Abstract
The host’s immune response to cervical human papillomavirus (HPV) infection is poorly understood. In a longitudinal cohort of women with cervical HPV infections, defined by PCR-based HPV DNA testing, we used exfoliated cervical cells and reverse transcription-PCR to examine the cervical mucosal mRNA expression of cytokines involved in regulating cell-mediated immunity. We identified seven HPV-positive subjects who were found to have cleared their HPV infections 4 months later. In all seven, a T-helper type 1 (Th1) cytokine pattern (expression of gamma interferon and absence of interleukin-4) preceded clearance. The more variable cytokine patterns seen in HPV-negative subjects suggest that the Th1 pattern in the women with subsequent clearance was a response to the HPV infection. This contention is supported by additional cross-sectional data showing a Th1 pattern in a majority of HPV-positive women. This study establishes a feasible means for assessing local cytokine expression in the cervical milieu and demonstrates that a Th1 cytokine response is associated with subsequent clearance of cervical HPV infection.
Cervical infection with human papillomavirus (HPV) usually results in a transient infection, with 70 to 90% of individuals showing clearance, as defined by repeated HPV DNA testing, within 12 to 24 months of detection (6, 9, 16). The importance of persistence of HPV in the remaining 10 to 30%, specifically with oncogenic types, is its strong association with the development of high-grade squamous intraepithelial lesions (9, 10, 16). Factors which contribute to viral persistence have not been elucidated but appear to have little or no association with sexual behavior (16). Evidence is accumulating that the host immune responses may be among the most influential factors in defining the natural history of HPV infection.
Although the immune response to cervical HPV infection is not well understood, evidence from murine and human infections by various intracellular pathogens has highlighted the roles of T-cell- and accessory-cell-derived cytokines in the initiation and maintenance of effective immune responses to such pathogens (17, 20, 21). In particular, a model of immune regulation by T helper (Th) cells has evolved in which antigen-experienced Th cells differentiate into phenotypically distinct cells which differ in the combinations of immunoregulatory cytokines they produce (18). The dichotomy between Th type 1 (Th1) cells, which promote cell-mediated immune (CMI) responses to intracellular pathogens and inhibit humoral immunity, and Th2 cells, which promote humoral responses and inhibit CMI responses, has provided insight into the natural history of leprosy, leishmaniasis, and other diseases (15, 22). This model may provide insight into HPV infection which, like leprosy and leishmaniasis, involves localization of infection by an intracellular pathogen to squamous epithelium.
The objective of the present study was to examine the association between cervical mucosal cytokine expression and the clearance of cervical HPV infection. Four cytokines were studied. Two cytokines produced principally by T cells (a Th1 cytokine, gamma interferon [IFN-γ], and a Th2 cytokine, interleukin-4 [IL-4]) were chosen for their roles in promoting or inhibiting CMI responses, respectively. We hypothesized that a Th1 cytokine response, defined as a pattern of cytokine production characterized by the expression of IFN-γ coupled with the absence of IL-4 expression, would be necessary for clearance of HPV infection and that failure to express and maintain this pattern would be associated with a persistent infection. Two other cytokines with important roles in promoting CMI responses were also examined: tumor necrosis factor (TNF), produced by a wide variety of cell types, and IL-12, produced by antigen-presenting cells. Using exfoliated cells collected by cervical cytology brush, we assessed cytokine expression at the mRNA level by reverse transcription (RT)-PCR in specimens collected from women with current or cleared cervical HPV infections. We report here that this method can be used to successfully assess cytokine production in exfoliated cervical cells and that our data suggest that a local Th1 cytokine pattern is associated with the clearance of cervical HPV infections and that its expression appears to be a response to the HPV infection. Also, while analysis of the cytokine patterns associated with viral persistence will require longitudinal study, preliminary observations suggest that women with persistent HPV infection may be unable to maintain a Th1 response.
(This work was presented as an abstract at the 17th International Papillomavirus Conference, January 1999, Charleston, S.C.)
MATERIALS AND METHODS
Study population and HPV detection by PCR.
Women participating in an ongoing natural history study of HPV were asked to participate in this study under the guidelines set forth by the Committee for Research on Human Subjects, University of California, San Francisco. Recruitment procedures for the longitudinal study have been described in detail elsewhere (16). Briefly, women aged 13 to 20 years who were attending one of two family planning clinics were screened for cervical HPV infection. Women found to be initially positive for HPV and who agreed to participate in the natural history study were seen at baseline and at 4-month intervals which included cervical testing for HPV. Cervical testing for Chlamydia trachomatis and Neisseria gonorrhoeae was also performed at the baseline and annual examinations and on women who had symptoms of lower genital tract infections at the interval visits. In addition, a random sample of women who had a negative screen for HPV were asked to participate as controls. These subjects were seen at baseline and at 6-month intervals unless they became HPV positive during follow-up, at which time they were asked to return every 4 months. HPV detection in this study was performed using the PCR technique described previously (25). The presence of amplified HPV product was separately sought in a dot blot format using an enhanced chemiluminescence method. A generic probe mix that determined the presence of one or more of 25 different HPV types nonspecifically was used. Samples were also probed for HPV types 6, 11, 16, 18, 31, 33, 35, 42, 43, 45, 51, 52, 56, and 58. For samples that were not typeable by these probes, additional typing was performed with a strip-based HPV detection kit kindly provided by Roche Diagnostics, Inc. (Alameda, Calif.).
Sample collection and RNA isolation.
Cervical cells were collected from study subjects via cervical cytology brush prior to all other samples to minimize contamination with peripheral blood. The cytology brush was immediately immersed in a 4 M guanidine thiocyanate-based denaturing solution (2). The lysates were stored at 4°C at the collection sites and sent to the laboratory for processing within 1 week of collection. Total RNA was isolated from the samples using the acid-phenol method (3) and quantified by measuring optical density at 260 nm.
Cytokine and CD4 RT-PCR.
RT was performed by using random-hexamer (Promega, Madison, Wis.) priming and Moloney murine leukemia virus reverse transcriptase (Promega) as previously described (1). Omission of sample RNA from one RT reaction in each test provided a negative control for the RT reactions and PCRs. PCR amplification was performed as previously described (11), using Taq DNA polymerase and primer sequences derived from the literature. The primer sequences, chosen so that amplified sequences span at least one intron, were as follows: IFN-γ, 5′-AATGCAGGTCATTCAGATGTAGCGG-3′ and 5′-GGATGAGTTCATGTATTGCTTTGCG-3′ (13); TNF, 5′-TCTCGAACCCCGAGTGACAA-3′ and 5′-TATCTCTCAGCTCCACGCCA-3′ (26); and IL-4, 5′-CAACTTTGTCCACGGACAC-3′ and 5′-TCCAACGTACTCTGGTTGG-3′ (23). Primers for the IL-12 p40 chain were a generous gift from Stan Wolf, Genetics Institute, Boston, Mass.; the sequences were 5′-CCAAGAACTTGCAGCTGAAG-3′ and 5′-TGGGTCTATTCCGTTGTGTC-3′. Amplification of message for CD4 was performed by using commercial primers (Clontech Laboratories, Inc., Palo Alto, Calif.). Positive control reactions were set up for each target sequence, using an identical sequence, in each PCR run. Message from the housekeeping gene for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also amplified from each sample to verify sample and cDNA integrity. PCRs were performed in a total volume of 50 μl in a reaction mix consisting of 2.5 μl of cDNA, deoxynucleoside triphosphate (dNTP) mix (0.2 M each [final concentration] dATP, dCTP, dGTP, and dTTP; Pharmacia, Uppsala, Sweden), 2.5 mM MgCl2, primers at 0.5 μM final concentration (each), reaction buffer (supplied with enzyme), and 1 U of Taq DNA polymerase (Promega). The enzyme was withheld until the reactions reached 94°C, to minimize mispriming. Amplification was carried out using 40 (or, for IL-4, 45) cycles of PCR in a Perkin-Elmer (Foster City, Calif.) thermocycler. The cycling program was 94°C denaturation for 1 min (5 min for first cycle), 60°C primer annealing for 1 min, and a 72°C extension for 2 min. Amplified product was resolved by electrophoresis on a 1.5% agarose gel and visualized and photographed using ethidium bromide staining. A DNA size marker (Pharmacia) was included on the gel to aid interpretation of product size. Bands were recorded as either present (positive result) or absent (negative result).
Data analysis.
Rates of cytokine expression between groups of subjects were compared by chi-square or two-sided Fisher’s exact test, where appropriate.
RESULTS
Detection of cytokine mRNA expression in exfoliated cervical cells.
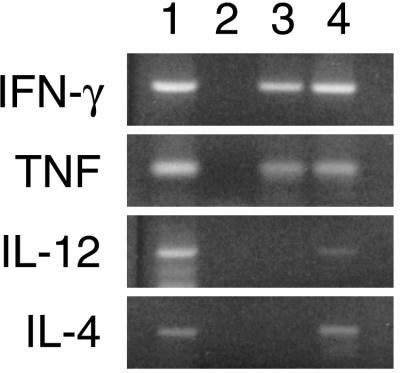
To examine in vivo cytokine expression in exfoliated cervical cells, we opted to perform our assessment on unstimulated cells. Cells were collected by cervical cytology brush and immersed immediately into a lysing and denaturing medium. Total cellular RNA was isolated from the samples, and RT-PCR was used to detect cytokine mRNA expression. All samples examined showed expression of the housekeeping gene for GAPDH, confirming sample and cDNA integrity. Figure 1 shows a typical cytokine RT-PCR result from two subjects.
FIG. 1.
Cytokine RT-PCR results for four cytokines from two subjects (lanes 3 and 4) and a positive (lane 1) and negative (lane 2) control for each cytokine. Gel photographs were scanned on a Hewlett-Packard flatbed scanner and composited with Adobe Photoshop 4.0; labels were added with Adobe Illustrator 8.0 on a Power Macintosh computer.
Cervical cytokine expression preceding clearance of HPV infection.
To examine cytokine profiles associated with subsequent clearance of infection, we identified seven subjects for whom we had RNA samples from a visit at which they were HPV positive, but who had cleared their infections by the time of the following study visit, 4 months later. All seven subjects showed a Th1 pattern of cytokine expression which preceded their clearance of HPV (Table 1). Samples from all seven subjects also showed expression of CD4 mRNA.
TABLE 1.
Cytokine expression preceding clearance of an HPV infection
| Subject | Visit no. | HPV type cleareda | Result of test
ford:
|
|||
|---|---|---|---|---|---|---|
| IFN-γ | IL-4 | TNF | IL-12 | |||
| a | 15 | MM9 | + | − | + | + |
| b | 12 | Untyp.b | + | − | + | − |
| c | 10 | Untyp. | + | − | + | + |
| d | 10 | 16 | + | − | + | − |
| e | 14 | 58 | + | − | + | − |
| f | 16 | 39 | + | − | + | + |
| g | 11 | Low-risk types,c 56 | + | − | + | + |
HPV type which was cleared by the following visit (4 months later).
Untyp., HPV positive by generic HPV probe but not positive for any of the types tested.
Low-risk types, positive on a probe mix which recognizes four low-risk HPV types (i.e., types 6, 11, 42, and 44).
Overall, 100% of these subjects had the Th1 cytokine pattern, defined as the expression of IFN-γ and absence of IL-4 by RT-PCR. Percentages of subjects expressing IFN-γ, IL-4, TNF, and IL-12 were 100, 0, 100, and 57%, respectively. +, positive; −, negative.
Further analysis of these subjects showed several interesting patterns. Subject e had had a history of intermittent positivity for HPV type 58, first at visit 2 in 1992, again at visit 9 in 1996, and once again at visit 14, as shown in Table 1. The fact that at the following visit (visit 15) she was found to be HPV negative suggests a role for Th1 cytokine production in maintaining suppression in some cases, rather than eradication.
Subjects b and f each had cytokine data available from three consecutive visits, which are detailed in Table 2. At the first of the three visits, subject b had been HPV negative for 2 years and subject f had been positive for HPV type 16 for 30 months. Neither of them had a Th1 pattern at this visit. At the following visit, each of them appeared to have acquired a new HPV type (untypeable for subject b and type 39 for subject f) and now showed a Th1 response. At the third visit, each had cleared the new HPV infection, although subject f remained HPV 16 positive. Also, neither maintained the Th1 pattern at the third visit. Subject b still showed expression of IFN-γ and TNF but now had expression of the CMI response-inhibiting cytokine IL-4 as well, reflecting a loss of a Th1 pattern. Subject f was positive only for TNF among the cytokines examined.
TABLE 2.
Longitudinal examination of cytokine expression in two subjects
| Subject | Visit no. | HPV test resultc | HPV type(s) | Result
of test forc:
|
|||
|---|---|---|---|---|---|---|---|
| IFN-γ | IL-4 | TNF | IL-12 | ||||
| b | 11a | − | − | ND | − | ND | |
| 12 | + | Untyp.d | + | − | + | − | |
| 13 | − | + | + | + | − | ||
| f | 15b | + | 16 | − | + | − | − |
| 16 | + | 16, 39 | + | − | + | + | |
| 17 | + | 16 | − | − | + | − | |
Subject b was HPV negative for 2 years (visits 7 to 11).
Prior to visit 15, subject f had been HPV 16 positive for a minimum of 30 months (visits 9 to 14, including one probable false negative on visit 10). By visit 17, she had been positive for at least 38 months.
ND, not determined; +, positive; −, negative.
Untyp., HPV positive by generic HPV probe but not positive for any of the types tested.
Cervical cytokine expression in HPV-negative subjects.
To investigate whether a Th1 pattern is a response to the HPV infection, as suggested by the longitudinal data from subjects b and f (described above) or represents constitutive cytokine production in resident cells of the cervical epithelium, we examined cytokine expression in 15 HPV-negative subjects (Table 3). All subjects had cleared a prior HPV infection at some time in the past and had remained HPV negative for various periods, as shown in Table 3. In contrast to the subjects in Table 1, of whom 100% had a Th1 cytokine pattern, only 23% (3 of 13) of these subjects had a Th1 pattern (P = 0.003; two subjects with incomplete data were excluded). IFN-γ expression was seen in only 67% (10 of 15) of these subjects compared with 100% in the group with subsequent clearance (P = 0.1), while 69% (9 of 13) showed expression of IL-4 compared with 0% (P = 0.005). The percentages of subjects expressing the accessory-cell products TNF and IL-12 were not significantly different between the two groups (P = 1 for both cytokines).
TABLE 3.
Cytokine expression in subjects who have previously cleared an HPV infectiona
| Subject | Months since the last positive HPV test | Result of test forb:
|
|||
|---|---|---|---|---|---|
| IFN-γ | IL-4 | TNF | IL-12 | ||
| h | 4 | + | + | + | + |
| i | 4 | + | − | + | + |
| j | 4 | + | + | + | + |
| k | 4 | − | − | + | − |
| l | 8 | + | − | + | + |
| m | 8 | + | + | + | − |
| n | 9 | − | NDc | + | − |
| o | 10 | + | ND | + | − |
| p | 12 | − | + | + | − |
| q | 17 | + | + | − | − |
| r | 17 | − | + | + | + |
| s | 21 | + | − | + | + |
| t | 22 | + | + | + | − |
| u | 39 | + | + | + | + |
| v | 75 | − | + | + | − |
All subjects shown in the table had a history of HPV infection and clearance and were HPV negative at the time of the cytokine assay. In cases where cytokine data was available for more than one HPV-negative visit by a subject, only the first visit with cytokine data was included in this cross-sectional analysis.
Overall, 23% of these subjects had the Th1 cytokine pattern, defined as the expression of IFN-γ and absence of IL-4 by RT-PCR. Due to incomplete data, subjects n and o were not included in determining the percentage of subjects with Th1 pattern. Percentages of subjects expressing IFN-γ, IL-4, TNF, and IL-12 were 67, 69, 93, and 47%, respectively.
ND, not determined.
Cervical cytokine expression in subjects with HPV infections of various duration.
To further investigate cytokine patterns associated with cervical HPV infection, we examined cross-sectional cytokine data from women with current positive HPV tests of various durations (Table 4) and compared them with data from the HPV-negative subjects (Table 3). Overall, a Th1 pattern was seen in 60% (9 of 15) of HPV-positive subjects compared with 23% (3 of 13) of subjects with cleared infections (P = 0.07). IFN-γ expression was seen in 80% (12 of 15) of HPV-positive subjects compared with 67% (P = 0.7), and IL-4 expression was seen in 20% (3 of 15) compared with 69% (P = 0.02). The percentages of subjects expressing the accessory-cell products TNF and IL-12 were not significantly different between the two groups (P = 1 for both cytokines).
TABLE 4.
Cytokine expression in HPV-positive subjectsa
| Subject | Minimum no. of months with positive HPV testb | HPV type | Result of
test forf:
|
|||
|---|---|---|---|---|---|---|
| IFN-γ | IL-4 | TNF | IL-12 | |||
| w | 0 (New)c | MM8 | + | + | + | + |
| x | 0 (New) | Untyp.e | + | + | + | + |
| y | 0 (New) | Untyp. | + | − | + | − |
| z | 0 (New) | MM8 | + | + | + | − |
| aa | 0 (New) | 18 | + | − | + | − |
| bb | 0 (New) | Untyp. | − | − | + | − |
| cc | 0 (New) | MM8 | + | − | + | + |
| dd | 4 | 16 | + | − | + | − |
| ee | 9 | 16 | + | − | + | + |
| ff | 12 | 16 | − | − | − | − |
| gg | 20 | 16 | + | − | + | − |
| hh | 22 | 58 | − | − | + | − |
| ii | 22 | 16 | + | − | + | + |
| jj | 46 | 31/33/35d | + | − | + | + |
| kk | 49 | 16 | + | − | + | + |
In cases where cytokine data was available for more than one visit by a subject, only the first visit with cytokine data was included in this cross-sectional analysis.
Since HPV infection was acquired at an indeterminate time prior to the first positive study visit, the actual duration of infection may be longer.
New, a newly positive test at the study visit shown.
Positive on a probe mix which recognizes these three types.
Untyp., HPV positive by generic HPV probe but not positive for any of the types tested.
Overall, 60% of these subjects had the Th1 cytokine pattern, defined as the expression of IFN-γ and absence of IL-4 by RT-PCR. Percentages of subjects expressing IFN-γ, IL-4, TNF, and IL-12 were 80, 20, 93, and 47%, respectively.
While no correlation between total duration of HPV infection and pattern of cytokine expression was apparent in this cross-sectional data set (Table 4), longitudinal data available from several subjects suggests that women with persistent infections may be unable to maintain Th1 cytokine patterns, as also suggested above by the data on subject f (Table 2). Subjects gg, ii, and kk each had cytokine data available from two study visits, the one shown in Table 4 and the following visit (not shown in the table). While all three of these women had Th1 patterns on the first of the two visits with cytokine data, two of them failed to maintain the Th1 pattern on the following visit, at which they continued to remain HPV positive for the same HPV type. Specifically, on the following visit, subjects gg and ii each showed expression of all four of the cytokines tested; only subject kk maintained a Th1 pattern, expressing only IFN-γ and TNF.
Additional testing for sexually transmitted diseases.
To assess the possible influence of other genital tract infections on cervical cytokine expression, we examined the most recent annual tests for C. trachomatis and N. gonorrhoeae in the subjects. None of the subjects was positive for either infection.
Correlation of cytokine patterns with the date of the last menstrual period.
To assess the possible influence of phases of the menstrual cycle on cervical cytokine expression, we evaluated cytokine patterns in those subjects who were HPV negative and not taking oral contraceptive pills. No apparent association was noted between cytokine patterns and the phases (proliferative, ovulatory, or follicular) of the cycle.
DISCUSSION
This study demonstrates the feasibility of using a sensitive RT-PCR method for the examination of local cytokine expression in the cervical milieu. The ease of sample collection and high sensitivity of this method allow for the much-needed investigation of the role of local cytokine expression in the natural history of cervical HPV infection in a longitudinal cohort. The method does not require invasive collection techniques, such as biopsy, nor extensive postcollection manipulation, such as cloning or in vitro stimulation. Furthermore, unlike immunoassays for detection of soluble protein, this method detects actual cellular production of cytokines, at the level of mRNA expression. The validity of this approach is affirmed by recent work demonstrating a strong correlation between detection of cytokine mRNA, by RT-PCR, and detection of protein secretion, by immunoassay methods, for IFN-γ and IL-4 (8). This is consistent with evidence that cytokine production is regulated principally at the level of transcription (14, 24).
Using this method, we found that in all seven women who were shown to clear an HPV infection, a Th1 pattern of cytokine expression preceded their clearance (Table 1), supporting our hypothesis that clearance requires a Th1 response. Several lines of evidence indicate that the observed Th1 pattern was a response to the HPV infection. First, the contrast between this pattern and the more variable patterns in the HPV-negative subjects argues strongly that the Th1 pattern observed in the subjects with subsequent clearance was neither a random finding nor a constitutional pattern of the cervical epithelium. The most compelling evidence is provided by the longitudinal data for the two subjects described in Table 2, who each appear to have responded to a new HPV infection by mounting a Th1 response, followed by clearance of the new infection. Lastly, although limited by its cross-sectional nature and small sample number, our data from HPV-positive subjects also showed that women with HPV appeared more likely to show a Th1 response than HPV-negative subjects.
We had also hypothesized that viral persistence would be associated with failure to express and maintain a Th1 cytokine pattern. As expected, many of the patients with a history of HPV persistence (Table 4) lacked a Th1 response. However, there did not appear to be a simple causal relationship between expression of a Th1 pattern and clearance, since several subjects with a prolonged history of infection had Th1 cytokine patterns. This exemplifies the complexity and heterogeneity of the immune response and highlights the importance of longitudinal analysis. It may be that patients with persistent infection are unable to maintain a Th1 response over successive visits, as suggested by the two women (gg and ii) who had Th1 patterns on one visit but not the following visit, even though they remained HPV positive. Furthermore, even if a Th1 response is required for clearance, it may be insufficient without sustained and competent effector mechanisms and may be type specific. Evidence for this possibility comes from our longitudinal data on subject f, who appeared to mount a Th1 response to an intercurrent infection with HPV type 39 but not to her longstanding HPV 16 infection (Table 2). This may suggest that certain HPV types, such as HPV 16, somehow evade the immune system and go unrecognized. We anticipate that these issues will become clearer as more longitudinal data is collected from our study cohort.
The interpretation of the varied cytokine responses seen in HPV-negative individuals (Table 3) is unclear but again highlights the heterogeneity of the immune response. The expression of IL-4 in several of the women with recent clearance of an HPV infection (Tables 2 and 3) may reflect a regulatory role for Th2 cytokines in inhibiting a prolonged inflammatory response and consequent immune-mediated tissue damage, following clearance of the pathogen. Also, it is possible that in some cases, the virus may actually be suppressed rather than eliminated, the suppression being associated with continued intermittent cytokine production. The intermittent HPV type 58 positivity observed in one subject underscores this possibility, since the recurrence at the visit for which we have cytokine data available was accompanied by a Th1 pattern and was followed by an HPV-negative visit.
A limitation of the RT-PCR method is the inability to determine the cellular sources of cytokine production. The four cytokines chosen for study include both T-cell products (IFN-γ and IL-4) and accessory cell products (TNF and IL-12). While our most compelling findings involve patterns of expression of IFN-γ and IL-4, it cannot be determined by our method whether Th cells were the main sources of these cytokines in our samples. The apparent exclusion of IL-4 production preceding viral clearance (Table 1) suggests that these women’s responses involved some degree of cross-regulation between IFN-γ and IL-4, which is a central feature of Th-cell-mediated immunoregulation (18). To begin investigating this, we were able to show that all seven patients in Table 1 also had expression of CD4 message, indicating the presence of Th cells in the samples. This points to Th cells as likely sources of IFN-γ in these samples but does not rule out a role for CD8+ cells and NK cells. Elucidation of the exact cellular sources of these cytokines will ultimately require single-cell-based assays. Still, it is apparent even from our bulk assay that examining the local mRNA expression of immunoregulatory cytokines, regardless of their cellular sources, provides greater understanding of the natural history of infection.
Another limitation of this method is the inability to assess potential contamination of the samples with peripheral blood, since cells are lysed at the time of collection. Our prior experience, however, had shown that the majority of samples collected with a cytology brush do not contain erythrocytes on microscopic examination if the sample is the first one collected during the patient visit. Moreover, detection of IFN-γ and IL-4 production in unstimulated peripheral blood leukocytes appears to be relatively uncommon, with some investigators reporting no detection by RT-PCR (12, 19) and others reporting that they did detect constitutive production of one or the other of these cytokines (4, 5, 7).
In summary, we have shown a feasible means for assessing an important aspect of local immune function in cervical HPV infection. The cytokine patterns demonstrated are not random, and a strong association between a Th1 pattern and subsequent clearance of infection was found, supporting our hypothesis that clearance requires Th1 cytokine production. It is anticipated that the collection of longitudinal data will allow more extensive correlation of cytokine patterns with subsequent course of infection. An understanding of the immunoregulatory mechanisms involved in control of HPV infection will prove valuable in assessment of vaccine and other prevention and treatment strategies.
ACKNOWLEDGMENTS
We thank Sepideh Farhat for her contribution in performing the HPV PCR testing.
This work was supported in part by National Cancer Institute grant R01CA51323 and National Institutes of Health grant M01RR01271.
REFERENCES
- 1.Beverley S M. Enzymatic amplification of RNA by PCR. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1992. pp. 15.14.11–15.14.16. [Google Scholar]
- 2.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Cluitmans F H, Esendam B H, Landegent J E, Willemze R, Falkenburg J H. Constitutive in vivo cytokine and hematopoietic growth factor gene expression in the bone marrow and peripheral blood of healthy individuals. Blood. 1995;85:2038–2044. [PubMed] [Google Scholar]
- 5.Diaz-Mitoma F, Kumar A, Karimi S, Kryworuchko M, Daftarian M P, Creery W D, Filion L G, Cameron W. Expression of IL-10, IL-4 and interferon-gamma in unstimulated and mitogen-stimulated peripheral blood lymphocytes from HIV-seropositive patients. Clin Exp Immunol. 1995;102:31–39. doi: 10.1111/j.1365-2249.1995.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis. 1995;171:1026–1030. doi: 10.1093/infdis/171.4.1026. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Nishanian P, Breen E C, McDonald M, Fahey J L. Cytokine gene expression in normal human lymphocytes in response to stimulation. Clin Diagn Lab Immunol. 1998;5:335–340. doi: 10.1128/cdli.5.3.335-340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods. 1997;204:57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 9.Ho G Y, Bierman R, Beardsley L, Chang C J, Burk R D. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 10.Koutsky L A, Holmes K K, Critchlow C W, Stevens C E, Paavonen J, Beckmann A M, DeRouen T A, Galloway D A, Vernon D, Kiviat N B. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992;327:1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- 11.Kramer M F, Coen D M. Enzymatic amplification of DNA by PCR: standard procedures and optimization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 15.11.11–15.11.19. [Google Scholar]
- 12.Kristensson K, Borrebaeck C A, Carlsson R. Human CD4+ T cells expressing CD45RA acquire the lymphokine gene expression of CD45RO+ T-helper cells after activation in vitro. Immunology. 1992;76:103–109. [PMC free article] [PubMed] [Google Scholar]
- 13.Lau A S, Sigaroudinia M, Yeung M C, Kohl S. Interleukin-12 induces interferon-gamma expression and natural killer cytotoxicity in cord blood mononuclear cells. Pediatr Res. 1996;39:150–155. doi: 10.1203/00006450-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Lewis D B, Yu C C, Meyer J, English B K, Kahn S J, Wilson C B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Investig. 1991;87:194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modlin R L. Th1-Th2 paradigm: insights from leprosy. J Investig Dermatol. 1994;102:828–832. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- 16.Moscicki A B, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh T M, Brescia R, Kanowitz S, Miller S B, Stone J, Hanson E, Palefsky J. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 18.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 19.Pisa E K, Pisa P, Hansson M, Wigzell H. OKT3-induced cytokine mRNA expression in human peripheral blood mononuclear cells measured by polymerase chain reaction. Scand J Immunol. 1992;36:745–749. doi: 10.1111/j.1365-3083.1992.tb03135.x. [DOI] [PubMed] [Google Scholar]
- 20.Prussin C. Cytokine flow cytometry: understanding cytokine biology at the single-cell level. J Clin Immunol. 1997;17:195–204. doi: 10.1023/a:1027350226435. [DOI] [PubMed] [Google Scholar]
- 21.Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15:121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 22.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 23.Sorg R V, Enczmann J, Sorg U R, Schneider E M, Wernet P. Identification of an alternatively spliced transcript of human interleukin-4 lacking the sequence encoded by exon 2. Exp Hematol. 1993;21:560–563. [PubMed] [Google Scholar]
- 24.Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- 25.Williams A B, Darragh T M, Vranizan K, Ochia C, Moss A R, Palefsky J M. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus-infected women. Obstet Gynecol. 1994;83:205–211. [PubMed] [Google Scholar]
- 26.Yamamura M, Wang X H, Ohmen J D, Uyemura K, Rea T H, Bloom B R, Modlin R L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–1475. [PubMed] [Google Scholar]