Dear Editor,
Mixed lineage kinase domain-like protein (MLKL) emerged as executioner of necroptosis1–3. The intrinsic nature of MLKL and how it induces plasma membrane permeabilization, forming a huge pore or a channel, remain an interesting conundrum for a long time4,5. Our previous study demonstrated that MLKL forms cation channels and its channel activity is a primary effector of necroptosis5,6. In the field of MLKL, a major unsettled issue is, if MLKL does serve as channels, how it ignites the necrotic or non-necrotic pathogenic progresses4,5. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) is a necessary cofactor of various ion channels7. The physical interaction between MLKL and phosphatidylinositol phosphates (PIPs), including PI(4,5)P2, facilitates MLKL-mediated liposome leakage and the necrotic membrane disruption8–10. These studies have been directed toward understanding whether PI(4,5)P2 is a direct modulator for the MLKL channel activity. The potential effects of PI(4,5)P2 on MLKL channels were thus evaluated electrophysiologically and the subsequent pathogenic influences were explored.
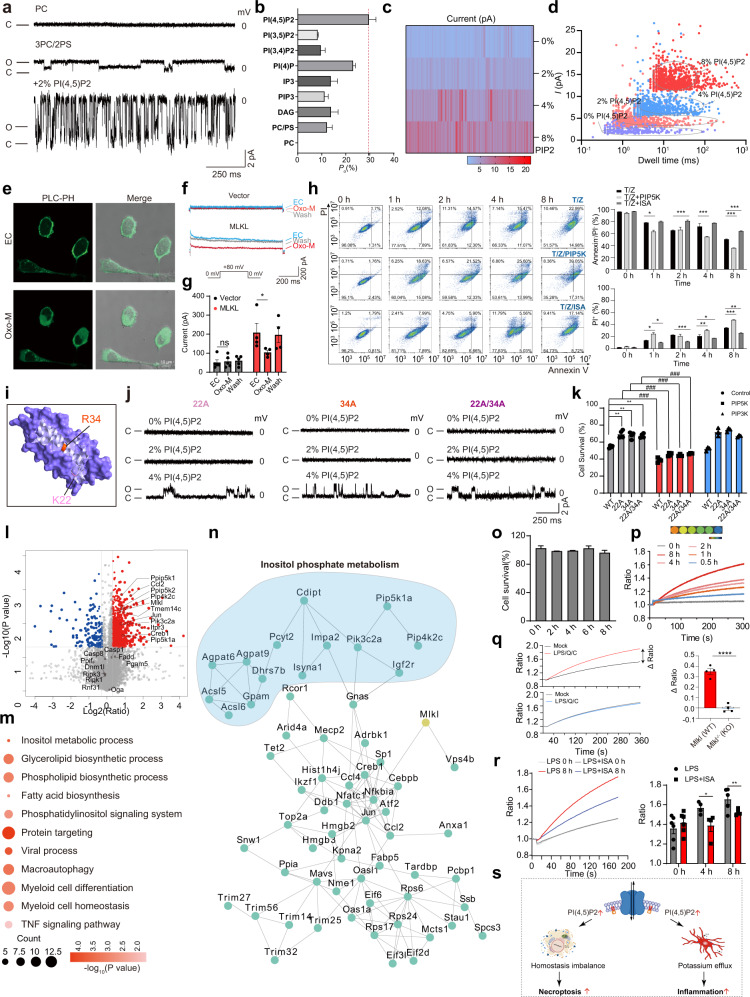
The four-helical bundle domain (4HBD) in the N-terminal region of MLKL (MLKLNT) is sufficient to induce oligomerization and trigger cell death9. Consistent with our previous study, MLKLNT protein exhibited channel activity, translocated onto plasma membrane, and caused cell death, similarly to full-length protein (MLKLFL) (Supplementary Figs. S1 and S2). We next asked whether lipids could regulate the channel activity. Interestingly, we found that the typical single-channel currents could only be recorded when the phosphatidylcholine (PC) bilayers were premixed with phosphatidylserine (PS). Different from the neutrally charged PC, PS is a type of negatively charged phospholipid (Fig. 1a; Supplementary Fig. S3a, b). Afterward, MLKL channel activity was tested with some other important negatively charged phospholipids. Under the identical experimental conditions, we observed much larger step-like signals in the 3PC/2PS lipids premixed with 2% PI(4,5)P2 compared with those without PI(4,5)P2 (Fig. 1a). The overall open probability (Po) was enhanced to 27.4% (Fig. 1b). These step-like currents exhibited two conductance states (Fig. 1b, c; Supplementary Fig. S3c). Influences of other lipids on MLKL channel activity were tested, including phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2) and phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2), phosphatidylinositol 4-phosphate (PI(4)P), 1,4,5-trisphosphate inositol (IP3) and diacylglycerol (DAG), phosphatidylinositol 3,4,5-trisphosphate (PIP3)11. Among these lipids, PI(4)P is the only one that increases MLKL channel open probability (Fig. 1b; Supplementary Fig. S3a, b). Collectively, these data indicate that the anionic phospholipids are essential for the channel function and the channel activity could be modulated by the PIPs, particularly PI(4,5)P2. Subsequently, we further tested MLKL channel activity in the presence of different concentrations of PI(4,5)P2. An increased concentration of PI(4,5)P2 induced more frequent and larger currents of MLKL channel (Fig. 1c, d; Supplementary Fig. S4a, b). To further evaluate whether the observed influences were due to the changes in PI(4,5)P2 level, a well-recognized approach was exploited to manipulate PI(4,5)P2 levels in live cells. Stimulation of the M1 muscarinic receptor can activate PLC-β which hydrolyzes PI(4,5)P2. PLC-β-PH-GFP (PLC-PH) construct, a PI(4,5)P2 reporter, is used to monitor the PI(4,5)P2 distribution on plasma membrane12. Here, MLKL, M1 receptor, and PLC-PH were co-transfected into HEK293 cells. With 5 μM oxotremorine M (Oxo-M) treatment, the PLC-PH probe dissociated from the plasma membrane to cytoplasm, showing PI(4,5)P2 hydrolyzation (Fig. 1e). Meanwhile, MLKL currents were rapidly inhibited and restored after Oxo-M withdrawal (Fig. 1f, g; Supplementary Fig. S4c). These data indicate that MLKL channel activity could be finely tuned by PI(4,5)P2.
Fig. 1. Enhanced MLKL channel activity by PI(4,5)P2 promotes necroptosis and inflammation.
a, b Single-channel current recordings and open probability of MLKLNT in different lipid compositions (n > 3). c The heatmap shows MLKLNT currents in various PI(4,5)P2 concentrations. d Scatterplots of MLKLNT currents vs. dwell times. e Representative subcellular localization of GFP-PH domain. Cells were treated with or without 5 μM Oxo-M. f The currents of MLKL channel with or without Oxo-M treatment. EC indicate the extracellular solution. g Statistical analysis of MLKL currents (n ≥ 3). h Necroptosis was detected through Annexin V-FITC (AV)/PI staining. L929 cells were treated with 20 ng/mL T and 20 μM Z after PIP5K transfection or ISA treatment (left). Statistical analysis of AV/PI staining (right). i Schematic representation of the residues examined by the solution MLKLNT structure. j Current traces of MLKL mutants 22A, 34A, and 22A/34A in the presence of different concentrations of PI(4,5)P2. k Cell viability of MLKL–/– HeLa cells after co-transfection with PIP5K or PIP3K and MLKL mutant constructs. l The volcano plots for the comparison between proteome patterns of BV2 cells treated with PBS or LPS. m Representative function enrichment analysis of upregulated DEGs. Functional terms were labeled and color-coded with log10 transformed P-value (Fisher’s exact test). n PPI network of proteins shown in Supplementary Fig. S8. Proteins participating in inositol phosphate metabolism are labeled with blue. o Cell viability of BV2 cells after LPS treatment. p, q Potassium efflux levels in LPS-treated BV2 cells and LPS/Q/C-treated BMDM cells (left). Statistical analysis results were shown (right). r Detection of K+ efflux level of LPS-treated BV2 cells with or without ISA (left). Statistical analysis results were shown (right). s Enhanced MLKL channel activity by PI(4,5)P2 promoted necroptosis and inflammation via disturbing ion homeostasis and accelerating potassium efflux.
Whether the PI(4,5)P2-induced augmentation of MLKL channel activity is correlated with cell death was examined. To elevate PI(4,5)P2 concentrations in the plasma membrane, PIP5K, one of the key PI(4,5)P2 biosynthetic enzymes, was transfected into L929 cells (Supplementary Fig. S5). A time-dependent increase in the number of fluorescent cells stained by Annexin V-FITC and prodidium iodide (PI) was observed in PIP5K-transfected L929 cells compared to non-transfected cells with tumor necrosis factor (TNF-α, T) plus pan-caspase inhibitor z-VAD-fmk (Z) treatment1 (Fig. 1h; Supplementary Fig. S6a). In addition, we found that either knockdown of PIP5K or administration of the PIP5K inhibitor ISA-2011B (ISA) efficiently suppressed the TNF-induced necroptosis in L929 cells (Fig. 1h; Supplementary Fig. S6b). Similar PI(4,5)P2 influences were observed in HeLa cells (Supplementary Fig. S6c, d). PIP3K was used as a negative control (Supplementary Fig. S5). Collectively, these results support that the elevated PI(4,5)P2 level aggravated MLKL-dependent necroptosis.
For many PI(4,5)P2-sensitive ion channels, PI(4,5)P2 binds with positive amino acid via “electrostatic interaction”7,13. We mutated the positive amino acids of MLKLNT to alanine and tested the channel activity (Supplementary Fig. S7a–c). Two of these mutations, K22A (22A) and R34A (34A), were found to abolish the channel function regardless with or without 2% PI(4,5)P2 (Fig. 1i, j). Notably, the channel functions were rescued under higher PI(4,5)P2 concentrations (4%) (Fig. 1j; Supplementary Fig. S7d). Whether these mutant channels could lead to cell death was then investigated. In comparison to WT MLKLNT, the capability of killing cells of the two mutant channels was lost or largely suppressed in MLKL–/– HeLa cells9 (Fig. 1k). Consistently, the two mutants became toxic to cells after overexpression of PIP5K that elevated PI(4,5)P2 levels on membranes. Of note, the double mutant K22A/R34A did not further reduce the PI(4,5)P2 sensitivity or abrogate the capability of killing cells after overexpression of PIP5K, suggesting that K22 and R34 may interact with PI(4,5)P2 independently (Fig. 1j, k).
MLKL could activate the innate immune receptor nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) in a cell-intrinsic manner before cell lysis, but its working model has not yet been clarified14. Thus, we further explored the potential function of PI(4,5)P2-enhanced MLKL channel activity in an inflammation model12 (Supplementary Table S1). The proteome profiling of mouse microglia (BV2 cells) treated with lipopolysaccharide (LPS) showed that expression of traditional MLKL-binding proteins related to cell death (such as RIPK1, RIPK3) was not influenced by LPS, indicating that this inflammatory process was independent of classical necroptosis pathway (Fig. 1l). Gene enrichment analysis on differentially expressed genes (DEGs) showed activation of inositol metabolism and phospholipid metabolic processes, as well as inflammatory responses induced by LPS treatment (Fig. 1m; Supplementary Fig. S8). Of particular relevance is that enzymes responsible for PI(4,5)P2 anabolism were upregulated by LPS treatment, along with the LPS-induced inflammation-related proteins (Fig. 1n; Supplementary Fig. S8). To investigate the molecular regulation network of the DEGs related to PI(4,5)P2 metabolism and inflammatory responses, we searched their protein–protein interactions (PPIs) through String database (https://string-db.org), and further revealed the close relationship between PI(4,5)P2, MLKL and LPS-induced inflammation (Fig. 1n).
Consistent with proteomic data, treatment with the lower concentration (10 ng/mL) of LPS significantly stimulated the secretion of inflammatory cytokines, but did not induce cell death, while the expression level of MLKL and PIP5K increased (Fig. 1o; Supplementary Fig. S9a, b). Accordingly, an essential role of MLKL-dependent K+ efflux in triggering inflammation has been proposed14,15. We therefore hypothesized that K+ efflux during inflammation is mediated by MLKL channels. A real-time, sensitive FluxOR™ assay was performed to monitor the K+ efflux during the LPS-induced inflammation. We found that the K+ efflux gradually increased in a time-dependent manner (Fig. 1p). The contribution of MLKL channel to the K+ efflux was further evaluated in a widely used MLKL-related inflammation model, bone marrow-derived macrophages (BMDMs) treated with LPS, Smac-mimetic compound (C) and a caspase inhibitor Q-VD-OPh (Q)14. Both the MLKL-mediated cytokine secretion and the LPS-induced K+ efflux were abrogated in MLKL-knockout (MLKL–/–) cells (Fig. 1q; Supplementary Fig. S9c, d). Whether inhibition of PI(4,5)P2 synthesis after ISA treatment suppresses K+ efflux was examined in two inflammation models. After administration of ISA, the K+ efflux was indeed decreased in both the LPS-induced and the classical NLRP3 activation inflammation models (Fig. 1r; Supplementary Fig. S9f). Since elevated PI(4,5)P2 level is a key factor for the MLKL-related inflammation, we used PIP5K-knockdown BV2 cells to detect cytokine secretion level (Supplementary Fig. S9g). The result showed that blocking the PI(4,5)P2 synthesis indeed suppressed the secretion of inflammatory cytokines (Supplementary Fig. S9h). These results link PI(4,5)P2 with K+ efflux in MLKL-related inflammation.
In summary, we show that MLKL channel activity is fine-tuned by PI(4,5)P2 in a dose-dependent manner for the first time (Fig. 1c–g). PI(4,5)P2 exhibits agonistic effects on MLKL channel activity and the enhanced channel activity, alone or with other factors together, may ignite necroptosis or inflammation under specific stimuli (Fig. 1s). Whether and how the fine-tuned MLKL channel activity by PI(4,5)P2 participates in more substantial biological functions of MLKL, such as immune escape, nerve regeneration and vesicle transport, warrants further investigation.
Supplementary information
Acknowledgements
We are grateful to the National Science Fund of Distinguished Young Scholars (81825021), Fund of Youth Innovation Promotion Association (2019285), the National Natural Science Foundation of China (31700732, 81773707, 92169202), the Linggang lab (LG202101-01-04), Fund of Shanghai Science and Technology (20ZR1474200, 22QA1411000).
Author contributions
Z.G. and B.X. conceived and designed the project. B.X. performed the electrophysiological recordings; X.S. and B.X. carried out the cell-based assays; S.W. and J.Q. performed proteomics experiment. Z.G., B.X., and S.W. wrote the manuscript.
Data availability
All proteome data generated in this study, including the raw files and quantitative data matrix of proteomes, have been deposited to PRIDE (https://www.ebi.ac.uk/pride) with accession number PXD030814 (and can be accessed with account: reviewer_pxd030814@ebi.ac.uk; Password: tLngFikB).
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bingqing Xia, Jingbo Qie
Contributor Information
Sheng Wang, Email: shengwang@fudan.edu.cn.
Zhaobing Gao, Email: zbgao@simm.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41421-022-00451-w.
References
- 1.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity. 2017;47:51–65.e57. doi: 10.1016/j.immuni.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, et al. Efficient engulfment of necroptotic and pyroptotic cells by nonprofessional and professional phagocytes. Cell Discov. 2019;5:39. doi: 10.1038/s41421-019-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan CN, Huang MC, Yang XJ, Hou J. MLKL: Functions beyond serving as the executioner of necroptosis. Theranostics. 2021;11:4759–4769. doi: 10.7150/thno.54072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores-Romero H, Ros U, Garcia-Saez AJ. Pore formation in regulated cell death. EMBO J. 2020;39:e105753. doi: 10.15252/embj.2020105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia B, et al. MLKL forms cation channels. Cell Res. 2016;26:517–528. doi: 10.1038/cr.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: How and why? Annu. Rev. Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai ZY, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dondelinger Y, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Quarato G, et al. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol. Cell. 2016;61:589–601. doi: 10.1016/j.molcel.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Z, et al. Activity-dependent PI4P synthesis by PI4KIIIα regulates long-term synaptic potentiation. Cell Rep. 2022;38:110452. doi: 10.1016/j.celrep.2022.110452. [DOI] [PubMed] [Google Scholar]
- 12.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HY, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Conos SA, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl Acad. Sci. USA. 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez KD, et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1beta independently of gasdermin-D. J. Immunol. 2017;198:2156–2164. doi: 10.4049/jimmunol.1601757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All proteome data generated in this study, including the raw files and quantitative data matrix of proteomes, have been deposited to PRIDE (https://www.ebi.ac.uk/pride) with accession number PXD030814 (and can be accessed with account: reviewer_pxd030814@ebi.ac.uk; Password: tLngFikB).