Abstract
Lifestyle risk behaviours such as smoking, physical inactivity, and unhealthy diet account for a considerable disease burden globally. These risk behaviours tend to cluster within an individual, which could have detrimental health effects. In this study, we aimed to examine the clustering effect of lifestyle risk behaviours on cardiovascular disease (CVD) and CVD risk among adults in the United Kingdom (UK). We performed a latent class (LC) analysis with distal outcomes using the UK Biobank baseline (2006–2010) data. First, we estimated LC measurement models, followed by an auxiliary model conditional on LC variables. We reported continuous (mean difference—MD) and binary (odds ratio—OR) outcomes with 95% confidence intervals. We included 283,172 and 174,030 UK adults who had data on CVD and CVD risk, respectively. Multiple lifestyle risk behaviour clustering (physically inactive, poor fruit & vegetable intake, high alcohol intake, and prolonged sitting) had a 3.29 mean increase in CVD risk compared to high alcohol intake. In addition, adults with three risk behaviours (physically inactive, poor fruit & vegetable intake, and high alcohol intake) had 25.18 higher odds of having CVD than those with two risk behaviours (physically inactive, and poor fruit and vegetable intake). Social deprivation, gender and age were also associated with CVD. Individuals' LC membership with two or more lifestyle risk behaviours negatively affects CVD. Interventions targeting multiple lifestyle behaviours and social circumstances should be prioritized to reduce the CVD burden.
Subject terms: Cardiology, Diseases, Health care, Medical research, Risk factors, Statistical methods
Introduction
Noncommunicable diseases (NCDs) are responsible for 71% of all deaths worldwide1. Lifestyle risk behaviours such as smoking, physical inactivity, alcohol consumption, and unhealthy diet increase the risk of dying from NCD1. Cardiovascular diseases (CVDs)1–3 account for most NCD deaths globally. In the UK, 11% of the population live with CVD, and CVD accounts for 25% of all deaths4. In 2010, the American Heart Association (AHA) recommended seven cardiovascular health behaviours (Life’s Simple 7) to reduce CVD morbidity and mortality in the general population, including smoking, diet, physical activity, body mass index, blood pressure, total cholesterol, and fasting glucose5. The 2019 AHA guideline on the primary prevention of CVD recommends that people engage in a healthy lifestyle throughout their life6.
Lifestyle risk behaviours, such as smoking, alcohol consumption, poor diet and physical inactivity are major risk factors for the development of CVD7,8. Physical inactivity and increased sedentary time are important modifiable risk factors for cardiometabolic disease9,10. Physical inactivity is also the fourth leading cause of disease and disability in the UK11. Sleeping too much or too little is also strongly associated with CVD12–14. People engaging in multiple risk behaviours tend to have poor health outcomes compared to those engaging in one risk behaviour15. Thus, identifying people with multiple risk behaviours provides insight into where policies should target to reduce inequalities in health16,17. While previous studies have investigated the clustering of lifestyle behaviours for people with CVD18,19 and the general population20, most did not include all major lifestyle behaviours, such as smoking, alcohol intake, fruit and vegetable consumption, physical activity, sitting behaviour, and sleep.
To analyse the co-occurrence of multiple health-related behaviours, different statistical approaches have been proposed in the literature21. However, these approaches are mainly focused on identifying clustering of risk behaviours and not estimating their effect on a distal outcome. Latent class (LC) analysis with a distal outcome is important for identifying how different lifestyle risk behaviours occur together among participants based on indicator variables, and to estimate the effect of LC membership on a distal outcome22–24. The effects of LC membership (clustering of lifestyle risk behaviours) on CVD and the risk of developing CVD have not yet been investigated. This study aimed to examine the prevalence of six lifestyle risk behaviours (smoking, poor fruit and vegetable consumption, alcohol intake, physical inactivity, prolonged sitting, and poor sleep), and clustering patterns of these lifestyle risk behaviours. In addition, we aimed to identify and estimate the effect of socio-demographic characteristics (age, gender), Townsend deprivation index and LC membership on CVD, and CVD risk.
Methods
UK Biobank has ethics approval from the North West Multi-centre Research Ethics Committee25. According to this approval, researchers do not require separate ethics applications. All participants provided written informed consent. This study was carried out in accordance with relevant guidelines and regulations.
Study population
We analysed data from the UK Biobank study that included more than 500,000 middle-aged (38–73 years) adults recruited from 22 sites across England, Wales, and Scotland. We used baseline data collected between 2006 and 201026,27. Socio-demographic, lifestyle (smoking, alcohol consumption, dietary intake, physical activity, sitting time and sleep duration) and medical history were collected using the touchscreen questionnaire27.
Disease categories
The UK Biobank collected self-reported medical information, such as CVD based on physician diagnosis. To define participants' CVD status, we used data on vascular/heart problems diagnosed by a doctor (Field ID = 6150). Under this Field ID, four CVDs were reported: heart attack, angina, stroke, and high blood pressure. For this analysis, participants who were reported to have at least one of these diseases were classified as having CVD, not otherwise. A total of 283,172 participants without missing data were included.
For the 174,030 participants without CVD, we computed a 10-year CVD risk score28 using the Framingham risk score function from the CVrisk package29 in R30. The variables included in the 10-year CVD risk calculation were age, gender, total cholesterol, HDL cholesterol, systolic blood pressure, blood pressure medication, smoking and diabetes status28.
Lifestyle behaviours
This analysis used six lifestyle behaviours: smoking, physical activity, fruit and vegetable consumption, alcohol intake, screen and driving time, and sleep duration.
Physical activity
The UK Biobank collected data on physical activity using adapted questions from the short International Physical Activity Questionnaire (IPAQ)31 that includes the frequency, intensity and duration of walking, moderate and vigorous activity. UK Biobank data on time spent on moderate and vigorous activity was added and converted to a metabolic equivalent of task (MET) score. Participants were classified as active if they had ≥ 750 MET min/week or inactive (< 750 MET min/week), based on the 2019 AHA guideline6.
Fruit and vegetable intake
The UK Biobank collected data on dietary intake using the Food Frequency Questionnaire32. The NHS guideline recommends every individual to eat at least 5 portions of a variety of fruit and vegetables every day33,34. Data on fresh fruit (pieces), dried fruit (pieces), salad/raw vegetable (heaped tablespoons) and cooked vegetable (heaped tablespoons) were combined to calculate portions. Participants consuming at least 5 portions of fruits and vegetables per day were considered to have adequate fruit and vegetable intake.
Alcohol intake
Participants were asked for the number of pints of beer, glasses of wine, and measures of spirit consumed in the last week. Since alcoholic drinks differ in the amount of alcohol content, we converted each drink into equivalent standard units (1 unit contains 10 ml of ethyl alcohol)35. We calculated total weekly units of alcohol consumption by adding the units of beer, wine, and spirits. Based on the NHS guidelines35, we grouped participants as low-risk drinkers (≤ 14 units per week) or high-risk drinkers (> 14 units per week).
Smoking
To measure smoking status, participants were asked, "Do you smoke tobacco now?". Response options were “Yes, on most or all days”, “Only occasionally” and “No”. Those who responded “yes” or “smoke occasionally” were coded as 1, current smoker, while those who responded as “no” were coded as 0, not a current smoker.
Prolonged sitting
Total sitting time was calculated from the sum of self-reported hours spent watching television, using the computer, and driving during a typical day. Based on the estimated total sitting time, participants were categorized as low risk sitting (< 8 h/day) or prolonged sitting (≥ 8 h/day)36,37. This was based on the evidence of greater mortality risk for each increased sitting time category compared with < 8 h/day36,37.
Sleep duration
To measure sleep duration, the UK Biobank asked participants ‘About how many hours sleep do you get in every 24 h? (please include naps)’. Sleep duration was split using predefined thresholds from the literature; < 7 h, 7–8 h and > 8 h13. Based on these cut points, participants were grouped as having ‘poor sleep’ (< 7 or > 8 h/night) and ‘good sleep’ (7–8 h/night).
Socio-demographic variables
Socio-demographic characteristics (age and gender), and Townsend deprivation index (TSDI) were included in the latent class analysis (LCA) model. TSDI was used to measure participants' deprivation38. The index combines information on housing, employment, car availability and social class, with higher values indicating greater deprivation38.
Statistical analysis
Descriptive statistics were performed on socio-demographic characteristics, lifestyle behaviours and medical conditions. The Mplus version 8.8 software39 was used to estimate a distal outcome model to identify latent classes (LCs) of lifestyle risk behaviours, and the association between LC membership and distal outcomes (CVD, and CVD risk) (Fig. 1). To select the number of LCs that best fit the data, we first fitted a two-class latent model and successively increased the number of LCs by one, up to a six-class latent model. Model evaluation was performed using the Bayesian Information Criterion (BIC) and Akaike information criterion (AIC)40. Model selection was made based on statistical criteria (with lower AIC and BIC) and interpretability of the estimated LCs40. Based on these criteria, four LCs for CVD, and three LCs for CVD risk were selected (see Supplementary Tables).
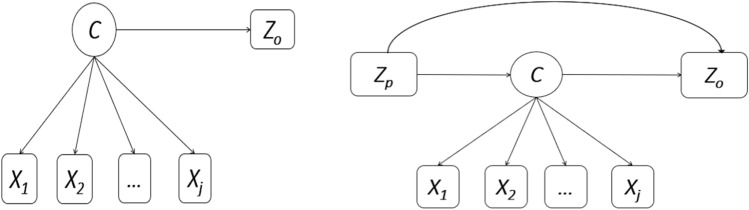
Figure 1.
Graphical representation of latent class model with distal outcome and covariate. Where C = latent class, X1, X2, …, XJ refer to latent class indicators of C, Zo = distal outcome, and Zp = covariate of the latent class variable C and distal outcome Zo.
LC analysis with a distal outcome22–24, covariate, and LC mediator41 was run to identify and estimate: (1) the effect of LC membership on distal outcomes (Fig. 1—left hand side), and (2) the direct, indirect (LC membership mediated effect) and total effect of covariate(s) on distal outcomes (Fig. 1—right hand side). Gender, age, and smoking were not used in the CVD risk distal outcome model—since we used them in the computation of the CVD risk score. For distal outcome models, the Bolck–Croon–Hagenaars (BCH) method22,42 outperforms other methods. In addition, the BCH approach gives more accurate mediation estimates in LC analysis mediation models41. The BCH method avoids shifts in LC in the final step and performs well when the variance of the auxiliary variable differs substantially across classes22. To estimate the model, the BCH method uses weights that reflect the measurement error of the LC variable22. Two versions of the BCH method were implemented in Mplus—the automatic and two-step manual BCH versions22. The automatic BCH method is restrictive. In this analysis, we used the manual BCH two-step approach to estimate auxiliary models with continuous (CVD risk) and categorical (CVD) distal outcomes. We estimated the LC measurement model and saved the BCH weights in the first step. The second step estimated the general auxiliary model conditional on the LC variable using the BCH weights. Continuous (mean difference—MD) and binary (odds ratio—OR) outcomes were reported with 95% confidence intervals (95% CI).
Results
Population characteristics
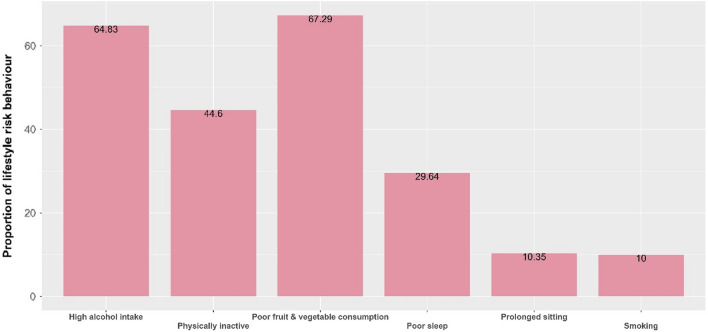
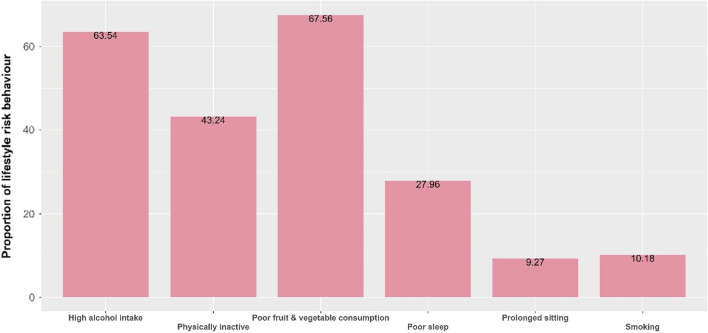
We included UK adults who had data on CVD status (n = 283,172) and CVD risk (n = 174,030) along with lifestyle risk behaviours. The mean (SD) age of participants was 56.39 (8.02) and 55.15 (8.05) years for CVD and for those at risk of developing CVD, respectively. Among adults with CVD and at risk of developing CVD, 52.64% and 49.27% were males, respectively. Among adults with CVD, 67.29% had poor fruit and vegetable consumption, followed by high alcohol intake (64.83%) and were physically inactive (44.60%) (Fig. 2). Similarly, among adults at risk of developing CVD, 67.56% had poor fruit and vegetable intake and 63.54% had high alcohol intake (Fig. 3). Males, except for physical inactivity, had the highest proportion of lifestyle risk behaviours among those with CVD and at risk of developing CVD (see Supplementary Figs. S1, S2).
Figure 2.
Lifestyle risk behaviour among UK adults with CVD data.
Figure 3.
Lifestyle risk behaviours among UK adults with CVD risk.
Lifestyle risk behaviours
A model with three LCs were selected for adults at risk of developing CVD and four LCs for CVD outcome data. For adults at risk of developing CVD, LC 1 was characterised by physical inactivity (53.70%), poor fruit and vegetable intake (83.00%), high alcohol intake (74.60%), and prolonged sitting (100.00%). Adults in LC 2 had a high probability of high alcohol intake (58.40%). LC 3 had high probabilities of poor fruit and vegetable intake (100.00%) and high alcohol intake (65.00%) (Table 1).
Table 1.
Lifestyle risk behaviour probabilities of UK adults at risk of developing CVD.
| Variable | LC 1 (6.27%) | LC 2 (31.37%) | LC 3 (62.46%) |
|---|---|---|---|
| Physically inactive | 0.54 | 0.34 | 0.47 |
| Poor fruit and vegetable consumption | 0.83 | 0.00 | 1.00 |
| High alcohol intake | 0.75 | 0.58 | 0.65 |
| Poor sleep | 0.38 | 0.28 | 0.27 |
| Prolonged sitting | 1.00 | 0.05 | 0.03 |
Probability values ≥ 0.50 are in bold.
For the CVD outcome, adults in the LC 1 had a high probability of being physically inactive (61.60%), poor fruit and vegetable consumption (83.70%) and high alcohol intake (77.50%). Participants in LC 2 had a high probability of high alcohol intake (64.80%). In LC 3, the probability of having poor fruit and vegetable intake and high alcohol intake were 91.60% and 80.60%, respectively. The LC 4 was characterised by physically inactive (52.00%) and poor fruit and vegetable intake (73.40%) (Table 2).
Table 2.
Lifestyle risk behaviour probabilities of UK adults with CVD outcome data.
| Variable | LC 1 (17.95%) | LC 2 (22.06%) | LC 3 (45.42%) | LC 4 (14.57%) |
|---|---|---|---|---|
| Physically inactive | 0.62 | 0.29 | 0.43 | 0.52 |
| Poor fruit and vegetable consumption | 0.84 | 0.00 | 0.92 | 0.73 |
| High alcohol intake | 0.78 | 0.65 | 0.81 | 0.00 |
| Poor sleep | 0.46 | 0.29 | 0.24 | 0.27 |
| Smoking | 0.23 | 0.05 | 0.11 | 0.00 |
| Prolonged sitting | 0.29 | 0.07 | 0.06 | 0.05 |
Probability values ≥ 0.50 are in bold.
Effects of latent class membership on CVD risk
LC membership had a statistically significant effect on CVD risk. Being in LC 1 (physically inactive, poor fruit & vegetable intake, high alcohol intake, and prolonged sitting) was associated with increased CVD risk compared with LC 2 (MD = 3.29 [3.12, 3.46]). Similarly, adults in LC 3 (poor fruit & vegetable intake, and high alcohol intake) had a 0.89 mean increased CVD risk relative to LC 2 (Table 3). Similarly, social deprivation measured in TSDI, except for total effect, showed a statistically significant effect on CVD risk (Table 3).
Table 3.
Latent class membership and TSDI associated with CVD risk: multivariable analyses (mean difference and 95% confidence intervals).
| Variables | MD (95% CI) |
|---|---|
| LC membership on CVD risk | |
| LC 1: physically inactive, poor fruit & vegetable intake, high alcohol intake, and prolonged sitting | 3.29 (3.12, 3.46) |
| LC 2: high alcohol intake | Reference |
| LC 3: poor fruit and vegetable intake, and high alcohol intake | 0.89 (0.82, 0.96) |
| TSDI effect on CVD risk | |
| Direct effect | − 0.02 (− 0.03, − 0.01) |
| Indirect (LC membership mediated effect) effect | 0.01 (0.01, 0.01) |
| Total effect | − 0.01 (− 0.02, 0.00) |
Significant values are in bold.
Effects of latent class membership on CVD status
The odds of developing CVD were significantly associated with an individual’s LC membership. UK adults who belonged to LC 1 (Physically inactive, poor fruit and vegetable intake, and high alcohol intake) had 25.18 higher odds of having CVD than those in LC 4 (Physically inactive, and poor fruit and vegetable intake). Similarly, compared to those in LC 4, the odds of having CVD was higher among those who were in LC 2 (7.70) and LC 3 (5.19). In addition, gender, age and TSDI showed statistically significant effects on the odds of developing CVD. The direct, indirect, and total effects of being male were 1.19, 1.37- and 1.63-times higher odds of having CVD than females, respectively. A single-year increase in the age of adults, except for the indirect effect, was significantly associated with higher odds of developing CVD. For UK adults, a one-point increase in the TSDI score was associated with higher odds of having CVD (Table 4).
Table 4.
Latent class membership and other factors associated with CVD: multivariable analyses (odds ratios and 95% confidence intervals).
| Variables | OR (95% CI) |
|---|---|
| LC membership on CVD status | |
| LC 1: physically inactive, poor fruit and vegetable intake, and high alcohol intake | 25.18 (7.02, 43.33) |
| LC 2: high alcohol intake | 7.70 (2.31, 13.08) |
| LC 3: poor fruit and vegetable intake, and high alcohol intake | 5.19 (1.55, 8.82) |
| LC 4: physically inactive, and poor fruit and vegetable intake | Reference |
| Direct effect of covariates on CVD status | |
| Male | 1.19 (1.04, 1.34) |
| Age | 1.08 (1.07, 1.09) |
| TSDI | 1.02 (1.01, 1.03) |
| Indirect (LC membership mediated effect) effect of covariates on CVD status | |
| Male | 1.37 (1.24, 1.50) |
| Age | 0.99 (0.99, 1.00) |
| TSDI | 1.08 (1.06, 1.10) |
| Total effect of covariates on CVD status | |
| Male | 1.63 (1.36, 1.91) |
| Age | 1.07 (1.06, 1.08) |
| TSDI | 1.11 (1.08, 1.13) |
Significant values are in bold.
Discussion
In this study, we demonstrated that clustering of multiple lifestyle risk behaviours in adults significantly increased the risk of CVD and being diagnosed with CVD. Individuals’ latent class membership with two or more lifestyle risk behaviours were significantly associated with CVD risk and being diagnosed with CVD. Socioeconomic characteristics were also associated with being diagnosed with CVD.
The likelihood of developing CVD within a given time depends on the number of risk factors. Individuals' latent class membership with multiple lifestyle risk behaviours showed a 3.29 mean increase in the risk of developing CVD relative to a single risky behaviour. Smoking, physical inactivity, alcohol use, and low fruit and vegetable intake had the highest effect on NCD development and death8. On the other hand, lifestyle modification can reduce individuals’ risk of developing CVD. Adherence to Life's Simple 7 metrics has been associated with a lower rate of cardiovascular events43. In a meta-analysis of prospective cohort studies on the effect of a combined healthy lifestyle (healthy diet, moderate alcohol consumption, non-smoking, physical activity, and optimal weight) on CVD risk, people with the highest number of healthy lifestyle factors had lower CVD risk relative to those with the lowest number of healthy lifestyle factors [pooled hazard ratio = 0.37 (95% CI 0.31–0.43)]44.
Clustering of two or more lifestyle risk behaviours could have a synergetic effect on CVD. Adults with clustering of three lifestyle risk behaviours (physically inactive, poor fruit and vegetable intake, and high alcohol intake) had 25.18 higher odds of having CVD than those with two lifestyle risk behaviours (physically inactive, and poor fruit and vegetable intake). In a systematic review of longitudinal observational studies, the combination of physical inactivity with smoking, high alcohol intake, poor diet, or sedentary behaviour showed increased CVD incidents, death due to CVD and/or any other cause45. However, smoking, and prolonged sitting did not show significant contributions to the LC membership and CVD status. It could be due to the small proportion, which needs further investigation.
Lifestyle risk behaviours are also associated with an increased risk of premature mortality. In a meta-analysis, years-of-life-lost due to high alcohol intake was 0.5 years, 2.4 years for physical inactivity, and 4·8 years for smoking46. In addition, the combination of multiple lifestyle risk behaviours showed increased risks of all-cause and/or cardiometabolic mortalities47,48. The co-occurrence of smoking, physical inactivity and poor social participation increased cardiometabolic mortality by 3.13 relative to no-risk behaviour47. On the contrary, meeting the cardiovascular health metrics and engaging in a healthy lifestyle were associated with lower incidences of CVD, cardiovascular mortality, and all-cause mortality49,50. Therefore, more emphasis should be placed on interventions targeting multiple lifestyle behaviours.
In addition, adults with increasing socioeconomic deprivation scores had an increased risk of being diagnosed with CVD. People living in socioeconomic deprived areas had multiple lifestyle risk behaviours, experience high CVD rates, and premature mortality46,51,52. In a study conducted in the UK, areas with higher socioeconomic deprivation had high coronary heart disease mortality53. Populations with socioeconomic deprivation are more likely to smoke, have a poor diet, and not exercise enough52. To reduce the effect of social deprivation on health, effective policies and strategies should be designed to modify socioeconomic circumstances and their consequences.
The odds of being diagnosed with CVD varied according to sex and age. In most cases, male and a single-year increase in the age of adults were significantly associated with the odds of being diagnosed with CVD. In a study conducted on gender-specific, lifestyle-related behaviours and 10-year CVD risk, males had higher CVD events—both first and recurrent CVD relative to females54. Regarding the effect of age, a meta-analysis reported that the hazard ratio of CVD increased with increasing age of adults44. Younger adults have more cardiovascular benefits from the combined effects of a healthy lifestyle44,50. Therefore, lifestyle interventions targeted towards younger people are needed to prevent CVD.
Our study has several strengths. LCA, a cross-sectional latent variable mixture modelling, has several advantages over other traditional methods used in lifestyle risk behaviour55,56. First, it uses maximum likelihood estimation to identify subgroups that are internally homogenous and externally heterogeneous57. Second, it is a model-based technique, which has an advantage over heuristic cluster techniques (e.g., k-means clustering) in that it provides fit statistics40. Fit statistics are useful in selecting the most appropriate model for the data58 and to compare models for hypothesis testing59. Third, LCA provides information on the probability that an individual is within a particular class58. This has significant importance for researchers and practitioners in the field to identify subgroups of lifestyle risk behaviours and a targeted approach to healthy lifestyle promotion. These subgroups can be studied further to investigate problems related to lifestyle risk behaviour classes that are commonly found in the general population, how prevalent they are, what causes them, what future outcomes they predict (distal outcomes), and whether lifestyle risk behaviour classes change over time.
Despite these strengths, our findings should be considered with the following limitations in mind. First, lifestyle risk behaviours were measured based on self-reported questionnaires, which could have recall bias or social desirability bias. Second, the measures of several lifestyle risk behaviours are under-specified; for example, the sleep measure was limited quantity only, without considering sleep quality. The smoking measure also did not consider past smoking.
Conclusion
Latent class membership with two or more lifestyle risk behaviours showed an increased risk of developing CVD and CVD events due to the potential synergetic relationships among lifestyle risk factors. Deprived populations are more likely to be affected by CVD from the wide combination of lifestyle risk behaviours. Early interventions targeting multiple lifestyle risk behaviours from young age should be prioritized to prevent future CVD events.
Supplementary Information
Acknowledgements
We would like to thank the UK Biobank for providing access to the data and Deakin University for creating the platform to access the data. We also thank Gavin Abbott and Katherine Livingstone for their help in facilitating and guiding us with UK Biobank registration and in accessing the data.
Author contributions
T.K.T., S.M.S.I. and R.M. conceptualized the design of the latent class analysis with the distal outcome. T.K.T. performed the data analysis and drafted the manuscript. T.K.T., S.M.S.I. and R.M. have participated in critical revision of the manuscript for important intellectual content. All authors read, provided feedback, and approved the final manuscript.
Data availability
All data relevant to the study are included in the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-22469-6.
References
- 1.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, Brauer M, Burnett R, Cercy K, Charlson FJ. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. The Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. The Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. The Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heart Statistics. BHF Statistics Factsheet: UK. https://www.bhf.org.uk/what-we-do/our-research/heart-statistics.
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;74(10):e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Group LPASW: Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization; 2009. [Google Scholar]
- 9.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A meta-analysis. JAMA. 2011;305(23):2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell JA, Hamer M, Batty GD, Singh-Manoux A, Sabia S, Kivimaki M. Combined effect of physical activity and leisure time sitting on long-term risk of incident obesity and metabolic risk factor clustering. Diabetologia. 2014;57(10):2048–2056. doi: 10.1007/s00125-014-3323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray CJ, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, Bennett D, Bernabé E, Blencowe H, Bourne R. UK health performance: Findings of the global burden of disease study 2010. The Lancet. 2013;381(9871):997–1020. doi: 10.1016/S0140-6736(13)60355-4. [DOI] [PubMed] [Google Scholar]
- 12.Covassin N, Singh P. Sleep duration and cardiovascular disease risk: Epidemiologic and experimental evidence. Sleep Med. Clin. 2016;11(1):81–89. doi: 10.1016/j.jsmc.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehn BM. Sleep Duration Linked to Cardiovascular Disease. American Heart Association; 2019. [DOI] [PubMed] [Google Scholar]
- 14.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease: A review of the recent literature. Curr. Cardiol. Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart CL, Smith GD, Gruer L, Watt GC. The combined effect of smoking tobacco and drinking alcohol on cause-specific mortality: A 30 year cohort study. BMC Public Health. 2010;10(1):1–11. doi: 10.1186/1471-2458-10-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens G, Fischer B, Kohler S, Park Y, Hollenbeck AR, Leitzmann MF. Healthy lifestyle behaviors and decreased risk of mortality in a large prospective study of US women and men. Eur. J. Epidemiol. 2013;28(5):361–372. doi: 10.1007/s10654-013-9796-9. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Bergmann MM, Boeing H, Li C, Capewell S. Healthy lifestyle behaviors and all-cause mortality among adults in the United States. Prev. Med. 2012;55(1):23–27. doi: 10.1016/j.ypmed.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233 110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open. 2016;6(3):e010038. doi: 10.1136/bmjopen-2015-010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol. 2018;3(8):693–702. doi: 10.1001/jamacardio.2018.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birch J, Petty R, Hooper L, Bauld L, Rosenberg G, Vohra J. Clustering of behavioural risk factors for health in UK adults in 2016: A cross-sectional survey. J. Public Health. 2019;41(3):e226–e236. doi: 10.1093/pubmed/fdy144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAloney K, Graham H, Law C, Platt L. A scoping review of statistical approaches to the analysis of multiple health-related behaviours. Prev. Med. 2013;56(6):365–371. doi: 10.1016/j.ypmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Using the BCH method in Mplus to estimate a distal outcome model and an arbitrary secondary model. Mplus Web Notes. 2014;21(2):1–22. [Google Scholar]
- 23.Bakk Z, Tekle FB, Vermunt JK. Estimating the association between latent class membership and external variables using bias-adjusted three-step approaches. Sociol. Methodol. 2013;43(1):272–311. doi: 10.1177/0081175012470644. [DOI] [Google Scholar]
- 24.Bakk Z, Kuha J. Relating latent class membership to external variables: An overview. Br. J. Math. Stat. Psychol. 2021;74(2):340–362. doi: 10.1111/bmsp.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UK Biobank Research Ethics Approval. https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics.
- 26.UK Biobank: A Large Scale Prospective Epidemiological Resource. https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf.
- 27.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 29.Victor Castro. CVrisk: Compute Risk Scores for Cardiovascular Diseases. R package version 1.1.0 (2021).
- 30.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2022. [Google Scholar]
- 31.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 33.The Eatwell Guide Booklet. https://www.gov.uk/government/publications/the-eatwell-guide.
- 34.5 A Day Portion Sizes: NHS Choices. https://www.nhs.uk/live-well/eat-well/5-a-day-portion-sizes/.
- 35.NHS. Alcohol Units. https://www.nhs.uk/live-well/alcohol-support/calculating-alcohol-units/.
- 36.Van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch. Intern. Med. 2012;172(6):494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 37.Henschel B, Gorczyca AM, Chomistek AK. Time spent sitting as an independent risk factor for cardiovascular disease. Am. J. Lifestyle Med. 2020;14(2):204–215. doi: 10.1177/1559827617728482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yousaf S, Bonsall A. UK Townsend Deprivation Scores from 2011 census data. UK Data Service; 2017. [Google Scholar]
- 39.Muthén LK, Muthén BO. Mplus User’s Guide. 8. Muthén & Muthén; 2022. [Google Scholar]
- 40.Weller BE, Bowen NK, Faubert SJ. Latent class analysis: A guide to best practice. J. Black Psychol. 2020;46(4):287–311. doi: 10.1177/0095798420930932. [DOI] [Google Scholar]
- 41.Hsiao Y-Y, Kruger ES, Lee Van Horn M, Tofighi D, MacKinnon DP, Witkiewitz K. Latent class mediation: A comparison of six approaches. Multivar. Behav. Res. 2021;56(4):543–557. doi: 10.1080/00273171.2020.1771674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nylund-Gibson K, Grimm RP, Masyn KE. Prediction from latent classes: A demonstration of different approaches to include distal outcomes in mixture models. Struct. Equ. Modeling. 2019;26(6):967–985. doi: 10.1080/10705511.2019.1590146. [DOI] [Google Scholar]
- 43.Díez-Espino J, Buil-Cosiales P, Babio N, Toledo E, Corella D, Ros E, Fitó M, Gómez-Gracia E, Estruch R, Fiol M. Impact of life's simple 7 on the incidence of major cardiovascular events in high-risk Spanish adults in the PREDIMED study cohort. Rev. Española Cardiol. 2020;73(3):205–211. doi: 10.1016/j.recesp.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Tsai M-C, Lee C-C, Liu S-C, Tseng P-J, Chien K-L. Combined healthy lifestyle factors are more beneficial in reducing cardiovascular disease in younger adults: A meta-analysis of prospective cohort studies. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-75314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacombe J, Armstrong ME, Wright FL, Foster C. The impact of physical activity and an additional behavioural risk factor on cardiovascular disease, cancer and all-cause mortality: A systematic review. BMC Public Health. 2019;19(1):1–16. doi: 10.1186/s12889-019-7030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, Guida F, Ricceri F, d'Errico A, Barros H, Bochud M. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 17 million men and women. The Lancet. 2017;389(10075):1229–1237. doi: 10.1016/S0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krokstad S, Ding D, Grunseit AC, Sund ER, Holmen TL, Rangul V, Bauman A. Multiple lifestyle behaviours and mortality, findings from a large population-based Norwegian cohort study: The HUNT Study. BMC Public Health. 2017;17(1):1–8. doi: 10.1186/s12889-016-3993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding D, Rogers K, van der Ploeg H, Stamatakis E, Bauman AE. Traditional and emerging lifestyle risk behaviors and all-cause mortality in middle-aged and older adults: Evidence from a large population-based Australian cohort. PLoS Med. 2015;12(12):e1001917. doi: 10.1371/journal.pmed.1001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michos ED, Khan SS. Further understanding of ideal cardiovascular health score metrics and cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2021;19(7):607–617. doi: 10.1080/14779072.2021.1937127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai M-C, Yeh T-L, Hsu H-Y, Hsu L-Y, Lee C-C, Tseng P-J, Chien K-L. Comparison of four healthy lifestyle scores for predicting cardiovascular events in a national cohort study. Sci. Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-01213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG. Socioeconomic status and cardiovascular outcomes: Challenges and interventions. Circulation. 2018;137(20):2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foster HM, Celis-Morales CA, Nicholl BI, Petermann-Rocha F, Pell JP, Gill JM, O'Donnell CA, Mair FS. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: A prospective analysis of the UK Biobank cohort. Lancet Public Health. 2018;3(12):e576–e585. doi: 10.1016/S2468-2667(18)30200-7. [DOI] [PubMed] [Google Scholar]
- 53.Theocharidou L, Mulvey MR. The effect of deprivation on coronary heart disease mortality rate. Biosci. Horiz. 2018;11:007. doi: 10.1093/biohorizons/hzy007. [DOI] [Google Scholar]
- 54.Kouvari M, Panagiotakos DB, Chrysohoou C, Georgousopoulou E, Notara V, Tousoulis D, Pitsavos C. Gender-specific, lifestyle-related factors and 10-year cardiovascular disease risk; the ATTICA and GREECS cohort studies. Curr. Vasc. Pharmacol. 2019;17(4):401–410. doi: 10.2174/1570161116666180608121720. [DOI] [PubMed] [Google Scholar]
- 55.Dumith SC, Muniz LC, Tassitano RM, Hallal PC, Menezes AM. Clustering of risk factors for chronic diseases among adolescents from Southern Brazil. Prev. Med. 2012;54(6):393–396. doi: 10.1016/j.ypmed.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuit AJ, van Loon AJM, Tijhuis M, Ocké MC. Clustering of lifestyle risk factors in a general adult population. Prev. Med. 2002;35(3):219–224. doi: 10.1006/pmed.2002.1064. [DOI] [PubMed] [Google Scholar]
- 57.Berlin KS, Williams NA, Parra GR. An introduction to latent variable mixture modeling (part 1): Overview and cross-sectional latent class and latent profile analyses. J. Pediatr. Psychol. 2014;39(2):174–187. doi: 10.1093/jpepsy/jst084. [DOI] [PubMed] [Google Scholar]
- 58.Vermunt J, Magidson J. Latent Class Cluster Analysis. Cambridge University Press; 2002. [Google Scholar]
- 59.Miettunen J, Nordström T, Kaakinen M, Ahmed A. Latent variable mixture modeling in psychiatric research–a review and application. Psychol. Med. 2016;46(3):457–467. doi: 10.1017/S0033291715002305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article.