Abstract
Background
Temporomandibular disorder (TMD) affects multiple tissues of the temporomandibular joint complex and manifests as orofacial pain and functional disturbance. While thread embedding acupuncture (TEA) is used for the treatment of TMD in clinical practice, sufficient clinical evidence is lacking. This pilot trial will explore the feasibility of a confirmatory randomized controlled trial (RCT) by considering the efficacy, safety, and cost-effectiveness of TEA to address pain, function, and quality of life in patients with TMD.
Methods
This randomized, assessor-blinded, controlled trial will include two parallel arms. Thirty patients with TMD and temporomandibular joint pain more severe than 40 mm on the 100-mm visual analog scale (VAS) and aged 19–70 years will be recruited and randomly allocated to either TEA or usual care groups. The TEA group will receive treatment at 14 predefined acupoints once weekly for 4 weeks. The usual care group will receive physical therapy consisting of transcutaneous electrical nerve stimulation and infrared therapy. The average VAS score over the last week for temporomandibular pain after four sessions will be assessed as the primary outcome. Furthermore, maximum pain VAS, vertical opening movement, Graded Chronic Pain version 2, Jaw Functional Limitation Scale 20, Patient Global Impression of Change, Korean version of Beck’s Depression Index, Short Form-12 Health Survey, EuroQol 5-Dimension 5-level, treatment expectation, rescue medication consumption, adverse events, and medical costs for economic evaluation will be measured and analyzed as secondary outcomes during four follow-up visits and after the termination of all sessions.
Conclusion
The results of this trial will help evaluate the feasibility of a confirmatory RCT considering efficacy, safety, and cost-effectiveness and verify the effect size required to determine an appropriate sample size.
Trial Registration Number
KCT0007421.
Keywords: jaw pain, visual analogue scale, vertical mouth opening, Jaw Functional Limitation Scale, polydioxanone thread, DC/TMD
Introduction
Temporomandibular disorders (TMD) are a collective term including clinical problems that involve the temporomandibular joint (TMJ), masticatory muscles, and associated structures.1 TMD include both functional disturbances and orofacial pain, in addition to various disorders affecting multiple tissues of the TMJ complex.2
In 2018, the National Institutes of Health (NIH) in the United States reported that TMD patients comprise an estimated 5% to 12% of the overall population.3 Moreover, the Health Insurance Review and Assessment Service in South Korea reported that the number of patients who visited the hospital due to TMD (K076 in Korean Standard Classification of Diseases) increased by 78% between 2011 and 2020, from 244,708 to 436,722.4
The clinical symptoms of TMD include joint noises; pain in the head, neck, and shoulder; inability to fully open/close the jaw; muscular tenderness; ear complaints; and psychosocial effects.5
Most patients improve with a combination of non-invasive therapies, including education, self-care, cognitive behavior therapy, pharmacotherapy, physical therapy, and occlusal devices. Nonsteroidal anti-inflammatory drugs and muscle relaxants are initially recommended, and benzodiazepines or antidepressants may be added for chronic cases.6
The prognosis of TMD is usually good since symptoms slowly improve and resolve over time.7 However, despite the conventional treatments mentioned above, persistent pain symptoms and physical disability can decrease patient quality of life.8
Therefore, alternative traditional Korean treatments have been applied for the treatment of TMD, mainly acupuncture, Chuna manual treatment, and electroacupuncture. Thread embedding acupuncture (TEA) has also been used in clinical practice.9
Various studies have been performed to assess the effect of acupuncture on TMD.10–12 Several randomized controlled trials have shown positive results such as reduced pain, improved masticatory function, and increased maximum interincisal opening.13 However, no studies have evaluated the effectiveness of TEA on TMD.
TEA is a method that treats diseases by inserting a foreign thread into acupoints to promote prolonged acupuncture-like stimulation.14 This method has been shown to be effective in chronic musculoskeletal diseases.15–17
Since chronic symptoms of TMD reduce patient quality of life, we assumed that persistent therapeutic stimulation with TEA would be more effective for TMD compared to short-term stimulation with acupuncture.
To develop an effective treatment based on evidence-based medicine, we aimed to design a clinical study that reflects the characteristics of TEA. Therefore, this pilot study will evaluate the feasibility of a confirmatory RCT considering efficacy, safety, and cost-effectiveness and verify the effect size required to determine an appropriate sample size.
Materials and Methods
Trial Design and Ethical Considerations
This pilot clinical RCT will include two parallel groups.
Thirty participants with TMJ pain will be allocated to the TEA and usual treatment groups in a 1:1 ratio.
This trial will be conducted according to the principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board(IRB) of Kyung Hee University Oriental Hospital at Gangdong (KHNMCOH 2021-12-002) and registered with the Clinical Research Information Service of the Republic of Korea (registration number: KCT0007421). The methodological details of TEA were established according to the revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) guidelines.18 The protocol items refer to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT).19
This trial began patient registration on 11 July 2022 and is currently ongoing.
Participants
Our researchers will recruit possible participants and explain our study to them. After obtaining informed consent, the participants will be screened using our inclusion/exclusion criteria. Eligible participants will then be randomly allocated to the experimental and control groups.
Inclusion Criteria
Participants fulfilling the following inclusion criteria will be included in this study: (1) regional pain in the unilateral or bilateral TMJ and masticatory muscles lasting >3 months intermittently or persistently; and (2) average pain intensity >40 mm on a 100 mm visual analogue scale (VAS) in the last week. (If the pain is on both sides, the one with the more pain; (3) local pain in the jaw, temple, inner ear, or front of the ear caused by jaw movement, function, or parafunction according to the diagnostic criteria for temporomandibular disorders (DC/TMD); (4) myalgia, myofascial pain, or arthralgia identified by palpation assessment of the examiner according to the DC/TMD; (5) adults aged 19–70 years who agree to participate in the clinical trial and provide written informed consent.
Exclusion Criteria
Participants meeting any of the following exclusion criteria will be excluded from this study: (1) pain caused or aggravated by traffic accidents or traumatic injuries; (2) history of surgery in the TMJ; (3) history of multiple pain disorders (eg, rheumatoid arthritis, etc.) or neurological diseases (eg, brain tumor, stroke, trigeminal neuralgia, etc.) that may interfere with the treatment effects or interpretation of results; (4) use of steroids, immunosuppressants, psychiatric drugs, or other medication that may affect the study results; (5) change in drug-taking history of analgesics, including nonsteroidal anti-inflammatory drugs, within the last 1 month; (6) history of TEA on the facial region within 1 month; (7) history of hypersensitivity to TEA or severe keloid scar; (8) not able to receive TEA due to skin diseases (inflammation, scar, seborrheic dermatitis, or psoriasis on the lesion) or blood coagulation disorders; (9) pregnant women, women who are planning to get pregnant, or women who are lactating; (10) participation in another clinical trial within 1 month before the date of selection, or plan to participate in another clinical trial during the treatment and follow-up period; (11) other conditions judged by the researchers to make participants ineligible for the clinical trial.
Procedures
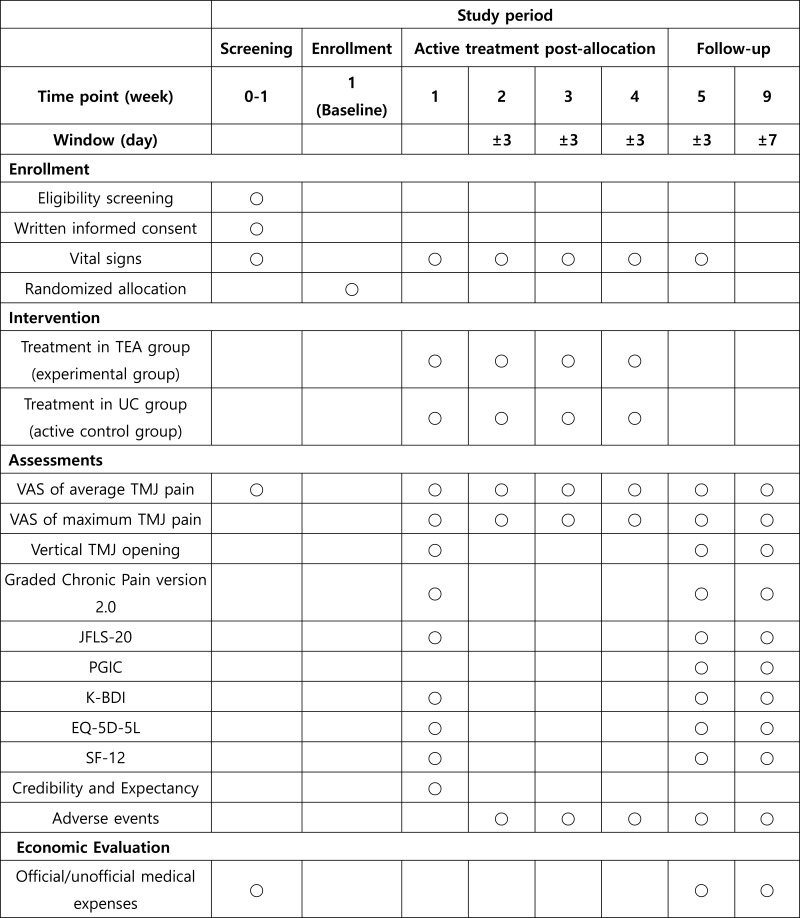
A total of 30 participants with TMJ pain will be recruited at Kyung Hee University Hospital, Gangdong. The recruited participants will be provided detailed study information in advance, and only those who voluntarily participate will be included in the study. Those who agree to participate and sign the informed consent form will be screened for eligibility. Participants fulfilling the inclusion and exclusion criteria will be randomly allocated to the TEA or usual care groups. Four treatment sessions for 4 weeks and two follow-up sessions will be conducted according to the 8-week appointed schedule (Figure 1).
Figure 1.
Planned trial schedule.
Abbreviations: TEA, thread embedding acupuncture; UC, usual care; VAS, visual analogue scale; TMJ, temporomandibular joint; K-BDI, Korean version of Beck’s Depression Index; JFLS, Jaw Functional Limitation Scale; PGIC, Patient Global Impression of Change; EQ-5D-5L, EuroQol 5- Dimension 5-Level; SF-12, Short Form-12 Health Survey.
Interventions
In both groups, the following intervention will be administered once weekly for 4 weeks.
TEA Group
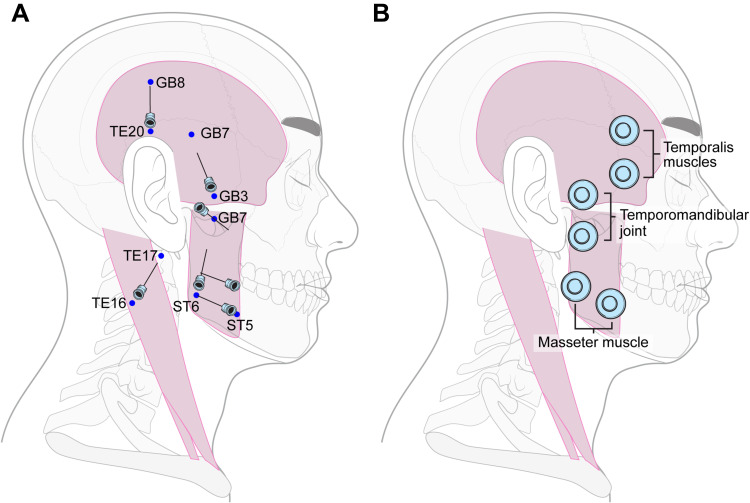
The TEA group will be treated with TEA. A total of 14 TEA (Dongbang Medical, Sungnam, South Korea) 29G x 25 mm needles with 30 mm threads will be inserted into seven acupoints on each side. The acupoints will be selected in advance by acupuncture specialists. The TEA procedure will be performed on both sides of the TMJ, regardless of the side of pain. Further details of the treatment group interventions such as the acupoints and the insertion method are described in the STRICTA checklist (Table 1), as well as in Figure 2A.
Table 1.
Details of the TEA Treatment According to the STRICTA 2010 Checklist
| Item | Detail |
|---|---|
| 1. Acupuncture rationale | 1a. Style of acupuncture: TEA |
| 1b. Reasoning for treatment provided: due to the consensus of a group of clinical experts and literature supporting the usefulness of TEA for TMJ disorders. | |
| 1c. Extent to which treatment was varied: No variation. | |
| 2. Details of needling | 2a. Number of needle insertions per subject per session: 14 |
| 2b and 2c. Names of points used and depth of insertion, based on a specified unit of measurement, or on a particular tissue level: 1. ST5→ST6: Transverse insertion from ST5 to ST6. 2. Near ST5→ST6: Transverse insertion on the horizontal line 5–10 mm from the ST5→ST6 line. 3. ST6→ST7: Transverse insertion from ST6 to ST7. 4. TE20→GB8: Transverse insertion from ST6 to ST7. 5. GB3→GB7: Transverse insertion from ST6 to ST7. 6. TE16→TE17: Transverse insertion from ST6 to ST7. 7. ST7: Oblique insertion from ST7 to the space between the zygomatic arch and mandibular notch. | |
| 2d. Response sought: De qi | |
| 2e. Needle stimulation: Thread-embedding | |
| 2f. Needle retention time: No retention time | |
| 2g. Needle type: TEA (29-gauge x 25 mm needle [30 mm thread] Dongbang Medical, Sungnam, South Korea) | |
| 3. Treatment regimen | 3a. Number of treatment sessions: Four |
| 3b. Frequency and duration of treatment sessions: Once weekly for 4 weeks | |
| 4. Other components of treatment | 4a. Details of other interventions administered to the acupuncture group: No other interventions for TMJ disorder, including moxibustion, cupping, electronic acupuncture, and administration of herbal medicine were permitted during the 4-week treatment phase. |
| 4b. Setting and context of treatment, including instructions to practitioners, and information and explanations to patients: Minimal conversations between practitioner and participant | |
| 5. Practitioner background | 5. Description of participating acupuncturists: Licensed acupuncture and moxibustion specialists |
| 6. Control or comparator interventions | 6a. Rationale for the control or comparator in the context of the research question, with sources that justify this choice: Physical therapy will be used as a comparator. TENS is commonly used for the treatment of TMD.31–33 |
| 6b. Precise description of the control or comparator. If sham acupuncture or any other type of acupuncture-like control is used: Sham acupuncture and any other type of acupuncture-like control are not used in this study. |
Abbreviations: STRICTA, Standards for Reporting Interventions in Clinical Trials of Acupuncture; TEA, thread-embedding acupuncture; TMJ, temporomandibular joint; TENS, transcutaneous electrical nerve stimulation.
Figure 2.
Location of intervention. (A) The locations of acupoints and insertion directions for the thread embedding acupuncture group are shown. (B) The locations in which transcutaneous electrical nerve stimulation was performed in the usual care group are shown. Three pairs of electrodes are attached, and infrared therapy will also be conducted in the same area.
Usual Care Group
This group will be treated with transcutaneous electrical nerve stimulation (TENS)(SPMI-330®, Stratek, Anyang, South Korea) and infrared (IR) therapy (IR-300®, Daekyung, Pocheon, South Korea) on both sides without TEA.
Physical therapy will be performed once weekly for 15 minutes for 4 weeks. TENS therapy will use current at 2 Hz and 100 Hz every 5 s for 15 min, at an intensity such that the patient feels electricity without pain. TENS electrodes will be attached to the bilateral temporal muscle, masseter muscle, and TMJ and a total of six spots will be stimulated (Figure 2B). In the same area, simultaneously with TENS treatment for 15 min, IR therapy will be applied at a distance of at least 20–30 cm, with an intensity within 250 W.
TMD Education
TMD education will be provided to all participants regardless of group assignment; this education will include materials on TMD causes, prevention, treatments, and self-corrective stretching methods.
Rescue Medication and Concurrent Treatment
Tylenol tablets (acetaminophen 500 mg/tablet) will be provided to the participants as a rescue medication. Patients feeling pain too severe to endure are allowed to take 1–2 tablets once at least every 4–6 h (up to 8 tablets per day). Thirty tablets will be provided in weeks 1, 2, and 3, while 50 tablets will be provided in week 5. A clinical research coordinator (CRC) will check the returned tablets at every visit to calculate consumption. Except for rescue medications, other medications or treatment in other medical institutions such as muscle relaxants, tricyclics, benzodiazepines, other analgesic drugs, injections, oral appliances, and acupuncture for TMD are not permitted. However, medications that the participants had been taking for 1 month before the screening are allowed. For the concurrent medication, the product name, component name, total daily dose, administration unit, administration route, start date of administration, end date of administration, and purpose of administration will be collected.
The participants are allowed to visit other medical institutions for the treatment of diseases not related to TMD but must inform the PI. Changes in concurrent treatments will be recorded at each visit.
Primary Outcome Measures
Average Pain VAS
The average intensity of TMJ pain over the last week will be evaluated using the 100-mm VAS at weeks 1 (baseline), 2, 3, 4, 5 (primary endpoint), and 9 (follow-up period).
The participants will be asked to record their average pain intensity within the last week on a 100-mm linear scale (0, absence of pain; 100, worst pain imaginable) on the day of their visits. The score in participants with unilateral pain or different intensities on each will be evaluated based on the more severe side. Week 5 (the first visit after treatment termination) is the primary endpoint.
Secondary Outcome Measures
The maximum pain intensity (100 mm VAS), vertical TMJ opening, Graded Chronic Pain 2.0, Jaw Functional Limitation Scale-20 (JFLS-20), Patient Global Impression of Change (PGIC), Korean version of Beck’s Depression Index (K-BDI), Short Form-12 Health Survey (SF-12), EuroQol 5- Dimension 5-level (EQ-5D-5L), rescue medication consumption, adverse events (AEs), and medical costs for economic evaluation will be evaluated as secondary outcome measures. Through this study, we will evaluate the efficacy, safety, and cost-effectiveness of TEA for TMD.
Maximum Pain VAS
The patient will point to the maximum TMJ pain over the last week on a 100-mm linear scale (VAS).
The maximum pain VAS will be assessed at weeks 1(baseline), 2, 3, 4, 5 (primary endpoint), and 9 (follow-up period).
Vertical TMJ Opening
For TMJ opening, pain-free opening and maximum unassisted opening will be measured using a TheraBite® (Atos Medical, Horby, Sweden) according to the DC/TMD evaluation method.
Vertical TMJ opening will be assessed at weeks 1 (baseline), 5 (primary endpoint), and 9 (follow-up period).
Graded Chronic Pain Version 2
The Graded Chronic Pain version 2 is officially provided by the DC/TMD.
It consists of eight questions on pain intensity, function, and number of days of pain. Based on the answers to these questions, the evaluation scale is derived into three categories: pain intensity (CPI), daily activity limitation days, and interference.
The characteristic pain intensity (CPI) is calculated based on answers to questions related to pain intensity.
The total disability points are derived from the sum of the limitation days points and interference points. Through this process, the graded chronic pain status will be derived following the scoring rule from the DC/TMD.
Graded Chronic Pain version 2 will be assessed at weeks 1 (baseline), 5 (primary endpoint), and 9 (follow-up period).
JFLS-20
The JFLS-20 comprises 20 items used to evaluate mastication, mobility, and communication over the past month. For each functional item, the responses range from 0 (no interference) to 10 (very severely compromised). The JFLS-20 will be assessed at weeks 1(baseline), 5 (primary endpoint), and 9 (follow-up period).
PGIC
The PGIC is used to evaluate the self-reported improvement of patients in seven categories. The scores are as follows: 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; 7, very much worse. The PGIC will be assessed at weeks 5 (primary endpoint) and 9 (follow-up period).
K-BDI
The K-BDI, the reliability and validity of which have been confirmed by Korean researchers, contains 21 items for the diagnosis of depression.20
Each item can be scored 0–3 points, in which the higher the score, the higher the tendency toward depression. The following cut-off points are will be used: 1–10 points, normal; 10–16 points, mild mood confusion; 17–20 points, threshold for clinical depression; 20–30 points, moderate depression; 30–40 points, severe depression; and ≥40 points, extreme depression. Score ≥17 points will be considered clinical depression. The K-BDI will be assessed at weeks 1 (baseline), 5 (primary endpoint), and 9 (follow-up period).
SF-12
The SF-12 comprises 12 questions in eight areas that evaluate health-related to health-related quality of life (HRQoL): physical functioning, physical role, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Higher scores indicate better HRQoL. The SF-12 is used to evaluate the functional health and well-being of patients and healthy people.21
The SF-12 will be assessed at weeks 1(baseline), 5 (primary endpoint), and 9 (follow-up period).
EQ-5D-5L
The EQ-5D-5L essentially consists of the EQ-5D descriptive system and EQ-VAS. The EQ-5D descriptive system comprises the following five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is rated from 1 to 5 (1, no problems; 2, slight problems; 3, moderate problems; 4, severe problems; 5, extreme problems). The EQ-VAS can be used to assess a patient’s current health status. It is a 20-cm scale numbered from 0 to 100 (0, the worst health imaginable; 100, the best health imaginable).22 EQ-5D-5L will be assessed at weeks 1 (baseline), 5 (primary endpoint), and 9 (follow-up period).
Treatment Expectation
Participants’ expectations for treatment will be evaluated using a 9-point Likert scale. At week 1, the participants will be asked, “How much do you think TEA and physical therapy will reduce your symptoms?” and to select their score as an answer (1, not at all; 5, somewhat, 9, very much).
Rescue Medication Consumption
The dose of the rescue medication taken during the study will be recorded.
Drug consumption will be assessed at weeks 2, 3, 4, 5 (primary endpoint), and 9 (follow-up period).
AEs
For AEs, information on symptoms, date of onset, disappearance date, severity, occurrence of serious AEs, association with undergoing clinical trial procedures, actions for clinical trial procedures, interventions for AEs, and AE-related outcomes will be collected.
The investigator will classify the severity of each AE in three categories using Spilker’s classifications: mild, no restriction on temporary or routine activity; moderate, some restricted activities; or severe, severely restricted activities or when medical treatment is absolutely required. The causal relationship between AEs and the intervention used in this clinical trial will be categorized into one of the following six criteria: 1, definitely related; 2, probably related; 3, possibly related; 4, probably not related; 5, definitely not related; and 6, unknown.
Should they occur, serious AEs should be reported to the principal investigator and IRB immediately, and the trial is to be promptly stopped. Further procedures to minimize AEs should be taken, and compensation should be given according to the clinical protocol.
Medical Costs for Economic Evaluation
We have developed a questionnaire to measure the direct/indirect medical costs of TMD to analyze its cost-effectiveness. The direct medical expenses include the costs for TMD prevention, treatment, and rehabilitation in medical institutions. The indirect medical expenses include the cost of purchasing functional foods and medical devices.
The medical costs will be assessed at weeks 1 (baseline), 5 (primary endpoint), and 9 (follow-up period).
Sample Size
Our calculation of the sample size according to the empirical method presented in the study on the calculation of the number of samples of pilot randomized trials using continuous variables showed that 12 people per group will be required.23,24 Considering a dropout rate of 20% during the study, 30 subjects will be needed for this study.
Fifteen participants will be assigned to each of the TEA and usual care groups.
Randomization and Allocation Concealment
Thirty participants will be randomly assigned to the TEA or usual care groups after block randomization in a 1:1 ratio. Random numbers will be generated by an independent statistician using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and random codes will be sealed in sequentially numbered opaque envelopes that will be managed by a CRC. After screening to confirm trial participation, the CRC will open the envelope and assign the participants to the allocated group according to the random code. Information regarding the allocation will be recorded in a separate log, access to which will only be provided to the researchers who will perform the intervention.
Blinding
Since it is impossible to blind the practitioner and participants, only the evaluators will be blinded.
Research nurses or residents who are blinded to group assignment will evaluate the results. Blinding may be removed on a case-by-case basis only when the principal investigator determines that it is essential for participant safety, such as in medical emergencies.
Statistical Methods
An independent statistician will perform statistical analysis using SAS version 9.4, with statistical significance set at P<0.05. In this study, intention-to-treat will be the main analysis method, and per-protocol will be conducted.
The primary endpoint is week 5 (the first visit after treatment termination). To compare the TEA and usual care groups, analysis of covariance (ANCOVA) will be used for continuous variables, and chi-square or Fisher’s exact tests will be used for categorical data. Paired t-tests will be used for analysis by time point within groups. Trends over time and time-by-treatment interactions will be analyzed using repeated-measures analysis of variance (RM ANOVA). Missing values will be addressed primarily by multiple imputations, and the last observation carrier (LOCF) will be used for sensitivity analysis.
According to the economic evaluation guidelines (2021) of the Health Insurance Review and Assessment Service (HIRA), direct and indirect medical expenses related to treatment will be estimated to analyze the cost-effectiveness of treatment from the perspective of the health care system. As base-case results, cost-effectiveness will be calculated as the incremental cost-effectiveness ratio (ICER), which represents the economic value of the additional effectiveness of additional costs. To analyze the uncertainty of the results caused by the uncertainty of each sampling-related parameter, we will perform 1) one-way sensitivity analysis, 2) Bayesian confidence interval estimation of ICER, and 3) cost-effectiveness curve (CEAC) estimation.
Data Collection and Management
Information on the participants and clinical trials will be recorded in the electronic medical record (EMR) system in our medical institution. Moreover, evaluation records written by patients and assessors will be kept as an evidence document, which is recorded in the EMR and paper and will be finally recorded in an electronic case report form (eCRF) provided by myTrial (Bethesda Soft Co., Ltd. Seoul, Korea).
We will give researchers appropriate permission to input, change, and view of data according to their role on this study. All of their activities will be logged in the eCRF system.
Quality Control
The study procedure and documents will be periodically monitored according to the KGCP guidelines to maintain the trial quality.
Discussion
The number of TMD patients is increasing in modern society due to stressful circumstances or other reasons. The longer the duration of pain related to TMD, the higher the restrictions on daily, social, and work activities. Medical expenses are also increasing.25
We will use the DC/TMD to select participants. The Research Diagnostic Criteria for Temporo-Mandibular Disorders (RDC/TMD) has been most widely used for TMD diagnosis since its publication in 1992. However, the RDC/TMD was only the beginning, and further research was needed to improve its validity and clinical utility. Therefore, the DC/TMD was published in 2004, which added detailed factors for diagnosis and headache types related to TMD.26
The DC/TMD provides a comprehensive list of symptom-related questionnaires, doctor examination protocols, and relevant assessment tools for TMD diagnosis. TMD is categorized into five types: pain-related, headache, intra-articular joint disorders, degenerative joint disorder, and subluxation. As TEA affects the muscle and fascia layers, participants with myalgia, myofascial pain, or arthralgia are included among those with pain-related TMD. The DC/TMD will be used to select those types of participants.
Based on their responses to the symptom questionnaire (SQ3 and SQ4) and the findings of the DC/TMD examination (E1a), we will check if participants meet our inclusion criteria (3) and (4) (SQ3: in the last 30 days, which of the following best describes any pain in your jaw, temple, in the ear, or in front of the ear on either side?, SQ4: In the last 30 days, did the following activities change any pain in your jaw, temple, in the ear, or in front of the ear on either side?, E1a: identifies pain areas for the last 30 days by verbal commands and palpation of the examiner.).
Graded chronic pain 2.0, and JFLS-20 are also provided in the DC/TMD and can be applied because they have been officially translated into Korean.
Initially, TMD was understood purely as a mechanical-based phenomenon such as malocclusion. In addition to malocclusion, it is limited to physical factors such as external injury, abnormal physical habits, and muscle abnormalities, which are the proposed causes of TMD.27 However, TMD has recently been described as a chronic pain biopsychosocial disease model. Thus, it is a multifactorial disease that includes biological, environmental, social, emotional, and cognitive triggers.6,28
TMD is a multifactorial disease, with various treatment methods, including painkillers, antidepressants, cognitive behavioral therapy (CBT), oral splints, physical therapy, and acupuncture.29
In addition to these treatment methods, TEA is used clinically in traditional Korean medicine for the treatment of TMD. First, TEA has been used for cosmetic purposes to improve wrinkles and skin elasticity. However, in recent years, it has also been widely used for the treatment of various diseases, especially musculoskeletal chronic pain. Clinical studies have provided evidence of the effects of TEA in musculoskeletal diseases.15–17 Thus, TEA newly develops connective tissue and increases vessel size.30 These effects can help subluxation of the TMJ due to degenerative changes and weakening of surrounding soft tissues.
TENS was used to manage TMD as physical therapy. TENS is commonly used to control pain and increase TMJ movement.31–33 We will use TENS at frequencies of 2 Hz and 100 Hz simultaneously. Alternating modes of TENS at low (2 Hz) and high (100 Hz) frequencies produce a synergistic interaction between dynorphin and enkephalin to produce a more potent analgesic effect compared to the use of a fixed stimulation frequency.34,35
Based on the premise that TEA can relieve pain intensity and improve patient quality of life, we designed this clinical study to explore the feasibility of further confirmatory RCT of TEA for TMD, considering efficacy, safety, and cost-effectiveness, and to determine the effect size to calculate the appropriate sample size for further RCT.
Although a previous study divided the study participants into TEA and sham TEA groups,36 we will instead set physical therapy as a comparative group instead of needling without thread. As in acupuncture, needle insertion may provide therapeutic stimulation even without thread.
This study had limitations in the design stage. Owing to its nature, TEA treatment must be performed by an experienced acupuncture expert. Hence, practitioner blinding is difficult.
Conclusions
We designed this pilot study to evaluate the feasibility of a large-scale clinical study and predict the appropriate number of participants.
The results of this trial will help to explore the feasibility of TEA for the treatment of patients with TMD, based on the efficacy, safety, and cost-effectiveness of this treatment.
Acknowledgments
The authors would like to thank Editage (www.editage.co.kr) for English language editing.
Funding Statement
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF21C0011).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ. 2008;72(8):930–947. doi: 10.1002/j.0022-0337.2008.72.8.tb04569.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumit Y, Yang Y, Dutra EH, Robinson JL, Wadhwa S. Temporomandibular joint disorders in the elderly and aging population. J Am Geriatr Soc. 2018;66(6):1213–1217. doi: 10.1111/jgs.15354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute of Dental and Craniofacial Research; Facial pain title. National Institute of Dental and Craniofacial Research; 2018. Available from: http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/FacialPain/. Accessed July 19, 2022. [Google Scholar]
- 4.The number of patients by year when K076 is entered in the disease code inquiry. Disease Subdivision (4-tier classification disease) Statistics. Health Insurance Review and Assessment Service; 2022. Available from: http://opendata.hira.or.kr/op/opc/olap4thDsInfo.do. Accessed July 18, 2022.
- 5.Durham J. Temporomandibular disorders (TMD): an overview. Oral Surg. 2008;1(2):60–68. doi: 10.1111/j.1752-248X.2008.00020.x [DOI] [Google Scholar]
- 6.Gauer R, Semidey MJ. Diagnosis and treatment of temporomandibular disorders. Am Fam Physician. 2015;91(6):378–386. [PubMed] [Google Scholar]
- 7.International Association for Dental Research. Temporomandibular disorders; 2015. Available from: https://www.iadr.org/science-policy/temporomandibular-disorders-tmd. Accessed July 22, 2020.
- 8.List T, Axelsson S. Management of TMD: evidence from systematic reviews and meta‐analyses. J Oral Rehabil. 2010;37(6):430–451. doi: 10.1111/j.1365-2842.2010.02089.x [DOI] [PubMed] [Google Scholar]
- 9.Kim C-E, Do H-J, Song H-S, et al. A web-based survey for assessment of Korean medical treatment clinical practice patterns for temporomandibular disorders. J Korean Med. 2018;28(1):73–84. [Google Scholar]
- 10.Seung-Hun Cho KMD, Whang W-W. Acupuncture for temporomandibular disorders: a systematic review. J Orofac Pain. 2010;24:152–162. [PubMed] [Google Scholar]
- 11.Jung A, Shin B-C, Lee MS, Sim H, Ernst E. Acupuncture for treating temporomandibular joint disorders: a systematic review and meta-analysis of randomized, sham-controlled trials. J Dent. 2011;39(5):341–350. doi: 10.1016/j.jdent.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 12.Dietrich L, Rodrigues IVS, de Assis Costa MDM, Carvalho RF, da Silva GR. Acupuncture in temporomandibular disorders painful symptomatology: an evidence-based case report. Eur J Dent. 2020;14(04):692–696. doi: 10.1055/s-0040-1716631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Touche R, Goddard G, De-la-Hoz JL, et al. Acupuncture in the treatment of pain in temporomandibular disorders: a systematic review and meta-analysis of randomized controlled trials. Clin J Pain. 2010;26(6):541–550. doi: 10.1097/AJP.0b013e3181e2697e [DOI] [PubMed] [Google Scholar]
- 14.Hong K. Comprehension of embedding therapy through meridian muscle system; focused on face. J Korean Acupunct Moxibustion Soc. 2008;25(3):215–219. [Google Scholar]
- 15.Lim SS, Sung HJ, Lee CK, Choi HY, Du Roh J, Lee EY. The effect of thread embedding acupuncture on lumbar herniated intervertebral disc patients: a retrospective study※. Acupunct. 2016;33(4):39–47. doi: 10.13045/acupunct.2016053 [DOI] [Google Scholar]
- 16.Lee JH, Yang TJ, Lee DG, Lee OJ, Wei TS. The effect of needle-embedding therapy on osteoarthritis of the knee combined with Korean medical treatment: report of five cases. J Acupunct Res. 2014;31(4):195–204. doi: 10.13045/acupunct.2014066 [DOI] [Google Scholar]
- 17.Yoo D-J, Jung J-Y, Chung S-H. Effects of the embedding acupuncture treatments for chronic low back pain patients. J Korean Med Rehabil. 2015;25(4):105–112. doi: 10.18325/jkmr.2015.25.4.105 [DOI] [Google Scholar]
- 18.MacPherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 2010;7(6):e1000261. doi: 10.1371/journal.pmed.1000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y-H, Song JY. A study of the reliability and the validity of the BDI, SDS, and MMPI-D scales. Korean J Clin Psychol. 1991;10(1):98–113. [Google Scholar]
- 21.Kim S-H, Jo M-W, Ahn J, Ock M, Shin S, Park J. Assessment of psychometric properties of the Korean SF-12 v2 in the general population. BMC Public Health. 2014;14(1):1–7. doi: 10.1186/1471-2458-14-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S-H, Ahn J, Ock M, et al. The EQ-5D-5L valuation study in Korea. Qual life Res. 2016;25(7):1845–1852. doi: 10.1007/s11136-015-1205-2 [DOI] [PubMed] [Google Scholar]
- 23.Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25(3):1057–1073. doi: 10.1177/0962280215588241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat J Appl Stat Pharm Ind. 2005;4(4):287–291. [Google Scholar]
- 25.Bu LX, Chen T, Chen X, Jing H, Li NY. Clinical observation of acupuncture and massage therapy for temporomandibular joint disorders. Shanghai Kou Qiang Yi Xue. 2011;20(3):292–295. [PubMed] [Google Scholar]
- 26.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28(1):6. doi: 10.11607/jop.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roh DH, Lee M, Literature A. Review of the Temporom andibular Joint(TMJ) Disorder. J Korean Med Rehabi. 2005;15(3):13–24. [Google Scholar]
- 28.Skármeta NP, Pesce MC, Saldivia J, Espinoza Mellado P, Montini F, Sotomayor C. Changes in understanding of painful temporomandibular disorders: the history of a transformation; 2019. [DOI] [PubMed]
- 29.Kapos FP, Exposto FG, Oyarzo JF, Durham J. Temporomandibular disorders: a review of current concepts in aetiology, diagnosis and management. Oral Surg. 2020;13(4):321–334. doi: 10.1111/ors.12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon JH, Kim SS, Oh SM, Kim BC, Jung W. Tissue changes over time after polydioxanone thread insertion: an animal study with pigs. J Cosmet Dermatol. 2019;18(3):885–891. doi: 10.1111/jocd.12718 [DOI] [PubMed] [Google Scholar]
- 31.Kato MT, Kogawa EM, Santos CN, Conti PCR. TENS and low-level laser therapy in the management of temporomandibular disorders. J Appl Oral Sci. 2006;14:130–135. doi: 10.1590/S1678-77572006000200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues D, Siriani AO, Bérzin F. Effect of conventional TENS on pain and electromyographic activity of masticatory muscles in TMD patients. Braz Oral Res. 2004;18:290–295. doi: 10.1590/S1806-83242004000400003 [DOI] [PubMed] [Google Scholar]
- 33.Shanavas M, Chatra L, Shenai P, et al. Transcutaneous electrical nerve stimulation therapy: an adjuvant pain controlling modality in TMD patients—A clinical study. Dent Res J. 2014;11(6):676. [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao-Hong C, Su-Fong G, Chung-Gwo C, Ji-Sheng H. Optimal conditions for eliciting maximal electroacupuncture analgesia with dense-and-disperse mode stimulation. Am J Acupunct. 1994;22(1):47–53. [Google Scholar]
- 35.Law P, Cheing G. Optimal stimulation frequency of transcutaneous electrical nerve stimulation on people with knee osteoarthritis. J Rehabil Med. 2004;36(5):220–225. doi: 10.1080/16501970410029834 [DOI] [PubMed] [Google Scholar]
- 36.Park YC, Goo BH, Lee CH, et al. Clinical effectiveness of thread-embedding acupuncture in the treatment of Bell’s palsy sequelae: a randomized, patient-assessor-blinded, controlled, clinical trial. Eur J Integr Med. 2020;37:101113. doi: 10.1016/j.eujim.2020.101113 [DOI] [Google Scholar]