [3]. It is estimated that 30% of infants are diagnosed with AD based on the rates of prescribed medications [4].
Various factors are involved in the development of AD, including genetic predisposition such as filaggrin mutations, a decline in barrier function of the skin, environmental factors and microbial dysbiosis [5]. This dysbiosis extends beyond the skin itself.
For several diseases, including AD and food allergy, patterns in the infant gut microbiome during its developmental stages have been detected [6]. These patterns include differences in overall microbial diversity, the relative prevalence of different phyla and the presence of specific strains of bacteria [7].
Studies have shown that the infant gut microbiome is seeded by maternal transfer of bacteria to offspring beginning in pregnancy [8, 9]. Bacterial DNA can be detected in amniotic fluid, in placental and fetal membranes and in umbilical cord blood [10–12]. Maternal transfer of bacteria also occurs during the birthing process and is directly affected by the make-up of the maternal microbiome [8]. Separately, the infant microbiome in early life has been repeatedly shown to alter infant uptake of breastmilk or formula, production of gastrointestinal metabolites and immune regulation [13, 14].
Since the 1990s, the use of probiotic supplementation from the prenatal period through early infancy has been studied as a method to support or optimize gut microbial composition and alter the risk of infant allergic disease. Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [15].
Previous meta-analyses suggest no benefit of oral probiotics in the treatment or prevention of atopic disease [16]. However, the high degree of heterogeneity between studies, which includes differences in probiotic strains, probiotic combinations, probiotic dosages, study populations (maternal vs infant vs both), duration of treatment, stage of intervention, lack of continuation of treatment throughout the perinatal period and outcomes measured impedes direct comparison of studies. This has been a highly debated topic for a number of years in the medical community.
Further, previous systematic reviews have come to a wide range of differing conclusions when probiotics are compared with a placebo in infants with high risk for allergy. One review found that the administration of probiotic microorganisms during pregnancy up until delivery did not have an effect on any outcomes evaluated (including AD) [17]. Similarly, two analyses found that the administration of probiotics may reduce the risk of AD but the evidence was judged to have a low quality [17, 18]. Another meta-analysis combined prenatal plus postnatal with postnatal only administration and found a statistically significant reduction in eczema but not in atopic eczema, with no definition of eczema or atopic eczema provided [19].
When considering bacterial strains, one review found significant risk reduction in atopy with the use of lactobacilli strains (various strains) as monotherapy during pregnancy and lactation [20]. Another analysis concluded that the administration of various genuses of probiotics administered during pregnancy only, in infants early life only or both had a risk reduction in AD when all were combined in a meta-analysis [21]; however, the findings were not statistically significant. Other systematic reviews have found that the administration of various genuses of probiotics administered prenatally and postnatally in infants had a positive and statistically significant effect of reducing the incidence of atopy [20].
These findings can be confusing to interpret as they seemingly contradict each other despite often including the same research. However, probiotic supplementation to a mother prenatally, when the infant’s and mother’s immune systems are effectively combined, is different from probiotic supplementation postnatally when the infant’s gut and immune system are developing independently [18]. Further, it is well understood that the effect of different bacterial strains, even within the same family, may differ [22]. Lastly, and perhaps most importantly, treatment of a disease is fundamentally different than prevention. Therefore, we postulated that the heterogeneity of conclusions in previous meta-analyses resulted largely from too broad a combination of administration protocols, bacterial strains and outcome measures.
Lactobacillus rhamnosus is the most extensively studied strain to date in the treatment of AD [23] and has also been explored for its potential in prevention of AD [24]. The intention of this systematic review and meta-analysis is to build upon a prior systematic review [20] and to focus specifically on the effect of L. rhamnosus when administered both prenatally and postnatally (in order to help remedy the above issues) on eczema, which to the author’s knowledge has not been examined to date. Additionally, this review will also assess the longitudinal effect of L. rhamnosus (± other probiotics) on AD over various timeframes.
Methods
This analysis followed the PRISMA guidelines for systematic reviews and meta-analyses [25] (see Appendix A—PRISMA checklist in the electronic supplementary material [ESM]). A systematic review of the literature was undertaken to identify randomized controlled trials (RCTs) of the oral administration of L. rhamnosus either alone or in conjunction with other probiotics during pregnancy and post-pregnancy in mothers and infants (with probiotic exposure via breast milk or oral supplementation) in order to determine its effect on AD and on adverse events.
The definition used for atopic eczema/dermatitis was extracted from the Mayo Clinic website and from the American Academy of Allergy, Asthma, and Immunology (AAAAI), and includes presence of dry skin, red to brownish patches on the body, small raised bumps which may leak fluid when scratched, thickened cracked scaly skin and raw, swollen skin from scratching (collectively a local inflammation of the skin) [26, 27]. As well, AD outcomes were included if they were moderate to severe only (as defined in each included study) by the Nottingham Eczema Severity Score (NESS) [28] and Eczema Area and Severity Index (EASI) [29] scoring. Studies that included other scoring systems (e.g. SCORAD) were also included where moderate to severe dermatitis was assessed. If any part of these definitions existed in the studies identified, they were included in the meta-analysis. Mild eczema/dermatitis was excluded due to the definition used for EASI (mild being barely perceptible) and the definitions found in each of the papers evaluated (AD—pruritis, chronic relapsing; excluding trivial rash, visible eczema, facial and extensor involvement). All of the definitions found in the studies more clearly mapped to moderate and severe eczema.
The following databases were searched from inception through December 8, 2021: PubMed, Cochrane Central Database of Controlled Trials (CENTRAL) and Cochrane Reviews using the search terms (((((((((((((probiotic) AND randomized) AND trial) AND infant) AND eczema)) AND placebo)) AND pregnancy)) AND Lactobacillus) AND rhamnosus)) AND HN001. Subsequent to this, the references of identified studies were hand searched for additional RCT publications.
Data collection was performed by two independent reviewers using Cochrane characteristics and risk of bias forms and then reviewed collectively to determine inclusion and exclusion of studies. Each assessed risk of bias independently and then convened to discuss and review their risk of bias assessments. Where differences existed, the more conservative risk assessment for bias was made (e.g. low to unclear, unclear; unclear to high, high; low to high, high). A PRISMA diagram was used to depict the distillation of included trials. Review manager (Version 5.3) from the Cochrane Collaboration was used in both the qualitative and quantitative assessments made in the current analysis [30].
Risk of bias assessment using Cochrane methodology was undertaken by one author, reviewed by another and then agreed to. The domains in risk of bias that were assessed included bias arising from the randomization process; bias due to deviations from intended interventions (allocation concealment); bias due to missing outcome data (attrition); bias in measurement of the outcome and who was aware of treatment allocation (blinding); bias in selection of the reported result; and bias related to potential conflicts of interest. Publication bias was assessed via funnel plots [31].
Studies were combined for meta-analytic purposes if two or more examined the same outcome during the same timeframe [32]. Statistics used in the analysis (for dichotomous outcomes, e.g. presence or absence of atopic dermatitis) was the Cochrane-Mantel-Haenszel random effects method (for combining results across studies), which is a statistical technique that generates an estimate of an association between an exposure and an outcome, after adjusting for or taking into account confounding [32]. The effect measure was evaluated using risk ratios. Additionally, if future studies examined patients longitudinally, the original study only was referenced.
Heterogeneity (diversity in outcomes) across studies was measured using the I2 statistic. Substantial heterogeneity was noted if the I2 statistic exceeded 50%. If high heterogeneity existed, sensitivity analysis was performed in order to determine which study(s) affected it and the possible reasons why the study was different from the others.
As alluded to above, studies on the evaluation of atopic eczema/dermatitis over time were grouped based on common timeframes for the evaluation on this outcome.
Lastly, a Grading of Recommendations, Assessment, Development and Evaluations (GRADE) was undertaken to assess the quality of the evidence. GRADE is a transparent framework for developing and presenting summaries of evidence and provides a systematic approach for making clinical practice recommendations [33].
Results
After duplicates were removed from database searching and hand searching of relevant references, 182 records were screened (i.e. abstracts reviewed). Of these, 59 articles were assessed for eligibility with 48 of these excluded with reasons (i.e. follow up longer term studies of Kalliomäki et al. [2001] [34], Wickens et al. [2008] [35] and Kukkonen et al. [2007] [36], total of six; six systematic reviews and meta-analyses which examined various probiotics on health; and 36 which examined other forms of Lactobacillus or other probiotics, where L. rhamnosus was not included). The PRISMA flow chart can be found in supplementary Fig. 1 (see ESM).
A total of 11 randomized, double-blind, placebo-controlled trials were identified (Table 1), reporting on the incidence of AD following prenatal and postnatal use of L. rhamnosus. Of these, ten studies reported on atopic dermatitis up to 2 years out (N = 2572 mother/infants), three studies up to 4–5 years (N = 1278), three studies up to 6–7 years (N = 588) and two studies up to 11 years (N = 999). Of the studies identified, five took place in Finland [34, 36–39], two in Norway [40, 41], two in New Zealand [37,[42], one in Germany [43] and one in Taiwan [44]. Five studies used L. rhamnosus solely compared with placebo [34, 35, 42–44] and six used L. rhamnosus combined with other probiotics versus placebo [36–41].
Table 1.
Summary of included studies
| Study | Maternal inclusion | Administration protocol |
L. rhamnosus (billions CFU) |
Probiotic combination | Follow-up age | Notes/country |
|---|---|---|---|---|---|---|
| Dotterud et al. (2010) [40] | All | 4 weeks prenatal, 12 weeks postnatal [mothers only] | 50 CFU |
L. rhamnosus G B. animalis subsp. lactis Bb-12 L. acidophilus La-5 |
2 y | Norway |
| Huure et al. (2008) [37] | All | ~10 weeks prenatal, through end of breastfeeding [mothers only] | 10 CFU |
L. rhamnosus GG B. animalis subsp. lactis Bb-12 |
1 y | Finland |
| Kalliomäki et al. (2001) [34] | Family history of allergic disease | 2–4 weeks prenatal, 24 weeks postnatal [mother prenatally then infant only postnatally] |
10 twice daily = 20 CFU |
L. rhamnosus GG | 2 y | Finland |
| Kalliomäki et al. (2003) [50] | Family history of allergic disease | 2–4 weeks prenatal, 24 weeks postnatal [mothers prenatally then infant only postnatally] |
10 twice daily = 20 CFU |
L. rhamnosus GG | 4 y | Follow-on study to Kalliomäki 2001; Finland |
| Kalliomäki et al. (2007) [51] | Family history of allergic disease | 2–4 weeks prenatal, 24 weeks postnatal [mothers prenatally then infant only postnatally] |
10 twice daily = 20 CFU |
L. rhamnosus GG | 7 y | Follow-on study to Kalliomäki 2001; Finland |
| Kopp et al. (2008) [43] | Family history of allergic disease |
4–6 weeks prenatal, 12 weeks mothers followed by 12 weeks infant postnatal [mothers AND infants] |
5 twice daily = 10 CFU |
L. rhamnosus GG | 2 y | Germany |
| Kukkonen et al. (2007) [36] | Family history of allergic disease | 2–4 weeks prenatal, 24 weeks postnatal infant only [mothers prenatally then infant only postnatally] |
5 twice daily = 10 CFU |
L. rhamnosus GG L. rhamnosus LC705 B. breve Bb99 P. freudenreichii ssp. shermanii |
2 y | Finland |
| Kuitunen et al. (2009) [52] | Family history of allergic disease | 4 weeks prenatal, 24 weeks postnatal infant only [mothers prenatally then infant only postnatally] |
5 twice daily = 10 CFU |
L. rhamnosus GG L. rhamnosus LC705 B. breve Bb99 P. freudenreichii ssp. shermanii |
5 y | Follow-on study to Kukkonen 2007; Finland |
| Ou et al. (2012) [44] | Sensitization and family history | 16 weeks prenatal, 24 weeks postnatal [mothers prenatally then infant only postnatally] | 10 CFU | L. rhamnosus GG | 18 mo | Taiwan |
| Peldan et al. (2017) [53] | Family history of allergic disease | 2–4 weeks prenatal, 24 weeks postnatal infant only [mother AND infant], in sequence |
5 twice daily = 10 CFU |
L. rhamnosus GG L. rhamnosus LC705 B. breve Bb99 P. freudenreichii ssp. shermanii |
10 y | Follow on study to Kukkonen 2007; Finland |
| Rautava et al. (2002) [38] | Family history of allergic disease | 2–4 weeks prenatal, 24 weeks postnatal [mothers only] |
10 twice daily = 20 CFU |
L. rhamnosus GG | 2 y | Finland |
| Rautava et al. (2012) [39] | Sensitization and family history | 8 weeks prenatal, 8 weeks postnatal [mothers only] | 1 CFU |
L. rhamnosus LPR B. longum BL999 |
2 y | Finland |
| Simpson et al. (2015) [41] | All | 4 weeks prenatal, 12 weeks postnatal [mothers only] | 50 CFU |
L. rhamnosus GG B. animalis subsp. lactis Bb-12 L. acidophilus La-5 |
6 y | Norway |
| Wickens et al. (2018) [43] | Family history of allergic disease | 24 weeks prenatal, 24 weeks postnatal [mothers only] | 6 CFU | L. rhamnosus HN001 | 12 mo | New Zealand |
| Wickens et al. (2008) [35] | Family history of allergic disease | 4 weeks prenatal, 2 years postnatal [mother AND infant] |
6 CFU prenatal, 12 postnatal |
L. rhamnosus HN001 | 2 y | New Zealand |
| Wickens et al. (2012) [54] | Family history of allergic disease | 4 weeks prenatal, 2 years postnatal [mother AND infant] |
6 CFU prenatal, 12 postnatal |
L. rhamnosus HN001 | 4 y | Follow-on study to Wickens 2008; New Zealand |
| Wickens et al. (2013) [55] | Family history of allergic disease | 4 weeks prenatal, 2 years postnatal [mother AND infant] |
6 CFU prenatal, 12 postnatal |
L. rhamnosus HN001 | 6 y | Follow-on study to Wickens 2008; New Zealand |
| Wickens et al. (2018) [43] | Family history of allergic disease | 4 weeks prenatal, 2 years postnatal [mother AND infant] |
6 CFU prenatal, 12 postnatal |
L. rhamnosus HN001 | 11 y | Follow-on study to Wickens 2008; New Zealand |
B animalis subsp. lactis BB-12 Bifidobacterium animalis subsp. BB-12, B. breve Bb99 Bifidobacterium breve Bb99, B. longum BL999 Bifidobacterium longum BL999, L. acidophilus La-5 Lactobacillus acidophilus La-5, L. rhamnosus GG Lactobacillus rhamnosus GG, L, rhamnosus HN001 Lactobacillus rhamnosus HN001, L. rhamnosus LC705 Lactobacillus rhamnosus LC705, L. rhamnosus LPR Lactobacillus rhamnosus LPR, P. freudenreichii ssp. shermanii Propionibacterium freudenreichii ssp. shermanii
Of the 11 studies included, eight studies focused on mother–infant dyads with a family history of atopy/allergies. The other three studies included a general population; however a majority of parents had a history of atopy/allergies, with a range of 70–80% [37, 40, 41].
There was a low risk of bias in five of the seven domains assessed: randomization of sequence generation (11/11); bias in measurement of the outcome (11/11) and who was aware of treatment allocation (11/11); non-selective reporting (11/11); and other biases such as conflicts of interest (10/11) (supplementary Figs 2 and 3, see ESM). There was a high risk of bias in incomplete outcome data (attrition of patients) in 9 out of 11 studies. For allocation concealment (e.g. patients entering treatment almost immediately following randomization), it was unclear in 10 of the 11 studies. Supplementary Fig. 4 is a funnel plot of studies examining the outcome of AD at 2 years. There is a noticeable symmetry in the scatter of the studies indicating a lack of publication bias. Appendix B in the ESM shows the risk of bias assessments for each study.
Outcome of Incidence of Atopic Eczema/Dermatitis
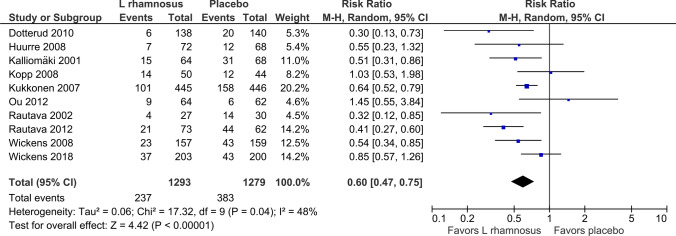
Figure 1 shows the forest plot for incidence of AD out to 2 years in the ten studies examined [34–40, 42–44]. The use of L. rhamnosus during pregnancy and thereafter in infants in the 2-year cohort demonstrated a statistically significant reduction in the incidence of AD (RR 0.60, 95% CI 0.47–0.75); p < 0.00001; I2 = 48%).
Fig. 1.
Forest plot incidence of atopic eczema/dermatitis, < 2 years out
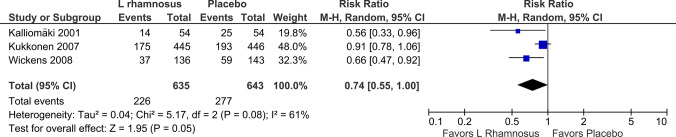
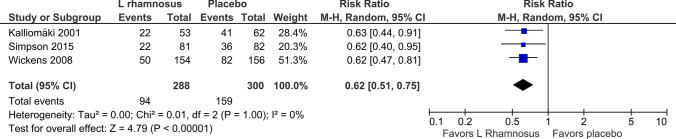
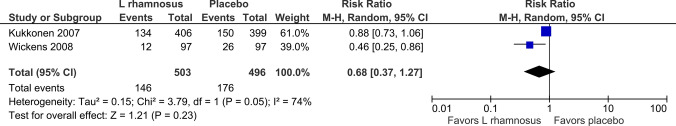
The use of L. rhamnosus during pregnancy and thereafter in infants in three studies [34–36] demonstrated a statistically significant reduction in the incidence of AD (RR 0.74, 95% CI 0.55–1.00; p = 0.05; I2 = 61%) 4–5 years out (Fig. 2). The use of L. rhamnosus during pregnancy and thereafter in infants in three studies [34, 35, 41] demonstrated a statistically significant reduction in the incidence of AD (RR 0.62, 95% CI 0.51–0.75; p < 0.00001; I2 = 0%) 6–7 years out (Fig. 3). The use of L. rhamnosus during pregnancy and thereafter in infants in two studies [35, 36] did not demonstrate a statistically significant reduction in the incidence of AD (RR 0.68, 95% CI 0.37–1.27; p = 0.23; I2 = 74%) out to 11 years (Fig. 4).
Fig. 2.
Forest plot incidence of atopic eczema/dermatitis, 4–5 years out
Fig. 3.
Forest plot incidence of atopic eczema/dermatitis, 6–7 years out
Fig. 4.
Forest plot incidence of atopic eczema/dermatitis, 11 years out
In a post-hoc analysis examining the modes of ingestion of probiotics, mothers and infants received probiotics via the following routes during the perinatal period: mothers only (prenatally and with infants receiving probiotics via breast feeding postnatally) [37–42]; mothers and infants both prenatally and postnatally [35, 42, 43, 53, 55]; and mothers prenatally and infants only postnatally [34, 36, 44, 50–52]. In a subgroup analysis of modes of ingestion for infants, the following was found: in infants who received probiotics prenatally and via breast milk postnatally, there was a significant reduction in AD (RR 0.52, 95% CI 0.28–0.72; p < 0.0001; I2 = 49%) (Supplementary Fig. 5, see ESM); in infants who received probiotics prenatally then ingested them postnatally via diet, there was a significant reduction in AD (RR 0.66, 95% CI 0.46–0.95; p = 0.02; I2 = 42%) (Supplementary Fig. 6, see ESM); and in infants who received probiotics prenatally and both the mother and infant continued to ingest them via diet postnatally, there was no statistical difference (RR 0.89, 95% CI 0.64–1.25; p = 0.51; I2 = 0%) (Supplementary Fig. 7, see ESM).
In a further post-hoc subgroup analysis of single-strain L. rhamnosus versus mixed strain at 2 years, the RR was 0.58 (95% CI 0.41–0.82; p = 0.002; I2 = 58%; Supplementary Fig. 8, see ESM) and 0.56 (95% CI 0.39–0.81; p = 0.002; I2 = 25%; Supplementary Fig. 9, see ESM), respectively.
Adverse Events
No adverse events were noted in three of the four studies where adverse events were evaluated [36, 38, 40]. One study noted gastrointestinal symptoms in 39% of the infants during the first 2 months of life in the probiotic arm and 34% in the placebo arm (p = 0.44) [39]. Eight of the studies did not report on adverse events.
Table 2 shows the GRADE profile. Overall, the quality of the evidence was low to moderate for each timeframe examined (≤ 2 years, 4–5 years, 6–7 years, 11 years); with a moderate finding owing mainly to a high attrition rate of patients in the studies. The quality of the evidence was determined to be low in one of the timeframes (11 years out) due to imprecision.
Table 2.
GRADE evidence profile: Incidence rate of atopic eczema with use of L. rhamnosus vs placebo
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | References | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | Incidence rate atopic eczema with L. rhamosus vs placebo | |||||
|
Incidence atopic eczema infants up to 2 years Follow-up: mean 2 years |
Study population | RR 0.6 (0.47–0.75) | 2572 (10 studies) |
⊕⊕⊕⊝ moderatea |
[34, 36–40, 42–45] | |
| 299 per 1000 | 180 per 1000 (141–225) | |||||
| Moderate | ||||||
| 272 per 1000 | 163 per 1000 (128–204) | |||||
|
Incidence eczema at 4–5 years Follow-up: mean 4–5 years |
Study population | RR 0.74 (0.55–1) | 1278 (3 studies) |
⊕⊕⊕⊝ moderatea |
[50, 52, 54] | |
| 431 per 1000 | 319 per 1000 (237–431) | |||||
| Moderate | ||||||
| 433 per 1000 | 320 per 1000 (238–433) | |||||
|
Incidence eczema at 6–7 years Follow-up: mean 6–7 years |
Study population | RR 0.62 (0.51–0.75) | 588 (3 studies) |
⊕⊕⊕⊝ moderatea |
[41, 51, 55] | |
| 530 per 1000 | 329 per 1000 (270–398) | |||||
| Moderate | ||||||
| 526 per 1000 | 326 per 1000 (268–394) | |||||
|
Incidence eczema at 10–11 years Follow-up: mean 11 years |
355 per 1000 | 241 per 1000 (131–451) | RR 0.68 (0.37–1.27) | 999 (2 studies) |
⊕⊕⊝⊝ Lowa,b |
[42, 53] |
Patient or population: patients with eczema or dermatitis
Intervention: Incidence rate atopic eczema with L. rhamosus vs placebo
CI confidence interval, RR risk ratio GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
*The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
aAttrition rate high in all studies leading to moderate assessment for quality of evidence
bIssue of imprecision in studies examining evidence 10–11 years out led to a low grading in the quality of evidence
Discussion
Overall, L. rhamnosus as a monotherapy or when used in conjunction with other probiotic strains during pregnancy through post-pregnancy demonstrated a significant risk reduction in AD in offspring over time; specifically at 2 years and 6–7 years. This finding is an extension of a prior systematic review on the use of monotherapy lactobacilli (various strains) and its risk reduction of AD in infants [20].
This finding is striking in light of previous meta-analyses on the use/administration of probiotics perinatally and their effect on atopic disease in offspring. The previous meta-analyses differ from this study by combining studies with probiotics used in pregnancy only, in infants post-pregnancy only and during pregnancy plus post-pregnancy by mother or infant [17, 20, 21, 23, 45–49]. The previous meta-analyses had also taken a broad perspective on probiotics, combining studies using Lactobacillus rhamnosus, L. acidophilus, L. paracasei, L. reuteri, L. salivarius, B. lactis, B. bifidum, B. longum and B. animalis. Specifically as it relates to ‘like’ meta-analyses, the findings herein are different than those of Szajewska and Horvath [50], who studied L. rhamnosus GG for the prevention of eczema in children and found that L. rhamnosus did not reduce the risk of eczema. Their meta-analysis of five studies included a study which evaluated the administration of L. rhamnosus GG during pregnancy only and a study which examined the administration of L. rhamnosus to infants only. Again, the current analysis included administration of L. rhamnosus with or without other probiotics during the perinatal period. Further, the current analysis builds on a prior 2012 meta-analysis which examined Lactobacilli [20] and not specifically the L. rhamnosus strain. The difference between the Doege et al. meta-analysis [20] and the current one is that Doege et al. combined years 2–7 (whereas the current study broke down the specific timeframes) and the Doege study included L. reuteri, whereas the current analysis only examined L. rhamnosus. Additionally, 11 studies were included in the present analysis whereas Doege et al. only examined four studies.
Even within this limited scope, the daily dosage of L. rhamnosus in billions of CFU varied considerably between studies, as noted in Table 1. Some of the highest dosages of L. rhamnosus were within probiotic preparations containing relatively large counts of other bacterial strains. To date, there have been no independent studies of the effects of interactions between bacterial strains within supplements, or of the minimum or maximum daily dosages of bacterial strains, including L. rhamnosus [48]. There was no noted difference in outcomes when considering correlating daily dosage of L. rhamnosus when used as a monotherapy versus a combination preparation, and thus the results are reported combined.
The 61% heterogeneity in the Fig. 6 analysis (4–5 years out) was examined in sensitivity analysis. If the Kukkonen et al. study [36] were excluded from this meta-analysis, the heterogeneity statistic was 0%. The main difference in the studies included in this 4–5-year out meta-analysis was that the participants in Kukkonen et al. [36] were treated with a combination/mixture of probiotics (2 lactobacilli, bifidobacterial and propionibacteria) while those in the other two studies (Kalliomäki et al. [34] and Wickens et al. [35]) were treated with Lactobacillus strains only. Additionally, by removing Kukkonen et al. [36], this 4–5-year timeframe becomes statistically different on the outcome of AD favoring L. rhamnosus (RR 0.63, 95% CI 0.47–0.84; p = 0.001; I2 = 0%). Further, the 74% heterogeneity in Fig. 8 was examined. The differences in the studies included a longer treatment period in Wickens et al. [35] of 2 years versus 6 months in Kukkonen et al. [36], and the use of Lactobacillus versus a combination/mixture of probiotics (2 lactobacilli, bifidobacterial and propionibacteria) in Kukkonen et al. [36].
The limitations of this analysis include a high attrition rate of those entered into the trials. However, as it relates to other potential biases in the trials, it was considered low. The GRADE assessments timeframes (< 2 years, 6–7 years) were considered moderate in nature due to this attrition rate and low due to attrition and imprecision in the 4–5-year and 11-year timeframes. Thus, the true effect is likely to be close to the estimated effect in the < 2-year and 6–7-year timeframes. There is a possibility that the true effect may be different [33]. However, the effects in new studies would need to be quite large in order to change the relative effect and confidence intervals included herein. Over 2500 patients were included in the cohort of < 2 years. Thus, a significant number of new patients would be required in future studies to affect these findings, even if the effects were smaller in nature.
The overall implications of this meta-analysis in comparison with previous research are that probiotics may have useful clinical effects, however, the administration protocol and the specific strains utilized should be carefully considered. Targeted inclusion criteria for those at higher risk of atopic disease might improve efficacy while reducing the need for longer interventions. More research is needed in these areas as the understanding and possibilities of probiotic supplementation continue to evolve.
Conclusion
Based on this analysis, the use of L. rhamnosus either solely or in conjunction with other probiotics during pregnancy and post-pregnancy in infants likely has a positive effect in reducing the incidence of AD. This finding was found in the 2-year and 6–7-year timeframes evaluated.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Jeff Voigt was partially paid to access, analyze and report on the use of L. rhamnosus in high-quality studies.
Conflicts of interest
Jeff Voigt is a paid evidence assessment expert for Lil Mixins, a manufacturer of probiotic supplements. Meenal Lele is an executive for Lil Mixins. Jeff Voigt was provided with financial support for the analysis and development of the manuscript.
Ethics approval
Not applicable.
Consent to participate/publish
Not applicable.
Availability of data and materials
All data was available through peer-reviewed publications. If data was missing, the authors of the publications were contacted and asked to report on any conflicting, missing or unclear material.
Code availability
Not applicable.
Author contributions
Both JV and ML contributed equally to the writing of the manuscript. Statistical analyses were performed by JV.
References
- 1.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6, Supplement):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Alduraywish SA, Lodge CJ, Campbell B, Allen KJ, Erbas B, Lowe AJ, et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71(1):77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 3.Shoda T, et al. Timing of eczema onset and risk of food allergy at 3 years of age: a hospital-based prospective birth cohort study. J Dermatol Sci. 2016;84(2):144–148. doi: 10.1016/j.jdermsci.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Mohn CH, et al. incidence trends of atopic dermatitis in infancy and early childhood in a nationwide prescription registry study in Norway. JAMA Netw open. 2018;1(7):e184145. doi: 10.1001/jamanetworkopen.2018.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pothmann A, et al. The microbiome and atopic dermatitis: a review. Am J Clin Dermatol. 2019;20(6):749–761. doi: 10.1007/s40257-019-00467-1. [DOI] [PubMed] [Google Scholar]
- 6.Lee E, Lee SY, Kang MJ, Kim K, Won S, Kim BJ, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117(1):91–2.e1. doi: 10.1016/j.anai.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Marrs T, et al. Gut microbiota development during infancy: Impact of introducing allergenic foods. J Allergy Clin Immunol. 2021;147(2):613–621.e9. doi: 10.1016/j.jaci.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller NT, Bakacs E, Combellick J, Girgoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker RW, Clemente JC, Peter I, Loos RJF. The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediat Obes. 2017;12(Suppl 1):3–17. doi: 10.1111/ijpo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenmalm MC, Björkstén B. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clin Exp Allergy. 2000;30:34–40. doi: 10.1046/j.1365-2222.2000.00771.x. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg M, Frykman A, Ernerudh J, et al. Cord blood cytokines and chemokines and development of allergic disease. Pediatr Allergy Immunol. 2009;20:519–527. doi: 10.1111/j.1399-3038.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 12.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9:565–576. doi: 10.1038/nrgastro.2012.144. [DOI] [PubMed] [Google Scholar]
- 13.Jenmalm MC, Duchen K. Timing of allergy-preventive and immunomodulatory dietary interventions - are prenatal, perinatal or postnatal strategies optimal? Clin Exp Allergy. 2013;43:273–278. doi: 10.1111/cea.12003. [DOI] [PubMed] [Google Scholar]
- 14.Douwes J, Cheng S, Travier N, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32:603–611. doi: 10.1183/09031936.00033707. [DOI] [PubMed] [Google Scholar]
- 15.Hill C, Guarner F, Reid G, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroentrol Hepatol 2014;11:506–14. [DOI] [PubMed]
- 16.Pérez-Castillo IM, Fernández-Castillo R, Lasserrot-Caudrado A, Gallo-Vellejo JL, Rojas-Carvajal AM, Agullar-Cordero MJ. Reporting of perinatal outcomes in probiotic randomized controlled trials. A systematic review and meta-analysis. Nutrients. 2021;13:256. doi: 10.3390/nu13010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn DA, Sinn JKH. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD006475.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamsson TR, Wu RY, Jenmalm MC. Gut microbiota and allergy: the importance of the pregnancy period. Ped Res. 2015;77(1):214–219. doi: 10.1038/pr.2014.165. [DOI] [PubMed] [Google Scholar]
- 19.Dang D, Zhou W, Lun ZJ, Mu X, Wang X, Wu H. Meta-analysis of probiotics and/or prebiotics for the prevention of eczema. J Int Med Res. 2013;4(5):1426–1436. doi: 10.1177/0300060513493692. [DOI] [PubMed] [Google Scholar]
- 20.Doege K, Grakecki D, Zyriax B-C, Detinkina E, zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Brit Jrl Nut. 2012;107:1–6. doi: 10.1017/S0007114511003400. [DOI] [PubMed] [Google Scholar]
- 21.Zuccotti G, Meneghlin F, Aceti A, Barone G, Callegari ML, Di Mauro A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 22.American Gastroenterological Association Consideration for use of probiotics in gastrointestinal diseases: clinical decision support tool. Gastroenterology. 2020;159(2):706. doi: 10.1053/j.gastro.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Makrgeorgou A, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:11 CD006135. doi: 10.1002/14651858.CD006135.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sestito S, et al. The role of prebiotics and probiotics in prevention of allergic diseases in infants. Front Pediatrics. 2020;8:583946. doi: 10.3389/fped.2020.583946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atopic dermatitis (eczema) - Symptoms and causes - Mayo Clinic. Accessed 1 July 2022
- 27.Johansson SGO, Bieber T, Dahl R, Friedman PS, Lanier BQ, et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 28.Nottingham Eczema Severity Score (NESS) - The University of Nottingham. Accessed 1 Sep 2022.
- 29.EASI score. Eczema area and severity index | DermNet NZ. Accessed 1 Sep 2022.
- 30.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 31.Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook. Accessed 9 Jan 2022.
- 32.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook. Accessed 9 Jan 2022.
- 33.GRADE handbook (gradepro.org). Accessed 1 Oct 2022
- 34.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Kokinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomized placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 35.Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, Purdie G, the Probiotic Study Group A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008;122:788–794. doi: 10.1016/j.jaci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Kukkonnen K, Savilahti E, Haahtela T, Juntenen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Huure A, Laitinen K, Rautava S, Korkeamäki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double blind placebo controlled study. Clin Exp Allergy. 2008;38:1342–1348. doi: 10.1111/j.1365-2222.2008.03008.x. [DOI] [PubMed] [Google Scholar]
- 38.Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodularity protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–121. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 39.Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130:1355–1360. doi: 10.1016/j.jaci.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Dotterud CK, Storrø O, Johnsen R, Øien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double blind trial. Br J Dermatol. 2010;163:616–623. doi: 10.1111/j.1365-2133.2010.09889.x. [DOI] [PubMed] [Google Scholar]
- 41.Simpson MR, Dotterud CK, Storrø O, Johnsen R, Øien T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015;15:13. doi: 10.1186/s12895-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickens K, Barthow C, Mitchell EA, Kang J, van Zyl N, Purdie G, Stanley T, Fitzharris P, Murphy R, Crane J. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Ped Allergy Immunol. 2018;29:808–814. doi: 10.1111/pai.12982. [DOI] [PubMed] [Google Scholar]
- 43.Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics. 2008;121(4):e850–e856. doi: 10.1542/peds.2007-1492. [DOI] [PubMed] [Google Scholar]
- 44.Ou C-Y, Wang L, Jsu T-Y, Chuang H, Liu C-A, Chang J-C, Yu H-R, Yang KD. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double-blind, placebo-controlled trial. Clin Exp Allergy. 2012;42:1386–1396. doi: 10.1111/j.1365-2222.2012.04037.x. [DOI] [PubMed] [Google Scholar]
- 45.Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Buach J-F, La Vecchia C. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis. A meta-analysis. Epidemiology. 2012;23:402–414. doi: 10.1097/EDE.0b013e31824d5da2. [DOI] [PubMed] [Google Scholar]
- 46.Zhang G-Q, Hu H-J, Liu C-Y, Zhang Q, Shakya S, Li Z-Y. Probiotics for prevention of atopy and food hypersensitivity in early childhood. Medicine. 2016;95(8):e2562. doi: 10.1097/MD.0000000000002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuang L, Jiang Y. Effect of probiotic supplementation in pregnant women: a meta-analysis of randomized controlled trials. Br J Nutr. 2020;123(8):870–880. doi: 10.1017/S0007114519003374. [DOI] [PubMed] [Google Scholar]
- 48.Tan-Lim CS, Esteban-Ipac NAR, Recto MST, Castor MAR, Casis-Hao R, Nano ALM. Comparative Effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: a systematic review and network meta-analysis. Ped Allergy Immunol. 2021;32:1255–1270. doi: 10.1111/pai.13514. [DOI] [PubMed] [Google Scholar]
- 49.Szajewska H, Horvath A. Lactobacillus rhamnosus GG in the primary prevention of eczema in children: a systematic review and meta-analysis. Nutrients. 2018;10:1319. doi: 10.3390/nu10091319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Kokinen P, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomized placebo-controlled trial. Lancet. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 51.Kalliomäki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(4):1019–1021. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 52.Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Turre T, Haahetela T, Savilathi E. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. 2009;123:335–341. doi: 10.1016/j.jaci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Peldan P, Jukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotics decreased eczema up to 10 years of age, but at 5–10 years, allergic rhino-conjunctivitis was increased. Clin Exp Allergy. 2017;47:975–979. doi: 10.1111/cea.12924. [DOI] [PubMed] [Google Scholar]
- 54.Wickens K, Black P, Stanley TV, Mitchell E, Barthow C, Fitzharris P, Purdie G, Crane J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy. 2012;42:1071–1079. doi: 10.1111/j.1365-2222.2012.03975.x. [DOI] [PubMed] [Google Scholar]
- 55.Wickens K, Stanley TV, Mitchell EA, BArthow C, Fitzharris P, Purdie G, Siebers R, Black PN, Crane J. Early supplementation with Lactobacillus rhamnosus Hn001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization? Clin Exp Allergy. 2013;43:1048–1057. doi: 10.1111/cea.12154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data was available through peer-reviewed publications. If data was missing, the authors of the publications were contacted and asked to report on any conflicting, missing or unclear material.