Abstract
BG24, a VRC01-class broadly neutralizing antibody (bNAb) against HIV-1 Env with relatively few somatic hypermutations (SHMs), represents a promising target for vaccine strategies to elicit CD4-binding site (CD4bs) bNAbs. To understand how SHMs correlate with BG24 neutralization of HIV-1, we report 4.1 Å and 3.4 Å single-particle cryo-EM structures of two inferred germline (iGL) BG24 precursors complexed with engineered Env-based immunogens lacking CD4bs N-glycans. Structures reveal critical Env contacts by BG24iGL and identify antibody light chain structural features that impede Env recognition. In addition, biochemical data and cryo-EM structures of BG24iGL variants bound to Envs with CD4bs glycans present provide insights into N-glycan accommodation, including structural modes of light chain adaptations in the presence of the N276gp120 glycan. Together, these findings reveal Env regions critical for germline antibody recognition and potential sites to alter in immunogen design.
Subject terms: Cryoelectron microscopy, Vaccines, Virus structures, Immunochemistry
VRC01-class bNAbs against the CD4 binding site of HIV-1 Env are targets of vaccine design. Here, the authors structurally characterized germline versions of the VRC01-class bNAb, BG24, bound to Env. They reveal mechanisms of germline binding, informing the design of VRC01-class targeting immunogens.
Introduction
Current strategies to engineer a vaccine towards preventing HIV-1 infection involve designing Env-mimetic immunogens that can elicit broadly neutralizing antibodies (bNAbs)1–4. The CD4-binding site (CD4bs) epitope is a target of immunogen design as bNAbs in this class have been shown to be among the most potent and broad5–9. Several studies have shown passive immunization using CD4bs bNAbs can confer protection from HIV-1 infection in animal models and human clinical trials, suggesting that immunization strategies to elicit these antibodies at effective concentrations would also be protective6,10–17. This includes the VRC01-class of bNAbs that are derived from the VH1-2*02 variable heavy chain gene segment and are characterized by a short 5 amino acid complementary determining region 3 (CDR3) in the antibody (Ab) light chain and a shortened or flexible CDRL15,18. These characteristics are necessary for VRC01-class bNAbs to accommodate the heavily N-glycosylated landscape of the CD4bs of HIV-1 Envs. Thus, VRC01-class bNAbs generally require high levels of somatic hypermutation (SHM), which is challenging to elicit through vaccination.
Germline precursors of bNAbs do not generally show detectable binding to non-engineered, natively-glycosylated HIV-1 Envs19,20, therefore, the germline-targeting approach to HIV-1 vaccine design involves efforts to engineer immunogens that can engage germline B-cell receptors (BCRs) and initiate bNAb development21. Inferred germline (iGL) versions of mature bNAbs derived from predicted germline gene segment sequences represented in the human B-cell repertoire22,23 are used for the germline-targeting approach. Analysis of VRC01-class iGLs has shown that the human VH1-2*02 heavy chain gene segment encodes signature residues that are required for breadth and potency18. Furthermore, germline VRC01-class precursors have been isolated from naïve individuals, and mature bNAbs have been identified from multiple HIV-1-infected human donors, suggesting that raising this class of bNAbs is not uncommon in natural infection24,25. Taken together, VRC01-class bNAbs are attractive targets for immunogen design.
The VRC01-class of bNAbs targets a particularly challenging epitope to elicit bNAbs against due to the presence of the CD4bs N-glycans that sterically obstruct interactions between Env and Ab CDRs26. The glycan at position N276gp120 is highly conserved and poses the greatest steric barrier to binding VRC01-class bNAb iGLs, as Ab residues in the iGL CDRL1 that interact with this region are typically 11–12 residues and cannot accommodate the N276gp120 glycan. Mature CD4bs Abs develop shortened or flexible CDRL1s to accommodate this glycan24,27,28. Thus, understanding the structural basis for how CD4bs iGL Abs mature to effectively accommodate the N276gp120 glycan is essential in efforts to develop effective immunogens to prime VRC01-class iGL precursors and shepherd antibody responses towards bNAb development. Furthermore, an overall structural understanding of VRC01-class iGL recognition of HIV-1 Envs and immunogens is limited as the only existing Fab-Env structures involving germline CD4bs Abs are complexed with gp120 or Env trimer immunogens lacking the N276gp120 glycan3,23,29. In addition, in the case of an iGL Fab complexed with an Env trimer, obtaining a structure required chemical cross-linking between the Env and Ab to form a stable complex22.
A VRC01-class bNAb isolated from an elite neutralizer, BG2430, is an attractive target for germline-targeting immunogen design. BG24 shows similar neutralization and breadth to other CD4bs bNAbs, but includes only 22.6% and 19.5% amino acid substitution by SHM in variable heavy and light chain genes, respectively30, as compared with higher levels of amino acid substitution in VRC01-class bNAbs7,9,28,31, with the exception of the PCIN63 lineage that has similar levels of SHM to BG2432. Structural characterization of BG24 bound to the clade A BG505 Env revealed a similar binding orientation to more mutated VRC01-class bNAbs, and signature contacts common to VRC01-class bNAbs30. Furthermore, neutralization studies using variants of BG24 that reverted variable heavy (VH) and variable light (VL) domain residues to germline counterparts showed that even fewer SHMs were necessary to maintain neutralization breadth30. Collectively, this suggests broad and potent neutralization targeting the CD4bs could be achieved through immunization without stimulating high levels of SHM.
In this work, we structurally characterize the binding of two versions of the BG24 iGL to the CD4bs germline-targeting immunogen BG505-SOSIPv4.1-GT13 (hereafter referred to as GT1), to better understand how the BG24 bNAb was elicited and inform VRC01-class immunogen design. We solve two single-particle cryo-electron microscopy (cryo-EM) structures of GT1 in complex with BG24iGLs containing either mature or iGL CDR3s at 4.1 and 3.4 Å resolution, respectively, in both cases in the absence of chemical crosslinking. Furthermore, to understand how N-glycans impact germline Ab recognition of Env, we conduct biochemical assays and solve cryo-EM structures of BG24iGL derivatives bound to Envs that included the N276gp120 glycan. The structures demonstrate that the CDRL1s of BG24iGLs can adopt conformations that accommodate the N276gp120 glycan, an important capability for a germline-targeting CD4bs immunogen. Collectively, these structures provide information regarding the physical characteristics of iGLs that recognize HIV-1 Env and provide a structural basis for the design of immunogens engineered to engage and mature germline Abs.
Results
Cryo-EM structures of GT1-BG24iGL-10-1074 complexes
To gain insight into how BG24 precursors interact with an HIV-1 Env-based immunogen, we created iGL versions of BG24 and used single-particle cryo-EM to structurally characterize them in complex with GT1, a CD4bs germline-targeting immunogen3. GT1 was modified from a soluble clade A BG505 SOSIP.664 native-like Env trimer33 to permit binding of VRC01-class germline precursors by including T278Rgp120 and G471Sgp120 substitutions and mutations to remove potential N-linked glycosylation sites (PNGSs) at positions N276gp120, N462gp120, N386gp120, and N197gp120 in the CD4bs3. Two iGL versions of BG24 Fab constructs were made starting with the VH1-2*02 and VL2-11*01 heavy and light chain germline gene segment sequences: one containing the CDR3s from mature BG24 (BG24iGL-CDR3mat) and the other containing the iGL CDR3s (BG24iGL-CDR3iGL) (Fig. 1a). Each BG24iGL was structurally characterized in complex with GT1 and the V3 bNAb 10-107434.
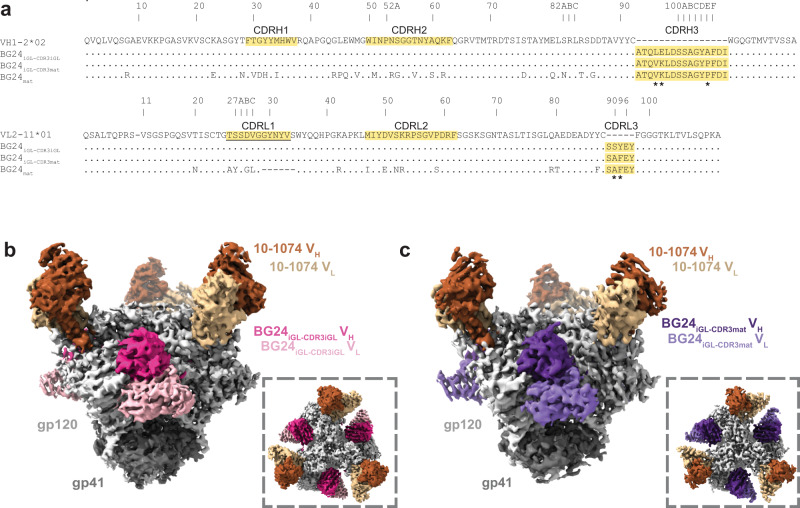
Fig. 1. BG24iGLs bind the CD4bs of the GT1 immunogen.
a Sequence alignment of VH and VL iGL precursors of BG24 (VH1-2*02 and VL2-11*01), BG24iGL-CDR3iGL, BG24iGL-CDR3mat, and BG24mat. CDRs are highlighted in yellow. Asterisks (*) indicate residue differences between mature and iGL CDR3s. Underlined CDRL1 indicates sequence used for the CDRL1 in the BG24CDRL1-iGL construct. b, c Side and top-down (inset) views of cryo-EM density of BG24iGL-CDR3iGL-GT1-10-1074 (b) and BG24iGL-CDR3mat-GT1-10-1074 (c) Highlighted in colors include: gp120 subunits (light gray), gp41 (dark gray), BG24iGL-CDR3iGL VH (bright pink) and VL (light pink) domains, BG24iGL-CDR3mat VH (dark purple) and VL (light purple) domains, and 10-1074 VH (dark brown) and VL (light brown) domains.
Cryo-EM structures of BG24iGL-CDR3iGL and BG24iGL-CDR3mat Fabs bound to GT1 were solved at 3.4 and 4.1 Å, respectively (Fig. 1b, c, Supplementary Fig. 1a–j, Supplementary Table 1). Both 3D cryo-EM reconstructions showed three BG24iGL and three 10-1074 Fabs bound per Env trimer. However, for the BG24iGL-CDR3iGL-GT1-10-1074 complex, a distinct 3D class contained two BG24iGL-CDR3iGL Fabs bound to the GT1 Env (Supplementary Fig. 1e, f, Supplementary Table 1). We also solved a 1.4 Å crystal structure of unbound BG24iGL-CDR3mat Fab (Supplementary Fig. 1k, Supplementary Table 2), which exhibited six disordered residues within CDRL1, but otherwise superimposed with a 1.3 Å root mean square deviation (rmsd; calculated for 225 VH-VL Cα atoms) with the Env-bound BG24iGL-CDR3mat Fab structure, suggesting no major structural differences upon Env binding.
BG24iGL Fabs recognize the modified CD4bs in GT1 Env
The GT1 complexes with BG24iGLs included density for CD4bs N-glycans attached to residues N234gp120, N363gp120, and N392 gp120 (Fig. 2a, b). These N-glycans were also observed in the crystal structure of BG505 Env complexed with a mature BG24 Fab30 (BG24mat) (PDB 7UCF), which also included densities for N-glycans at N197gp120, N276gp120, and N386gp120 that are not present in GT1 (Fig. 2c). Despite additional glycans in BG505 compared with GT1, the CDR loops in the GT1-bound iGL Fabs showed similar orientations and positions as in the BG505-bound BG24mat Fab, except for CDRL1, which is six residues longer in BG24iGL than in BG24mat (Figs. 1a, 2d–f).
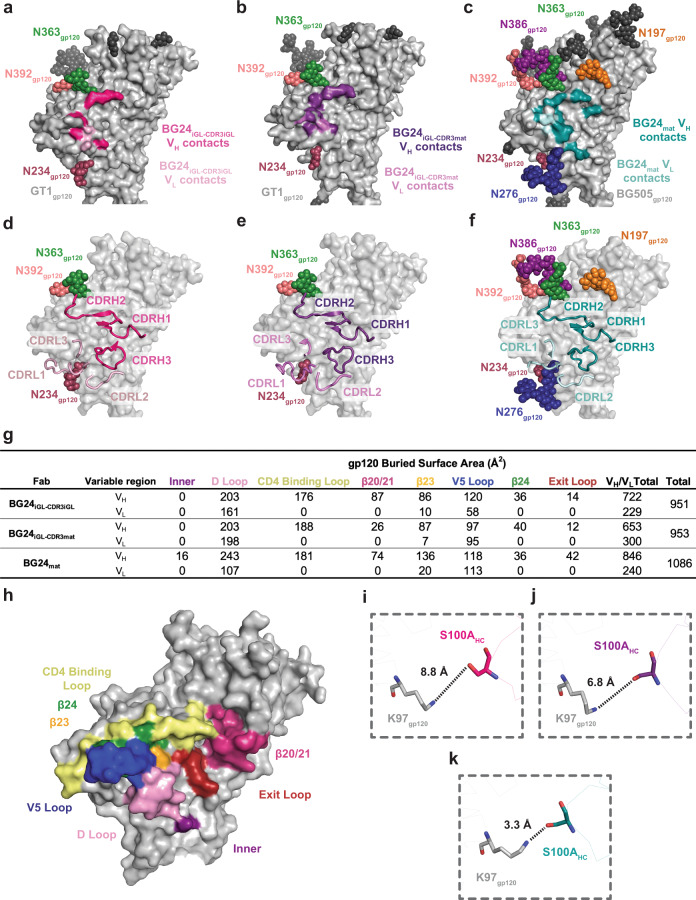
Fig. 2. Comparison of BG24iGL and BG24mat CD4bs epitopes.
Surface contacts made by BG24iGL-CDR3iGL VH (bright pink) and VL (light pink) on GT1 gp120 (light gray) (a), BG24iGL-CDR3mat VH (dark purple) and VL (light purple) on GT1 gp120 (light gray) (b), and BG24mat VH (deep teal) and VL (light teal) on BG505 gp120 (light gray) (PDB 7UCF). (c) Surface representation of gp120 (light gray) with cartoon representations of BG24iGL-CDR3iGL (d), BG24iGL-CDR3mat (e), and BG24mat (f) CDR loops. VH and VL CDR loops are colored the same as in a–c. g Summary table of gp120 buried surface area (BSA) (Å2) calculations for BG24iGL-CDR3iGL, BG24iGL-CDR3mat, and BG24mat at the inner domain (inner), D loop, CD4bs loop, β20/21, β23, V5 loop, β24, and exit loop of the CD4bs. BSA calculations were conducted for gp120 peptide components and did not include glycan interactions. h Surface representation of gp120 (PDB 5T3Z) with CD4bs motifs colored and labeled (inner domain—purple, D loop—light pink, CD4bs loop—yellow, β20/21—bright pink, β23—orange, V5 loop—blue, β24—green, and exit loop—red). Distance measurements between K97gp120 and S100AHC for i BG24iGL-CDR3iGL-GT1gp120, j BG24iGL-CDR3mat-GT1gp120, and k BG24mat-BG505gp120 structures. The distances between atoms are represented by black dotted lines.
BG24iGL-CDR3mat and BG24iGL-CDR3iGL buried comparable surface areas on GT1 gp120 (953 and 951 Å2, respectively) as compared with only slightly larger surface area (1086 Å2) buried on BG505 gp120 in the BG24mat-BG505 structure (PDB 7UCF) (Fig. 2g). We hypothesize that, although a germline precursor antibody presumably exhibits fewer contacts to an antigen than its counterpart somatically mutated bNAb, the overall interface BSA values for the gp120 peptide components of the BG24iGL-GT1 and BG24mat-BG505 structures were similar because the modifications in GT1 (both amino acid substitutions and removals of N-glycans) allowed increased contacts between BG24iGLs and the GT1 gp120. However, differences in interactions between the BG24iGL-GT1 and BG24mat-BG505 structures suggest that SHM substitutions enrich interactions in particular regions within the CD4bs (Fig. 2g, h). For example, in the BG24mat-BG505 complex, BG24mat residue S100AHC hydrogen bonds with the gp120 inner domain residue K97gp120 (Fig. 2g–j). K97gp120 is ~90% conserved among HIV-1 Envs, making this a crucial interaction of broad and potent CD4bs bNAbs18. Residue S100AHC is a germline-encoded residue, however, in both BG24iGL-GT1 structures, is not within the distance to form a hydrogen bond with K97gp120 (Fig. 2I, j). Compared to BG24iGL-GT1, BG24mat-BG505 also showed increased VH buried surface area (BSA) in the gp120 exit loop (gp120 residues 472–476). Together, this analysis demonstrated differences in the distribution of BSA among BG24mat and BG24iGLs in CD4bs regions.
BG24 somatic hypermutation and germline features play a role in CD4bs recognition
We next compared how differences in BG24iGL and BG24mat contribute to their recognition of GT1 and BG505, respectively. BG24iGL contains a germline 11-residue CDRL1 that can recognize the mostly aglycosylated CD4bs in GT1, whereas the BG24mat CDRL1 is six residues shorter and includes a glycine to create a more flexible loop that can accommodate the N276gp120 glycan30. In the BG24mat-BG505 structure, the five-residue BG24 CDRL1 is oriented adjacent to the N276gp120 glycan (Fig. 3a). The CDRL1 interface with GT1 in the BG24iGL-CDR3iGL and BG24iGL-CDR3mat structures showed the longer CDRL1s in the germline precursor VL domains in different conformations, demonstrating CDRL1 flexibility (Fig. 3b, c) consistent with cryo-EM data processing. Indeed, the local resolutions for the CDRL1 in these structures were poor and resolved only after iterative rounds of focused classification and local refinements (Supplementary Fig. 2a, b). Overlaying the BG24iGL CDRL1s with the gp120 region surrounding the N276gp120 glycan from the BG24mat-BG505 structure showed steric clashes, consistent with SHM being necessary for N276gp120 glycan accommodation by BG24 (Fig. 3b, c).
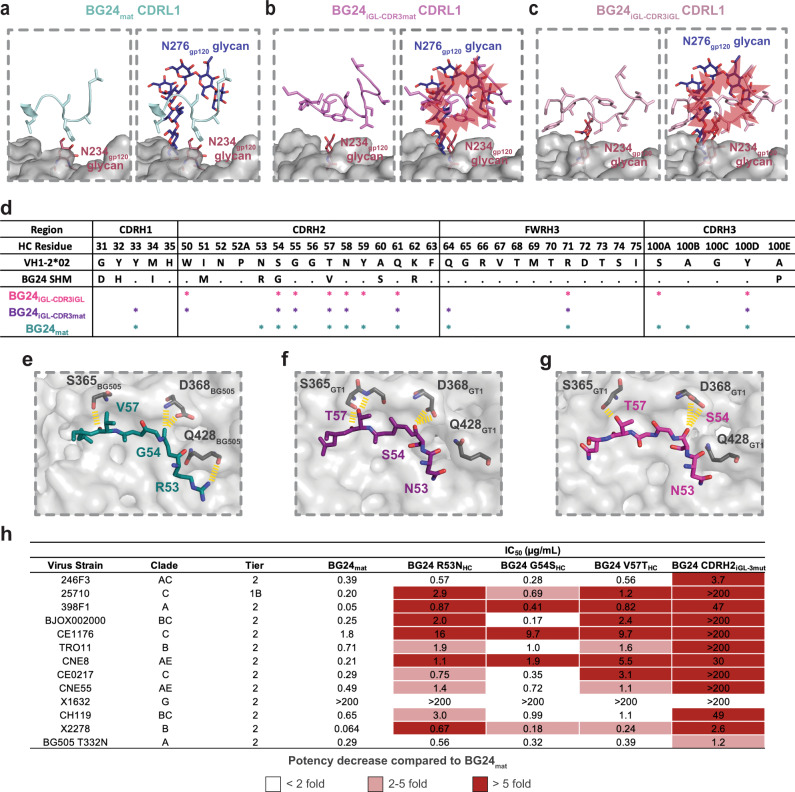
Fig. 3. Somatic hypermutation and germline features play a role in BG24 recognition of the CD4bs interface.
gp120 (light gray) surface in the vicinity of the CD4bs with cartoon representation main chain/stick side chains for the CDRL1s of a BG24mat (light teal), b BG24iGL-CDR3mat (light purple), and c BG24iGL-CDR3iGL (light pink) overlaid with the N276gp120 N-glycan (dark blue) from the BG24mat-BG505 complex (PDB 7UCF). Steric clashes are represented with red bursts. d Table summarizing HC paratope residues in BG24iGL-CDR3iGL-GT1, BG24iGL-CDR3mat-GT1, and BG24mat-BG505 structures. The paratope was defined by Ab residues that make contacts with gp120 within 4 Å for each structure. Stick representations of the CDRH2 residues from e BG24mat (deep teal), f BG24iGL-CDR3mat (dark purple), and g BG24iGL-CDR3iGL (bright pink) interacting with BG505 or GT1 gp120 residues. Yellow dashed lines indicate Ab-gp120 interactions within 4 Å. h Neutralization data of BG24 CDRH2 mutants against a global 12 virus panel35 and BG505 T332N33. IC50 values represent the average mean from duplicate neutralization measurements. A heatmap describes <2-fold (white), 2–5-fold (light red), and >5-fold (dark red) potency decreases compared to BG24mat.
The role of SHMs in Env recognition is summarized in Fig. 3d, where BG24iGL-CDR3iGL, BG24iGL-CDR3mat, and BG24mat HC paratope interactions are mapped to individual Ab residues within 4 Å of gp120. Paratope contacts were limited to CDR H1, H2, and H3, as well as framework region 3 in the heavy chain (FWRH3), with most contacts in CDRH2. Previous studies showed neutralization by an engineered BG24 minimal construct that contained germline-reverted SHMs in FWRs, CDRH1, and CDRL2, but maintained most SHMs in CDRH2, suggesting the importance of SHMs in this region30. The structure of BG24mat-BG505 showed a CDRH2 SHM (N53RHC) interacted with Q428gp120 in gp120 β20/21 (Figs. 1a, 3e). β20/21 interactions with germline-encoded N53HC were absent in BG24iGL-CDR3iGL-GT1 and BG24iGL-CDR3mat-GT1 (Fig. 3f, g). This demonstrates the direct impact of SHM in creating favorable interactions with Env. Other BG24mat somatically hypermutated residues in CDRH2 also interacted with the CD4bs loop (gp120 residues 364–375); e.g., residue T57VHC makes a backbone interaction with S365gp120, and S54GHC interacts with D368gp120, a highly conserved Env residue (Fig. 3d)18. For BG24iGL, germline-encoded residues at positions T57HC and S54HC, maintain similar interactions with GT1 residues S365gp120 and D368gp120, respectively (Fig. 3f, g).
To understand the functional role of these CDRH2 SHMs, we tested the neutralization activity of BG24mat constructs in which individual SHMs were reverted to the corresponding germline residue (BG24 R53NHC, BG24 G54SHC, and BG24 V57THC,) and a combined construct with all three mutations reverted (BG24 CDRH2iGL-3mut) against a 12-strain global panel of HIV-1 strains35 plus BG505 T332N33, and compared potencies and breadth against BG24mat (Fig. 3h). For BG24 constructs with R53NHC and V57THC single mutations, we observed a greater than 5-fold decrease in potency against 6 of the 13 strains tested compared to BG24mat. BG24 G54SHC most closely compared to BG24mat, showing a more than five-fold decrease in potency against only 3 strains. BG24 CDRH2iGL-3mut, was more strongly affected by the SHM reversions, showing more than a five-fold decrease in potency against almost all strains compared the BG24mat. These results demonstrate that SHMs in CDRH2 shown to interact favorably with Env also play a functional role by contributing to neutralization potency and breadth.
Signature residues encoded by the VH1-2*02 germline gene in VRC01-class bNAbs interact with conserved gp120 residues and are correlated with neutralization potency18. These interactions have been structurally characterized in the context of VRC01-class iGLs bound to monomeric gp120s22,23,29, but there are no known structures of VRC01-class iGLs bound to a trimeric Env, except when the iGL was chemically cross-linked to Env22. To evaluate and verify VRC01-class VH1-2*02 germline-encoded interactions with an Env trimer, we compared these interactions in the BG24iGL-GT1 and BG24mat-BG505 structures (Supplementary Fig. 2c–f). Specifically, as previously described in structures involving gp120 monomers22,23,29, germline-encoded R71HC in the BG24iGL-GT1 and BG24mat-BG505 structures formed a salt bridge with the conserved D368gp120 side chain, an Ab interaction that mimics the interaction of host receptor residue R59CD4 with D368gp120 (Supplementary Fig. 2c). In the gp120 D loop, there were interactions between the backbone and side chain of N280gp120 with Y100DHC and germline-encoded W50HC side chains (Supplementary Fig. 2d). In the V5 loop, interactions between the conserved R456gp120 residue and germline-encoded N58HC are conserved in both structures (Supplementary Fig. 2e). In BG24iGL-CDR3mat-GT1, atoms within these Fab residues were separated by more than 5 Å from atoms within gp120 residues; thus, this is not defined as an interaction. In the light chain, E96LC interacted with the backbone of G459gp120 and the side chain of N280gp120 (Supplementary Fig. 2f).
GT1 CD4bs glycan modifications affect BG24 binding
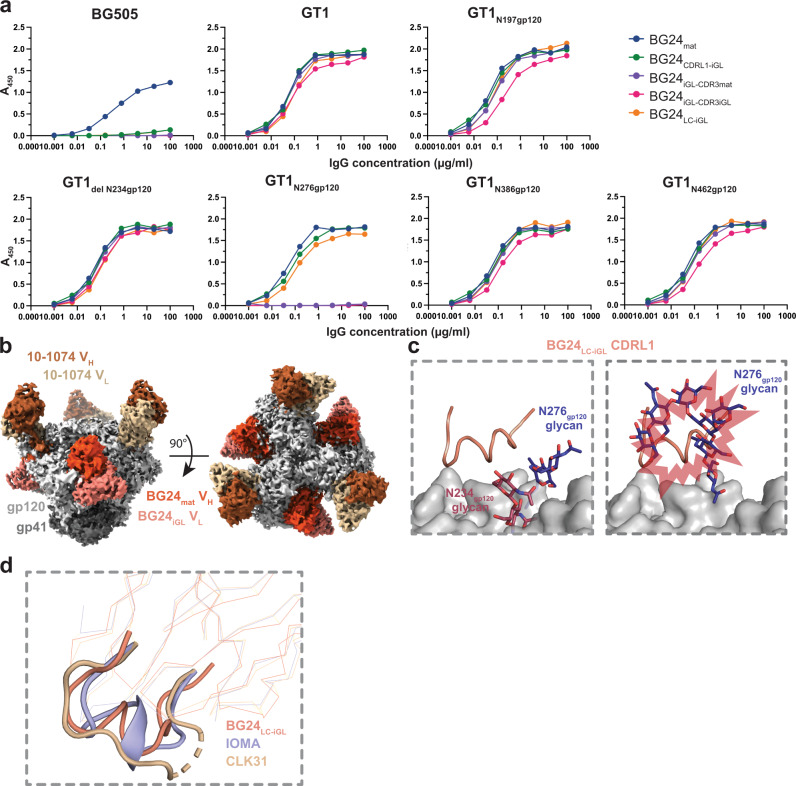
To evaluate how glycan modifications in the GT1 immunogen contributed to BG24iGL binding, we evaluated the binding of BG24 constructs to GT1 with Env PNGSs either restored to or removed from the CD4bs (Fig. 4a). The BG24 constructs included BG24mat, BG24 with germline CDRL1 (BG24CDRL1-iGL) (Fig. 1a), BG24iGL-CDR3mat, BG24iGL-CDR3iGL, and BG24 with an iGL light chain (BG24LC-iGL). PNGSs were individually restored at positions N197gp120, N276gp120, N386gp120, and N462gp120 and removed at N234gp120 to create five GT1 constructs with altered glycan landscapes (GT1N197gp120, GT1N276gp120, GT1N386gp120, GT1N462gp120, and GT1del N234gp120, respectively). BG505 and GT1 binding was evaluated by enzyme-linked immunosorbent assays (ELISAs). Restoring Env PNGSs at positions N197gp120, N386gp120, and N462gp120 and removing the PNGS at N234gp120 did not greatly affect the binding of BG24 IgG constructs (Fig. 4a). BG24iGL constructs did not bind detectably to GT1 with a PNGS at N276gp120; however, BG24mat and mature BG24 constructs with iGL LC features (BG24CDRL1-iGL, BG24LC-iGL) showed comparable binding to each other on GT1N276gp120 (Fig. 4a). BG24mat was the only Ab that showed substantial binding to BG505, which unlike the GT1 Env, included all PNGSs. We conclude that BG24 constructs with a long, germline CDRL1 can accommodate the N276gp120 glycan on Envs that have been engineered to have a limited glycan landscape in the CD4bs. These results contribute to existing studies of N276gp120 glycan accommodation by germline CDRL1 (e.g., ref. 36).
Fig. 4. BG24iGL binding is mediated by CD4bs glycans.
a ELISA to access binding of the indicated BG24 Abs to BG505, GT1, and GT1 SOSIP Envs with altered N-glycans in the CD4bs. Streptavidin plates were coated with randomly biotinylated SOSIPs and incubated with IgGs at increasing concentrations. Values are shown as mean of two individual biological replicates (n = 2). Source data are provided as a Source Data file. b Side and top-down views of cryo-EM density of BG24LC-iGL-GT1N276gp120-10-1074. Highlighted in colors include: gp120 subunits (light gray), gp41 (dark gray), BG24mat VH (deep coral), and VL (light coral) domains, and 10-1074 VH (dark brown) and VL (light brown) domains. c Cartoon representation of the CDLR1 of BG24LC-iGL (left) (light coral) and overlaid with the N276gp120 N-glycan (dark blue) from a BG24mat-BG505 (PDB 7UCF) (right). Predicted steric clashes are indicated by red bursts. d Alignment of BG24LC-iGL (from the BG24LC-iGL-GT1N276gp120−10-1074 structure) (light coral), IOMA (PDB 5T3Z) (lilac), and CLK31 (PDB 6D2P) (wheat) LC with CDRL1s represented in cartoon.
To gain insight into BG24 CDRL1-iGL interactions with GT1 containing an N276gp120 glycan, we solved a single-particle cryo-EM structure of BG24LC-iGL bound to GT1 containing the restored N276gp120 PNGS (GT1N276gp120) (Fig. 4b, Supplementary Fig. 3a–h, Supplementary Table 1). We identified three unique 3D volumes containing either one, two, or three bound BG24LC-iGL Fabs, with the highest resolution complex (4.2 Å) being C3 symmetric with three bound BG24LC-iGL Fabs (Supplementary Fig. 3a–h, Supplementary Table 1). Electron density in the Fab CDRL1 was not optimal after cryo-EM processing; therefore, side chains were not modeled (Supplementary Fig. 3i).
The BG24LC-iGL-GT1N276gp120 complex structure showed that the Fab CDRL1 main chain residues adopted a helix-like conformation to accommodate the N276gp120 glycan (Fig. 4c). Available crystallographic and cryo-EM Env structures demonstrate that the N276gp120 glycan is conformationally heterogeneous6,27,30,37,38 (Supplementary Fig. 3h). Indeed, the N276gp120 glycans in the GT1 and BG505 Envs exhibited different conformations (Fig. 4c). Thus, after superimposing the gp120 residues in the BG24LC-iGL-GT1N276gp120 and BG24mat-BG505 structures, it was evident that the N276gp120 glycan in BG505 showed steric clashes with the CDRL1-iGL in BG24LC-iGL (Fig. 4c). Flexibility of the N276gp120 glycan on BG505 may be more constrained than the counterpart glycan on GT1, as GT1 contains fewer N-glycans in the CD4bs, allowing for increased N276gp120 glycan flexibility. This assumption is consistent with the ELISA results showing that BG24LC-iGL bound to GT1, but not to BG505 Env trimers with an N276gp120 glycan (Fig. 4a).
The only other known CD4bs-targeting bNAb with a helical CDRL1 is IOMA, another VH1-2*02-derived bNAb27. IOMA contains features that distinguish it from VRC01-class bNAbs, including a normal length (8-residue) CDRL3 and a 13-residue CDRL1, which adopts a short α-helix to accommodate the glycan at N276gp120. However, CLK31, an IOMA-like Ab isolated from naïve human B cells using a VRC01 germline-targeting immunogen, did not include a helical CDRL122. Alignment of the LCs of BG24LC-iGL, IOMA, and CLK31 showed that each CDRL1 adopts a different configuration (Fig. 4d). These observations suggest CDRL1 helical conformations are diverse and have only been observed when bound to gp120s that contain the glycan at N276gp120.
BG24CDRL1-iGL accommodates the N276gp120 glycan in a non-engineered Env trimer
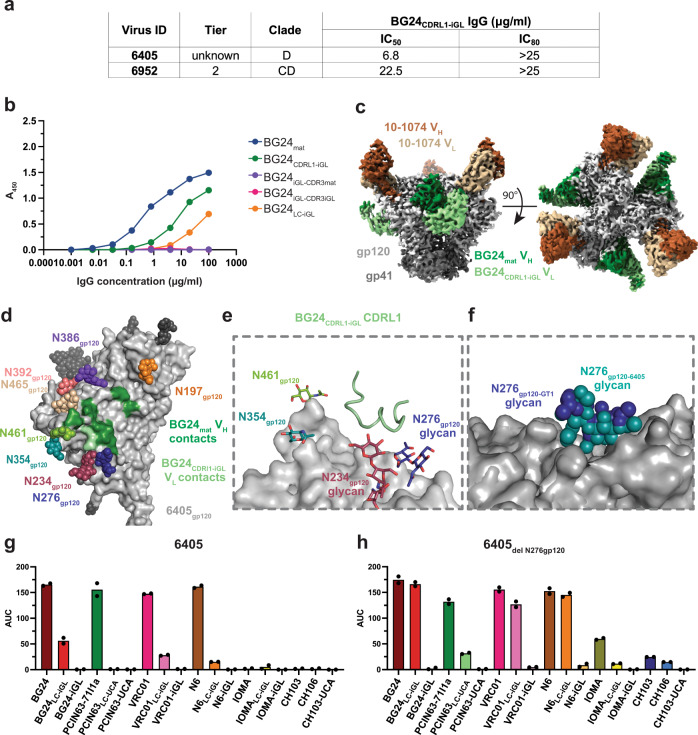
A longitudinal study that tracked the development of a VRC01-class lineage (PCIN63) found that bNAb development branched into two types of N276gp120 glycan engagement: one that interacted with and depended on the presence of the N276 gp120 glycan, and one in which CD4bs binding was diminished by the presence of the N276 gp120 glycan32. In the absence of longitudinal data for BG24mat development, an engineered BG24 intermediate, BG24CDRL1-iGL, was tested in previous work for neutralization against a 119-virus cross-clade panel to better understand how the germline BG24 CDRL1 interacted with HIV-1 Envs bearing the N276 gp120 glycan30. BG24CDRL1-iGL exhibited neutralization activity against two viruses that contained PNGSs at N276gp120: clade D 6405.v4.c34 (6405) and clade CD 6952.v1.c20 (6952) (Fig. 5a)30. The 6405 Env was selected for further investigation by creating a soluble 6405 SOSIP.664 trimer. Sequence alignment of 6405 and BG505 gp120s showed the amino acid identity in the CD4bs and V4 loops differed by more than 50% between BG505 and 6405 Envs (Supplementary Fig. 4). The 6405 gp120 sequence included similar CD4bs PNGSs as BG505, except for the absence of a PNGS at position 363gp120 and an added PNGS at position 465gp120 (Supplementary Fig. 4). We note that the degree to which each PNGS is utilized, the chemical and structural heterogeneity of N-glycans that are attached, and potential cell line and virus versus soluble Env trimer differences in glycosylation are not known for 6405. The binding of BG24 Fab constructs to 6405 was assessed by ELISA. Consistent with neutralization results (Fig. 5a), ELISAs showed that BG24CDRL1-iGL and BG24mat each bound the 6405 SOSIP, whereas BG24LC-iGL bound 6405 to a lesser extent (Fig. 5b). BG24iGLs did not bind detectably to 6405 (Fig. 5b).
Fig. 5. Non-engineered 6405 SOSIP recognizes BG24CDRL1-iGL.
a Summary of neutralization of 6405 and 6952 pseudoviruses by BG24CDRL1-iGL IgGs. b ELISA to access binding of BG24-derived Abs to 6405 SOSIP. Streptavidin plates were coated with randomly biotinylated SOSIPs and incubated with BG24-derived IgGs, at increasing concentrations. Values are shown as mean of two individual biological replicates (n = 2) with associated. c Side and top-down views of cryo-EM density of BG24CDRL1-iGL−6405-10-1074. Highlighted in colors include: gp120 subunits (light gray), gp41 (dark gray), BG24CDRL1-iGL VH (dark green), and VL (light green) domains, and 10-1074 VH (dark brown) and VL (light brown) domains. d Surface contacts made by BG24CDRL1-iGL VH (dark green) and VL (light green) on 6405 gp120 (light gray). e Cartoon representation for the CDRL1 of BG24CDRL1-iGL (light green). f Alignment of GT1N276gp120 (light gray) and 6405 gp120s (light gray) in surface representation and N276 glycans (dark blue and deep teal) in sphere representation. g, h Summary for area under the curve (AUC) values derived from ELISAs that accessed binding of CD4bs IgGs to g 6405 and h 6405delN276gp120 SOSIPs. Streptavidin plates were coated with randomly biotinylated SOSIPs and incubated with CD4bs IgGs at increasing concentrations. Values are shown as mean of two individual biological replicates (n = 2). Source data are provided as a Source Data file.
To understand how the germline CDRL1 of BG24 could be accommodated by a non-engineered Env trimer, we characterized interactions between BG24CDRL1-iGL and 6405 SOSIP by solving a 3.4 Å cryo-EM structure of a BG24CDRL1-iGL-6405 complex (Fig. 5c, Supplementary Fig. 5a–d, Supplementary Table 1). As expected, BG24CDRL1-iGL recognized the CD4bs of 6405, which contained N-glycans at positions N197gp120, N234gp120, N276gp120, N354gp120, N386gp120, N392gp120, N461gp120, and N465gp120 (Fig. 5d). Again, side chains were not modeled for CDRL1 residues (Supplementary Fig. 5e). As also observed in the BG24LC-iGL-GT1N276 structure, the CDRL1-iGL in the BG24CDRL1-iGL-6405 complex formed a helical conformation, although the CDRL1 confirmations in the two Fabs in these complexes were not identical (Supplementary Fig. 5f). By aligning gp120s from GT1N276 and 6405, we found that the well-ordered portions of the N276gp120 glycan occupied similar positions (Fig. 5f), suggesting that the position of N276gp120 in these Envs is conducive to CDRL1 accommodation for BG24iGLs.
We also evaluated the binding of other CD4bs bNAbs to 6405 and a 6405delN276gp120 Env to determine if the CD4bs glycan landscape in 6405 was conducive to interactions with germline CDRL1s in other CD4bs bNAbs (Fig. 5g, h, Supplementary Fig. 6). In this experiment, we included mature versions of the BG24, VRC01, N6 and IOMA bNAb Fabs, chimeric bNAb Fabs including an iGL LC, and complete iGL Fabs. PCIN63 and CH103 and intermediates were compared with their unmutated common ancestors (UCAs), as identified from longitudinal studies32,39, instead of iGLs. The ELISA revealed that the 6405 Env interacted with VRC01LC-iGL and N6LC-iGL in addition to BG24LC-iGL. Binding for all species increased for 6405del N276gp120, indicating the N276gp120 glycan can sterically impede the CDRL1 of the iGLs tested. We conclude that the 6405 Env tolerates the binding of some germline VRC01-class CDRL1s.
Discussion
VRC01-class bNAbs are promising targets for germline-targeting immunogen design as germline-encoded residues make signature contacts with gp120 that contribute to the impressive breadth and potency5,18,22,23. However, challenges in eliciting VRC01-class bNAbs through a germline-targeting vaccine regimen include explicitly selecting for the VH1-2*02 germline gene, overcoming CD4bs glycan barriers, and stimulating high levels of SHM40,41. Despite these challenges, progress has been made in developing a VRC01-class bNAb germline-targeting approach21,41,42, which is initiated by engineering an immunogen that binds to the germline precursors of VRC01 bNAbs. Priming immunogens are engineered to interact with specific germline-encoded residues and lack CD4bs glycans that obstruct germline recognition3,29,40,43. VRC01-class priming immunogens include monomeric gp120 cores25,40,44, SOSIP-based trimers3, and anti-idiotypic antibodies that recognize target BCRs with VH1-2*02 gene segments45,46. Furthermore, selecting a particular strain of Env and a gp120- versus trimeric Env-based platform to engineer priming characteristics that have proven to impact germline BCR selection in vivo47. Thus, identifying and developing the optimal priming immunogen for VRC01-like bNAb elicitation will require a robust understanding of the structural and biophysical nature of Env recognition by germline precursors.
In a sequential immunization approach, boosting immunogens are introduced to shape the development of a germline precursor into a bNAb by stimulating favorable SHMs41. Example boosting immunogens reintroduce native Env glycans and heterogenous Env strains to develop bNAbs capable of overcoming steric glycan barriers and have heterologous-neutralizing activity41. The N276gp120 glycan on HIV-1 Env provides a particularly difficult roadblock, as VRC01-like germline CDRL1s must become shorter or more flexible through SHM to avoid steric clashes23,27,28,32,48. Several iterations of this approach have been tested in animal models; however, the elicitation of heterologous-neutralizing activity has not yet been accomplished40,49.
BG24mat represents a VRC01-class bNAb that can be targeted for germline-targeting approaches30. BG24mat has a fraction of the SHMs found in VRC01 and other VRC01-class bNAbs and maintains notable breadth and potency. Together with previous studies of the VRC01-class PCIN63 lineage and construction of a minimally mutated VRC01, our studies of BG24 suggest that high levels of SHM are not absolutely required for the development of VRC01-class Abs. Our cryo-EM structures of the iGL precursors of BG24 bound to the priming immunogen GT1 contribute to understanding how VRC01-class bNAb precursors interact with immunogens. We found that VH1-2*02 germline-encoded residues make the predicted signature contacts with gp120, and the long germline CDRL1 is accommodated in the absence of the N276gp120 glycan in GT1, rationalizing removal of this glycan in a priming immunogen since modeling suggested the germline CDRL1 conformation would clash with the N276gp120 glycan. These observations validate the design of priming immunogens that nurture interactions with germline residues and remove the N276gp120 glycan from the CD4bs epitope. We further investigated how the glycan landscape of an immunogen affects germline binding, finding that BG24iGL-LC can evade clashes with the N276gp120 glycan when the BG24 HC includes bNAb features and the CD4bs epitope is only minimally glycosylated. Based on these observations, we propose that boosting immunogens might first aim to target mature HC features, and then introduce the N276gp120 glycan in a limited CD4bs glycan landscape before moving to a fully glycosylated Env landscape.
We also characterized the binding of BG24CDRL1-iGL to the clade D 6405 Env, which suggested that some non-engineered HIV-1 Envs can accommodate some germline VRC01-class CDRL1s. In the case of BG24CDRL1-iGL, accommodation of the N276gp120 glycan occurred through a helix-like conformation in the iGL CDRL1. Furthermore, ELISA data suggested that other VH1-2*02-derived bNAbs with iGL LCs can also bind to the 6405 Env. Taken together, we propose further investigation of the 6405 Env to identify properties of the CD4bs that led to the structural and biochemical observations reported here, which could be applicable to the design of CD4bs-targeting immunogens.
Methods
BG24iGL constructs design
Genes encoding the IGHV1-2*02 and IGLV2-11*02 germline sequences with mature CDR3 loops were used to generate the BG24iGL-CDR3mat Fab construct. For the BG24iGL-CDR3iGL construct, amino acids in D- and J- gene regions were reverted based on inferred sequences using IMGT/V-QUEST. Mutations to BG24 IgG and Fab sequences were generated using QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent).
Protein expression and purification
Fabs and IgGs were expressed and purified as previously described50. Briefly, Fabs were expressed by transient transfection using the Expi293 expression system (ThermoFisher). Fab expression vectors contained genes of LC and the C-terminally 6x-His-tagged HC. The Fab and IgG proteins were purified from cell supernatants by Ni2+-NTA (GE Healthcare) and protein A affinity chromatography (GE Healthcare), respectively, followed by size exclusion chromatography (SEC) using a Superdex 200 10/300 column (GE Healthcare).
SOSIP.664 Env constructs contained the disulfide mutations 501 C and 605 C (SOS), I559P (IP), and the furin cleavage site mutated to six arginine residues (6 R)33. Genes encoding BG505 SOSIP.664v4.1-GT1 and 6405 SOSIPs were expressed using transient transfection of Expi293 cells (ThermoFisher) and purified as described previously51. The 6405 SOSIP construct contained gp120 residues 46–477 from the 6405 sequence, with the remaining gp120 residues derived from BG505 and the extracellular portion of the BG505 gp4152. Trimeric Env was separated from cell supernatants by PGT145 immunoaffinity chromatography and SEC using a Superose 6 10/300 column (GE Healthcare), as described53.
X-ray crystallography
Purified BG24iGL-CDR3mat Fab was concentrated to 8–15 mg/mL. Matrix crystallization screens were performed at room temperature using the sitting drop vapor diffusion method by mixing equal volumes of protein sample and reservoir using a TTP LabTech Mosquito robot and commercially-available screens (Hampton Research and Qiagen). Initial hits were optimized and crystals were obtained in 20% PEG 3350 at 20 °C. Crystals were cryo-protected in glycerol stepwise until 20% before being cryopreserved in liquid nitrogen.
X-ray diffraction data were collected to 1.4 Å for BG24iGL-CDR3mat Fab at the Stanford Synchroton Radiation Lightsource (SSRL) beamline 12-2 on a Pilatus 6 M pixel detector (Dectris). Data from a single crystal were indexed and integrated into XDS54 and merged with AIMLESS in the CCP4 software suite55. Structures were determined by molecular replacement in PHASER56 using coordinates of the BG24mat Fab (PDB 7UCE), after the removal of CDR loops and independent searches of the VHVL and CHCL domains. Models were refined using rigid body and B-factor refinement in Phenix57, followed by several cycles of iterative manual building in Coot58 and real-space refinement with TLS groups in Phenix57,59 (Supplementary Table 2).
Assembly of protein complexes and cryo-EM sample preparation
Protein complexes for cryo-EM were generated by incubating a purified BG24iGL Fab and the 10-1074 Fab with an Env trimer in a 3:1 Fab:trimer molar ratio and incubating at 4 °C overnight. The complex was then SEC purified over a Superdex 200 1/150 column (GE Healthcare). The peak corresponding to the complex was pooled and concentrated to 1.0 mg/ml. Quantifoil R2/2 400 mesh cryo-EM grids (Ted Pella) were prepared by glow-discharging for 1 min at 20 mA using a PELCO easiGLOW (Ted Pella). Fab-Env complexes (3 μL) were then applied to grids and blotted with Whatman No. 1 filter paper for 3–4 s at 100% humidity at room temperature. The grids were vitrified by plunge-freezing in liquid ethane using a Mark IV Vitrobot (ThermoFisher).
Cryo-EM data collection and processing
Data for single-particle cryo-EM were collected on either a Talos Arctica (BG24iGL-CDR3mat-GT1-10-1074, BG24iGL-CDR3iGL-GT1N276gp120-10-1074, BG24CDRL1-iGL-6405-10-1074) or a Titan Krios (BG24iGL-CDR3iGL-GT1-10-1074) transmission electron microscope, operating at 200 and 300 kV, respectively. Movies were collected with beam-image shift over a single exposure per hole in a 3-by-3 pattern of 2 μm holes. For datasets collect on the Talos Arctica, movies were recorded in super-resolution mode on a Falcon III camera (Thermo Fisher) at 1.436 Å•pixel−1 or a K3 camera (Gatan) at 0.4345 Å•pixel−1. Movies obtained from samples on the Titan Krios were collected in super-resolution mode on a K3 camera (Gatan) equipped with an BioQuantum energy filter (Gatan) with a 20 eV slit width at 0.4327 Å•pixel−1. The defocus range was set from 1.0 to 3.0 μm for each dataset.
The data processing workflow described below was performed similarly for all datasets using RELION60,61. Movies were motion-corrected using MotionCor262 after binning. GCTF63 was used to estimate CTF, and micrograph power spectra that showed poor CTF fits or bad ice were removed. A subset of particles was manually picked and used for reference-free 2D classification. Classes representing the defined complex were used as references for RELION AutoPicking60,61 to generate 2D classes. Subsequent 2D classes were inspected, and 2D classes representing a defined complex were selected for 3D classification. An ab initio model was generated using cryoSPARC64 using a subset of particles for each dataset and used as a reference in 3D classification, which assumed C1 symmetry. 3D classes representing a defined complex were selected for 3D auto-refinement and post-processing in Relion. Particles used in 3D refinement were then re-extracted and un-binned. Particles were then subjected to 3D classification with the map generated with un-binned particles used as a reference. Distinct classes representing a particular defined complex (C1 or C3 symmetric) were selected for 3D auto-refinement after masking out Fab CHCL domains. Iterative rounds of particle CTF refinement, particle polishing, 3D auto-refinement, and post-processing were used for each class to generate final maps. To improve the resolution of FabLC CDRL1s, a soft mask surrounding the Fab VH-VL-gp120 interface was created in chimera and used for local refinements in cryoSPARC to improve density in this region and allow for CDRL1 fitting and refinement. Resolutions were calculated in RELION using the gold-standard FSC 0.143 criterion65. FSCs were generated by the 3DFSC program66.
Cryo-EM model building and refinement
Model coordinates were generated by fitting reference gp120 (PDB 5T3Z), gp41(PDB 5T3Z), 10-1074 (PDB 5T3Z), and BG24-derivative Fabs (this study) chains into cryo-EM density with UCSF Chimera67. Initial models were refined using the Phenix command phenix.real_space_refine57,59. Sequence updates to the model and further manual refinement was conducted with Coot58. Iterative rounds of Phenix auto-refinement and manual refinements were done to generate the final models (Supplementary Table 1).
Structural analyses
Structure figures were made using PyMol (Schrödinger LLC), UCSF Chimera67, and UCSF ChimeraX68,69. PyMol was used to calculate r.m.s.d. values after the pairwise alignment of Cα atoms. PDBePISA70 was used to calculate buried surface areas using a 1.4 Å probe. Calculations for gp120 BSA were for peptide components of gp120 and did not include glycan interactions. Defined interactions were assigned tentatively due to the low resolution of complexes using the following criteria: hydrogen bonds were assigned pairwise interactions that were less than 4.0 Å and with an A-D-H angle >90°, and van der Waals interactions were assigned as distances between atoms that were less than 4.0 Å.
TZM.bl neutralization assay
The neutralizing activities of BG24 CDRH2 mutant IgGs were measured using a luciferase-based TZM.bl assay according to standard protocols71. Each assay was performed in duplicate. Data were analyzed using Antibody Database (v2.0)72. Five-parameter curve fitting was used to determine 50% inhibitory concentrations (IC50s), and nonspecific activity was detected by testing against murine leukemia virus (MuLV).
Enzyme-linked immunosorbent assay
SOSIP trimers were randomly biotinylated following the manufacturer’s guidelines using the EZ-Link NHS-PEG4-Biotin kit (Thermo Fisher Scientific). The Pierce Biotin kit (Thermo Fisher Scientific) was used to quantify biotin molecules per SOSIP protomer: biotin estimations ranged from 1 to 10 biotin molecules per SOSIP protomers. Streptavidin-coated 96-well plates (Thermo Fisher Scientific) were coated with 5 µg/mL of randomly biotinylated SOSIPs diluted in 3% BSA in TBS-T (20 mM Tris, 150 mM NaCl, 0.1% Tween20) and incubated at room temperature (RT) for 2 h. Plates were washed to remove unbound SOSIPs. Serial dilutions of IgGs were made in 3% BSA in TBS-T and applied to the plates. After a 2 h incubation at RT, plates were washed twice in TBS-T. Goat anti-human IgG Fc conjugated to horse-radish peroxidase (Southern BioTech) was added at 1:8000 dilution for 30 min, followed by three washes with TBS-T. 1-Step™ Ultra TMB-ELISA Substrate Solution (ThermoFisher Scientific) was added for colorimetric detection, and color development was quenched with 1 N HCl. Absorbance was measured at 450 nm. Two independent, biological replicates (n = 2) were performed.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank J. Vielmetter, P. Hoffman, and the Protein Expression Center in the Beckman Institute at Caltech for expression assistance. Electron microscopy was performed in the Caltech Cryo-EM Center with assistance from S. Chen and A. Malyutin. We thank the Gordon and Betty Moore and Beckman Foundations for gifts to Caltech to support the Molecular Observatory (Dr. Jens Kaiser, Director) and the beamline staff at SSRL for data collection assistance. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-c76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. C.O.B. was supported by a Burroughs Wellcome PDEP fellowship and HHMI Hanna Gray Fellowship. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) Grant HIVRAD P01 AI100148 (to P.J.B. and M.C.N.), the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (CAVD) grant INV-002143 (P.J.B. and M.C.N.), and NIH P50 AI150464 (P.J.B.).
Source data
Author contributions
K.A.D., C.O.B., H.B.G., T.S., M.C.N., and P.J.B. designed the research. K.A.D. performed protein purification, structural studies, and ELISA experiments. P.N.P.G. performed in vitro neutralization assays. K.A.D., C.O.B., and H.B.G. analyzed results. K.A.D. and P.J.B. wrote the manuscript with input from co-authors.
Peer review
Peer review information
Nature Communications thanks Quentin Sattentau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The atomic model generated for the X-ray crystallography structure of the BG24iGL-CDR3mat Fab in this study has been deposited in the Protein Data Bank (PDB) under accession code 7UGM. The cryo-EM maps and atomic structures have been deposited in the PDB and/or Electron Microscopy Data Bank (EMDB) under accession codes 7UGN and EMD-26490 for BG24iGL-CDR3iGL-GT1-10-1074 Class I, EMD-26491 for BG24iGL-CDR3iGL-GT1-10-1074 Class II, 7UGO and EMD-26492 for BG24iGL-CDR3mat-GT1-10-1074, 7UGP and EMD-26493 for BG24iGL-LC-GT1N276gp120−10-1074 Class I, EMD-26494 for BG24iGL-LC-GT1N276gp120−10-1074 Class II, EMD-26495 for BG24iGL-LC-GT1N276gp120−10-1074 Class III, and 7UGQ and EMD-26496 for BG24CDRL1-iGL−6405-10-1074. Local refinement maps used to model CDRL1s of BG24-derivatives have been deposited with PDB and EMDB accession codes for each respective structure. The ELISA data generated in this study are provided in the Supplementary Information and Source Data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33860-2.
References
- 1.Escolano A, et al. Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques. Nature. 2019;570:468–473. doi: 10.1038/s41586-019-1250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Crotty S. HIV vaccinology: 2021 update. Semin. Immunol. 2021;51:101470. doi: 10.1016/j.smim.2021.101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medina-Ramírez M, et al. Design and crystal structure of a native-like HIV-1 envelope trimer that engages multiple broadly neutralizing antibody precursors in vivo. J. Exp. Med. 2017;214:2573–2590. doi: 10.1084/jem.20161160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders RW, Moore JP. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol. Rev. 2017;275:161–182. doi: 10.1111/imr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou, T. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science329, 1–8 (2010). [DOI] [PMC free article] [PubMed]
- 6.Schommers P, et al. Restriction of HIV-1 escape by a highly broad and potent neutralizing antibody. Cell. 2020;180:471–489.e22. doi: 10.1016/j.cell.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, et al. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity. 2016;45:1108–1121. doi: 10.1016/j.immuni.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar KJ, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N. Engl. J. Med. 2016;375:2037–2050. doi: 10.1056/NEJMoa1608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-On Y, et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat. Med. 2018;24:1701–1707. doi: 10.1038/s41591-018-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miner, M. D., Corey, L. & Montefiori, D. Broadly neutralizing monoclonal antibodies for HIV prevention. J. Int. AIDS Soc. 24, 59–65 (2021). [DOI] [PMC free article] [PubMed]
- 13.Mendoza, P. et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature10.1038/s41586-018-0531-2 (2018). [DOI] [PMC free article] [PubMed]
- 14.VRC 602 Study Team. et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin. Exp. Immunol. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch, R. M. et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci. Transl. Med. 7, 1–14 (2015). [DOI] [PMC free article] [PubMed]
- 16.Mayer KH, et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: results of a phase 1 randomized trial. PLoS Med. 2017;14:e1002435. doi: 10.1371/journal.pmed.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escolano A, Dosenovic P, Nussenzweig MC. Progress toward active or passive HIV-1 vaccination. J. Exp. Med. 2017;214:3–16. doi: 10.1084/jem.20161765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West Jr, A. P. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. PNAS10.1073/pnas.1208984109/-/DCSupplemental (2012). [DOI] [PMC free article] [PubMed]
- 19.Xiao X, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: Implications for evasion of immune responses and design of vaccine immunogens. Biochem. Biophys. Res. Commun. 2009;390:404–409. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonsignori M, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J. Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton DR. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 2019;19:77–78. doi: 10.1038/s41577-018-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borst, A. Germline VRC01 antibody recognition of a modified clade C HIV-1 envelope trimer and a glycosylated HIV-1 gp120 core. eLife10, 1–32 (2018). [DOI] [PMC free article] [PubMed]
- 23.Scharf L, et al. Structural basis for HIV-1 gp120 recognition by a germ-line version of a broadly neutralizing antibody. Proc. Natl Acad. Sci. USA. 2013;110:6049–6054. doi: 10.1073/pnas.1303682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou T, et al. Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell. 2015;161:1280–1292. doi: 10.1016/j.cell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardine JG, et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351:1458–1463. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou T, et al. Quantification of the impact of the HIV-1-glycan shield on antibody elicitation. Cell Rep. 2017;19:719–732. doi: 10.1016/j.celrep.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gristick HB, et al. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat. Publ. Group. 2016;23:906–915. doi: 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou T, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jardine JG, et al. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349:156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes CO, et al. A naturally arising broad and potent CD4-binding site antibody with low somatic mutation. Sci. Adv. 2022;8:eabp8155. doi: 10.1126/sciadv.abp8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang D, et al. B cells expressing authentic naive human VRC01-class BCRs can be recruited to germinal centers and affinity mature in multiple independent mouse models. Proc. Natl Acad. Sci. USA. 2020;117:22920–22931. doi: 10.1073/pnas.2004489117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umotoy J, et al. Rapid and focused maturation of a VRC01-class HIV broadly neutralizing antibody lineage involves both binding and accommodation of the N276-glycan. Immunity. 2019;51:141–154.e6. doi: 10.1016/j.immuni.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders RW, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618–e1003620. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl Acad. Sci. USA. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.deCamp A, et al. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2014;88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonsignori M, et al. Inference of the HIV-1 VRC01 antibody lineage unmutated common ancestor reveals alternative pathways to overcome a key glycan barrier. Immunity. 2018;49:1162–1174.e8. doi: 10.1016/j.immuni.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scharf, L. et al. Broadly neutralizing antibody 8ANC195 recognizes closed and open states of HIV-1 Env. Cell162, 1–13 (2015). [DOI] [PMC free article] [PubMed]
- 38.Lee JH, et al. A broadly neutralizing antibody targets the dynamic hiv envelope trimer apex via a long, rigidified, and anionic β-hairpin structure. Immunity. 2017;46:690–702. doi: 10.1016/j.immuni.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao H-X, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parks KR, et al. Overcoming steric restrictions of VRC01 HIV-1 neutralizing antibodies through immunization. Cell Rep. 2019;29:3060–3072.e7. doi: 10.1016/j.celrep.2019.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatatos L, Pancera M, McGuire AT. Germline-targeting immunogens. Immunol. Rev. 2017;275:203–216. doi: 10.1111/imr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimitrov DS. Therapeutic antibodies, vaccines and antibodyomes. mAbs. 2010;2:347–356. doi: 10.4161/mabs.2.3.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuire AT, et al. Specifically modified Env immunogens activate B-cell precursors of broadly neutralizing HIV-1 antibodies in transgenic mice. Nat. Commun. 2016;7:10618. doi: 10.1038/ncomms10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jardine J, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seydoux E, et al. Development of a VRC01-class germline targeting immunogen derived from anti-idiotypic antibodies. Cell Rep. 2021;35:109084. doi: 10.1016/j.celrep.2021.109084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dosenovic P, et al. Anti-idiotypic antibodies elicit anti-HIV-1–specific B cell responses. J. Exp. Med. 2019;216:2316–2330. doi: 10.1084/jem.20190446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin Y-R, et al. HIV-1 VRC01 germline-targeting immunogens select distinct epitope-specific B cell receptors. Immunity. 2020;53:840–851.e6. doi: 10.1016/j.immuni.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jardine JG, et al. Minimally mutated HIV-1 broadly neutralizing antibodies to guide reductionist vaccine design. PLoS Pathog. 2016;12:e1005815–e1005833. doi: 10.1371/journal.ppat.1005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briney B, et al. Tailored immunogens direct affinity maturation toward HIV neutralizing antibodies. Cell. 2016;166:1459–1470.e11. doi: 10.1016/j.cell.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Z, Wang H, Liu AZ, Gristick HB, Bjorkman PJ. Asymmetric opening of HIV-1 Env bound to CD4 and a coreceptor-mimicking antibody. Nat. Struct. Mol. Biol. 2019;26:1167–1175. doi: 10.1038/s41594-019-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escolano A, et al. Sequential immunization of macaques elicits heterologous neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env. Sci. Transl. Med. 2021;13:eabk1533. doi: 10.1126/scitranslmed.abk1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joyce MG, et al. Soluble prefusion closed DS-SOSIP.664-Env trimers of diverse HIV-1 strains. Cell Rep. 2017;21:2992–3002. doi: 10.1016/j.celrep.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Cupo A, et al. Optimizing the production and affinity purification of HIV-1 envelope glycoprotein SOSIP trimers from transiently transfected CHO cells. PLoS ONE. 2019;14:e0215106. doi: 10.1371/journal.pone.0215106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winn MD, et al. Overview of the CCP 4 suite and current developments. Acta Crystallogr. D. Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCoy AJ, et al. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afonine PV, et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. Sect. Struct. Biol. 2018;74:531–544. doi: 10.1107/S2059798318006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zivanov J, et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheres SHW. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 65.Scheres SHW, Chen S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods. 2012;9:853–854. doi: 10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan YZ, et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods. 2017;14:793–796. doi: 10.1038/nmeth.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pettersen EF, et al. UCSF Chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 68.Goddard TD, et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis: UCSF ChimeraX Visualization System. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pettersen EF, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 71.Sarzotti-Kelsoe M, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods. 2014;409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.West, A. P. et al. Computational analysis of anti–HIV-1 antibody neutralization panel data to identify potential functional epitope residues. PNAS. 110, 10598–10603 (2013). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic model generated for the X-ray crystallography structure of the BG24iGL-CDR3mat Fab in this study has been deposited in the Protein Data Bank (PDB) under accession code 7UGM. The cryo-EM maps and atomic structures have been deposited in the PDB and/or Electron Microscopy Data Bank (EMDB) under accession codes 7UGN and EMD-26490 for BG24iGL-CDR3iGL-GT1-10-1074 Class I, EMD-26491 for BG24iGL-CDR3iGL-GT1-10-1074 Class II, 7UGO and EMD-26492 for BG24iGL-CDR3mat-GT1-10-1074, 7UGP and EMD-26493 for BG24iGL-LC-GT1N276gp120−10-1074 Class I, EMD-26494 for BG24iGL-LC-GT1N276gp120−10-1074 Class II, EMD-26495 for BG24iGL-LC-GT1N276gp120−10-1074 Class III, and 7UGQ and EMD-26496 for BG24CDRL1-iGL−6405-10-1074. Local refinement maps used to model CDRL1s of BG24-derivatives have been deposited with PDB and EMDB accession codes for each respective structure. The ELISA data generated in this study are provided in the Supplementary Information and Source Data file. Source data are provided with this paper.