Abstract
The etiology of triple-negative breast cancers (TNBCs) is poorly understood. As many TNBCs develop prior to the initiation of breast cancer screening or at younger ages when the sensitivity of mammography is comparatively low, understanding the etiology of TNBCs is critical for discovering novel prevention approaches for these tumors. Further, the higher incidence rate of estrogen receptor (ER)-negative breast cancers, and specifically, of triple-negative breast cancers (TNBCs), among young African American women (AAW) versus white women is a source of racial disparities in breast cancer mortality. Whereas immune responses to TNBCs have received considerable attention in relation to prognosis and treatment, the concept that dysregulated immune responses may predispose to the development of TNBCs has received limited attention. We present evidence that dysregulated immune responses are critical in the pathogenesis of TNBCs, based on the molecular biology of the cancers and the mechanisms proposed to mediate TNBC risk factors. Further, proposed risk factors for TNBC, especially childbearing without breastfeeding, high parity, and obesity, are more prevalent among AAW than white women. Limited data suggest genetic differences in immune responses by race, which favor a stronger T-helper type 2 (Th2) immune response among AAW than white women. Th2 responses contribute to wound healing processes, which are implicated in the pathogenesis of TNBCs. Accordingly, we review data on the link between immune responses and TNBC risk and consider if the prevalence of risk factors that result in dysregulated immunity is higher among AAW than white women.
Incidence of TNBC
TNBCs are clinically aggressive breast cancers that do not express estrogen receptor (ER) or progesterone receptor (PR), and do not overexpress human epidermal growth factor receptor 2 (HER2)(1, 2), and thus do not respond to hormonal or HER2 targeted therapeutics. The incidence rate of TNBC among AAW is approximately double that among white women, with much of the disparity occurring at early ages (3, 4). Data indicate that the relative percentage of TNBCs is highest in Sub-Saharan West African countries of Ghana and Nigeria than among AAW and lower in East African countries; however, further epidemiological data are needed to confirm these results (5, 6). It has been proposed that genetic admixture between white Americans and Ghanaian/west Africans as a result of west Africa-based trans-Atlantic slave trade accounts for the intermediate prevalence of TNBC in AAW (5, 7). Improved registry infrastructure in Africa would enable estimation of age-adjusted rates, as would be needed to definitively compare rates with AAW and white women. If confirmed, the higher incidence of TNBCs among African and AAW may suggest a genetic predisposition to TNBC.
TNBC and immune responses in tissues
TNBCs are diverse with regard to clinicopathological features and prognosis (8), and immune factors figure prominently in the sub-classification of TNBCs (particularly definition of the numerically predominant subtype of TNBC, basal breast cancer). Medullary carcinoma, a morphologically distinctive form of TNBC, is defined by the presence of a prominent immune infiltrate, and more intense immune responses predict improved survival (9). Further, Casbas-Hernandez et al. (10) demonstrated that benign breast tissues adjacent to different molecular subtypes of breast cancer show different RNA expression patterns. Notably, the ontology of genes expressed in benign tissues surrounding TNBCs prominently include functions such as activation of leukocytes, proliferation of mononuclear leukocytes, cell movement of leukocytes, interferon signaling, hepatic fibrosis, T-helper cell differentiation or antigen presentation pathways. Although an effect of the tumor on its microenvironment cannot be excluded, the benign tissues analyzed in this study were grossly separated from the cancers, suggesting a possible role for immune responses in their development.
Lehmann et al. (2011) (11) initially classified TNBC into six molecular subtypes, but later revised categorization to four molecular subtypes: basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M), and luminal androgen receptor (LAR) (11). Recently, Davis et al. (2018) (12) correlated androgen receptor (AR) expression with TNBC subtypes and race, based on Lehman’s initial TNBC subtyping and reported that the majority of TNBCs (75% – 90%) did not express AR (quadruple negative (QNBC)), and that the TNBCs that expressed AR had a better prognosis than QNBCs. TNBCs among AAW included a higher percentage of AR-negative BCs within each TNBC subtype. Furthermore, this study identified genes associated with immune pathways, including E2F1, NFKBIL2, CCL2, TGFB3, CEBPB, PDK1, IL12RB2, IL2RA and SOS1, which were differentially expressed in QNBC among AAW versus white women and could differentially impact immune responses in these racial groups. They concluded that these genes may represent molecular drivers within IL12, CCR5, and B-cell response pathways that reflect immunologic differences linked to racial disparities in these breast cancers. Other findings from this investigation included a higher overall frequency of AR-negative TNBCs among AAW (81%) versus white women (56%) and upregulation of the immune checkpoint inhibitors PD-1, PD-L1, and CTLA-4 in QNBC vs non-QNBC, and in AAW with QNBC vs white women with QNBC (12).
Our provisional analysis of data from The Cancer Genome Atlas (TCGA) revealed that while overall mutation burden (defined by Thorsson et al. 2019(13)) did not differ significantly by race for breast cancer overall or by molecular subtype or stage (Figure 1A), the degree of microsatellite instability (defined by Bonneville et al. 2019(14)) in basal breast cancers (expressed as a continuous variable) among women ages 26–50 years was significantly higher among AAW (p<0.01) (Figure 1B). Microsatellite instability reflects a predisposition to mutation from impaired DNA mismatch repair, and accumulation of tumor cells with microsatellite instability could indicate deficiencies in tumor immunosurveillance and failure to eliminate such clones prior to cancer development (15). Consistent with this possibility, the TIL percent area, as defined by Saltz et al. 2019 and Thorsson et al. 2019 (13, 16), was significantly higher in basal breast cancer among white women than AAW (p<0.01) (Figure 1C). These results are provocative because augmentation of immune responses in tumors with microsatellite instability represents a possible new treatment approach (17); however expanded studies of basal breast cancers from AAW using state-of-the science methods are needed to more clearly define molecular features that distinguish basal breast cancers among white and AAW.
Figure 1.
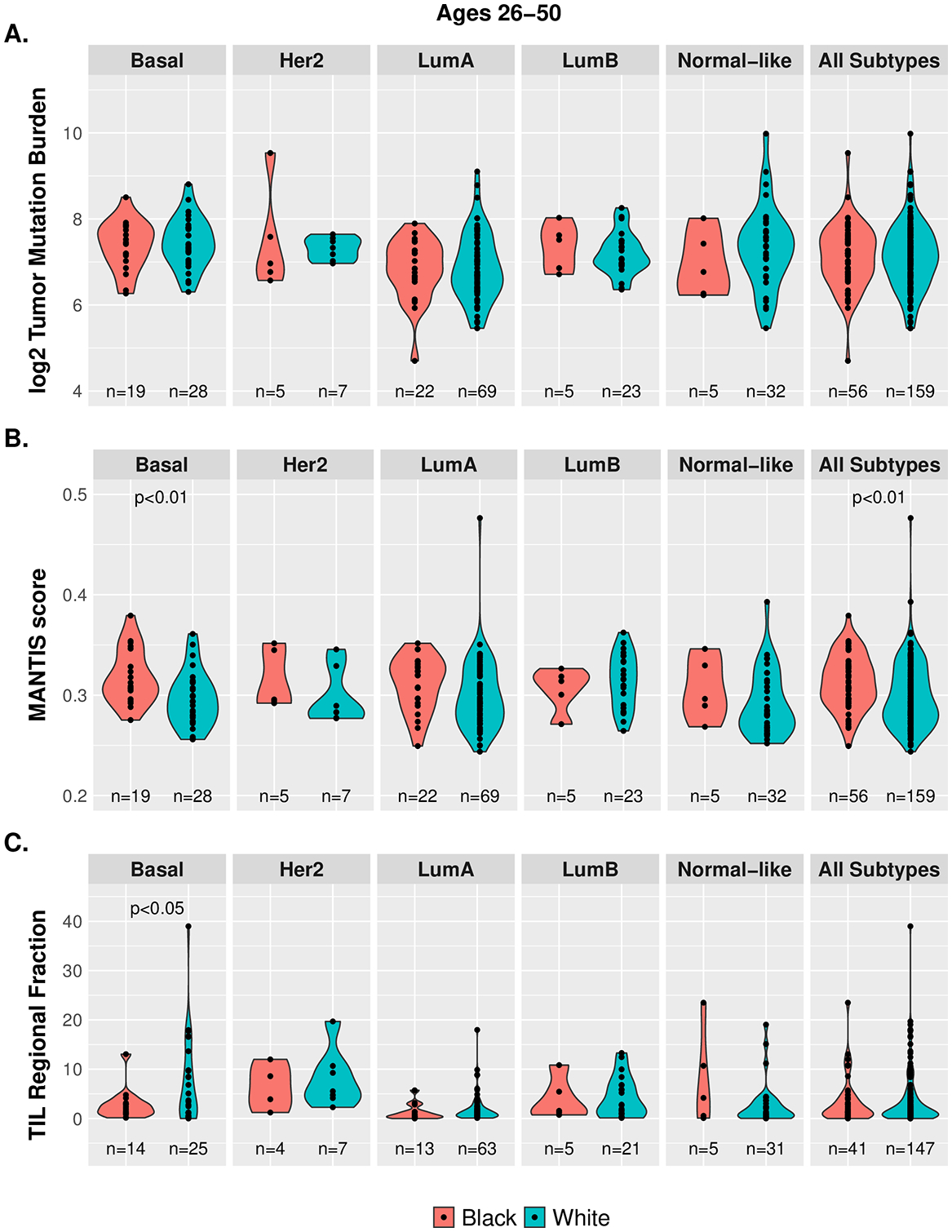
Racial and molecular subtype differences in tumor mutational burden, microsatellite instability, and TIL regional fraction in breast cancer. TCGA data does not show significant racial differences in mutation burden (1A), whereas microsatellite instability levels (continuous) are suggestively higher among AAW ages 26–50 years with basal breast cancer (1B), and TIL regional fraction is higher in basal breast cancer among white women (1C). TIL: tumor infiltrating lymphocyte; TCGA: the cancer genome atlas.
TNBC risk factors are associated with deleterious inflammation and susceptibility to DNA damage
Factors associated with increased risk of TNBC include obesity, multiparity with lack of sustained breastfeeding, and mutations in genes such as BRCA1 and BRCA2, which have important functions in DNA repair (18–20). These factors may affect the immune microenvironment in the breast, leading to deleterious inflammation that can result in cancer causing mutations or failure to eliminate mutated cells (19–25).
Data for associations between obesity and TNBC risk are inconsistent, with probably the strongest support for a link found among premenopausal AAW with abdominal obesity (26, 27). A meta-analysis showed that pre-menopausal women with a BMI greater than 30 Kg/m2 were at increased risk of TNBC versus non-obese women (odds ratio (OR): 1.43; 95% confidence interval (CI): 1.23 – 1.65)(19). A recent investigation by Schoemaker et al. (2018) (28) found that premenopausal weight gain was inversely correlated with breast cancer risk overall among women less than age 55 years, although associations with TNBC were not significant. Gaudet et al. also reported a non-significant direct association between BMI and TNBC among women ages <55 years in a pooled analysis of prospective studies (29). Obesity is associated with increased levels of serum and tissue inflammatory cytokines such as IL-6, IL-8, and TNF-α, which can activate various signaling pathways, including JAK/STAT, NF-κB, and Wnt/EZH2 that promote cell proliferation, invasion, and metastasis, which are hallmarks of aggressive cancers, including TNBC (30–33). In short, epidemiological data generally support a link between adiposity and TNBC risk, which is bolstered by mechanistic evidence implicating immune factors.
Whereas multiparity has been associated with a modest reduction in risk for ER-positive breast cancers (OR 0.92, 95% CI = 0.81 – 1.03 relative to nulliparity), parity increases the risk of TNBC (OR 1.37, 95% CI = 1.06 – 1.70) (34). Data indicate that childbirth itself is associated with a transient increase in risk of ER-positive breast cancers for over two decades and a persistent increase in ER-negative breast cancers (34, 35). Multiparity is related to increased risk of TNBC, but this effect is mitigated by breastfeeding, as originally reported in the Carolina Breast Cancer Study, and later confirmed in the African American Breast Cancer Epidemiology and Risk (AMBER) Consortium (36, 37). We hypothesize that the impact of parity on TNBC risk and the mitigation of this risk by breastfeeding may partly reflect the effects of postpartum involution (PPI), the process that returns the breast to a baseline state after weaning (38).
Elegant preclinical studies by Lyons et al. and others (39–42) have demonstrated that breast re-modeling after weaning results in an immune suppressed microenvironment resembling wound healing, characterized by immune cells expressing PD1/PD-L1 and increases in M2 macrophages, T regulatory cells, cyclooxygenase-2 expression and collagen synthesis. While PPI is a physiological process required to return the lactating breast to a baseline state (38), PPI may provide a favorable microenvironment for progression of mutated cells. Thus, MCF10 DCIS cells placed into the fat pad of dams that have been weaned develop rapidly growing metastatic TNBCs; treatment of such mice with non-steroidal anti-inflammatory agents (NSAIDs) inhibits TNBC development (39). Parity appears to expand breast lobules from which breast cancers arise (43), and we hypothesize that PPI may produce DNA damage, especially in the absence of breastfeeding.
Given the limited availability of benign post-partum human breast tissues, studies have analyzed breastmilk to understand PPI among women. Murphy et al. (44) analyzed convenience samples of breastmilk from 130 AAW and 162 white women for 15 analytes to compare the breast immune milieu by race. In multivariable analyses, samples donated by AAW demonstrated higher levels of IL-1β and leptin, whereas samples from obese women showed elevated levels of IL-1β, BFGF, EGF, leptin and FASL. Factor analysis identified two main discriminating factors related to: 1) angiogenesis and apoptosis and 2) inflammation. The level of the inflammation factor was higher among AAW than white women and declined with duration of breastfeeding (44). In another analysis, measurement of 80 cytokines in breastmilk donated by 15 women at 1, 4, 5 and 12 weeks post-partum revealed that levels of 9 inflammatory cytokines decreased with time since childbirth (45). These preliminary data suggest that the post-partum milieu of AAW and obese women may be more inflammatory than that of white and lean women, respectively, and that sustained breastfeeding may be associated with reduced inflammation in the breast.
Molecular alterations occur frequently in normal breast epithelium and breast cancer precursors (reviewed by Danforth (46, 47)); however, intrinsic cellular mechanisms (e.g. senescence, DNA repair) and extrinsic immune responses prevent most such changes from progressing to breast cancer. Deleterious inflammation may produce DNA damage, but the impact of this effect is mitigated by competent DNA repair. Homologous recombination repair deficiencies associated with germline BRCA1 or BRCA 2 mutations are associated with approximately 20% of TNBCs and somatic alterations that reduce function of these genes occurs in about 40% of TNBCs (18). Mutations in other genes with DNA repair functions, including BARD1, PALB2 and RAD51D are also associated with increased risk of TNBC (48), irrespective of race (49). TNBCs are associated with mutations in TP53 and DNA repair genes, a high mutational burden and a high neoantigen load, which would be anticipated to evoke robust immune responses (50, 51). Subsets of TNBCs are associated with abundant tumor infiltrating lymphocytes (TILs) (21, 52–56). However, studies of normal breast epithelium that interrelate DNA damage, neoantigen production and immune responses are technically challenging to perform and have not been conducted. Nonetheless, limited data evaluating immune responses in benign breast biopsies in relation to breast cancer risk (overall) have identified that decreased B cells in normal lobules are related to increased risk (57).
Prevalence of risk factors for TNBC among AAW
Several TNBC risk factors are higher for AAW. These include higher birth rates: in 2018, birth rates for AAW were 13.6/1,000 for non-Hispanic AAW versus 10.0/1,000 for non-Hispanic white women(58). Multiparity is also higher among AAW versus white women (59). The prevalence of obesity among AAW is approximately 58% versus 38% among white women in the U.S (60, 61), and 64.9% of AAW were overweight or obese at time of giving birth as compared with 51.1% of white women. These factors are linked, as multiparity is associated with an increased prevalence of obesity among parous women (62). Data from the National Longitudinal Survey of Youth Cohort showed that the 5-year incidence rate of obesity was 11.3/100 parous women versus 4.5/100 among nulliparous women; among parous women, the incidence of obesity was 15.1/100 in AAW versus 9.1/100 among white women (63). AAW also have higher pre-pregnancy BMI than white women and are more likely to retain weight post-delivery (62), and AAW participate less in breastfeeding than white women (31% vs 58%) (20). A study by McKinney et al. reported that analysis of data from the Community and Child Health Network study (N=1636) revealed that African American mothers were less likely to initiate breastfeeding compared to white mothers (61% vs 78%), and had lower intention to breastfeed (57% vs 77%) (23). Thus, obesity, weight gain after childbirth and limited breastfeeding are more prevalent among AAW than white women, and may significantly contribute to increased inflammation and high TNBC incidence in AAW.
Data for the prevalence of pathogenic mutations in DNA repair genes by race are limited. Several studies show that BRCA1/2 mutation prevalence is highest among the Ashkenazi Jews, while not significantly different in other ethnic groups (64–66). John et al. (2007) found that the prevalence of BRCA1 mutations was highest among African-Americans younger than 35 years of age (16.7 %) as compared with other racial/ethnic groups (67) in Northern California, although these data may not be representative of the broader United States. A larger study conducted by Hall MJ et al. (2009) found that the risk of BRCA1/2 mutations in breast cancer patients was similar across diverse racial/ethnic groups (68). Limited available data suggest that approximately 50% of BRCA1/2 pathogenic variants are likely or possibly of African origin, with many suggesting distinctive functions versus those detected among white women, suggesting the need for further studies among AAW (49)
Variation in genetic markers and circulating cytokine levels by race in relation to breast cancer risk
The principal arms of adaptive immunity designated as type 1 and type 2 T-helper (Th1 and Th2) each play distinctive but pivotal roles in anti-tumor responses, with potential implications for breast cancer development and progression. However, more complex differentiation states among macrophages and CD4+ T-helper cells are now acknowledged, and it is unclear how these differentiation states might impact immunity (69). Th1 immune response is operationalized through IL2, TNFα, IFNγ, M1-polarized macrophages, and natural killer (NK) cells, and results in activation of CD8 positive cytotoxic T cells that can potentially eliminate breast cancer cells. Th2 immunity is characterized by production of IL-4, IL-5, IL-9, IL-10, IL-13, with concomitant suppression of cytotoxic T cell function, resulting in a microenvironment that is proposed to be more conducive to breast cancer development. Studies suggest that distinctive evolutionary pressures contributed to genetically determined differences in immune responses by race, with heightened Th2 responses among AAW (70, 71). TH2 responses are tumor suppressive, suggesting that the breast immune microenvironment of AAW may contribute to increased incidence of TNBC.
The AMBER consortium identified single nucleotide polymorphisms (SNPs) in IL2RB, TLR6, and IL8 to be associated with the risk of ER-negative breast cancer among premenopausal AAW (72). In another study, Quan L et al. (2014) (73) in The Women’s Circle of Health Study (WCHS) tested the association between breast cancer risk and 47 SNPs in 26 cytokine related genes of the adaptive immune system using a two-stage approach. The first stage included 650 white women and 864 AAW, followed by a confirmatory study including 1307 white women, and 1365 AAW. Multivariable logistic regression analyses identified genetic variants in the adaptive immune response pathway associated with breast cancer risk, which differed by ancestry groups, menopausal status, and estrogen receptor (ER) status (73). This study identified five SNPs in genes important for T helper (Th) immunity: IFNGR2 rs1059293, IL15RA rs2296135, LTA rs1041981, IL4R rs1801275 for Th2 immunity, and TGFB1 rs1800469 for T regulatory cell-mediated immunosuppression, which were associated with breast cancer risk among AAW, and related to a statistically significant aggregate effect among AAW (p-trend=0.0005). However, the effects were not significant after adjustment for multiple comparisons. A notable finding from the same study was that the SNP rs746868 in the LTA gene, which was associated with increased risk for ER-positive BC among white women, conferred increased risk for ER-negative breast cancer among AAW. Of the 47 SNPs in 26 genes tested in this study, the genotype frequencies of 41 SNPs (87%) differed significantly between AAW and white women who were controls. This study had multiple strengths, which included racial comparisons, relatively large sample size, and in-person interviews; nonetheless, larger validation studies are needed. Numerous other studies have also reported racial differences in SNP variants with immune response regulatory functions (see table 1) (73–84). These differences in immune response genes are potential sources of differences in breast cancer incidence and mortality, leading to breast cancer disparity.
Table 1.
Differences in genetic variants of SNPs in genes related to cytokines by race
| Study first author, year | Number of subjects | Study design | Gene – SNP variants that differ by race | Main findings |
|---|---|---|---|---|
| Cox ED et al., 2001 (74) | CA (n = 102) AA (n = 43) |
Prospective cohort |
IL6-174G IL2-330T |
|
| Lazarus R et al., 2002 (75) | CA (n = 23) AA (n = 24) HA (n = 24) |
Retrospective cohort | IL10-1117 |
|
| Hoffmann SC et al., 2002 (76) | CA (n = 216) AA (n = 58) |
Prospective cohort |
IL2-2330G IL6-174G IL10-1084G |
|
| Martin AM et al., 2003 (77) | CA (n = 74) AA (n = 84) |
Prospective cohort |
TNFαR-28T→C TNFαR-36G→A TNFαR-7979C→T IL-1α-2121C→T IL-1β-3406C→T |
|
| Hassan MI et al., 2003 (78) | CAW (n = 81) AAW (n = 42) |
Prospective cohort |
IL6–174C/G
IFNγ-873T/A |
|
| Ness RB et al., 2004 (79) | CAW (n = 396) AAW (n = 179) |
Prospective cohort |
IL1A-4845G/G IL1A-889T/T IL1B-3957C/C IL1B-511A/A IL6-174G/G IL10-819T/T IL10-1082A/A |
|
| Rady PL et al., 2004 (80) | CA (n = 91) AA (n = 97) |
Retrospective cohort | IL10-1082 A |
|
| Zabaleta J et al., 2008 (81) | CA (n = 299) AA (n = 294) |
Retrospective cohort |
IL1B-511T IL1B-31C IL10-1082A IL10-592A TNF-308A IL1R2 |
|
| Van Dyke AL et al., 2009 (82) | CAW (n = 380) AAW (n = 103) |
Prospective cohort |
IL1B rs16944 IL2 rs2069763 IL8 rs4073 IL15 rs1057972 IL15RA rs2228059 IFNGR2 rs1059293 |
|
| Gong Z et al., 2013 (83) | CAW (n = 650) AAW (n = 864) |
Case - Control | TNFA-rs1799724 |
|
| Murray JL et al., 2013 (84) | CAW (n = 739) AAW (n = 141) |
Retrospective cohort |
NFKB1 rs230532 IL13 rs1800925 NFKB1 rs3774932 IL4R rs3024543 |
|
| Quan L et al., 2014 (73) | CAW (n = 1307) AAW (n = 1365) |
Case - Control |
IFNGR2 rs1059293 IL15RA rs2296135 LTA rs1041981 IL4R rs1801275 TGFB1 rs180469 |
|
Th2-associated cytokines have also been shown to activate innate immune cells leading to chronic inflammation and diminished anti-tumor responses (85). The variations in cytokine expression have been observed in different populations, races and ethnic groups (see table 2) (44, 86–89). In a study of 914 AAW and 855 white women, Yao et al. (2014) (89) examined plasma levels of 14 cytokines involved in innate and adaptive immunity, including those implicated in chronic inflammation. After adjusting for age, obesity, smoking, and other potential confounding factors, they found that the cytokines CCL11, CCL2, IL-10, IL-4, IFNG, and IL-1B were significantly higher in white women, while IFNα2, IL-1RA, CX3CL1, IL-5, TNFα, and CXCL10 were significantly higher in AAW (89). The data from Multi-Ethnic Study of Atherosclerosis (MESA) support the proposal that circulating biomarkers might provide insights into racial disparities in risks of chronic diseases among AAW versus white women (90). Unpublished data from MESA suggest that soluble IL-2R (sIL-2R) levels are significantly higher in white women as compared with AAW, which is of interest because of the protean role of this cytokine on immune functions (Suzette Bielinski, personal communication). The contribution of these potential differences to racial disparities in immune responses and disparities in breast cancer incidence and mortality remain undefined, and require further investigation.
Table 2.
Differences in measured circulating levels of cytokines by race
| Study first author, year | Number of subjects studied | Study design | Differentially expressed cytokines | Main findings |
|---|---|---|---|---|
| Negi SI et al., 2012(86) | CA (n = 596) AA (n = 488) |
Nested case-cohort | IL-18 - higher in white women |
|
| Deshmukh SK et al., 2015(87) | CA (n = 5) AA (n = 5) |
Retrospective cohort | IL-6 - higher in AAW |
|
| Gillespie SL et al. 2016(88) | CAW (n = 43) AAW (n = 41) |
Prospective cohort | IL-6 – higher in AAW IL-1β – higher in white women |
|
| Yao S et al., 2018(89) | CAW (n = 855) AAW (n = 914) |
Case-Control | CCL2, CCL11, IL4, IL10 – higher in white women IL1RA, IFN-α2 – higher in AAW |
|
| Murphy J et al., 2018(44) | CAW (n = 162) AAW (n = 130) |
Prospective cohort | IL1-β - higher in white women |
|
CA; Caucasian American, CAW; Cucasian American women, AA; African American, AAW; African-American women
Proposed Model of the Immune Pathogenesis of TNBC
We propose a life course model to describe the manner in which immune dysregulation may increase TNBC risk, especially among AAW (Figure 2). Bagby et al. (91) summarized evidence that genetic factors and deleterious exposures at young ages (e.g. psychosocial stress, toxins and poor nutrition) may increase cortisol levels, alter macrophage and T cell cytokine secretion and cause metabolic dysregulation, among other mechanisms, resulting in a “disease-susceptible phenotype”. Repeated exposures to adverse events pose greatest disease risks (i.e. high allostatic load) (91). Further, adverse early life events and African American race are associated with earlier onset of puberty, obesity and metabolic dysregulation, which may increase breast cancer risk (92). After puberty, lobules undergo proliferation (and differentiation) with each menstrual cycle, which favors the accumulation of mutations. Pregnancy is associated with proliferation of breast epithelium and differentiation (43, 93); during nursing, immune cells increase in milk in response to infections among mothers or infants (94). At weaning, the breast undergoes PPI, which re-establishes the baseline state of the breast (38–40). During PPI, best studied in animal models, activation of inducible COX-2 leads to up-regulation of inflammatory and cell survival pathways with deposition of fibrillar collagen and increases in M2 macrophages, creating an immune suppressed wound healing microenvironment that drives the progression of mutated cells to aggressive breast cancer, including TNBCs, and can be inhibited with anti-inflammatory agents (39–42). Limited data from women suggest that immune cell populations in the postpartum breast return to baseline within months to a few years of weaning (42); however, benign tissues of parous women show durable alterations in gene expression, including up-regulation of immune and inflammatory pathways (95). We hypothesize that dysregulated immune function could lead to intense or prolonged PPI related inflammation, increasing TNBC risk among women. Normal breast tissues and breast cancer precursors may contain a range of mutations (46) and the frequent presence of mutations in TP53 or DNA repair genes, as in TNBC mutations, favors persistence and progression of mutated cells. The growth promoting effects of pregnancy and the tumor-promoting effects of PPI may increase risk particularly among individuals with a large burden of occult mutated cells. These effects may be opposed by neoantigen expression on precancerous cells, which may evoke protective immune responses that result in “elimination” of mutated cells; however, if unrecognized by the immune system or situated within cancer promoting microenvironments, such cells may remain dormant and then escape.
Figure 2.
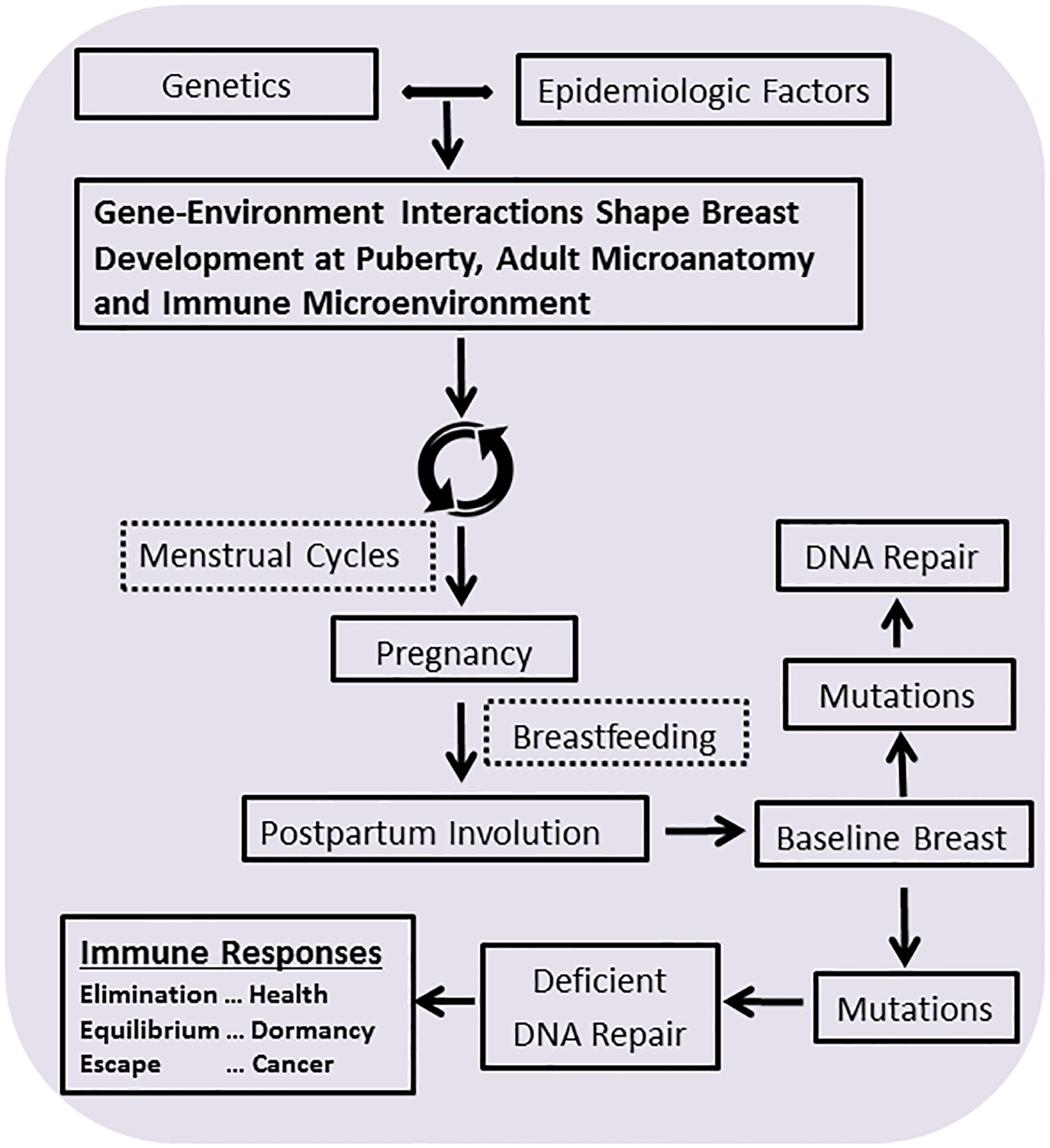
Gene environment interactions influence the microanatomy of the pre-pubescent and pubertal breast, both with regard to lobular architecture and the immune microenvironment. After puberty, menstrual cycles and other breast cancer risk factors alter this baseline state; however, the most dramatic changes occur with pregnancy and then postpartum involution, two factors that dramatically impact breast anatomy and cancer risk. Breastfeeding impacts the immune component of the breast, protecting the breast from infection, contributing to the developing immune system of the infant, and influencing breast cancer risk. Postpartum involution restores the baseline state of the beast after weaning and may contribute importantly to breast cancer risk or health.
Outstanding questions and future directions for research
Large definitive studies using optimal analytical methods are needed to determine TNBC subtypes by race, and the associations of these subtypes with exposures, genetic variants, and mutations that may be related to immunity. Determining attributable fractions of potentially modifiable TNBC risk factors, such as obesity, weight gain and failure to breastfeed could have potential to drive public health interventions to improve “immune health” and lower rates of TNBC among young mothers. Identifying features of pregnancies that link dysregulated PPI to increased TNBC risk could point to early detection strategies, increase understanding of mechanisms that mediate TNBC risk and evaluation of short-term low risk interventions, such as use of anti-inflammatory agents, as suggested by elegant preclinical studies by Lyons et al. (39) and subsequent research (96, 97). Efforts to improve health messaging about breastfeeding and healthy weight maintenance among young AAW are important, especially with digital health strategies and online support groups. Studies to characterize and compare immune responses among women by race in relation to risk of TNBC and other cancers and chronic diseases with suggested immunologic etiologies could suggest public health efforts to improve immune health in African American communities.
Conclusion
Data suggest that racial differences in immune health may contribute to increased risks of TNBC among AAW. We propose that increased understanding of the immunologic mechanisms that define high-risk AAW represent important steps towards early detection and prevention of these aggressive breast cancers.
Acknowledgments
The authors would like to thank Suzette Bielinski and the Multi-Ethnic Study of Atherosclerosis (MESA) for providing us with data on the expression of soluble IL-2R in different racial groups, and NCI for financial support. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA229811. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare no potential conflict of interest
References
- 1.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52(1):108–18. [DOI] [PubMed] [Google Scholar]
- 2.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nature reviews Clinical oncology. 2010;7(12):683–92. [DOI] [PubMed] [Google Scholar]
- 3.Zahnd WE, Sherman RL, Klonoff-Cohen H, McLafferty SL, Farner S, Rosenblatt KA. Disparities in breast cancer subtypes among women in the lower Mississippi Delta Region states. Cancer causes & control : CCC. 2019. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA: a cancer journal for clinicians. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Jiagge E, Jibril AS, Chitale D, Bensenhaver JM, Awuah B, Hoenerhoff M, et al. Comparative Analysis of Breast Cancer Phenotypes in African American, White American, and West Versus East African patients: Correlation Between African Ancestry and Triple-Negative Breast Cancer. Annals of surgical oncology. 2016;23(12):3843–9. [DOI] [PubMed] [Google Scholar]
- 6.Newman LA, Kaljee LM. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA surgery. 2017;152(5):485–93. [DOI] [PubMed] [Google Scholar]
- 7.Newman LA. Disparities in breast cancer and african ancestry: a global perspective. The breast journal. 2015;21(2):133–9. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. The Journal of pathology. 2014;232(2):142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marginean F, Rakha EA, Ho BC, Ellis IO, Lee AH. Histological features of medullary carcinoma and prognosis in triple-negative basal-like carcinomas of the breast. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23(10):1357–63. [DOI] [PubMed] [Google Scholar]
- 10.Casbas-Hernandez P, Sun X, Roman-Perez E, D’Arcy M, Sandhu R, Hishida A, et al. Tumor intrinsic subtype is reflected in cancer-adjacent tissue. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(2):406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of clinical investigation. 2011;121(7):2750–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis M, Tripathi S, Hughley R, He Q, Bae S, Karanam B, et al. AR negative triple negative or “quadruple negative” breast cancers in African American women have an enriched basal and immune signature. PloS one. 2018;13(6):e0196909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The Immune Landscape of Cancer. Immunity. 2019;51(2):411–2. [DOI] [PubMed] [Google Scholar]
- 14.Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, et al. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO precision oncology. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature reviews Immunology. 2006;6(10):715–27. [DOI] [PubMed] [Google Scholar]
- 16.Saltz J, Gupta R, Hou L, Kurc T, Singh P, Nguyen V, et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell reports. 2018;23(1):181–93.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L, Chang M, Chang HM, Chang F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Applied immunohistochemistry & molecular morphology : AIMM. 2018;26(2):e15–e21. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino B, Musolino A, Llop-Guevara A, Serra V, De Silva P, Hlavata Z, et al. Homologous Recombination Repair Deficiency and the Immune Response in Breast Cancer: A Literature Review. Translational oncology. 2020;13(2):410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast cancer research and treatment. 2013;137(1):307–14. [DOI] [PubMed] [Google Scholar]
- 20.Shinde SS, Forman MR, Kuerer HM, Yan K, Peintinger F, Hunt KK, et al. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer. 2010;116(21):4933–43. [DOI] [PubMed] [Google Scholar]
- 21.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nature reviews Cancer. 2015;15(4):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anstey EH, Shoemaker ML, Barrera CM, O’Neil ME, Verma AB, Holman DM. Breastfeeding and Breast Cancer Risk Reduction: Implications for Black Mothers. American journal of preventive medicine. 2017;53(3s1):S40–s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinney CO, Hahn-Holbrook J, Chase-Lansdale PL, Ramey SL, Krohn J, Reed-Vance M, et al. Racial and Ethnic Differences in Breastfeeding. Pediatrics. 2016;138(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietze EC, Chavez TA, Seewaldt VL. Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology. The American journal of pathology. 2018;188(2):280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief. 2015(219):1–8. [PubMed] [Google Scholar]
- 26.Laudisio D, Muscogiuri G, Barrea L, Savastano S, Colao A. Obesity and breast cancer in premenopausal women: Current evidence and future perspectives. European journal of obstetrics, gynecology, and reproductive biology. 2018;230:217–21. [DOI] [PubMed] [Google Scholar]
- 27.Agurs-Collins T, Ross SA, Dunn BK. The Many Faces of Obesity and Its Influence on Breast Cancer Risk. Frontiers in oncology. 2019;9:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM, et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA oncology. 2018;4(11):e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaudet MM, Gierach GL, Carter BD, Luo J, Milne RL, Weiderpass E, et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer research. 2018;78(20):6011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nature reviews Cancer. 2015;15(8):484–98. [DOI] [PubMed] [Google Scholar]
- 31.Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends in cell biology. 2012;22(11):557–66. [DOI] [PubMed] [Google Scholar]
- 32.Simone V, D’Avenia M, Argentiero A, Felici C, Rizzo FM, De Pergola G, et al. Obesity and Breast Cancer: Molecular Interconnections and Potential Clinical Applications. The oncologist. 2016;21(4):404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer research. 2013;73(11):3470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. Journal of the National Cancer Institute. 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols HB, Schoemaker MJ, Cai J, Xu J, Wright LB, Brook MN, et al. Breast Cancer Risk After Recent Childbirth: A Pooled Analysis of 15 Prospective Studies. Annals of internal medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295(21):2492–502. [DOI] [PubMed] [Google Scholar]
- 37.Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer causes & control : CCC. 2014;25(3):309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Molecular carcinogenesis. 2004;41(4):207–20. [DOI] [PubMed] [Google Scholar]
- 39.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nature medicine. 2011;17(9):1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace TR, Tarullo SE, Crump LS, Lyons TR. Studies of postpartum mammary gland involution reveal novel pro-metastatic mechanisms. Journal of cancer metastasis and treatment. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schedin P, O’Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. Journal of mammary gland biology and neoplasia. 2007;12(1):71–82. [DOI] [PubMed] [Google Scholar]
- 42.Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast cancer research : BCR. 2014;16(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figueroa JD, Pfeiffer RM, Patel DA, Linville L, Brinton LA, Gierach GL, et al. Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. J Natl Cancer Inst. 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy J, Pfeiffer RM, Lynn BCD, Caballero AI, Browne EP, Punska EC, et al. Pro-inflammatory cytokines and growth factors in human milk: an exploratory analysis of racial differences to inform breast cancer etiology. Breast cancer research and treatment. 2018;172(1):209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chollet-Hinton LS, Stuebe AM, Casbas-Hernandez P, Chetwynd E, Troester MA. Temporal trends in the inflammatory cytokine profile of human breastmilk. Breastfeeding medicine : the official journal of the Academy of Breastfeeding Medicine. 2014;9(10):530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danforth DN Jr. Genomic Changes in Normal Breast Tissue in Women at Normal Risk or at High Risk for Breast Cancer. Breast cancer : basic and clinical research. 2016;10:109–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danforth DN. Molecular profile of atypical hyperplasia of the breast. Breast cancer research and treatment. 2018;167(1):9–29. [DOI] [PubMed] [Google Scholar]
- 48.Shimelis H, LaDuca H, Hu C, Hart SN, Na J, Thomas A, et al. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. Journal of the National Cancer Institute. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friebel TM, Andrulis IL, Balmana J, Blanco AM, Couch FJ, Daly MB, et al. BRCA1 and BRCA2 pathogenic sequence variants in women of African origin or ancestry. Human mutation. 2019;40(10):1781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loibl S, Sinn BV, Karn T, Untch M, Sinn H-P, Weber KE, et al. Exome analysis of oncogenic pathways and tumor mutational burden (TMB) in triple-negative breast cancer (TNBC): Results of the translational biomarker program of the neoadjuvant double-blind placebo controlled GeparNuevo trial. Journal of Clinical Oncology. 2019;37(15_suppl):509-. [Google Scholar]
- 51.Ren Y, Cherukuri Y, Wickland DP, Sarangi V, Tian S, Carter JM, et al. HLA class-I and class-II restricted neoantigen loads predict overall survival in breast cancer. Oncoimmunology. 2020;9(1):1744947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Current molecular medicine. 2012;12(1):96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harano K, Wang Y, Lim B, Seitz RS, Morris SW, Bailey DB, et al. Rates of immune cell infiltration in patients with triple-negative breast cancer by molecular subtype. PloS one. 2018;13(10):e0204513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diana A, Franzese E, Centonze S, Carlino F, Della Corte CM, Ventriglia J, et al. Triple-Negative Breast Cancers: Systematic Review of the Literature on Molecular and Clinical Features with a Focus on Treatment with Innovative Drugs. Current oncology reports. 2018;20(10):76. [DOI] [PubMed] [Google Scholar]
- 55.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nature reviews Clinical oncology. 2016;13(11):674–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25(8):1544–50. [DOI] [PubMed] [Google Scholar]
- 57.Degnim AC, Hoskin TL, Arshad M, Frost MH, Winham SJ, Brahmbhatt RA, et al. Alterations in the Immune Cell Composition in Premalignant Breast Tissue that Precede Breast Cancer Development. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(14):3945–52. [DOI] [PubMed] [Google Scholar]
- 58.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final Data for 2018. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2019;68(13):1–47. [PubMed] [Google Scholar]
- 59.Ursin G, Bernstein L, Wang Y, Lord SJ, Deapen D, Liff JM, et al. Reproductive factors and risk of breast carcinoma in a study of white and African-American women. Cancer. 2004;101(2):353–62. [DOI] [PubMed] [Google Scholar]
- 60.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307(5):491–7. [DOI] [PubMed] [Google Scholar]
- 61.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstetrics and gynecology. 2015;125(1):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis EM, Zyzanski SJ, Olson CM, Stange KC, Horwitz RI. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. American journal of public health. 2009;99(2):294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malone KE, Daling JR, Thompson JD, O’Brien CA, Francisco LV, Ostrander EA. BRCA1 mutations and breast cancer in the general population: analyses in women before age 35 years and in women before age 45 years with first-degree family history. Jama. 1998;279(12):922–9. [DOI] [PubMed] [Google Scholar]
- 65.Modan B, Hartge P, Hirsh-Yechezkel G, Chetrit A, Lubin F, Beller U, et al. Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. The New England journal of medicine. 2001;345(4):235–40. [DOI] [PubMed] [Google Scholar]
- 66.Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer research. 2006;66(16):8297–308. [DOI] [PubMed] [Google Scholar]
- 67.John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. Jama. 2007;298(24):2869–76. [DOI] [PubMed] [Google Scholar]
- 68.Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115(10):2222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carbo A, Hontecillas R, Andrew T, Eden K, Mei Y, Hoops S, et al. Computational modeling of heterogeneity and function of CD4+ T cells. Frontiers in cell and developmental biology. 2014;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. The Journal of clinical investigation. 2008;118(4):1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye CJ, Feng T, Kwon HK, Raj T, Wilson MT, Asinovski N, et al. Intersection of population variation and autoimmunity genetics in human T cell activation. Science (New York, NY). 2014;345(6202):1254665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong CC, Sucheston-Campbell LE, Liu S, Hu Q, Yao S, Lunetta KL, et al. Genetic Variants in Immune-Related Pathways and Breast Cancer Risk in African American Women in the AMBER Consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2018;27(3):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quan L, Gong Z, Yao S, Bandera EV, Zirpoli G, Hwang H, et al. Cytokine and cytokine receptor genes of the adaptive immune response are differentially associated with breast cancer risk in American women of African and European ancestry. International journal of cancer. 2014;134(6):1408–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox ED, Hoffmann SC, DiMercurio BS, Wesley RA, Harlan DM, Kirk AD, et al. Cytokine polymorphic analyses indicate ethnic differences in the allelic distribution of interleukin-2 and interleukin-6. Transplantation. 2001;72(4):720–6. [DOI] [PubMed] [Google Scholar]
- 75.Lazarus R, Klimecki WT, Palmer LJ, Kwiatkowski DJ, Silverman EK, Brown A, et al. Single-nucleotide polymorphisms in the interleukin-10 gene: differences in frequencies, linkage disequilibrium patterns, and haplotypes in three United States ethnic groups. Genomics. 2002;80(2):223–8. [DOI] [PubMed] [Google Scholar]
- 76.Hoffmann SC, Stanley EM, Cox ED, DiMercurio BS, Koziol DE, Harlan DM, et al. Ethnicity greatly influences cytokine gene polymorphism distribution. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2(6):560–7. [DOI] [PubMed] [Google Scholar]
- 77.Martin AM, Athanasiadis G, Greshock JD, Fisher J, Lux MP, Calzone K, et al. Population frequencies of single nucleotide polymorphisms (SNPs) in immuno-modulatory genes. Human heredity. 2003;55(4):171–8. [DOI] [PubMed] [Google Scholar]
- 78.Hassan MI, Aschner Y, Manning CH, Xu J, Aschner JL. Racial differences in selected cytokine allelic and genotypic frequencies among healthy, pregnant women in North Carolina. Cytokine. 2003;21(1):10–6. [DOI] [PubMed] [Google Scholar]
- 79.Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and White Americans. American journal of epidemiology. 2004;160(11):1033–8. [DOI] [PubMed] [Google Scholar]
- 80.Rady PL, Matalon R, Grady J, Smith EM, Hudnall SD, Kellner LH, et al. Comprehensive analysis of genetic polymorphisms in the interleukin-10 promoter: implications for immune regulation in specific ethnic populations. Genetic testing. 2004;8(2):194–203. [DOI] [PubMed] [Google Scholar]
- 81.Zabaleta J, Schneider BG, Ryckman K, Hooper PF, Camargo MC, Piazuelo MB, et al. Ethnic differences in cytokine gene polymorphisms: potential implications for cancer development. Cancer immunology, immunotherapy : CII. 2008;57(1):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Dyke AL, Cote ML, Wenzlaff AS, Land S, Schwartz AG. Cytokine SNPs: Comparison of allele frequencies by race and implications for future studies. Cytokine. 2009;46(2):236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gong Z, Quan L, Yao S, Zirpoli G, Bandera EV, Roberts M, et al. Innate immunity pathways and breast cancer Risk in African American and European-American women in the Women’s Circle of Health Study (WCHS). PloS one. 2013;8(8):e72619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murray JL, Thompson P, Yoo SY, Do KA, Pande M, Zhou R, et al. Prognostic value of single nucleotide polymorphisms of candidate genes associated with inflammation in early stage breast cancer. Breast cancer research and treatment. 2013;138(3):917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Current opinion in immunology. 2007;19(2):209–16. [DOI] [PubMed] [Google Scholar]
- 86.Negi SI, Pankow JS, Fernstrom K, Hoogeveen RC, Zhu N, Couper D, et al. Racial differences in association of elevated interleukin-18 levels with type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes care. 2012;35(7):1513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, et al. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6(13):11231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gillespie SL, Porter K, Christian LM. Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. Journal of reproductive immunology. 2016;114:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao S, Hong CC, Ruiz-Narvaez EA, Evans SS, Zhu Q, Schaefer BA, et al. Genetic ancestry and population differences in levels of inflammatory cytokines in women: Role for evolutionary selection and environmental factors. PLoS genetics. 2018;14(6):e1007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 91.Bagby SP, Martin D, Chung ST, Rajapakse N. From the Outside In: Biological Mechanisms Linking Social and Environmental Exposures to Chronic Disease and to Health Disparities. American journal of public health. 2019;109(S1):S56–s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bleil ME, Booth-LaForce C, Benner AD. Race disparities in pubertal timing: Implications for cardiovascular disease risk among African American women. Population research and policy review. 2017;36(5):717–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russo J, Russo IH. Development of the human breast. Maturitas. 2004;49(1):2–15. [DOI] [PubMed] [Google Scholar]
- 94.Hassiotou F, Geddes DT. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Advances in nutrition (Bethesda, Md). 2015;6(3):267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rotunno M, Sun X, Figueroa J, Sherman ME, Garcia-Closas M, Meltzer P, et al. Parity-related molecular signatures and breast cancer subtypes by estrogen receptor status. Breast cancer research : BCR. 2014;16(4):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fornetti J, Martinson HA, Betts CB, Lyons TR, Jindal S, Guo Q, et al. Mammary gland involution as an immunotherapeutic target for postpartum breast cancer. Journal of mammary gland biology and neoplasia. 2014;19(2):213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinson HA, Lyons TR, Giles ED, Borges VF, Schedin P. Developmental windows of breast cancer risk provide opportunities for targeted chemoprevention. Experimental cell research. 2013;319(11):1671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


