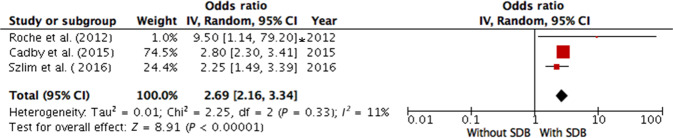Abstract
Sleep-disordered breathing (SDB) is characterized by repeated breathing pauses during sleep. The prevalence of SDB varies widely between studies. Some longitudinal studies have found an association of SDB with incident or recurrent cardiovascular events. We sought to systematically describe the current data on the correlation between SDB and cardiovascular pathology. Studies were included if they were original observational population-based studies in adults with clearly diagnosed SDB. The primary outcomes include all types of cardiovascular pathology. We carried out pooled analyses using a random effects model. Our systematic review was performed according to the PRISMA and MOOSE guidelines for systematic reviews and was registered with PROSPERO. In total, 2652 articles were detected in the databases, of which 76 articles were chosen for full-text review. Fourteen studies were focused on samples of an unselected population, and 8 studies were focused on a group of persons at risk for SDB. In 5 studies, the incidence of cardiovascular pathology in the population with SDB was examined. In total, 49 studies described SDB in patients with cardiovascular pathology. We found an association between SDB and prevalent /incident cardiovascular disease (pooled OR 1.76; 95% CI 1.38–2.26), and pooled HR (95% CI 1.78; 95% CI 1.34–2.45). Notably, in patients with existing SDB, the risk of new adverse cardiovascular events was high. However, the relationship between cardiovascular disease and SDB is likely to be bidirectional. Thus, more large-scale studies are needed to better understand this association and to decide whether screening for possible SDB in cardiovascular patients is reasonable and clinically significant.
Subject terms: Epidemiology, Respiratory tract diseases
Introduction
Sleep-disordered breathing (SDB) is characterized by repeated breathing pauses during sleep1. According to the American Academy of Sleep Medicine (AASM), the main types of respiratory events are obstructive apnea or hypopnea, central apnea and mixed apnea2. A respiratory event is scored as an apneic episode if it meets the following criteria: a drop of the oronasal flow ≥90%, the duration of the event is over 10 s, and it is associated with continued inspiratory effort2. In obstructive hypopnea, the duration of the ≥30% drop in oronasal flow lasts ≥10 s and is associated with ≥3% oxygen desaturation from a pre-event baseline or with an arousal2. The event is defined as central if it meets apnea criteria and is associated with a lack of inspiratory effort2. If the event meets the apnea criteria and is associated with a lack of inspiratory effort at the beginning of the event, followed by resumption of inspiratory effort in the second portion of the event, it is classified as mixed apnea2. In the AASM guidelines, there are three additional types of respiratory events that are less prevalent: respiratory effort-related arousals (RERA), hypoventilation and periodic breathing. SDB is labeled as mild, moderate or severe based on the number of apnea and/or hypopnea episodes per hour of sleep, known as the apnea-hypopnea index (AHI)3. The prevalence of SDB varies widely because of the heterogeneity of the study methodology, different diagnostic standards (full polysomnography or portable sleep monitoring) and different AHI cut-offs. The prevalence of SDB has been reported to range between 17 and 49% for AHI ≥ 54,5 and between 4.6 and 43.7% for AHI ≥ 156,7.
Some longitudinal studies have found an association of SDB with incident or recurrent cardiovascular events: heart failure, myocardial infarction, stroke/transient ischemic attack8–12. Previous studies7,13,14 have shown that there is a dose-response relationship between the severity of SDB and the risk of various clinical manifestations of cardiovascular disease (CVD). These studies were conducted predominantly in cardiology patients or patients with an established diagnosis of SDB. Only a few studies focusing on this association in an unselected population have been published14–16.
SDB influences CVD through numerous pathophysiological mechanisms. The most likely causal pathway through which SDB causes CVD is thought to be intermittent hypoxia, endothelial dysfunction and inflammation, repetitive arousal from sleep, large intrathoracic pressure variations and increased sleeping blood pressure17–19. While the acute, unfavorable effects of sleep disorders on cardiovascular physiology have been well characterized, less research is available on how strong the effect of SDB is on symptomatic CVD. Previous systematic reviews were mostly based on a particular type of cardiovascular pathology or cardiovascular outcome20–22.
The main objective of this systematic review is to systemize the current data on the association between SDB and cardiovascular pathology.
Methods
Search question and search strategy
Our systematic review was performed according to the PRISMA and MOOSE guidelines for systematic reviews23,24. A systematic literature review was added to the PROSPERO register (registration number is CRD42018082314).
A ‘PACO’ (Patient-Type of Association-Comparison-Outcome) analogous to the ‘PICO’ (Patient-Intervention-Comparison-Outcome) for systematic reviews of interventional studies, was created to guide our systematic review of observational studies.
Our basic aim was to review the association between SBD and prevalent and/or incident CVD. Our research question was further operationalised as: (P) Patients with a documented SBD (A): cross-sectional or longitudinal associations (C) Persons without SBD, (O) Prevalent/incident CVD.
Since we aimed to investigating the association between SBD and CVD in a bidirectional way, we decided to search for complementary studies that investigated the prevalence and/or incidence of SBD in patients with an established CVD.
The search was made in PubMed, Ovid and EMBASE databases in February 2022 without limitations on language, publication year or country. The search strategy included only terms relating to or describing the association of SDB and cardiovascular pathology. The search terms were adapted for use with bibliographic databases separately and in combination with database-specific filters.
The search terms were obstructive sleep apnea syndrome, obstructive sleep apnea, sleep apnea, obstructive sleep apnea hypopnea syndrome, sleep-disordered breathing, heart failure, cardiac insufficiency, heart insufficiency, atrial fibrillation, cerebral vascular accident, stroke and myocardial infarction.
The identified articles were screened by title and abstract and selected for full text review if they met the following inclusion criteria developed according to the objectives of the review.
Original observational (including cross-sectional, cohort and case–control studies) that were population-based and concerned the adult population.
Studies in hospital cardiovascular departments, in sleep centers and studies of unselected populations.
The diagnosis of SDB was based on the apnea-hypopnea index or respiratory disturbance index (RDI), which were obtained by the gold standard for diagnosing full polysomnography or portable home sleep apnea testing2.
The primary outcome concerned the following types of cardiovascular pathology: heart failure, cardiovascular disease, coronary heart disease, atrial fibrillation, stroke, myocardial infarction.
A diagnosis of cardiovascular pathology was based on the clinical evidence, laboratory data and/or functional methods of examination.
Exclusion criteria.
Studies on children and pregnant women.
The diagnosis of SDB was based on a questionnaire or ICD-9 diagnosis code.
If only risk factors for CVD were studied as an outcome.
Data extraction
Titles and/or abstracts of studies retrieved by the search strategy were screened independently by two reviewers (AH and EA) to identify studies that potentially met the inclusion criteria. The full text versions of these potentially eligible studies were retrieved and independently assessed for eligibility by two review team members. Any disagreement between them over the eligibility of studies was resolved through discussion with a third reviewer (JD). A standardized form was used to extract data from the included studies for assessment of study quality and evidence synthesis. Extracted information included study setting; study population and participant demographics and baseline characteristics; study design and methodology; type of SDB and cardiovascular pathology; observation time, if possible; and results (prevalence in percentage, odds ratios and hazard ratios, if possible). Two reviewers extracted data independently; discrepancies were identified and resolved through discussion (with a third author when necessary). Missing data were requested from study authors.
Quality assessment
Two reviewers (AH and EA) independently assessed the methodological quality of the selected studies with the Quality Assessment Tool for Case-Control, Cohort and Cross-sectional Studies, depending on the study design (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools).
Statistical analysis
We looked at data from cross-sectional studies and cohort studies separately to estimate the effect of SDB on the risk of cardiovascular pathology. We collected multivariable-adjusted (if possible) hazard ratios (HR) or odds ratios (OR) from the original studies. When a study did not report the OR of the outcomes of interest, we calculated an unadjusted OR based on the original data of events. We performed random effects analysis to quantify the dispersion of effect sizes in a meta-analysis. We pooled the HR and OR separately with a 95% confidence interval (CI) for adverse cardiovascular outcomes within a random effects model (DerSimonian-Laird) that incorporates between-study heterogeneity. The Cochran Q test (at a significance level of p < 0.10) and the I2 statistic were used to examine statistical heterogeneity across studies. We planned to assess publication bias using visual evaluation of the funnel plots. All analyses were performed with Cochrane Review Manager software (version 5.3).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
Study selection and characteristics
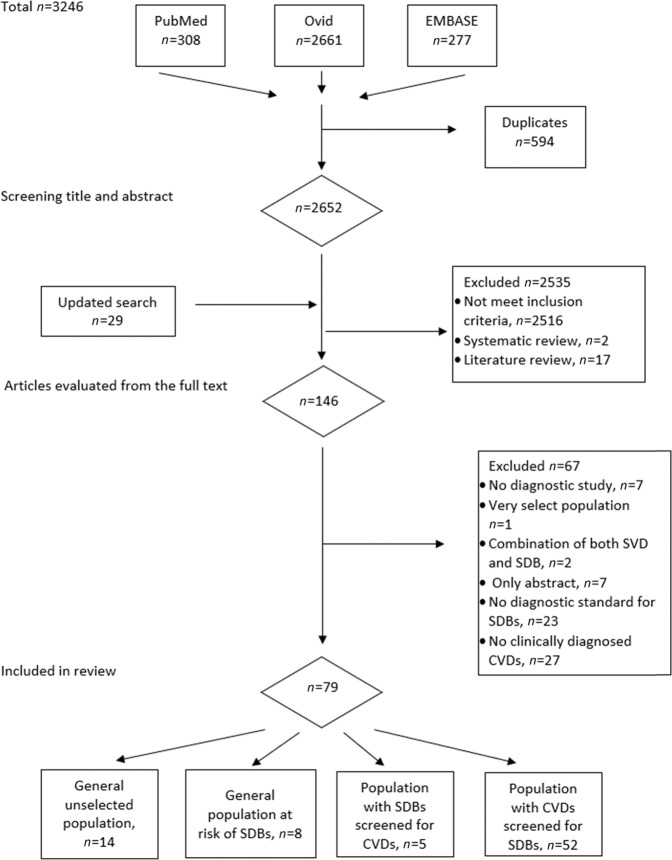
Using the above-mentioned search terms, 2681 articles were selected from the PubMed, Ovid and EMBASE databases after deduplication. We excluded 2535 articles for the following reasons: did not relate to the correlation of SDB and cardiovascular pathology (n = 2516); were literature reviews (n = 17) and were systematic reviews (n = 2). One hundred forty-six articles were eligible for full text evaluation. Of these articles, 7 were not diagnostic, 1 was performed on a very select population (combination of atrial fibrillation and erectile dysfunction), 3 were on patients with both CVD and SDB, in 23 the diagnostic standard for SDB was not used, in 27 there were no clinical diagnoses of CVD made, and 7 did not contain full texts. In 86 out the 2681 initially screened studies disagreement between reviewers appeared around their eligibility; After discussion with a third reviewer 2534 were excluded. After reviewing the full text articles no further disagreement emerged. Seventy-nine articles were eligible based on the aforementioned criteria. Some studies were excluded for more than one reason.
Fourteen studies were performed on samples of an unselected population, and eight were performed on a group of persons at risk for SDB. In five studies, the incidence of cardiovascular pathology in the population with SDB was examined. Forty-nine studies described SDB in patients with cardiovascular pathology. The flow chart of the literature search is shown in Fig. 1. A total 38 studies were cross-sectional, 33 studies were cohort studies, and 5 studies were case-controls. The characteristics of the studies grouped by type of population are listed in Tables 1 to 5.
Fig. 1. Flow chart of the study selection process for meta-analysis.
SDB sleep-disordered breathing, CVD cardiovascular disease.
Table 1.
Association of sleep-disordered breathing and cardiovascular disease in an unselected population: study characteristics, outcomes and results.
| First author | Year | Country | Study Design | Sample Size | Age | Diagnostic Standard | Outcome | Follow-up Period | AHI cut-off | Overall prevalence | Odds ratio (95% CI) | Hazard ratio (95% CI) | Adjusted for | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roca G.Q. et al. | 2015 | USA | cohort | 1645 | 62.5 ± 5.5 | PSG | All-cause mortality, incident CHD and HF | 13.6 ± 3.2 years | 15 | Men 23% | – | – | Age, BMI, prevalent hypertension and diabetes, systolic BP, smoking status, and use of medications. | |
| Women 10.4% | OR: 1.25 (1.02–1.52) | HR: 1.33 (1.03–1.74) | ||||||||||||
| Javaheri S. et al. | 2015 | USA | cohort | 2865 | 76.3 ± 5.5 | PSG | Incident HF | 7.3 years | AHI > 15 | 43.7% | OR: 1.9 (1.4–2.6) | – | Clinic, age, race, BMI, history CAD, HF, stroke, diabetes, hypertension, smoking, alcohol use, and physical activity. | |
| CAI > 5 | 7.3% | OR: 2.16 (1.4–3.4) | HR: 1.79 (1.16–2.77) | |||||||||||
| Redline S. et al. | 2010 | USA | cohort | 5422 | median 72 | PSG | Incident stroke (nonfatal or fatal) | 8.7 years | 15 | 20.2% | OR: 2.26 (1.45–3.52) | HR: 2.64 (1.01–6.88) | Age, BMI, smoking status, systolic BP, use of medications, diabetes status, and race. | |
| Gottlieb D.J. et al. | 2010 | USA | cohort | 4422 | 64 (57, 72) men | PSG | CHD, HF | Median of 8.7 years | 15 | 24% men | – |
CHD HR: 1.10 (1.00–1.21) HF HR: 1.13 (1.02–1.26) |
Age, race, BMI, smoking, total and HDL cholesterol, lipid- lowering meds, diabetes mellitus, systolic BP, diastolic BP, and anti- hypertensive medications. | |
| 66 (58, 74) women | 11% women | |||||||||||||
| Munoz R. et al. | 2007 | Spain | cohort | 394 | median 77, 28 years | PSG | Ischemic stroke | 6 years | 30 | 24.1% | – | HR: 2.52 (1.04–6.01), P 0.04 | Sex | |
| Marshall N.S. et al. | 2014 | Australia | cohort | 400 | 55.1 ± 8.2 | PSM | All-cause mortality (CVD, CHD, Stroke) | 20 years | 5 | 20.6% | – | HR: 0.5 (0.27–0.99) | Age, sex, BMI, smoking status, total cholesterol, HDL cholesterol, mean arterial pressure, diabetes, angina, and history of CVD. | |
| 15 | 4.6% | HR: 4.2 (1.9–9.2) | ||||||||||||
| Stone K.L. et al. | 2015 | USA | cohort | 2872 | 76.3 ± 5.5 | PSG | Incident stroke | 7.3 years | 5 | – | – | HR: 1.83 (1.12–2.98) | Age, clinic, race, BMI, and smoking | |
| Munoz R. et al. | 2012 | Spain | cohort | 394 | median 77.3 | PSG | Ischemic stroke | 4.5 years | CAI > 1 | – | – | HR: 2.65 (1.08–6.49) | AF | |
| CAI > 3 | HR: 3.08)1.26–7.52) | AF and sex. | ||||||||||||
| Hla Khin Mae et al. | 2015 | USA | cohort | 1280 | 47 ± 8 | PSG | CHD, HF | 24 years | 5–15 | 14% | _ | HR: 1.9 (1.05–3.5) | Age, sex, BMI, and smoking. | |
| 15–30 | 5% | HR: 1.8 (0.85–4.0) | ||||||||||||
| >30 | 4% | HR: 2.6 (1.1–6.1) | ||||||||||||
| May A. M. et al. | 2016 | USA | cohort | 843 | 75 ± 5 | PSG | Incident AF | 65 ± 0.7 years | CSA > 5 | 6% | OR: 9.97 (2.72–36.50) | _ | Age, clinic, race, BMI, history of CVD, hypertension, diabetes, stroke, COPD, pacemaker placement, total cholesterol, use of medications, and alcohol use. | |
| AHI > 15 | 41,7% | OR: 2.64 (1.16–6.00) | ||||||||||||
| Tung P. et al. | 2017 | USA | cohort | 2912 | 62.8 ± 11.2 | PSG | Incident AF | 5.3 years | AHI | 5 | 49% | – | _ | Age, clinic, race, BMI, history of CVD, hypertension, diabetes mellitus, stroke, COPD, pacemaker placement, total cholesterol, use of cardiovascular medications, and alcohol. |
| 15 | 19% | – | ||||||||||||
| 30 | 7% | – | ||||||||||||
| CAI > 5 | 2.5% | OR: 3.00 (1.40–6.44) | ||||||||||||
| Kwon Y. et al. | 2015 | USA | cross-sectional | 2048 | 68.4 ± 9.2 | PSG | AF | – | 15 | 33.74% | OR: 1.23 (1.01–1.50) | _ | Age, field center, race/ethnicity, sex, BMI, height, smoking status, diabetes, systolic BP, and medications. | |
| Arzt M. et al. | 2005 | USA | cross-sectional | 1475 | 47 ± 8 | PSG | Stroke | – | 5 | 17% | – | |||
| 20 | 7% | OR: 3.83 (1.17–12.56) | Age, sex, BMI, alcohol, smoking, diabetes, and hypertension. | |||||||||||
| Cho E.R. et al. | 2013 | Korea | cross-sectional | 746 | 59.3 ± 7.2 | PSG | Cerebral infarction | – | 15 | 12.06% | SCI OR: 2.44 (1.03–5.80) | – | Age, hypertension and diabetes mellitus. | |
| Lacunar infarction OR: 3.48 (1.31–9.23) | ||||||||||||||
AF atrial fibrillation, AHI apnea-hypopnea index, BMI body mass index, BP blood pressure, CAD coronary artery disease, CAI central apnea index, CHD coronary heart disease, COPD chronic obstructive pulmonary disease, CVD cardiovascular disease, HDL high density lipoprotein, HF heart failure, HR hazard ratio, OR odds ratio, PSM portable sleep monitor, PSG polysomnography, SCI silent cerebral infarction.
Table 5.
Association of sleep-disordered breathing and cardiovascular disease in patients with established SDB.
| Author | Year | Country | Study Design | Sample Size | Diagnostic Standard | Outcome | Results |
|---|---|---|---|---|---|---|---|
| Gunbatar H. et al. | 2016 | Turkey | cross-sectional | 56 | PSG | Silent prestroke damage | OR: 3.7 (1.2–11.9) |
| Davies C.WH et al. | 2000 | UK | case-control | 90 | PSM | Arterial hypertension |
High SBP OR: 9.2 (2.3–16.1) High DBP OR: 7.2 (3.7−10.6) |
| Chang Chih-Cheng et al. | 2014 | Taiwan | case-control | 149805 | PSG | New diagnosis of stroke, and death. | HR: 1.19 (1.09–1.30) |
| Mansukhani M.P. et al. | 2013 | USA | case-control | 108 | PSG | Ischemic stroke | OR: 5.34 (1.79–17.29) |
| Won C.H. et al. | 2012 | USA | cohort | 281 | PSG | All-cause mortality | HR: 1.72 (1.01–2.91) |
Study characteristics, outcomes and results.
DBP diastolic blood pressure, HR hazard ratio, OR odds ratio, PSM portable sleep monitor, PSG polysomnography, SBP systolic blood pressure.
SDB was assessed by overnight polysomnography in 51 studies and by validated portable diagnostic devices in 28 others. All studies screened their study populations for SDB prior to the outcomes being obtained.
The definition of SDB was based on a standardized assessment of AHI in all studies except one, where RDI was used7. The AHI cut-off value varied across studies from 5 to 51. In most studies, AHI ≥ 15 was used as the cut-off value for moderate-to-severe SDB.
The median follow-up duration for cohort studies and case-controls was from 6 months to 24 years. The number of patients varied between 28 and 30719, with a mean age range from 47 ± 8 to 76.3 ± 5.5 years. We did not consider whether SDB was treated during the study period. Most of the studies reported adjusted risk estimates for the primary endpoint. The potential confounding factors were age, sex, body mass index, hypercholesterolemia, hypertension, diabetes, smoking and others. In studies when authors reported both the unadjusted and adjusted risk, we used only the adjusted risk for the pooled analysis.
The types of cardiovascular pathology studied were atrial fibrillation, coronary artery disease, coronary heart disease, heart failure, myocardial infarction, resistant hypertension, transient ischemic attack (TIA) and stroke. Longitudinal studies assessed adverse cardiovascular outcomes and all-cause mortality.
Quality assessment
Supplementary Table 1 reports the evaluation of each item from the Quality Assessment Tool for Studies (see Supplementary Information file). The quality of the reports was heterogeneous, with scores between 4–9 for cross-sectional studies, 8–12 for cohort studies and 7–10 for case-controls. In many reports, several items were missed, such as a sample size justification and power description, a definition of the potential risk of bias and a description if the outcome assessors were blinded to the exposure status of the participants.
Association of SDB and CVD in an unselected population
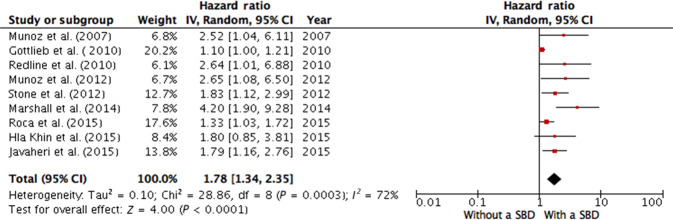
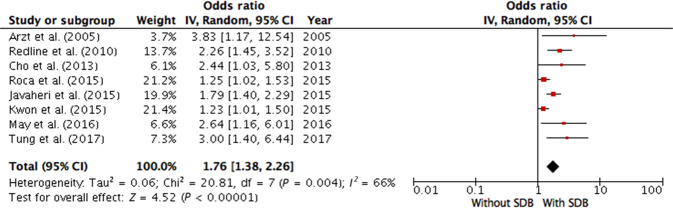
Fourteen studies with 27718 participants (the mean age ranged from 47 to 76.3 years) were included in the meta-analysis to explore the associations of SDB with CVD in a general unselected population4,5,7,13–16,25–31 (Table 1). Due to the clinical and statistical heterogeneity of the studies, their reported risk ratios could not be pooled into a meta-analysis into investigate the power of association. Nine studies provided adequate information on the HR and eight on the OR; they were included in two separate pooled analysis. Analyzing relative risks, we found that SDB was significantly associated with the risk of cardiovascular pathology in the general unselected population (overall HR 1.78; 95% CI 1.34–2.35), and the values of the HR varied from 1.10 to 4.20. Evidence of moderate between-study heterogeneity was found (I2 = 72%, p < 0.000, Fig. 2). After pooling the ORs, we found a similar association (overall OR 1.76; 95% CI 1.38–2.26, I2 = 66%, p < 0.000, Fig. 3) with a minimum OR of 1.23 and maximum OR of 3.83.
Fig. 2. Meta-analysis of association between sleep-disordered breathing and risk of cardiovascular disease (hazard ratios) in an unselected general population.
Munoz et al. (2007) AHI cut-off 30, outcome—ischemic stroke; Gottlieb et al. (2010) AHI cut-off 15, outcome—coronary heart disease; Redline et al. (2010) AHI cut-off 15, outcome—incident stroke; Munoz et al. (2012) CAI cut-off 1, outcome—ischemic stroke; Stone et al. (2012) AHI cut-off 5, outcome—incident stroke; Marshall et al. (2014) AHI cut-off 15, outcome—all-cause mortality; Roca et al. (2015): AHI cut-off 15, outcome—all-cause mortality; Hla Khin et al. (2015) AHI cut-off 15, outcome—coronary heart disease and heart failure; Javaheri et al. (2015) CAI cut-off 5, outcome—incident heart failure. AHI apnea-hypopnea index, CAI central apnea index, CI confidence interval, IV inverse variance, SDB sleep-disordered breathing.
Fig. 3. Meta-analysis of association between sleep-disordered breathing and risk of cardiovascular disease (odds ratios) in an unselected general population.
Arzt et al. (2005) AHI cut-off 20, outcome—incident stroke; Redline et al. (2010) AHI cut-off 15, outcome—incident stroke; Cho et al. (2013) AHI cut-off 15, outcome—cerebral infarction; Roca et al. (2015): AHI cut-off 15, outcome—all-cause mortality; Javaheri et al. (2015) CAI cut-off 5, outcome—incident heart failure; Kwon et al. (2015) AHI cut-off 15, outcome—incident atrial fibrillation; May et al. (2016) AHI cut-off 15, outcome—incident atrial fibrillation; Tung et al. (2017) CAI cut-off 5, outcome—incident atrial fibrillation. AHI apnea-hypopnea index, CAI central apnea index, CI confidence interval, IV inversed variance, SDB sleep-disordered breathing.
Association of SDB and CVD in a population at risk of sleep apnea
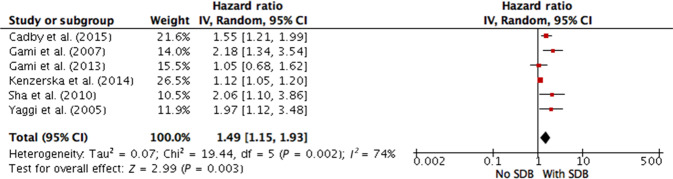
Eight studies32–39 were performed in patients with a high probability of having SDB. Six of the studies reported the HR, and three of the studies reported the OR; they were included in a separate pooled analysis (Table 2). SDB was also found to be associated with comorbid cardiovascular pathology in the random effects analysis (overall HR 1.49; 95% CI 1.15–1.93; overall OR 2.62; 95% CI 2.09–3.16). There was moderate evidence of between-study heterogeneity for the HR (I2 = 74%, p = 0.003, Fig. 4) and no evidence of heterogeneity for the OR (I2 = 11%, p < 0.000, Fig. 5).
Table 2.
Association of sleep-disordered breathing and cardiovascular disease in a population at risk of SDB: study characteristics, outcomes and results.
| Author | Year | Country | Study Design | Sample Size | Age | Outcome | Follow-up period | AHI cut-off | Prevalence | OR (95% CI) | HR (95% CI) | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cadby G. et al. | 2015 | Australia | cohort | 6841 | 48.3 ± 12.5 | AF | 11.9 years | 5 | 63.6% | OR: 2.8 (2.2–3.6) | HR: 1.55 (1.21–2.00) | Age, sex, height, BMI, hypertension, valvular disease, stroke/TIA, coronary or peripheral artery disease, COPD, chronic renal disease, HF, and diabetes. |
| Gami A.S. et al. | 2013 | USA | cohort | 10,701 | 53 ± 14 | Sudden cardiac death | 5.3 years | 5 | 78% | – | – | |
| 20 | – | HR 1.05 (1.00–1.09) | Univariate analyses | |||||||||
| Shah N.A. et al. | 2010 | USA | cohort | 1436 | 60 | CVD | 2.8 years | 5 | 71% | – | HR: 2.06 (1.10–3.86) | Age, race, sex, smoking, alcohol use, BMI, AF, hypertension, hyperlipidemia, and diabetes. |
| Yaggi H. K. et al. | 2005 | USA | cohort | 1022 | 60,9 | Incident stroke/TIA or all-cause death | 3.4 years | 5 | 68% | – | HR: 1.97 (1.12–3.48) | Age, sex, race, smoking, alcohol use, BMI, presence of diabetes mellitus, hyperlipidemia, AF, and hypertension. |
| Gami A.S. et al. | 2007 | USA | cohort | 3542 | 49 ± 14 | Incident AF | 4.7 years | 5 | 74% | – | HR: 2.18 (1.34–3.54) | Univariate analyses |
| Kendzerska T. et al. | 2014 | Canada | cohort | 10,149 | 49,9 ± 14,1 | MI, stroke, CHF, revascularization procedure, all-cause death | 68 months | 5 | 79.2% | – | HR: 1.12 (1.05–1.2) | BMI, age, sex, smoking, hypertension, diabetes, MI, stroke, and HF. |
| Selim B.J. et al. | 2016 | USA | cross-sectional | 697 | 58.7 ± 12.1 | Nocturnal cardiac arrhythmias | – | 5 | 77% | – | – | |
| 15 | 56% | OR: 2.24 (1.48–3.39) | Age, BMI, sex, and CVD. | |||||||||
| Roche F. et al. | 2002 | Switzerland | cross-sectional | 147 | 54.5 ± 10.7 | Nocturnal paroxysmal asystole | 0 | 10 | 44.9% | OR: 9.5 (1.14–79.2) | – | Not adjusted |
AF atrial fibrillation, AHI apnea-hypopnea index, BMI body mass index, COPD chronic obstructive pulmonary disease, CVD cardiovascular disease, HF heart failure, HR hazard ratio, MI myocardial infarction, OR odds ratio, PSM portable sleep monitor, PSG polysomnography, TIA transient ischemic attack.
Fig. 4. Meta-analysis of association between sleep-disordered breathing and risk of cardiovascular disease (hazard ratios) in a population at high risk of SDB.
Cadby et al. (2015) AHI cut-off 5, outcome—atrial fibrillation; Gami et al. (2007) AHI cut-off 5, outcome—incident atrial fibrillation; Gami et al. (2013) AHI cut-off 20, outcome—sudden cardiac death; Kenzerska et al. (2014) AHI cut-off 5, outcome—myocardial infarction, stroke, congestive heart failure, revascularization procedure, all-cause death; Sha et al. (2010) AHI cut-off 5, outcome—cardiovascular disease; Yaggi et al. (2005) AHI cut-off 5, outcome—incident stroke/TIA or all-cause mortality. AHI apnea-hypopnea index, CI confidence interval, IV inversed variance, SDB sleep-disordered breathing.
Fig. 5. Meta-analysis of association between sleep-disordered breathing and risk of cardiovascular disease (odds ratios) in a population at high risk of SDB.
Roche et al. (2002) AHI cut-off 10, outcome—nocturnal paroxysmal asystole; Cadby et al. (2015) AHI cut-off 5, outcome—atrial fibrillation; Szlim et al. (2016) AHI cut-off 15, outcome—nocturnal cardiac arrhythmias. AHI apnea-hypopnea index, CI confidence interval, IV inversed variance, SDB sleep-disordered breathing. *-calculated manually.
The prevalence and risk of SDB in patients with an established CVD diagnosis
We included fifty one studies8–10,12,40–85, in patients with various cardiovascular pathology in our review (Tables 3 and 4). The prevalence of SDB reported in these studies varied widely. Overall, SDB measured as AHI > 5 events/h ranged from 19%43 to 93.4%54. At the clinically important AHI level over 15 events/h, the prevalence of SDB in cardiovascular patients varied from 37.9%56 to 65.7%47. The highest prevalence was found in patients with myocardial infarction47, coronary artery disease61 and atrial fibrillation54. The incidence of major adverse events was significantly higher in cardiovascular patients with comorbid SDB than in those with only CVD (HR ranged from 1.029 to 11.0844. Due to the clinical heterogeneity of the studies of various cardiovascular diseases, their reported prevalence and power of association could not be compared.
Table 3.
Association of sleep-disordered breathing and cardiovascular disease in patients with an established cardiovascular pathology.
| First Author | Year | Country | Sample Size | Age | Diagnostic standard | Type of CVD | Outcome | Follow-up period | AHI cut-off | Prevalence | Odds ratio (95% CI) | Hazard ratio (95% CI) | Adjusted for | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sano K. et al. | 2013 | Japan | 178 | 71.4 ± 1.3 | PSG | CHF | Death from CV causes (worsening HF, ventricular tachyarrhythmia systemic embolism, stroke, acute MI, or aortic dissection) | 22 months | CAI >7.5 | 38.7% | AF OR: 1.03 (1.02–2.51) | HR: 1.29 (1.16–2.32) |
For OR: age, sex, BMI, NYHA class, LVEF, brain natriuretic peptide, CAI, minimum SpO2, duration of SpO2 <90%, C-reactive protein, and the use of a beta-blocker. For HR: age, NYHA class, LVEF, C-reactive protein, brain natriuretic peptide, and minimum SpO2. |
|
| Sinus pause OR: 1.12 (1.08–1.35) | ||||||||||||||
|
Nonsustained ventricular tachycardia daytime OR: 1.22 (1.00–6.92), nighttime OR: 3.57 (1.06–13.1) | ||||||||||||||
| Mooe T. et al. | 2001 | Sweden | 408 | < 70 years | PSG | CAD | Composite of death, CV events, and MI | 5.1 years | ≥10 | 34% | composite end point OR: 1.62 (1.09–2.41) | HR: 2.98 (1.43–6.20) | Age, sex, BMI, and hypertension. | |
| CV events OR: 3.41 (1.73–6.71) | ||||||||||||||
| Silvia L. et al. | 2016 | Portugal | 73 | 63.5 ± 10.3 | PSM | Acute Coronary Syndrome | All-cause mortality, MI, and myocardial revascularization | 75 months | >5 | 63% | – | – | Sex | |
| ≥15 | – | HR: 3.58 (1.09 −17.73) | ||||||||||||
| Shah R.V. et al. | 2014 | USA | 403 | median 57 | PSG | AF | All-cause mortality/HF hospitalization | 3.3 ± 0.5 years | >5 | 19% | – | HR: 2.14 (1.16–3.98) | Age, male sex, BMI, history of HF, hyperlipidemia, hypertension, diabetes, left ventricle mass-to-volume ratio, left ventricular end-systolic volume index, left ventricular myocardial infarction, and right ventricular ejection fraction. | |
| Ponsaing L.B. et al. | 2017 | Denmark | 63 | median 67.5 | PSG | Stroke/ TIA | Mortality | 19–37 months | >24 | – | – |
stroke severity HR: 10.95 (1.25–95.14 |
Age, disability measured with the modified Barthel index, and atrial fibrillation were nonsignificant. | |
| disability HR: 11.08 (1.23–99.52) | ||||||||||||||
| Emdin M. et al. | 2017 | Italy | 525 | 66 ± 12 | PSM | Systolic HF | Cardiac mortality | median 34 month | >5 | CSA 38.2% | nighttime 49.9% | – | HR: 1.02 (1.01–1.04) | Age, N-terminal pro–B-type natriuretic peptide, estimated glomerular filtration rate, and LVEF. |
| daytime 28.4% | HR: 1.03 (1.01–1.06) | |||||||||||||
| OSA 4.5% | nighttime 9% | HR: 1.02 (1.01–1.04) | ||||||||||||
| daytime 1.5% | ||||||||||||||
| Lee Chi-Hang et al. | 2011 | Singapore | 120 | 52.7 ± 9.8 | PSM | Acute MI | Death, reinfarction, stroke, unplanned target vessel revascularization, and HF requiring hospitalization. | 18 months | <30 | 58% | – | – | Age, and BMI. | |
| ≥30 | 42% | HR: 5.36 (1.01–28.53) | ||||||||||||
| Javaheri S. et al. | 2010 | USA | 30,719 | 67.1 ± 12.1 | PSG | Chronic HF | Incidence, treatment, outcomes, and economic cost of sleep apnea in new-onset HF. | 2-year survival rate | >5 | 97% | – | HF HR: 0.33 (0.21–0.51) | Age, sex, and Comorbidities. | |
| Khayat R. et al. | 2014 | USA | 1117 |
CSA 60.3 ± 14.7 OSA 60.3 ± 13.0 |
PSM | Acute HF | Mortality | 3 years | >15 | CSA − 31% | – | HR: 1.61 (1.1–2.4) | LVEF, age, BMI, sex, race, creatinine, diabetes, type of cardiomyopathy, CAD, chronic kidney disease, discharge systolic BP < 110, hypertension, discharge medications, initial length of stay, admission sodium, hemoglobin, and blood urea nitrogen. | |
| OSA − 47% | HR: 1.53 (1.1–2.2) | |||||||||||||
| O. Parra A. et al. | 2004 | Spain | 161 | 72 ± 9 | PSM | Stroke/ TIA | Death and time of survival since the neurological event. | 22.8 months | >10 | 72% | – | HR: 1.05 (1.01–0.08) | Age, middle cerebral artery involvement, and coronary disease. | |
| >20 | 47.2% | |||||||||||||
| >30 | 28% | |||||||||||||
| >40 | 11.2% | |||||||||||||
| >50 | 5% | |||||||||||||
| Hang L.C. et al. | 2016 | Singapore, China, Brazil, India, Myanmar | 1311 | 58.2 ± 10.3 | PSM | Percutaneous coronary intervention | MACCEs, secondary end points: all-cause mortality, target vessel revascula-rization, stent thrombosis, and hospitalization for HF. | 1.9 years | ≥15 | 45.3% | – | HR: 1.57 (1.10–2.24) | Age, sex, ethnicity, BMI, diabetes mellitus, and hypertension. | |
| Bolotova M.N. et al. | 2008 | Russia | 120 | 57.5 ± 1.2 | PSG | HPT | Death stroke, MI, HF, and AF. | 4.1 years | >15 | 53% | Stroke OR: 1 (0.32–11) | – | Not adjusted | |
| MI OR: 0.56 (0.17–1.42) | ||||||||||||||
| HF OR: 1.44 (0.36–1.33) | ||||||||||||||
| AF OR: 1.54 (0.63–3.79) | ||||||||||||||
| Uchoa C. et al. | 2015 | Brazil | 67 | 58 ± 8 | PSG | CAD | MACCEs, Secondary end points (individual MACCEs, typical angina, and arrhythmias). | 4.5 years | >15 | 56% |
MASSE OR: 4.10 (1.94–385.24) |
– | Age, Male sex, waist circumference, statins, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, and LVEF. | |
| New vascularization OR: 2.02 (1.21–64.22) | ||||||||||||||
|
Typical angina OR: 10.05 (1.12–62.25) | ||||||||||||||
|
Atrial fibrillation OR: 12.56 (1.44–159.21) | ||||||||||||||
| Szymanski F.M. et al. | 2015 | Poland | 251 | 57.6 ± 10 | PSM | AF | Reoccurrence of the AF | 30 months | >5 | 45.4% | OR: 2.58 (1.52–4.38) | – | Multivariate logistic regression analysis. | |
| Zhao Liang-Ping et al. | 2015 | Singapore | 41 | 52.2 ± 9.6 | PSM | Acute MI | Cardiac death, nonfatal MI, hospitalization for angina and/or congestive HF. | 5 years | >5 | 34.1% | 15-30 22.0% | OR: 1.044 (1.003–1.086) | – | – |
| >30 34.1% | ||||||||||||||
| Fan Jingyoa et al. | 2019 | China | 804 | 57 ± 10.2 | PSM | Acute CoronarySyndrome | MACCE | 1 Year | >15 | 50.1% |
HR: 1.55 [0.94–2.57] after 1 Year HR: 3.87 [1.20–12.46] |
Age, sex, BMI, HT, diabetes, PCI procedure and minimum oxygen saturation. | ||
| Yuhui Huang et al. | 2020 | China | 382 |
No Osa: 51 ± 16 Osa: 57± |
PSM | Decompensated HF | Death, heart transplantation or implantation of LVAD. Unplanned hospitalization for worsening HF, ACS, significant arrhythmias, Stroke | 19.7 months | >15 | 49.5% | HR: 1.14 [0.859–1.532] | No | ||
AF atrial fibrillation, AHI apnea-hypopnea index, BMI body mass index, BP blood pressure, CAD coronary artery disease, CAI central apnea index, CHF congestive heart failure, CV cardiovascular, CSA central sleep apnea, HF heart failure, HPT hypertension, HR hazard ratio, LVEF left ventricle ejection fraction, MACCEs major adverse cardiac and cerebrovascular events, MI myocardial infarction, OSA obstructive sleep apnea, OR odds ratio, PSM portable sleep monitor, PSG polysomnography.
Cohort studies: characteristics, outcomes and results.
Table 4.
Association of sleep-disordered breathing and cardiovascular disease in patients with established cardiovascular pathology.
| Author | Year | Country | Sample Size | Age | Diagnostic standard | Type of CVD | AHI cut-off | Prevalence | OR (95% CI) | Adjusted for | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vazir A. et al. | 2006 | UK | 55 | 61 ± 12 | PSG | CHF | 5 | 80% | – | – | |
| 15 | 53% | ||||||||||
| 30 | 22% | ||||||||||
| Otero L. et al. | 2016 | Colombia | 834 | 40–80 | PSG | CAD. AF | 5 | overall 91% | OR: 5.52 (2.9–10.7) for OSA | Not adjusted | |
| OR: 2.44 (1.2–5.2) for CSA | |||||||||||
| Strotmann J. et al. | 2018 | Germany | 211 | 68.7 ± 8.6 | PSM | AF | 5 | 93.4% | – | – | |
| 15 | 59.7% | ||||||||||
| Losurdo A. et al. | 2018 | Italy | 140 | 66.9 ± 11.9 | PSM | Ischemic stroke | 10 | 51.40% | – | – | |
| Zhao L.P. et al. | 2014 | Singapore | 162 | 58.6 ± 0.8 | PSM | CAD | 15 | 37.9% | 35.0% men | – | – |
| 40.3% women | |||||||||||
| Logan A.G. et al. | 2001 | Canada | 41 | 57.2 ± 1.6 | PSG | RHTN | 10 | 82.9% | 95.8% men | – | – |
| 64.7% women | |||||||||||
| Gessner V. et al. | 2017 | Germany | 223 | 63.2 ± 11.2 | PSM | Acute MI | 5 | 85.6% | 40.8% OSA | – | – |
| 7% CSA | |||||||||||
| 3.1% mixed | |||||||||||
| Prinz C. et al. | 2011 | Germany | 63 | 59.5 ± 13.0 | PSM | Hypertrophic Cardiomyopathy | 5 | 82.5% | 61.9% OSA | – | – |
| 20.6% CSA | |||||||||||
| Lee C.H. et al. | 2009 | Singapore | 105 | 53 ± 10 | PSM | Acute MI | 15 | 65.70% | – | – | |
| Bazan V. et al. | 2013 | Spain | 56 | 66 ± 11 | PSG | AF | 5 | 82% | – | – | |
| 30 | 45% | ||||||||||
| Glantz H. et al. | 2013 | Sweden | 662 | 64.1 ± 8.7 | PSM | CAD | 15 | 63.7% | – | – | |
| 30 | 24.6% | ||||||||||
| Strotmann J. et al. | 2017 | Germany | 211 | 68.7 ± 8.5 | PSM | AF | 15 | 57.9% | 55.9% OSA | – | – |
| 36.5% CSA | |||||||||||
| Muxfeldt E. et al. | 2014 | Brazil | 422 | 62.4 ± 9.9 | PSG | RHTN | 5 | 82.2% | – | – | |
| 15 | 55.5% | ||||||||||
| Paulino A. et al. | 2008 | France | 316 | 59 ± 3 | PSM | CHD | 10 | 81% | 56% OSA | – | – |
| 25% CSA | |||||||||||
| NorAdina A.T. et al. | 2006 | Malaysia | 28 | 60.3 ± 8.9 | PSM | Ischemic stroke | 5 | 92.8% | – | – | |
| 10 | 78.5% | ||||||||||
| 15 | 44.8% | ||||||||||
| 20 | 37.7% | ||||||||||
| Redeker N.S. et al. | 2010 | USA | 170 | 60.3 ± 16.8 | PSG | CHD | 5 | 84.1% | – | – | |
| Albuquerque F.N. et al. | 2011 | USA | 151 | 69.1 ± 11.7 | PSG | AF | 5 | 78.1% | – | – | |
| 15 | 52.3% | ||||||||||
| 30 | 29.1% | ||||||||||
| Brooks D. et al. | 2010 | USA | 45 | 67 ± 12 | PSG | Stroke | 10 | 91% | – | – | |
| Lutohin G.M. | 2016 | Russia | 54 | 66 (57; 72) | PSM | Ischemic stroke | 5 | 92% | 81.5% OSA | – | – |
| 11.1% CSA | |||||||||||
| Abumuamar A.M. et al. | 2018 | Canada | 100 | 63.6 ± 13.3 | PSG | AF | 5 | 85% | – | – | |
| Boulos M.I. et al. | 2016 | Canada | 102 | 68.7 ± 13.7 | PSM | Stroke/TIA | 5 | 63.40% | – | – | |
| Hoyer F.F. et al. | 2010 | Germany | 46 | 65 ± 7 | PSM | AF | 5 | 67% | – | – | |
| Cai A. et al. | 2018 | China | 1157 | 56.6 ± 11.7 | PSG | RHTN | 5 | 33.1% | OR: 1.049 (1.021–1.079) | Age, male sex, neck girth, BMI, mean SaO2 level, serum uric acid level, presence of diabetes mellitus and CHD. | |
| Koo B. B. et al. | 2016 | USA | 164 | 62 ± 11.3 | PSG | Ischemic stroke | 5 | 80.20% | men OR: 1.04 (1.00–1.09) | Age, diabetes, AF, and PHQ-8 score. | |
| women OR: 0.88 (0.78–0.99) | |||||||||||
| Shah N. et al. | 2013 | USA | 136 | Median 57.2 | PSM | Acute MI | 5 | 77% | – | Age, sex, race, smoking, hyperlipidemia, hypertension, CVD history, diabetes mellitus, and baseline creatinine. | |
| 30 | 10% | OR: 0.038 (0.002–0.610) | |||||||||
| Pedrosa R. et al. | 2010 | Brazil | 80 | 47 | PSM | AF | 15 | 40% | OR: 1.07 (1.01–1.13) | Multivariate analysis | |
| 30 | 21% | ||||||||||
| Geovanini G. et al. | 2016 | Brazil | 80 | 62 ± 10 | PSG | Refractory angina | 5 | 75% | – | Not adjusted | |
| 51 | 25% | OR: 4.00 (1.17–13.73) | |||||||||
| Kohno T. et al. | 2018 | Japan | 197 | 60 ± 9 | PSM | AF | 10 | 68.5% | 60.9%-OSA | Hyp OR: 2.6 (1.3–5.1) | Not adjusted |
| 7.6%-CSA | |||||||||||
| Sin D.D. et al. | 2002 | Canada | 301 | CSA 67.2 ± 0.9. OSA 59.4 ± 1.1 | PSG | HF | 10 | 40% | OR: 2.89 (1.25–6.73) | BMI, age, sex, mean and minimum SaO2, and LVEF. | |
| Grimm W. et al. | 2014 | Germany | 267 | 60 ± 14 | PSG | Systolic HF | 15 | 43% | – | Age, male sex, arterial hypertension, chronic kidney disease, brain natriuretic peptide, left atrial diameter, NYHA heart failure class, the use of digitalis, the lack of angiotensin-converting enzyme, inhibitors or angiotensin II receptor blockers | |
| 30 | 25% | AF OR: 5.21 (1.67–16.27) | |||||||||
| Kumar R. et al. | 2017 | India | 50 | 54.6 ± 12.49 | PSG | Stroke | 5 | 78% | OR: 1.14 (1.03–1.25) | Age, sex, BMI, and stroke severity. | |
| 15 | 46% | ||||||||||
| 30 | 18% | ||||||||||
| Macdonald M. et al. | 2007 | USA | 108 | 57 ± 11 | PSM | CHF | 15 | 61% | 30% OSA |
AF: OR: 11.56 (1.43–93.02) worse functional class of HF: OR: 2.77 (1.14–6.73) |
Male sex, age >60 years, BMI, and LVEF. |
| 31% CSA | |||||||||||
| Cadilhac D. A. et al. | 2005 | Australia | 78 | 63.5 ± 14.7 | PSG | Stroke | 5 | 81% | – | Age, neck circumference and stroke severity. | |
| 15 | 64.4% | OR: 4.15 (1.05–16.38) | |||||||||
| Braga B. et al. | 2007 | Brazil | 84 | 60.5 ± 9.5 | PSG | AF | 10 | 81.60% | OR: 2.87 (1.07–7.70) | Not adjusted | |
| Bekfani T. et al. | 2020 | Germany | 111 | 67.6 ± 10.2 | PSG | HF | 5 | 66.7% (OSA 42.3%,CSA 21.6%, Mixed 2.7%) | |||
AF atrial fibrillation, AHI apnea-hypopnea index, BMI body mass index, CAD coronary artery disease, CAI central apnea index, CHD coronary heart disease, CHF congestive heart failure, CSA central sleep apnea, CVD cardiovascular disease, HF heart failure, HR hazard ratio, LVEF left ventricle ejection fraction, MACCEs major adverse cardiac and cerebrovascular events, MI myocardial infarction, OSA obstructive sleep apnea, OR odds ratio, PHQ-8 eight-item Patient Health Questionnaire depression scale, PSM portable sleep monitor, PSG polysomnography, RHTN resistant hypertension, TIA transient ischemic attack.
Cross-sectional studies: characteristics, outcomes and results.
The risk of CVD in patients with an established SDB diagnosis
We found five studies11,86–89 in patients with SDB (Table 5). In patients with established SDB diagnoses according to the validated instrumental measurement, there was a high risk for the incident cardiovascular events of different kinds. The results could not be pooled due to the important clinical heterogeneity.
Publication bias
It was difficult to assess for publication bias due to the low number of studies in each subgroup. We tested for evidence of publication bias based on visual inspection of the funnel plot (Supplementary Figs. 1–3). There was no evidence of publication bias for the association of SDB and CVD in the general population (HR and OR). However, the funnel plot was asymmetrical for the association of SDB and CVD in the population at risk. We could therefore not rule out publication bias.
Discussion
Our main finding was that SDB was strongly associated with CVD. In the general population as well as in patients with an already diagnosed cardiovascular pathology, the presence of SDB significantly increases the risk of adverse cardiovascular outcomes and all-cause mortality. Considering the higher prevalence of SDB in cardiovascular patients (up to 93–96%54,57) and the higher risk of adverse cardiovascular events, it may be reasonable to screen for possible SDB in cardiovascular patients.
Our results suggest that the presence of SDB increases the risk of cardiovascular events 1.5 times in the general population and 2.6 times in the population at risk. The prevalence of OSA90 in the general population, as well as the correlation between OSA and risk of certain cardiovascular events, has already been established in earlier reviews20–22. In the general population, OSA was significantly associated with stroke and IHD. However, these literature reviews presented data only on one type of SDB and studied a particular type of cardiovascular pathology as an outcome and/or the risk in patients with preexisting cardiovascular pathology. In contrast, the present meta-analysis focused on all types of cardiovascular pathology and SDB. Regarding adverse cardiovascular outcomes and mortality, our results were consistent with those reported in the general population20,91. However, due to the methodological and clinical heterogeneity of the studies, the results could not be pooled in most of the meta-analyses.
In our review, we found that in patients with established cardiovascular pathology, SDB significantly increased the risk of new adverse cardiovascular events (up to 3.58 times). The results are consistent with other recent literature reviews and meta-analyses, with four-fold higher odds for AF92 and a threefold higher risk for ischemic stroke93. We did not find a high-quality meta-analysis on other types of cardiovascular pathology.
The pathophysiological mechanisms of SDB and associated cardiovascular risk are complex and vary between patients. Obstructive and central apnea may induce severe intermittent hypoxemia and CO2 retention during sleep, with oxygen saturation sometimes dropping to 60%, disrupting the normal structured autonomic and hemodynamic responses to sleep94. Apnea occurs repetitively through the night and is accompanied by increases in sympathetic activity to peripheral blood vessels and consequent vasoconstriction95. Nocturnal apnea initiates a range of pathophysiological mechanisms that may act to promote cardiac and vascular disease. These mechanisms include sympathetic activation, oxidative stress, cardiovascular variability, increased release of vasoactive and trophic substances, inflammation, endothelial dysfunction and thrombosis96. Thus, sleep apnea may initiate, promote, or exacerbate hypertension, coronary artery disease, arrhythmias, stroke, and heart failure97. Compared with the general population, the prevalence of SDB is higher in patients with cardiovascular conditions, such as hypertension (33–83%57,73), heart failure (40–61%78,81), atrial fibrillation (67–93.4%54,72), and stroke (63–92%65,71). However, the coexistence of these conditions with SDB does not prove causality, and potential confounding variables may intervene.
In the European Society of Cardiology Guidelines for the management of atrial fibrillation98, it is recommended that all AF patients be interrogated for symptoms and clinical signs of OSA. In addition, the treatment of an associated OSA should be optimized to reduce AF recurrences and improve AF treatment results (class of recommendation IIa). In addition, the updated Guideline for the Management of Heart Failure (American College of Cardiology/American Heart Association)99 stated that in patients with NYHA class II–IV HF and suspicion of SDB or excessive daytime sleepiness, a formal sleep assessment is reasonable (class of recommendation IIa). Thus, it is necessary to screen for possible SDB in patients with severe associated cardiovascular pathology, although the evidence of treatment benefits is still scarce.
This is the first comprehensive systematic review of the literature on the association between SDB and all kinds of cardiovascular pathology in adults in all regions of the world. Previous systematic reviews were limited to certain types of CVD or SDB or to selected populations.
The most significant strength of our systematic review is that we included both types of studies: those that measured the association in an unselected general population (or age-/sex-specific subgroups) and in a population at risk of SDB; and those that focused on a select population (with suspected sleep disorder or with diagnosed CVD). We included studies in which sleep apnea was measured using standard objective procedures.
Due to heterogeneity across studies and outcomes, there was a limited scope for conducting a meta-analysis. We have generally relied on adjusted data, but in some reports, risk indices were not adjusted for traditional risk factors or were adjusted for only some risk factors. We realize that confounding and differences in patient selection comprise a possible source of bias. Another limitation is that the range of AHI cut-off values varies across studies. Unfortunately, it was not possible to conduct separate subgroup meta-analyses for the associations between SDB severity (i.e., mild, moderate, and severe, according to AHI) and cardiovascular outcomes because of small sample sizes. When data concerning SDB severity were reported, we included in the pooled analysis the HR and OR related to the most severe forms of SDB (i.e., those with the highest AHI cut-off value).
In conclusion, we found an association between sleep-disordered breathing and cardiovascular disease. Notably, in patients with existing SDB pathology, the risk of new adverse cardiovascular events is high. However, the relationship between CVD and SDB is likely to be bidirectional. Thus, more large-scale studies are needed to better understand this association and to decide whether screening for possible SDB in cardiovascular patients is indicated and clinically relevant.
Supplementary information
Author contributions
Study design: A.H., J.D. Literature search: A.H., E.A., and J.D. Data interpretation: A.H., E.A., and J.D. Manuscript writing: A.H. Critical review of the manuscript for important intellectual content: E.A., and J.D. All authors read and approved the final manuscript.
Data availability
The search concepts are available in the supplement.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41533-022-00307-6.
References
- 1.Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Berry RB, et al. AASM scoring manual version 2.2 updates: new chapters for scoring infant sleep staging and home sleep Apnea testing. J. Clin. Sleep. Med. 2015;11:1253–1254. doi: 10.5664/jcsm.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 4.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am. J. Respiratory Crit. Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung P, et al. Obstructive and central sleep Apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J. Am. Heart Association. 2017;6:e004500. doi: 10.1161/JAHA.116.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaheri S, et al. Association between obstructive sleep apnea and left ventricular structure by age and gender: The multi-ethnic study of atherosclerosis. Sleep. 2016;39:523–529. doi: 10.5665/sleep.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall NS, Wong KKH, Cullen SRJ, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J. Clin. Sleep. Med.: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2014;10:355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khayat R, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur. Heart J. 2015;36:1463–1469. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emdin M, et al. Prognostic significance of central Apneas throughout a 24-hour period in patients with heart failure. J. Am. Coll. Cardiol. 2017;70:1351–1364. doi: 10.1016/j.jacc.2017.07.740. [DOI] [PubMed] [Google Scholar]
- 10.Shah N, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep. Breath. = Schlaf Atm. 2013;17:819–826. doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 11.Chang C-C, et al. High incidence of stroke in young women with sleep apnea syndrome. Sleep. Med. 2014;15:410–414. doi: 10.1016/j.sleep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Parra O, et al. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur. Respiratory J. 2004;24:267–272. doi: 10.1183/09031936.04.00061503. [DOI] [PubMed] [Google Scholar]
- 13.Hla KM, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38:677–684. doi: 10.5665/sleep.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb, D. et al. Prospective study of obstructive sleep Apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation122, 352–360 (2010). [DOI] [PMC free article] [PubMed]
- 15.Stone KL, et al. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39:531–540. doi: 10.5665/sleep.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca, G. Q. et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort. Circulation132, 1329–1337 (2015). [DOI] [PMC free article] [PubMed]
- 17.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am. J. Respir. Crit. Care Med. 2008;177:1150–1155. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somers VK, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189420. [DOI] [PubMed] [Google Scholar]
- 19.Duran-Cantolla J, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ (Clin. Res. ed.) 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 20.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circulation Cardiovascular Qual. Outcomes. 2012;5:720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 21.Porto F, Sakamoto YS, Salles C. Association between obstructive sleep Apnea and myocardial infarction: a systematic review. Arquivos Brasileiros de. Cardiologia. 2017;108:361–369. doi: 10.5935/abc.20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie W, Zheng F, Song X. Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2014;93:e336. doi: 10.1097/MD.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, et al. Meta-analysis of observational studies in epide.miology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.Javaheri, S. et al. Sleep-disordered breathing and incident heart failure in older men. Am J Respir. Crit. Care Med.193, 561–568 (2016). [DOI] [PMC free article] [PubMed]
- 26.Redline S, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am. J. Respir. Crit. Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz R, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 28.Munoz R, et al. Central sleep apnea is associated with increased risk of ischemic stroke in the elderly. Acta Neurologica Scandinavica. 2012;126:183–188. doi: 10.1111/j.1600-0404.2011.01625.x. [DOI] [PubMed] [Google Scholar]
- 29.May AM, et al. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am. J. Respiratory Crit. Care Med. 2016;193:783–791. doi: 10.1164/rccm.201508-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho ER, et al. Obstructive sleep apnea as a risk factor for silent cerebral infarction. J. Sleep. Res. 2013;22:452–458. doi: 10.1111/jsr.12034. [DOI] [PubMed] [Google Scholar]
- 31.Kwon, Y. et al. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax70, 873–879 (2015). [DOI] [PMC free article] [PubMed]
- 32.Cadby, G. et al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest148, 945–952 (2015). [DOI] [PubMed]
- 33.Gami AS, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep. Breath. = Schlaf Atm. 2010;14:131–136. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 35.Yaggi HK, et al. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 36.Gami AS, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 37.Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long Historical Cohort Study. PLoS Med. 2014;11:e1001599. doi: 10.1371/journal.pmed.1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selim BJ, et al. The association between nocturnal cardiac arrhythmias and sleep-disordered breathing: The DREAM study. J. Clin. Sleep. Med. 2016;12:829–837. doi: 10.5664/jcsm.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche F, et al. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin. Electrophysiology: Pace. 2003;26:669–677. doi: 10.1046/j.1460-9592.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 40.Sano K, et al. Central sleep apnoea and inflammation are independently associated with arrhythmia in patients with heart failure. Eur. J. Heart Fail. 2013;15:1003–1010. doi: 10.1093/eurjhf/hft066. [DOI] [PubMed] [Google Scholar]
- 41.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am. J. Respiratory Crit. Care Med. 2001;164:1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 42.Leao S, et al. Effect of obstructive sleep apnea in acute coronary syndrome. Am. J. Cardiol. 2016;117:1084–1087. doi: 10.1016/j.amjcard.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 43.Shah RV, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am. Heart J. 2014;167:620–626. doi: 10.1016/j.ahj.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponsaing LB, Iversen HK, Jennum P. Polysomnographic indicators of mortality in stroke patients. Sleep. Breath. 2017;21:235–242. doi: 10.1007/s11325-016-1387-z. [DOI] [PubMed] [Google Scholar]
- 45.Lee C-H, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J. Clin. Sleep. Med.: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2011;7:616–621. doi: 10.5664/jcsm.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am. J. Respir. Crit. Care Med. 2011;183:539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 47.Lee C-H, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–1495. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 48.Bolotova MN, et al. Obstructive sleep apnea and a cardiovascular risk in patients with arterial hypertension. Vestn. Rentgenologii i Radiologii. 2008;2–3:26–32. [PubMed] [Google Scholar]
- 49.Uchoa C, et al. Impact of OSA on cardiovascular events after coronary artery bypass surgery. Chest. 2015;147:1352–1360. doi: 10.1378/chest.14-2152. [DOI] [PubMed] [Google Scholar]
- 50.Szymanski FM, et al. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations - a long-term prospective, cross-sectional cohort study. Sleep. Breath. = Schlaf Atm. 2015;19:849–856. doi: 10.1007/s11325-014-1102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao L-P, et al. Relationship between severity of obstructive sleep apnea and adverse cardiac outcomes in non-diabetic patients presenting with myocardial infarction. Eur. Arch. Oto-Rhino-Laryngol.: Off. J. Eur. Federation Oto-Rhino-Laryngological Societies (EUFOS): affiliated Ger. Soc. Oto-Rhino-Laryngol. - Head. Neck Surg. 2015;272:2527–2533. doi: 10.1007/s00405-015-3537-5. [DOI] [PubMed] [Google Scholar]
- 52.Vazir A, et al. A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. Eur. J. Heart Fail. 2007;9:243–250. doi: 10.1016/j.ejheart.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Otero L, Hidalgo P, González R, Morillo CA. Association of cardiovascular disease and sleep apnea at different altitudes. High. Alt. Med. Biol. 2016;17:336–341. doi: 10.1089/ham.2016.0027. [DOI] [PubMed] [Google Scholar]
- 54.Strotmann J, et al. Characteristics of sleep-disordered breathing in patients with atrial fibrillation and preserved left ventricular ejection fraction. Clin. Res. Cardiology: Off. J. Ger. Card. Soc. 2018;107:120–129. doi: 10.1007/s00392-017-1163-5. [DOI] [PubMed] [Google Scholar]
- 55.Losurdo A, et al. Dysphagia and obstructive sleep apnea in acute, first-ever, Ischemic stroke. J. Stroke Cerebrovasc. Dis.: Off. J. Natl. Stroke Assoc. 2018;27:539–546. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 56.Zhao L-P, et al. Effects of gender on the prevalence of obstructive sleep apnea in patients with coronary artery disease. J. Clin. Sleep. Med.: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2014;10:1279–1284. doi: 10.5664/jcsm.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Logan A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J. Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 58.Gessner V, Bitter T, Horstkotte D, Oldenburg O, Fox H. Impact of sleep-disordered breathing in patients with acute myocardial infarction: a retrospective analysis. J. Sleep. Res. 2017;26:657–664. doi: 10.1111/jsr.12540. [DOI] [PubMed] [Google Scholar]
- 59.Prinz C, et al. Incidence of sleep-disordered breathing in patients with hypertrophic cardiomyopathy. Congest Heart Fail. 2011;17:19–24. doi: 10.1111/j.1751-7133.2010.00196.x. [DOI] [PubMed] [Google Scholar]
- 60.Bazan V, et al. Obstructive sleep apnea in patients with typical atrial flutter: prevalence and impact on Arrhythmia control outcome. Chest. 2013;143:1277–1283. doi: 10.1378/chest.12-0697. [DOI] [PubMed] [Google Scholar]
- 61.Glantz H, et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann. Am. Thorac. Soc. 2013;10:350–356. doi: 10.1513/AnnalsATS.201211-106OC. [DOI] [PubMed] [Google Scholar]
- 62.Strotmann J, et al. Predominant obstructive or central sleep apnea in patients with atrial fibrillation: influence of characterizing apneas versus apneas and hypopneas. Sleep. Med. 2017;37:66–71. doi: 10.1016/j.sleep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Muxfeldt E, Margallo V, Guimaraes G, Salles G. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am. J. Hypertens. 2014;27:1069–1078. doi: 10.1093/ajh/hpu023. [DOI] [PubMed] [Google Scholar]
- 64.Paulino A, et al. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch. Cardiovascular Dis. 2009;102:169–175. doi: 10.1016/j.acvd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Noradina AT, Hamidon BB, Roslan H, Raymond AA. Risk factors for developing sleep-disordered breathing in patients with recent ischaemic stroke. Singap. Med. J. 2006;47:392–399. [PubMed] [Google Scholar]
- 66.Redeker NS, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33:551–560. doi: 10.1093/sleep/33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albuquerque F, et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141:967–973. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brooks D, et al. Sleep-disordered breathing in patients enrolled in an inpatient stroke rehabilitation program. Arch. Phys. Med. Rehabilitation. 2010;91:659–662. doi: 10.1016/j.apmr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 69.Lutokhin, G. M., Geraskina, L. A. & Fonyakin, A. V. [Sleep-disordered breathing syndrome in acute ischemic stroke]. Синдром нарушения дыхания во сне при ишемическом инсульте. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova116, 14–20 (2016). [DOI] [PubMed]
- 70.Abumuamar AM, Dorian P, Newman D, Shapiro CM. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin. Cardiol. 2018;41:601–607. doi: 10.1002/clc.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boulos MI, et al. Unattended Hospital and home sleep apnea testing following cerebrovascular events. J. Stroke Cerebrovasc. Dis.: Off. J. Natl. Stroke Assoc. 2017;26:143–149. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Hoyer FF, et al. High prevalence of obstructive sleep apnea in patients with resistant paroxysmal atrial fibrillation after pulmonary vein isolation. J. Interventional Card. Electrophysiology: Int. J. Arrhythm. Pacing. 2010;29:37–41. doi: 10.1007/s10840-010-9502-8. [DOI] [PubMed] [Google Scholar]
- 73.Cai A, et al. Joint effects of obstructive sleep apnea and resistant hypertension on chronic heart failure: a cross-sectional study. Int. J. Cardiol. 2018;257:125–130. doi: 10.1016/j.ijcard.2017.10.089. [DOI] [PubMed] [Google Scholar]
- 74.Koo BB, et al. Observational study of obstructive sleep apnea in wake-up stroke: The SLEEP TIGHT study. Cerebrovasc. Dis. 2016;41:233–241. doi: 10.1159/000440736. [DOI] [PubMed] [Google Scholar]
- 75.Pedrosa R, et al. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010;137:1078–1084. doi: 10.1378/chest.09-2335. [DOI] [PubMed] [Google Scholar]
- 76.Geovanini G, et al. Obstructive sleep apnoea is associated with myocardial injury in patients with refractory angina. Heart. 2016;102:1193–1199. doi: 10.1136/heartjnl-2015-309009. [DOI] [PubMed] [Google Scholar]
- 77.Kohno T, et al. Prevalence and clinical characteristics of obstructive- and central-dominant sleep apnea in candidates of catheter ablation for atrial fibrillation in Japan. Int J. Cardiol. 2018;260:99–102. doi: 10.1016/j.ijcard.2018.01.103. [DOI] [PubMed] [Google Scholar]
- 78.Sin DD, et al. Relationship of systolic BP to obstructive sleep apnea in patients with heart failure. Chest. 2003;123:1536–1543. doi: 10.1378/chest.123.5.1536. [DOI] [PubMed] [Google Scholar]
- 79.Grimm W, et al. Severe central sleep apnea is associated with atrial fibrillation in patients with left ventricular systolic dysfunction. Pacing Clin. Electrophysiology: Pace. 2015;38:706–712. doi: 10.1111/pace.12495. [DOI] [PubMed] [Google Scholar]
- 80.Kumar R, Suri JC, Manocha R. Study of association of severity of sleep disordered breathing and functional outcome in stroke patients. Sleep. Med. 2017;34:50–56. doi: 10.1016/j.sleep.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 81.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J. Clin. Sleep. Med.: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 82.Cadilhac DA, et al. Sleep disordered breathing in chronic stroke survivors. A study of the long term follow-up of the SCOPES cohort using home based polysomnography. J. Clin. Neurosci. 2005;12:632–637. doi: 10.1016/j.jocn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 83.Braga B, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep. Med. 2009;10:212–216. doi: 10.1016/j.sleep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Bekfani T, et al. Heart failure and sleep-disordered breathing: susceptibility to reduced muscle strength and preclinical congestion (SICA-HF cohort) ESC Heart Fail. 2020;7:2063–2070. doi: 10.1002/ehf2.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan J, et al. Association of obstructive sleep apnea with cardiovascular outcomes in patients with acute coronary syndrome. J. Am. Heart Assoc. 2019;8:e010826. doi: 10.1161/JAHA.118.010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gunbatar H, et al. A silent pre-stroke damage: Obstructive sleep apnea syndrome. Int. J. Clin. Exp. Med. 2016;9:3481–3488. [Google Scholar]
- 87.Davies C, et al. Case-control study of 24 h ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000;55:736–740. doi: 10.1136/thorax.55.9.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mansukhani MP, et al. The association between atrial fibrillation and stroke in patients with obstructive sleep apnea: a population-based case-control study. Sleep. Med. 2013;14:243–246. doi: 10.1016/j.sleep.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Won CH, et al. Severe obstructive sleep apnea increases mortality in patients with ischemic heart disease and myocardial injury. Sleep. Breath. = Schlaf Atm. 2013;17:85–91. doi: 10.1007/s11325-012-0653-y. [DOI] [PubMed] [Google Scholar]
- 90.Senaratna CV, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep. Med. Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Wang X, Fan JY, Zhang Y, Nie SP, Wei YX. Association of obstructive sleep apnea with cardiovascular outcomes after percutaneous coronary intervention: a systematic review and meta-analysis. Medicine. 2018;97:e0621. doi: 10.1097/MD.0000000000010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geovanini GR, Lorenzi-Filho G. Cardiac rhythm disorders in obstructive sleep apnea. J. Thorac. Dis. 2018;10:S4221–S4230. doi: 10.21037/jtd.2018.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.King S, Cuellar N. Obstructive sleep apnea as an independent stroke risk factor: a review of the evidence, stroke prevention guidelines, and implications for neuroscience nursing practice. J. Neurosci. Nurs.: J. Am. Assoc. Neurosci. Nurses. 2016;48:133–142. doi: 10.1097/JNN.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 94.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 95.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J. Appl. Physiol. (Bethesda, Md: 1985). 1989;67:2101–2106. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 96.Somers VK, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J. Am. Coll. Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet (Lond., Engl.). 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 98.Kirchhof P, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 99.Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart failure society of America. J. Card. Fail. 2017;23:628–651. doi: 10.1016/j.cardfail.2017.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The search concepts are available in the supplement.