Abstract
Introduction:
Growing evidence implicates air pollution as a risk factor for dementia, but prior work is limited by challenges in diagnostic accuracy and assessing exposures in the decades prior to disease development. We evaluated the impact of long-term fine particulate matter (PM2.5) exposures on incident dementia (all-cause, Alzheimer’s disease [AD], and vascular dementia [VaD]) in older adults.
Methods:
A panel of neurologists adjudicated dementia cases based on extensive neuropsychological testing and magnetic resonance imaging. We applied validated fine-scale air pollutant models to reconstructed residential histories to assess exposures.
Results:
An interquartile range increase in 20-year PM2.5 was associated with a 20% higher risk of dementia (95% confidence interval [CI]: 5%, 37%) and an increased risk of mixed VaD/AD but not AD alone.
Discussion:
Our findings suggest that air pollutant exposures over decades contribute to dementia and that effects of current exposures may be experienced years into the future.
Keywords: air pollution, Alzheimer’s disease, dementia, longitudinal cohort study, vascular dementia
1 |. INTRODUCTION
Dementia is a highly prevalent disease and is expected to increase dramatically in the coming decades as the population ages.1 Individuals with dementia experience cognitive decline and lose their ability to function independently, affecting their own quality of life and health as well as the well-being of their caregivers and family members.2 Few avenues for treatment or prevention have been convincingly demonstrated.
Air pollution is a pervasive and modifiable exposure. Decades of research have shown that air pollution is an important contributor to respiratory and cardiovascular morbidity and mortality.3 Evidence is building that air pollution may contribute to dementia,4–12 which led the 2020 Lancet Commission on dementia prevention, intervention, and care to add air pollution to the list of potentially modifiable risk factors for dementia.1 This growing evidence base highlights the need for careful consideration of methodological issues common in epidemiologic studies of dementia and air pollution.13–15 The major barriers to studying this association include the lack of populations with rigorous, prospective dementia characterization to allow for both accurate diagnosis and precise ascertainment of the timing of disease onset and the difficulty of assessing air pollution exposures during the critical window of exposure, which likely is many years before the disease develops.16 Constructing residential histories and exposure profiles is challenging because address histories prior to study entry may be unknown, and people with dementia may not recall their prior residences. Furthermore, most air pollution exposure assessment approaches have not estimated exposure before 1999 due to the absence of widespread monitoring. In addition, most prior studies have relied on administrative records.14 Although this approach permits the inclusion of large, geographically diverse populations, administrative records are not accurate with regard to diagnosis or precise with regard to disease onset.14,17 These records also have limited information on key confounding factors, such as education and smoking, or potential effect modifiers, such as the apolipoprotein E (APOE) ε4 allele, the strongest genetic predictor of Alzheimer’s disease (AD), which may increase susceptibility to the impacts of air pollution exposure.9,18
We report findings from the Ginkgo Evaluation of Memory Study (GEMS), a clinical trial of Ginkgo biloba (G. biloba) supplementation specifically designed to characterize the development of dementia in older adults via rigorous dementia ascertainment, coupled with a long-term residential history in participants that accounts for residential mobility prior to baseline and a novel approach to characterizing individual-level outdoor pollutant concentrations back to 1980. We draw on this unique study resource to investigate the impact of long-term fine particulate matter (less than 2.5 μm in diameter) (PM2.5) and nitrogen dioxide (NO2) exposures on incident dementia overall and by subtype, and to investigate whether the impact is modified by APOE genotype.
2 |. METHODS
2.1 |. Study design and participants
GEMS has been described previously.19 Briefly, GEMS was a randomized controlled trial (RCT) to evaluate the efficacy of G. biloba for the prevention of dementia and AD. G. biloba did not have a protective effect,20 but the study provides a setting to evaluate risk factors for dementia, including air pollution, due to the exceptional dedication of resources to detecting subtle incident neurocognitive changes. Between 2000 and 2002, a total of 3069 adults 75 years of age and older were recruited from four sites in the United States—Winston-Salem, North Carolina (NC); Hagerstown, Maryland (MD); Sacramento, California (CA); and Pittsburgh, Pennsylvania (PA)—using methods relying primarily on voter registration and other regional mailing lists.21 All participants identified three proxies including one who agreed to provide information should the participant be unable to do so. Those with prevalent dementia or other neurological or neurodegenerative diseases at baseline were excluded. Prevalent dementia was defined as meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for dementia22 or having a Clinical Dementia Rating (CDR) scale score greater than 0.5.23 In addition, participants taking cognitive enhancers, treatments for AD, or supplements or medications that could interfere with the action of G. Biloba supplements were excluded. Signed informed consent was obtained from participants and their proxies for the RCT. The University of Washington Institutional Review Board approved this study.
2.2 |. Procedures for dementia ascertainment
GEMS participants were evaluated prospectively for dementia every six months from randomization until the end of follow-up in 2008. At each semi-annual exam, participants were administered three dementia screening examinations: the Modified Mini-Mental State Examination (3MSE),24 the cognitive subscale of the Alzheimer Disease Assessment Scale (ADAS-Cog),25 and the CDR.23 A decline in a pre-specified number of points on two of three of these cognitive assessments, a report by participant or proxy of the onset of a new memory or other cognitive problem, or a diagnosis of dementia or prescription for dementia medication by a private physician since the prior visit triggered administration of an extensive neuropsychological battery (NPB) consisting of 12 tests in six cognitive domains. Following the NPB, participants classified as having incident dementia based on a previously described algorithm then underwent a neurological evaluation and brain magnetic resonance imaging (MRI).20 A dementia adjudication panel confirmed the diagnosis and determined dementia subtype by consensus according to diagnostic criteria from the National Institute of Neurological and Communication Disorders and Stroke, Alzheimer’s Disease and Related Disorders Association, the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences, and the Alzheimer’s Disease Diagnostic and Treatment Centers.20 Each dementia case was categorized as either Alzheimer’s disease (AD), vascular dementia (VaD) with no AD, VaD and AD (here called “mixed” type), or dementia of other etiology (eg, dementia with Lewy bodies). Due to the small number of participants with incident VaD with no AD, we grouped these cases with VaD/AD mixed type dementia. Only 15 participants developed dementia classified as “other.” As a result, we did not perform analyses evaluating the impact of air pollution on dementia classified as “other.”
2.3 |. Exposure assessment
Long-term exposures to PM2.5 and NO2 at home prior to study entry were of primary interest. We based exposure on participant residential addresses, which were documented and updated during semi-annual follow-up visits and by telephone contact between visits. We constructed residential history up to 20 years prior to study entry using LexisNexis (LN), a commercial credit-reporting company (Supplement 1).26 LN applies proprietary algorithms and public information (eg, mortgage records) to obtain address history.26 We refer to this comprehensive approach to address history reconstruction as “enhanced history (EH)” and to the approach that assumes no residential mobility prior to study enrollment as “baseline history (BH).”
Once address history from 1980 forward was reconstructed, we geocoded each residential location using parcel-based geocoding methods available with the Business Analyst tool in ArcGIS10.6.1 using the TeleAtlas Dynamap 2000 v.16.1 road network (Boston, MA). For each geocoded location, we calculated more than 300 spatiotemporal geographic predictors of air pollutant exposure. Because dementia is believed to develop over decades, we hypothesized that long-term mean exposure is most important in the development of this chronic disease.27 We applied a validated PM2.5 spatiotemporal model to estimate long-term PM2.5 exposure at each residential location between 1980 and 1999.28 Long-term NO2 exposure between 1990 and 1999 was estimated with a validated national prediction model.29 Exposure metrics were: (1) mean PM2.5 exposure in the 5, 10, 15, and 20 years prior to GEMS enrollment; and (2) mean NO2 exposure in the 5 and 10 years prior to GEMS enrollment. Each air pollution averaging period includes mean exposures up to 1999. For example, the 10-year average was calculated as the mean of the annual averages from 1990 through 1999. These intervals were selected because they occurred prior to GEMS enrollment.
2.4 |. Statistical analyses
We performed descriptive analyses of individual- and neighborhood-level characteristics by study community and examined differences using analysis of variance or chi-square tests, as appropriate. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) describing associations between long-term air pollutant exposure and incident all-cause dementia, AD, and VaD/AD mixed in those who were free from dementia and mild cognitive impairment (MCI) at baseline. PM2.5 and NO2 and their respective averaging periods were evaluated in separate analyses. HRs were reported per interquartile range (IQR) increase in air pollutant exposure. Follow-up time began at age at randomization and ended at the age halfway between the last examination without dementia and the examination at which the diagnosis was made.20 Censoring occurred at the age of last contact or death.20
Analyses were adjusted for potential confounders including year of randomization, GEMS study site, treatment assignment, sex, education, occupational history, smoking status, pack-years smoking, percent of life exposed to secondhand smoke (SHS), and neighborhood deprivation index (NDI; Supplement 2), since research has shown that the neighborhood social environment is an important confounder of associations between air pollution and health.30 We also included APOE genotype as a precision variable in all analyses. We included a multiplicative interaction term for air pollutant exposure and APOE genotype to evaluate APOE genotype as an effect modifier. To account for missing APOEε4 carrier information, we used the mice package31 in the R statistical computing language (R Core Team) to perform multiple imputation of missing APOE status and missing covariate values (Supplement 3).
2.5 |. Sensitivity analyses
We performed a number of sensitivity analyses (Supplement 4). We altered both the air pollutant and NDI exposure periods by including up to the year of randomization to evaluate the robustness of results to alternative averaging periods. We also evaluated the impact of peak annual exposure in the 20 years prior to the year 2000. In addition, we examined modification of the effect of air pollution on dementia risk by gender, study site, G. biloba treatment assignment, and reported history of cardiovascular disease and diabetes mellitus (see Supplement 2). Finally, we examined the impact of additional potential confounders including body mass index, physical activity, and alcohol consumption, all of which were assessed at the examination closest in time to the baseline visit.15
3 |. RESULTS
A total of 3069 individuals participated in GEMS. Of these, we excluded 482 who had MCI at baseline and an additional 23 who were missing information on air pollution exposure. Thus, the final analytic sample included 2564 participants with mean (SD) follow-up of 5.7 years (1.5); 324 (12.6%) developed dementia of any type (Table 1). AD comprised 65% of these cases. A mixture of dementia with features of both VaD and AD comprised 30% of the cases. Mean age at randomization was 78 years (3.2). Overall, 54% of participants were male with similar distributions across study sites. The cohort was nearly 97% White. Of the 80% of the cohort with information on APOE ε4 carrier status, 23% had at least one copy of the ε4 allele.
TABLE 1.
GEMS participant characteristics overall and by study community presented as n and percent except where indicated (N = 2564)a
| Winston-Salem, NC | Sacramento, CA | Hagerstown, MD | Pittsburgh, PA | |||
|---|---|---|---|---|---|---|
| All | N = 579 | N = 781 | N = 386 | N = 818 | P-valueb | |
| All-cause dementia | 324 (12.6) | 65 (11.2) | 108 (13.8) | 66 (17.1) | 85 (10.4) | 0.005 |
| AD only | 212 (8.3) | 48 (8.3) | 66 (8.5) | 41 (10.6) | 57 (7.0) | 0.20 |
| VaD/AD mixed | 97 (3.8) | 17 (2.9) | 34 (4.4) | 22 (5.7) | 24 (2.9) | 0.061 |
| Follow-up time, y, mean (SD) | 5.7 (1.5) | 5.5 (1.5) | 5.7 (1.4) | 5.7 (1.6) | 5.7 (1.5) | 0.082 |
| Age at randomization, y, mean (SD) | 78.4 (3.2) | 78.3 (3.3) | 78.5 (3.1) | 78.7 (3.4) | 78.2 (3.0) | 0.036 |
| Male | 1,394 (54.4) | 302 (52.2) | 449 (57.5) | 213 (55.2) | 430 (52.6) | 0.15 |
| Non-White | 84 (3.3) | 18 (3.1) | 42 (5.4) | 1 (0.3) | 23 (2.8) | <0.001 |
| Placebo assignment | 1,285 (50.1) | 293 (50.6) | 392 (50.2) | 181 (46.9) | 419 (51.2) | 0.56 |
| Education | <0.001 | |||||
| High school or less | 930 (36.3) | 191 (33.0) | 244 (31.2) | 208 (53.9) | 287 (35.1) | |
| Some college | 628 (24.5) | 177 (30.6) | 202 (25.9) | 76 (19.7) | 173 (21.1) | |
| College graduate | 425 (16.6) | 105 (18.1) | 117 (15.0) | 42 (10.9) | 161 (19.7) | |
| Postgraduate | 581 (22.7) | 106 (18.3) | 218 (27.9) | 60 (15.5) | 197 (24.1) | |
| Smoking status | 0.060 | |||||
| Never | 1,017 (40.4) | 213 (37.4) | 288 (38.0) | 175 (45.9) | 341 (42.2) | |
| Former | 1,391 (55.2) | 327 (57.4) | 441 (58.2) | 188 (49.3) | 435 (53.8) | |
| Current | 110 (4.4) | 30 (5.3) | 29 (3.8) | 18 (4.7) | 33 (4.1) | |
| Pack-years smoking | 0.022 | |||||
| 0 | 1030 (43.4) | 218 (40.0) | 294 (41.5) | 175 (48.6) | 343 (45.0) | |
| >0 to 24 | 692 (29.1) | 185 (33.9) | 213 (30.1) | 84 (23.3) | 210 (27.6) | |
| >24 | 653 (27.5) | 142 (26.1) | 201 (28.4) | 101 (28.1) | 209 (27.4) | |
| Percent of life exposed to SHS | 0.009 | |||||
| ≤1.14 | 615 (24.6) | 129 (22.6) | 185 (24.4) | 110 (29.2) | 191 (24.0) | |
| >1.14 to ≤24.39 | 616 (24.6) | 138 (24.2) | 207 (27.3) | 81 (21.5) | 190 (23.8) | |
| >24.39 to ≤46.43 | 637 (25.4) | 137 (24.0) | 206 (27.1) | 81 (21.5) | 213 (26.7) | |
| >46.43 | 636 (25.4) | 167 (29.2) | 161 (21.2) | 105 (27.9) | 203 (25.5) | |
| NDI, mean (SD) | ||||||
| 5 year | −0.08 (3.09) | −0.55 (3.37) | 0.09 (3.05) | 1.73 (1.99) | −0.77 (2.99) | <0.001 |
| 10 year | −0.08 (3.05) | −0.60 (3.35) | 0.11 (3.01) | 1.68 (1.99) | −0.72 (2.93) | <0.001 |
| 15 year | −0.08 (3.01) | −0.62 (3.32) | 0.10 (2.95) | 1.63 (2.03) | −0.68 (2.90) | <0.001 |
| 20 year | −0.09 (2.99) | −0.61 (3.31) | 0.09 (2.89) | 1.60 (2.08) | −0.68 (2.88) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 27.2 (4.2) | 27.0 (4.3) | 27.2 (4.3) | 27.5 (4.1) | 27.3 (4.3) | 0.25 |
| Alcoholic drinks/wk, mean (SD) | 3.7 (6.6) | 3.3 (6.0) | 5.0 (7.6) | 2.1 (4.7) | 3.5 (6.4) | <0.001 |
| Occupation History | 0.001 | |||||
| Professional | 1484 (57.9) | 357 (61.7) | 473 (60.6) | 206 (53.4) | 448 (54.8) | |
| Sales or clerical | 436 (17.0) | 95 (16.4) | 128 (16.4) | 57 (14.8) | 156 (19.1) | |
| Manual labor | 325 (12.7) | 50 (8.6) | 88 (11.3) | 73 (18.9) | 114 (13.9) | |
| Homemaker | 263 (10.3) | 65 (11.2) | 72 (9.2) | 44 (11.4) | 82 (10.0) | |
| Other | 56 (2.2) | 12 (2.1) | 20 (2.6) | 6 (1.6) | 18 (2.2) | |
| APOE ε4 carrier status | 472 (22.9) | 94 (20.8) | 140 (23.0) | 81 (25.0) | 157 (23.1) | 0.58 |
Abbreviations: AD, Alzheimer’s disease; APOE, apolipoprotein E.; NDI, neighborhood deprivation index; SD, standard deviation; SHS, secondhand smoke; VaD, vascular dementia.
Information was missing for the following covariates: APOE ε4 carrier status (n = 500), body mass index (n = 9), alcohol number (n = 38), smoking (n = 46), passive smoking percentage (n = 60), pack-years (n = 189), and NDI (n = 2).
Differences by study community were examined using analysis of variance or chi-square tests, as appropriate.
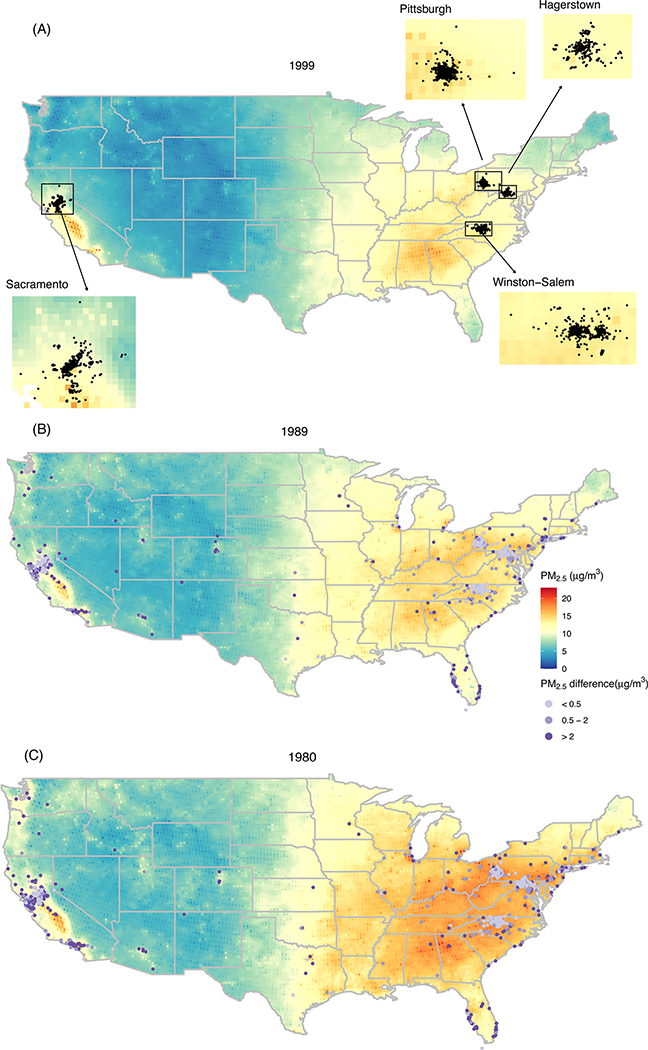
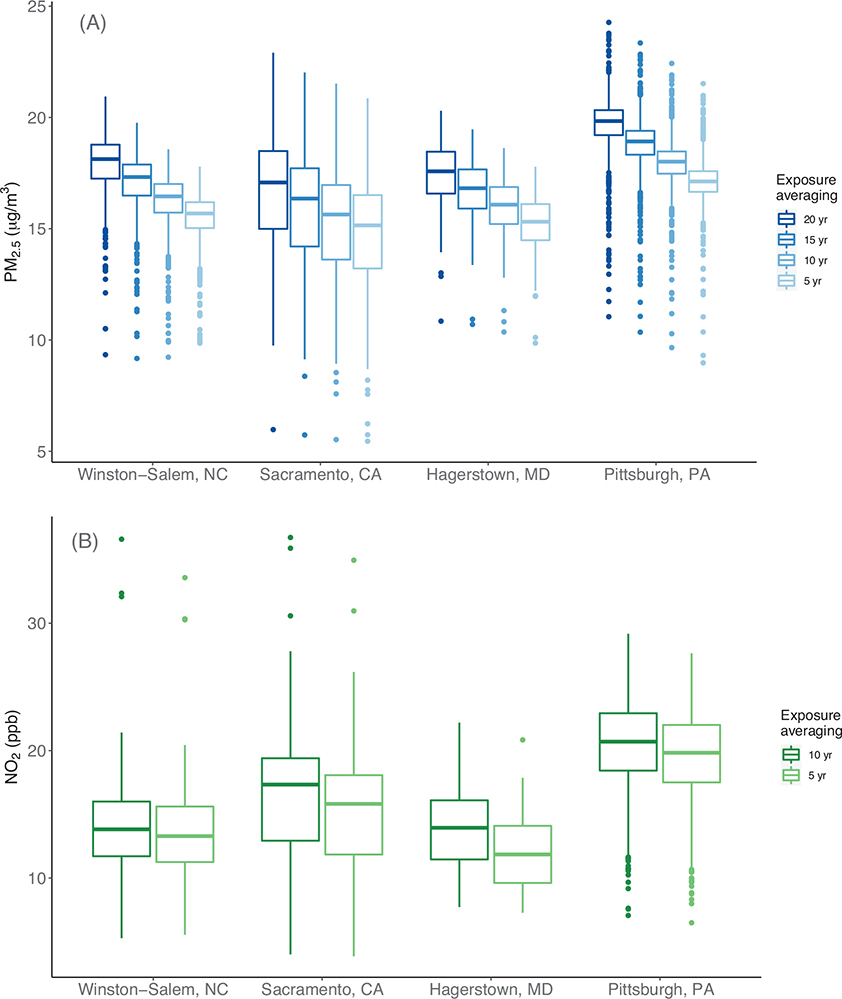
Participant home addresses were clustered around GEMS clinics at baseline (Figure 1A) but were dispersed widely in the two decades preceding enrollment (Figure 1B–C). Figures 1B and 1C indicate that the PM2.5 declined substantially between 1980 and 1989 and also that assuming participants lived in the same address 20 years prior to enrollment in GEMS influenced estimated long-term exposure. For example, the absolute value of the difference between EH- and BH-estimated PM2.5 exposure exceeded the IQR of 2 μg/m3 for 7% of participants in 1989 (Figure 1B) and 12% of participants in 1980 (Figure 1C). Figure 2 shows the distributions of estimated long-term PM2.5 and NO2 exposure for GEMS participants by averaging period and study community using the EH-based approach.
FIGURE 1.
Ginkgo Evaluation of Memory Study (GEMS) participant residential locations and estimated fine particulate matter (PM2.5) concentrations in the contiguous USA in 1999 (A), 1989 (B), and 1980 (C). Points indicate the absolute value of the difference between PM2.5 exposure estimated from the enhanced history (EH) and the approach that assumed the address ascertained at the GEMS baseline history (BH) visit was the residential address in 1989 (B) or 1980 (C)
FIGURE 2.
Long-term fine particulate matter (PM2.5, A) and nitrogen dioxide (NO2, B) exposure by study community and selected exposure averaging periods using the enhanced history (EH) approach for constructing residential history
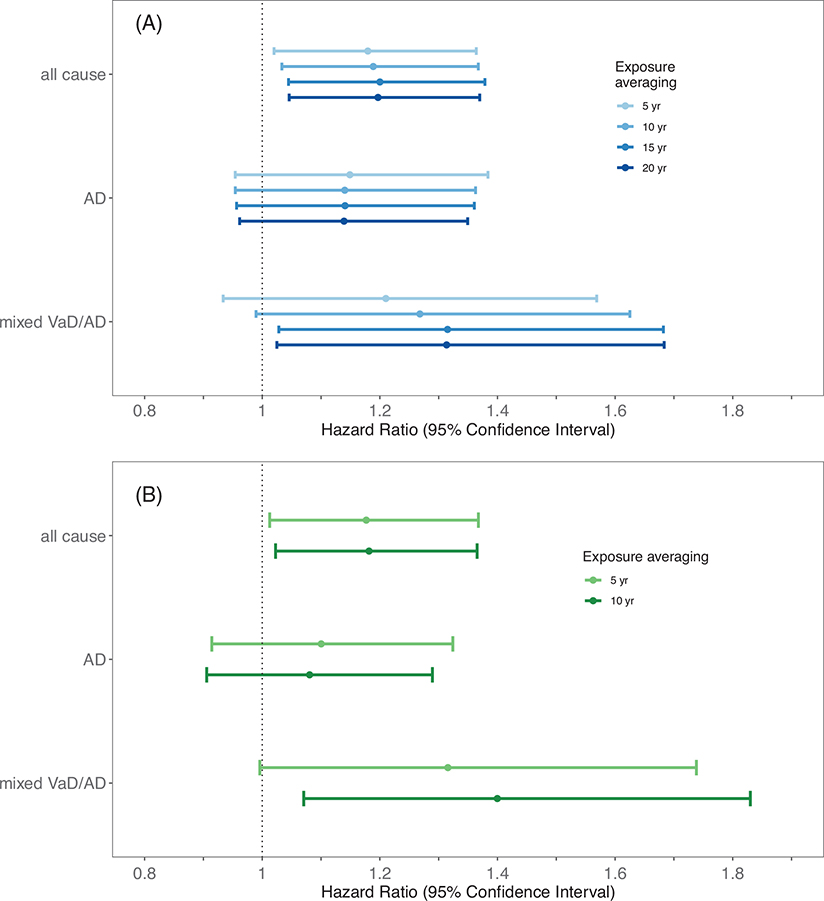
In adjusted analyses, we observed significant associations between PM2.5 exposure and all-cause dementia regardless of the exposure averaging period (Figure 3). A 2 μg/m3 higher PM2.5 exposure (IQR) in the 20 years prior to enrollment in GEMS was associated with a 1.20-fold higher (95% CI: 1.05, 1.37) risk of dementia. Results were similar for a 5-ppb IQR higher 10- and 5-year NO2 exposure, respectively (HR: 1.18, 95% CI: 1.02, 1.37; HR: 1.18, 95% CI: 1.01, 1.37). HRs describing air pollutant associations with mixed VaD/AD generally were larger in magnitude, with statistical significance varying by pollutant and averaging period. Relationships with AD were slightly smaller in magnitude and more imprecise than those observed for all-cause dementia. We saw no evidence on the multiplicative scale that the impact of air pollutant exposure differed by APOE ε4 carrier status (P > 0.58 for interaction), AD (P > 0.27 for interaction), or mixed VaD (P > 0.37 for interaction) regardless of pollutant or averaging period. Sensitivity analyses did not change overall conclusions (Figures S2–S5). Although the point estimates for the HRs varied somewhat between study sites, the CIs were wide and overlapping, and we did not see evidence that the impact of air pollution exposure on dementia risk varied significantly by study site (Figures S6 and S7). The impact of air pollution on dementia risk also did not vary by gender, G. biloba treatment assignment, or reported history of cardiovascular disease or diabetes mellitus reported at baseline (Figures S6–S9).
FIGURE 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) describing associations between long-term fine particulate matter (PM2.5, A) and nitrogen dioxide (NO2, B) exposure and incident all cause dementia, Alzheimer’s disease (AD) only, and mixed vascular dementia (VaD)/AD. Age is the time scale in analyses adjusted for year of randomization, study community, treatment assignment, sex, education, occupational history, neighborhood deprivation index (NDI), smoking status, pack-years of smoking, secondhand smoke (SHS) exposure, and apolipoprotein E (APOE) genotype
4 |. DISCUSSION
We found strong associations between long-term PM2.5 and NO2 exposure and incident dementia in a large cohort of older adults with repeated and rigorous follow-up for dementia over a mean of nearly six years. We used detailed and updated residential history information and state-of-the-art air pollutant models to assess exposure two decades prior to study entry, the period most closely matching when the underlying disease processes are hypothesized to begin. Results were consistent across pollutant and averaging period and robust to various stages of adjustment for confounders and additional sensitivity analyses and supported the hypothesis that the earliest assessed exposure period was most important to subsequent dementia development. Associations were stronger for mixed VaD/AD than for AD but were only statistically significant for the longest exposure averaging periods. We saw no evidence that the impact of air pollution varied by APOE ε4 carrier status. Overall, findings provide strong evidence that air pollution exposure increases the risk of dementia, and that important pollutant effects likely precede dementia onset by several years if not decades.
Our findings are consistent with previous work demonstrating associations between air pollutant exposure and dementia.14,32,33 The magnitude of the effect we observed is similar to what others have found. In a notable exception, compared to the 1.20-fold increase in all-cause dementia that we found, Grande and colleagues observed a stronger effect of an IQR increase in PM2.5 exposure in an urban Swedish setting in the 5 years prior to dementia diagnosis (ie, HR = 1.54 per 0.88 μg/m3 increase; 95% CI: 1.33, 1.78). More pronounced effects of an increase in PM2.5 in relatively low air pollutant settings have been found in recent work,8,34 and the comparatively high exposures in the GEMS cohort may explain, in part, the smaller effect estimates observed in our study. Similar to Grande and colleagues, we observed larger magnitudes of effect for VaD and no significant effects for AD. This is consistent with findings from a community-based cohort in a US metropolitan area, in which no association between PM2.5 and AD neuropathology was found.35
The importance of VaD relative to AD in our study may explain in part why we did not observe significant effect modification by APOE ε4 carrier status, which is more strongly linked to AD. Air pollution has well-established impacts on cardiovascular health,36 and cardiovascular disease is associated with a higher risk of dementia.37 As such, our findings related to VaD are expected. Air pollution also may cause dementia through oxidative stress, inflammation, disruption of the blood-brain barrier, or direct particle translocation to the brain along the olfactory tract.38 We must acknowledge that even with the rigorous methods for assessing dementia including brain MRI, misclassification of dementia subtype is likely in the absence of postmortem evaluation.39 As a result, it is possible that the association between air pollution and AD may be weaker than what we observed, particularly if vascular pathology is driving the increased risk of dementia.
Our study had a number of strengths. We had comprehensive, rigorous, and frequent prospective assessments for dementia, a necessary strategy to accurately assess dementia in the absence of dementia registries or surveillance systems.14 The vast majority of previous studies on this topic ascertained dementia cases from administrative databases5,12,40 such as Medicare claims34,41 or hospitalization data,11,42 each of which relies on health care service utilization, which favors the identification of more severe cases and those with comorbidities. In the few studies that prospectively assessed dementia,4,8,9,33 researchers did not have access to brain MRI, a critical tool for distinguishing subtypes. In contrast, dementia was the primary end point of the GEMS RCT, and extensive resources were directed toward prospective and accurate dementia ascertainment. In addition to screening every six months during follow-up, GEMS participants regularly underwent detailed NPB testing and, if indicated, had a full neurological evaluation and brain MRI to determine dementia subtype, with final diagnosis made by an expert adjudication panel. This allowed for highly accurate classification of dementia and temporally resolved estimates of disease onset. In addition, most previous studies assumed that recent or current air pollution exposure was a reasonable proxy for exposure during the critical window of development of dementia4,9,43 and that participants were living in the same place during this window.10,41,42 Our exploration of residential history reveals substantial residential mobility with potentially meaningful consequences for air pollution exposure assessment. The findings reported here are among the first to account for residential mobility and exposures in the decades prior to disease development, the time period hypothesized to be most important.14 Longer averaging periods and the incorporation of lags between air pollution exposure and dementia ascertainment are critical to examine, as the onset of changes associated with dementia can be seen many years before diagnosis.15 Moreover, our detailed residential history from a prospective follow-up of participants combined with a finely resolved air pollution model enabled us to generate residence-specific rather than postal code-5,10,34 or city district-level42 estimates of air pollution exposure. Our study also benefitted from the inclusion of four geographically diverse sites in the United States, standardized data collection across sites, and extensive information on potential confounders including smoking, education, and neighborhood-level socioeconomic status.
We acknowledge several limitations to our study. Residential history misclassification and exposure measurement error are a concern; we expect this error was not associated with dementia status, which would imply attenuation toward the null on average under repeat sampling. Effects of air pollutants were dependent on participation in GEMS. Those experiencing cognitive decline are less likely to participate in research studies and more likely to be lost to follow-up,44,45 which likely would attenuate associations between air pollution and dementia.14 Studies of dementia are particularly subject to selective attrition.46 However, because dementia was the primary end point in the GEMS clinical trial and procedures were in place to mitigate this effect, selective attrition was less of a concern here.20 All participants, even those who died during follow-up, were classified as having or not having dementia at the time of their deaths. It is possible that competing events could have muted observed effects if air pollution exposure was related to the cause of death, a potential bias of particular concern in deaths related to cardiovascular disease. Finally, our study may not be generalizable to other study populations. Although special efforts were made to recruit minorities into the cohort,21 only 3% non-Whites were enrolled, and participants were relatively well educated.
5 |. CONCLUSION
Although many high-income countries have successfully reduced pollutant concentrations, these reductions are not uniform, and increased concentrations continue to afflict many communities as well as many rapidly industrializing countries. Given the apparent sustained impact of pollutant exposures on dementia risk decades later, air pollution exposure reductions may be urgently needed to reduce the burden of dementia risk in older adults for years to come.
Supplementary Material
HIGHLIGHTS.
There is an urgent need to identify modifiable risk factors for dementia.
Twenty-year average air pollution is linked to dementia risk in older adults.
Air pollutant effects likely precede dementia onset by decades.
RESEARCH IN CONTEXT.
1. Systematic review:
We searched PubMed for human studies on air pollution and dementia through July 2021. Most previous studies have relied on administrative records to assess dementia cases, which is efficient but lacks accuracy in diagnosis, dementia subtype classification, and timing of disease onset. In addition, most prior work has evaluated the impact of recent air pollution exposure on risk without characterizing long-term residential mobility, and as a result could not accurately examine exposures during the time of most interest, when the underlying neurodegenerative disease processes occur.
2. Interpretation:
We detected strong associations between long-term air pollutant exposure and incident dementia, provide compelling evidence for pollutant effects on dementia with an underlying vascular etiology, and demonstrate the importance of exposure in middle age on the development of dementia in later life.
3. Future directions:
Future research should investigate the biological mechanisms underpinning the associations to inform potential targets for interventions.
ACKNOWLEDGEMENTS
We are grateful to the study volunteers who made this work possible. This research was supported by the National Institute on Aging (NIA RF1AG057033); the National Center for Complementary and Integrative Health [(NCCIH) previously the National Center for Complementary and Alternative Medicine (NCCAM)], and the Office of Dietary Supplements (U01 AT000162); National Heart, Lung, and Blood Institute (NHLBI); the University of Pittsburgh Alzheimer’s Disease Research Center (P50AG05133); the Roena Kulynych Center for Memory and Cognition Research; the National Institute of Neurological Disorders and Stroke; the National Institute of General Medical Sciences (NIGMS, P20GM130418); the National Institute of Environmental Health Sciences (NIEHS, 5T32ES015459–08); and the US Environmental Protection Agency (US EPA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, the National Institutes of Health, or the US EPA. The funders of this study had no role in data collection, analysis, interpretation, presentation of study findings, or the decision to submit the article for publication.
DECLARATION OF INTERESTS
Funding to support the effort for this submission for Drs. Semmens, Fitzpatrick, Adam, Hajat, and Kaufman and Ms. Leary and Ms. Park was provided to the authors’ respective institutions by the NIA (RF1AG057033). Support for Dr. Ilango’s effort was provided by the University of Washington Biostatistics, Epidemiologic, and Bioinformatic Training in Environmental Health grant (NIEHS, 5T32ES015459) to her institution. In addition to the funding support listed above, Dr. Semmens received support from the National Institutes of Health Office of the Director Environmental Influences on Child Health Outcomes IDeA States Pediatric Clinical Trials Network (8UG1OD024952), the National Institute for Occupational Safety and Health (5R21OH011385), and grants from the NIGMS American Indian-Alaska Native Clinical and Translational Research Program. Dr. Fitzpatrick received support from the National Institutes of Health (NIH D43 TW011596, U19 AG057377, U01HL130114, NHLBI-HC-9515, R01AG058969, R01AG059727) and US Centers for Disease Control and Prevention (BAA75D301_20_R-68024, CDC SIP02). Dr. Hajat received support from the NIA (R01AG060011) and the NIEHS (R00 ES023498). Dr. Kaufman received support from several NIH grants, one from the US EPA, one subcontract on a grant from the US Department of Defense to Michigan State University, and one subcontract on a grant from the Global Alliance for Clean Cookstoves (UN Foundation) to the University of California, Berkeley. Except where noted, all of the above were paid to the authors’ respective institutions.
Dr. DeKosky received royalty payments from UpToDate for Dementia. Dr. Lopez received consulting fees from Grifols and Biogen. Dr. Hajat received consulting fees from the Pew Charitable Trusts and honoraria for NIH grant review. Dr. DeKosky received payment for expert testimony from Blood, Hurst, & O’Reardon, LLP. Travel support was provided to Dr. Semmens, Ms. Leary, and Dr. Adam from NIH grants to respective author institutions. Dr. DeKosky received payments for serving on the medical advisory boards or data safety and monitoring boards for Acumen Pharmaceuticals, Biogen, Cognition Therapeutics, Prevail Pharmaceuticals, and Vaccinex. Dr. Kaufman participated on an external advisory board to NIH-funded trial: Household Air Pollution Intervention Network. Dr. Adam served as an unpaid member on the Society for Epidemiologic Research Student & Post-Doc Committee Board, and Dr. Kaufman served as an unpaid Executive Council Member of the International Society for Epidemiology. Dr. DeKosky holds stock options for Acumen Pharmaceuticals. Dr. DeKosky received payment for serving as an Associate Editor for Neurotherapeutics. No other disclosures were reported.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 2020;396:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahoney R, Regan C, Katona C, Livingston G. Anxiety and depression in family caregivers of people with Alzheimer disease: the LASER-AD study. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2005;13(9):795–801. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. 10.1016/s0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oudin A, Forsberg B, Adolfsson AN, et al. Traffic-Related air pollution and dementia incidence in Northern Sweden: a longitudinal study. Environ Health Perspect. 2016;124(3):306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Kwong JC, Copes R, et al. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int. 2017;108:271–277. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Kwong JC, Copes R, et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet. 2017;389(10070):718–726. [DOI] [PubMed] [Google Scholar]
- 7.Shi L, et al. Long-term effects of PM2·5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grande G, Ljungman PLS, Eneroth K, Bellander T, Rizzuto D. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol. 2020;77(7):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cacciottolo M, Wang X, Driscoll I, et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7(1):e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey IM, Anderson HR, Atkinson RW, et al. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open. 2018;8(9):e022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerza F, Renzi M, Gariazzo C, et al. Long-term exposure to air pollution and hospitalization for dementia in the Rome longitudinal study. Environ Health. 2019;18(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smargiassi A, Sidi EAL, Robert L-E, et al. Exposure to ambient air pollutants and the onset of dementia in Quebec. Canada Environ Res. 2020;190:109870. [DOI] [PubMed] [Google Scholar]
- 13.Weuve J, Proust-Lima C, Power MC, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11(9):1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 2016;56:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weuve J, Bennett EE, Ranker L, et al. Exposure to air pollution in relation to risk of dementia and related outcomes: an updated systematic review of the epidemiological literature. Environ Health Perspect. 2021;129(9):096001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2020 Alzheimer’s disease facts and figures. Alzheimers Dement, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulick ER, Elkind MSV, Boehme AK, et al. Long-term exposure to ambient air pollution, APOE-epsilon4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ Int. 2020;136:105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekosky ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27(3):238–253. [DOI] [PubMed] [Google Scholar]
- 20.Dekosky ST. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2008;300(19):2253–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick AL, Fried LP, Williamson J, et al. Recruitment of the elderly into a pharmacologic prevention trial: the Ginkgo Evaluation of Memory Study experience. Contemporary clinical trials. 2006;27(6):541–553. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11): 2412.2–2412-a. [DOI] [PubMed] [Google Scholar]
- 24.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 25.Mohs RC. The Alzheimer’s disease assessment scale. Int Psychogeriatr. 1996;8(2):195–203. [DOI] [PubMed] [Google Scholar]
- 26.Hurley S, et al. Tracing a path to the past: exploring the use of commercial credit reporting data to construct residential histories for epidemiologic studies of environmental exposures. Am J Epidemiol. 2017;185(3):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S-Y, Olives C, Sheppard L, et al. Historical prediction modeling approach for estimating long-term concentrations of PM2.5 in cohort studies before the 1999 implementation of widespread monitoring. Environ Health Perspect. 2017;125(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young MT, Bechle MJ, Sampson PD, et al. Satellite-Based NO2 and model validation in a national prediction model based on Universal Kriging and land-use regression. Environ Sci Technol. 2016;50(7):3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajat A, Diez-Roux AV, et al. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect. 2013;121(11–12):1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):1–67. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 32.Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air Pollution and Dementia: a Systematic Review. J Alzheimers Dis. 2019;70(s1):S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortamais M, Gutierrez LA, De Hoogh K, et al. Long-term exposure to ambient air pollution and risk of dementia: results of the prospective Three-City Study. Environ Int. 2021;148:106376. [DOI] [PubMed] [Google Scholar]
- 34.Shi L, Wu X, Danesh Yazdi M, et al. Long-term effects of PM2.5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health. 2020:e557–e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaffer RM, Ge Li, Adar SD, et al. Fine particulate matter and markers of Alzheimer’s Disease neuropathology at autopsy in a community-based cohort. J Alzheimers Dis. 2021;79(4):1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(17):2054–2070. [DOI] [PubMed] [Google Scholar]
- 37.de Roos A, van der Grond J, Mitchell G, Westenberg J. Magnetic resonance imaging of cardiovascular function and the brain: is dementia a cardiovascular-driven disease? Circulation. 2017;135(22):2178–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33(5):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84(1):10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ran J, Schooling CM, Han L, et al. Long-term exposure to fine particulate matter and dementia incidence: a cohort study in Hong Kong. Environ Pollut. 2021;271:116303. [DOI] [PubMed] [Google Scholar]
- 41.Lee M, Schwartz J, Wang Y, Dominici F, Zanobetti A. Long-term effect of fine particulate matter on hospitalization with dementia. Environ Pollut. 2019;254(Pt A):112926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C-Yi, Li CH, Martini S, Hou WH. Association between air pollution and risk of vascular dementia: a multipollutant analysis in Taiwan. Environ Int. 2019;133(Pt B):105233. [DOI] [PubMed] [Google Scholar]
- 43.Delgado-Saborit JM, Guercio V, Gowers AM, Shaddick G, Fox NC, Love S. A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci Total Environ. 2021;757:143734. [DOI] [PubMed] [Google Scholar]
- 44.Weuve J Invited commentary: how exposure to air pollution may shape dementia risk, and what epidemiology can say about it. Am J Epidemiol. 2014;180(4):367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snitz BE. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302(24):2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haneuse S, Schildcrout J, Crane P, et al. Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology. 2009;32(3):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.