Abstract
Introduction
L-DOS47, a targeted urease–anti-CEACAM6 immunoconjugate, alters the acidity of the tumor microenvironment by increasing local ammonia production. In vitro, the cytotoxic effects of L-DOS47 were additive when combined with pemetrexed and carboplatin.
Methods
This phase I, open-label, dose-escalation study evaluated the safety and tolerability of up to four cycles of L-DOS47 (administered on days 1, 8, and 15 of each cycle at doses ranging from 0.59 to 9.0 μg/kg) combined with pemetrexed and carboplatin in patients with stage IV nonsquamous NSCLC. Continued L-DOS47 treatment after the fourth cycle was allowed at the treating physicians’ discretion.
Results
A total of 14 patients received at least one dose of L-DOS47. Overall, L-DOS47 was well tolerated. Grade greater than or equal to 3 adverse events (AEs) were typically neutropenia related. Two grade greater than or equal to 3 AEs and no serious AEs were considered at least possibly related to L-DOS47. No dose-limiting toxicities were reported, so the maximum tolerated dose was not reached. The objective response rate was 41.7% with a median duration of response of 187 days. Clinical benefit was observed in 75.0% of the patients. After the first dose, L-DOS47 systemic exposure increased in a generally dose-proportional manner but decreased substantially with repeat dosing. Anti–L-DOS47 antibodies were detectable in 13 of 14 patients by cycle 2 with titers typically increasing with continued treatment. There was an apparent association between best overall response rate and highest anti–L-DOS47 antibody titer measured.
Conclusions
L-DOS47 combined with standard pemetrexed and carboplatin chemotherapy is well tolerated in patients with recurrent or metastatic nonsquamous NSCLC at doses up to 9.0 μg/kg.
Keywords: Non–small cell lung cancer, L-DOS47, Immunotherapy, Pemetrexed, Carboplatin
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1 The outlook is particularly challenging for those with advanced stage IV NSCLC without driver alterations or who have exhausted targeted therapy options; for such patients, platinum-based doublet chemotherapy is recommended, often in combination with a PD-L1 inhibitor, as clinically indicated.2,3
Manipulation of the tumor microenvironment (TME) has the potential to improve treatment options for advanced NSCLC. Solid tumors exist in an hypoxic environment owing to inadequate vascularization and altered glycolysis metabolism, resulting in accumulation of lactic acid in the TME.4 Cancer cells maintain a glycolytic phenotype even in the presence of oxygen (the so-called Warburg effect), causing further acidification of the TME.5 The hypoxic environment and accumulation of lactate contribute to tumor plasticity and heterogeneity and facilitate immune evasion, extracellular matrix remodeling, angiogenesis, and metastasis.4,5
L-DOS47, a targeted therapeutic immunoconjugate, consists of an anti-CEACAM6 camelid single-chain monoclonal antibody conjugated to jack bean urease. L-DOS47 binds to its cell surface ligand CEACAM6, which is overexpressed in a range of cancers,6 whereas the urease enzyme converts urea, an abundant natural metabolite, into ammonia, which results in an increase in TME pH. The combined effect of ammonia toxicity and pH increase is cytotoxic to cancer cells.7 L-DOS47 has been found to be safe and well tolerated at doses up to 13.55 μg/kg in a previous phase 1 clinical trial.
In in vitro studies, the cytotoxic effects of L-DOS47 against the A549 human lung adenocarcinoma cell line were additive when combined with pemetrexed and carboplatin.8 This study was designed to identify the maximum tolerated dose (MTD) of L-DOS47 in combination with pemetrexed and carboplatin as a treatment for NSCLC.
Materials and Methods
This was a phase 1, open-label, dose-escalation study to evaluate the safety and tolerability of escalating doses of L-DOS47 in combination with standard doublet chemotherapy of pemetrexed (500 mg/m2) plus carboplatin (area under the curve [AUC] 6 mg/mL) in patients with stage IV (TNM M1a and M1b) nonsquamous NSCLC. The study used a standard “3 + 3” design for cohorts 1, 2, 6, and 7 and an accelerated “1 + 2” design for cohorts 3 to 5.9 The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines, along with applicable local regulatory requirements and laws. The study protocol and patient information documents were approved by the institutional review board or independent ethics committee at each study center. All patients provided written, informed consent.
Patients
Adults (at least 18 y old) with histologically or cytologically confirmed stage IV (TNM M1a or M1b) nonsquamous NSCLC who were either chemotherapy naive or experiencing a recurrence after previous surgery, radiation, with or without adjuvant chemotherapy, and for whom pemetrexed plus carboplatin would be an appropriate therapy were eligible to participate. Patients also had to have at least one site of measurable disease per Response Evaluation Criteria in Solid Tumors version 1.1, an Eastern Cooperative Oncology Group performance status of 0 or 1, a life expectancy of at least 3 months, adequate bone marrow, cardiac, renal, and liver functions, and to test negative for human immunodeficiency virus and hepatitis B and C. Patients with an uncontrolled serious medical condition or mental illness or with peripheral neuropathy were excluded from participation, as were those with recurrent disease within 6 months of completing previous adjuvant chemotherapy. Patients with treated and stable (≥1 mo) brain metastases per magnetic resonance imaging had to be off steroids and not taking antiepileptics.
Study Design
Study participants received up to four cycles of L-DOS47 (on days 1, 8, and 15 of each cycle) in combination with pemetrexed and carboplatin (on day 1 of each cycle). Patients whose disease had not progressed by the end of the fourth cycle and who had experienced no unacceptable toxicities had the opportunity to receive continued L-DOS47 on the same schedule until there was no longer a clinical benefit. Patients who were unable to complete the four cycles owing to pemetrexed and carboplatin-related toxicity had the opportunity to continue receiving L-DOS47 after discontinuation of pemetrexed and carboplatin while there remained clinical benefit without unacceptable toxicity.
End Points
The primary end point was treatment-emergent adverse events (TEAEs), with severity graded according to the National Institutes of Health Common Terminology Criteria for Adverse Events version 4.0. Adverse events, performance status, vital signs, and clinical laboratory parameters were assessed regularly throughout the study (see Supplementary Table 1 for the schedule). Other end point included tumor response, assessed according to Response Evaluation Criteria in Solid Tumors version 1.1 at baseline, the start of each of the first four treatment cycles and every other cycle thereafter, and 7 days after the end of cycle 4. The objective response rate (ORR) and proportion of patients achieving a complete response (CR), partial response (PR), or stable disease (SDi) were derived from these measures. For patients to be assessable for SDi, the duration of SDi had to be at least 42 days from the first dose of study treatment. Exploratory evaluations included L-DOS47 pharmacokinetics, anti–L-DOS47 antibody titer, and plasma CEACAM6 levels. Venous blood samples for pharmacokinetic analysis and for anti–L-DOS47 antibody testing were drawn on days 1 and 8 of cycles 1 to 4. Anti–L-DOS47 testing was also performed at the start of each subsequent cycle, 7 days after the end of cycle 4, and in cases of early termination. Assessment of CEACAM6 levels was performed on day 1 of cycle 2 and day 8 of cycle 4. Further details of the timing of assessments and assay methods are provided in Supplementary Table 1.
L-DOS47 Dose Selection
L-DOS47 was administered by a 30-minute intravenous infusion on days 1, 8, and 15 of each 21-day treatment cycle at planned doses ranging from 0.59 to 12.0 μg/kg (Table 1). Body weight was determined on day 1 of each cycle and used to calculate dose for that cycle. All patients in each dose-level cohort had to complete cycle 1 before dosing of the next cohort could proceed, and intracohort patient dosing occurred at least 24 hours apart to allow for assessment of possible infusion-related or allergic reactions. The dose level for the next cohort was determined by the Safety Review Committee after a review of the available safety data, and dose escalation was based on evaluation of dose-limiting toxicities (DLTs).
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristics | Overall |
|---|---|
| Number of patients, N (%) | 14 (100.0) |
| Mean (SD) age, (y) | 63.5 (7.4) |
| Female, n (%) | 7 (50.0) |
| Race, n (%) | |
| White | 11 (78.6) |
| Black or African American | 2 (14.3) |
| Asian | 1 (7.1) |
| ECOG performance status, n (%) | |
| 1 | 10 (71.4) |
| 0 | 4 (28.6) |
| Histopathology, n (%) | |
| Adenocarcinoma, NOS | 9 (64.3) |
| Nonsmall cell carcinoma | 5 (35.7) |
| Median (range) duration of disease, (mo) a | 8.9 (13.4) |
| Reason for discontinuation of study drug, n (%) | |
| Death | 2 (14.3) |
| Disease progression | 7 (50.0) |
| Unacceptable toxicityb | 2 (14.3) |
| Investigator decision | 1 (7.1) |
| Other | 2 (14.3) |
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified.
Duration of disease is calculated from the date of first diagnosis to the date of informed consent.
One patient was taken off-study treatment owing to unacceptable toxicity (superior vena cava syndrome), which was assessed as not related to the study treatment but related to the disease under study.
Further information, including definitions for DLTs, is provided in the Supplementary Data. Within each cohort, dose delays and modifications were proscribed for subsequent treatment cycles if relevant safety criteria were met (Supplementary Tables 2-6). Patient concomitant medications are provided in Supplementary Table 7. The MTD was defined as the highest dose level at which less than or equal to one of six patients experienced a DLT during the first treatment cycle. Once the MTD for L-DOS47 was defined, a confirmation cohort would be enrolled at that dose to assess its appropriateness as the dose for evaluation in a subsequent phase 2 study.
Statistical Analysis
The study was designed with the intention of enrolling up to 37 patients.
The intent-to-treat (ITT) study population was defined as all patients enrolled in the study regardless of whether they met the eligibility criteria or received the study treatment. The safety population consisted of those patients in the ITT population who received any L-DOS47 or pemetrexed premedication. The efficacy population was those patients in the safety population with baseline measurable disease who completed a minimum of two cycles of study therapy and had at least one postbaseline tumor assessment. Patients in the safety population who completed less than two cycles of study therapy but had clear evidence of clinical progression were also included. End points were analyzed descriptively using counts and percentages for binary or categorical variables and mean, SD, median, quartiles, minimum, and maximum for quantitative variables. Time-to-event data were analyzed using the method of Kaplan-Meier.
Results
Study Population
A total of 14 patients were enrolled between April 2015 and July 2019 and were included in the ITT and safety study populations. Of these, 12 patients met the criteria for inclusion in the efficacy population. The mean (SD) age was 63.5 (7.42) years, 50% were female, and most were white (Table 1). Previous cancer therapies consisted of chemotherapy (three patients), immunotherapy (three patients), radiotherapy (two patients), and surgery (13 patients). The study was terminated in August 2019 for slow recruitment owing to immunotherapy becoming part of standard of care, at which time all patients had discontinued study treatment (Table 1).
Duration of Exposure
Patients were exposed to a median of four cycles of the study drug over a median of 78 days (Table 2). Six patients continued to receive L-DOS47 beyond the end of the four protocol-mandated cycles for up to 19 treatment cycles. There was no apparent relationship between dose level and time on study drug.
Table 2.
Overall Summary of TEAEs, by Cohort
| Treatment Emergent Adverse Events | L-DOS47 Dose Cohort (μg/kg) + Pemetrexed and Carboplatin |
Overall (N = 14) | |||
|---|---|---|---|---|---|
| 0.59 (n = 3) | 0.78 (n = 6) | 1.5/3.0/6.0a (n = 3) | 9.0 (n = 2) | ||
| Duration of exposure, d, median (range) | 2 (1–8) | 8 (2–19) | 2 (2–6) | 5 (2–8) | 4 (1–19) |
| Number of cycles, median (range) | 36 (15–177) | 165 (30–398) | 57 (50–127) | 102.5 (43–162) | 78 (15–398) |
| Percentage compliance, median (range) | 99.0 (95.2–100.0) | 97.3 (83.2–101.8) | 100.1 (100.0–100.1) | 89.5 (83.3–95.7) | 98.4 (83.2–101.8) |
| Number of patients with ≥1 TEAE, n (%) | 3 (100) | 6 (100) | 3 (100) | 2 (100) | 14 (100) |
| Number of patients with ≥1 L-DOS47–related TEAE,a n (%) | 2 (66.7) | 5 (83.3) | 0 | 0 | 7 (50.0) |
| Number of patients with ≥1 TEAE, n (%) | 3 (100) | 5 (83.3) | 3 (100) | 1 (50.0) | 12 (85.7) |
| Number of patients with ≥1 grade ≥ 3 L-DOS47–related TEAE,b n (%) | 0 | 2 (33.3) | 0 | 0 | 2 (14.3) |
| Number of patients with ≥1 serious TEAE | 0 | 3 (50.0%) | 2 (66.7%) | 1 (50.0%) | 6 (42.9%) |
| Number of patients with ≥1 serious L-DOS47–related TEAEb | 0 | 0 | 0 | 0 | 0 |
| Number of patients who died due to a TEAE | 0 | 0 | 0 | 0 | 0 |
| Number of patients who discontinued any study drug owing to TEAEs, n (%) | 2 (66.7) | 0 | 1 (33.3) | 0 | 3 (21.4) |
| Number of patients who discontinued L-DOS47 owing to ≥1 related TEAE,a n (%) | 1 (33.3) | 0 | 0 | 0 | 1 (7.1) |
Note: TEAEs were defined as all AEs that occurred from the first dose until 30 days after the last dose of study medication. Event severity was graded using the National Institutes of Health Common Terminology Criteria for Adverse Events version 4.0.
AE, adverse event; TEAE, treatment-emergent adverse event.
Dose levels grouped for convenience of reviewing.
Considered to be at least possibly related to L-DOS47 administration or definitely related to carboplatin or pemetrexed administration.
Safety
All 14 patients in the safety population experienced at least one TEAE, and 12 (85.7%) experienced at least one event that was grade greater than or equal to 3 in severity (Table 2). The grade greater than or equal to 3 TEAEs observed in more than or equal to 20% of patients were neutrophil count decreased (four patients, 28.6%), white blood cell count decreased (three patients, 21.4%), and neutropenia (three patients, 21.4%). The most frequent TEAEs considered at least possibly related to L-DOS47 were pyrexia, rash, and constipation (two patients each; 14.3%). Two patients experienced grade greater than or equal to 3 events considered at least possibly related to L-DOS47: one with neutropenia and one with hypertension (Supplementary Tables 8–10). Six patients (42.9%) experienced a serious TEAE, none of which were considered at least possibly related to L-DOS47, and no patient died owing to a TEAE.
Three patients had TEAEs leading to discontinuation (one case of pneumonitis, considered possibly related to L-DOS47, one case each of superior vena cava syndrome and neutropenia, both considered unrelated to L-DOS47). There were no notable trends observed in clinical and laboratory parameters, vital signs, and electrocardiogram or physical examination findings.
No DLTs were reported during the study.
Efficacy
Of the 12 patients with assessable efficacy data, five (41.7%) had a PR, four (33.3%) had SDi as their best response to therapy, and three (25.0%) had progressive disease (Fig. 1). The ORR (CR + PR) was 41.7% (95% confidence interval [CI]: 15.2%–72.3%) with a median duration of response of 187 days (95% CI: 92–337 d). Clinical benefit (CR + PR + SDi) was observed in 75.0% (95% CI: 42.8%–94.5%) of patients with a median duration of 141 days (95% CI: 54–337 d) (Supplementary Table 11). There was no apparent relationship between L-DOS47 dose level and overall response. Of the five patients with PR, four were chemotherapy naive and one had two previous lines of chemotherapy; two of the patients with SDi were chemotherapy naive, another one had previous treatment with pembrolizumab, and a fourth had one previous line of chemotherapy in combination with pembrolizumab. The three remaining patients who experienced progressive disease were all chemotherapy naive. Two patients did not meet the efficacy assessable criteria owing to posttreatment radiologic assessment being less than 42 days from the start of the study treatment.
Figure 1.
Best percentage change in lesion size from baseline. Individual results for the 12 patients in the efficacy-assessable population are presented, including best overall response assessed using the RECIST v1.1. RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Pharmacokinetics
Overall, systemic L-DOS47 exposure (maximum observed plasma concentration after dosing, or Cmax, and the area under the plasma concentration versus time curve from the start of dose administration to the time after dosing at which the last quantifiable concentration was observed, or AUCtlast) increased with increasing dose on cycle 1 day 1 (Supplementary Table 12). Exposure was dose proportional except between 0.78 and 1.5 μg/kg, where the increase was greater than dose proportional. Although the low number of patients with quantifiable data for subsequent cycles made interpretation challenging, AUCtlast decreased substantially for most patients with repeat dosing, with individual accumulation ratios ranging from 0.00765 to 0.987. Individual T1/2 ranged from 6.59 to 9.21 hours, and Tmax occurred between 0.5 and 1.0 hours postdose for all dose levels and treatment cycles evaluated (Supplementary Table 12).
Immunogenicity
Anti–L-DOS47 antibodies were undetectable in all patients before the first dosing and were detectable in 13 of 14 patients (92.9%) by the start of cycle 2. There was no apparent relationship between L-DOS47 dose level or specific batch of L-DOS47 drug product and antibody titer. Antidrug antibody (ADA) titer typically increased over time, although in four of the six patients who continued on-study beyond four cycles, the titer dropped just before the patient went off-study or at the follow-up visit. ADA levels were not followed long enough after the end of the study to determine whether this drop was sustained, however, as the patients who went off-study did so due to disease progression or death. Interestingly, an apparent association was observed between best overall response (BORR) and highest ADA titer measured (Fig. 2). None of the patients whose ADA levels were in the lowest quartile were able to complete four treatment cycles, and patients who made the strongest responses typically also exhibited the highest ADA titers. It should be noted that the ADA assay measured total binding antibodies to L-DOS47 but was not specific for neutralizing antibodies (Supplementary Data).
Figure 2.
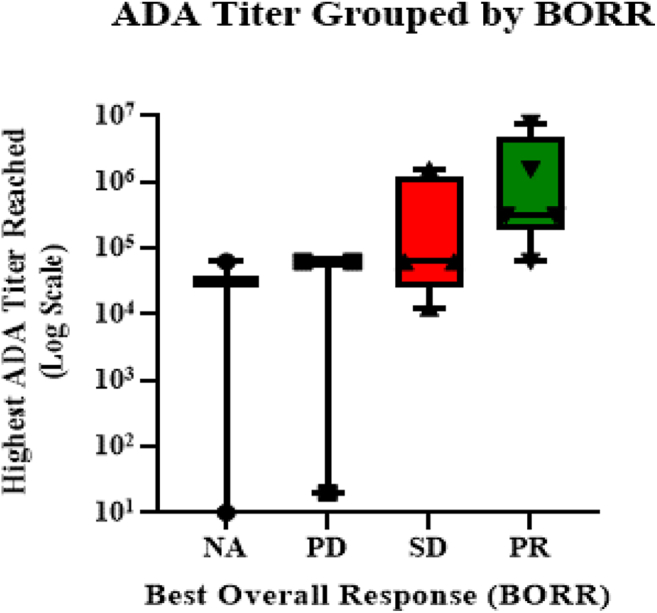
Association was observed between BORR and highest ADA titer measured. The line and box represent the median (interquartile range) ADA titer, and the whiskers represent the minimum and maximum titer values per BORR. ADA, antidrug antibody; BORR, best overall response; NA, not assessable; PD, progressive disease; PR, partial response; SDi, stable disease.
Tumor Antigen (CEACAM6)
Samples for assessment of circulating levels of the tumor antigen and ligand for L-DOS47, CEACAM6, were collected on cycle 2 day 1 and on cycle 4 day 8 or the end of the treatment visit (whichever occurred sooner) for patients in all dosing cohorts. Plasma levels of CEACAM6 were below the limit of assay quantitation (20 ng/mL) for all samples collected.
Discussion
L-DOS47 is a novel antibody-urease immunoconjugate designed to increase local ammonia production in CEACAM6-expressing solid tumors, applying both a direct cytotoxic effect and constraining the normally acidic TME. In this study, repeat doses of L-DOS47 ranging from 0.59 to 9.0 μg/kg, administered to patients with recurrent or metastatic nonsquamous NSCLC in combination with the standard doublet chemotherapy of pemetrexed plus carboplatin, were well tolerated with no DLTs reported. Most L-DOS47–related TEAEs reported were grade less than or equal to 2 and manageable with supportive care or dose interruptions, consistent with the results from a previous phase 1/2 trial as monotherapy in a similar patient population.
The ORR and clinical benefit rates reported here (41.7% and 75%, respectively) are encouraging. Previously, ORRs in the range of 20% to 51% have been reported for pemetrexed plus carboplatin alone in similar patient populations.10, 11, 12, 13, 14, 15 In patients with previously untreated metastatic nonsquamous NSCLC, the addition of pembrolizumab to pemetrexed-platinum chemotherapy increases overall efficacy.12
A robust, nondose-dependent immunogenic response to L-DOS47 was detected in most patients by the start of cycle 2, with antibody titers typically increasing as treatment progressed. This is consistent with the results of the previous phase 1/2 monotherapy study and is also expected given the structure of L-DOS47, as there is no human endogenous counterpart to urease and while single-chain antibodies may not be highly immunogenic individually, the immune response could be increased in this case owing to the presence of multiple antibody copies per L-DOS47 molecule. The apparent correlation between ORR and ADA titer was unexpected, and the significance is unclear. In previous in vitro studies, L-DOS47 reduced PD-L1 expression on acidified MDA-MB-231 breast cancer cells and reduced programmed cell death protein 1 levels on acidified primary T cells.16 Taken together, these findings suggest that L-DOS47 may also contribute to the ability of the patient’s immune system to respond to the cancer and suggests an intriguing potential avenue of research for L-DOS47 in combination with checkpoint inhibitors.
The study has some limitations. It was not designed or powered to determine efficacy, though the activity found is encouraging. Owing to slow patient enrollment, recruitment was stopped during dosing cohort level 9 μg/kg, and therefore, MTD was not reached. This is consistent with the results from a previous phase 1 trial of L-DOS47 monotherapy in which MTD was not reached at the maximum dose tested (13.55 μg/mL).17 In addition, although the patients were monitored for infusion-related hypersensitivity reactions, because L-DOS47 was administered after pemetrexed and carboplatin on day 1 of each cycle, it was challenging to attribute causality for any reactions observed. Capture of any potential delayed hypersensitivity reactions was also limited to standard monitoring of adverse events. Furthermore, premedication with oral dexamethasone from the day before until the day after each pemetrexed dose may have minimized infusion reactions. It should be noted, however, that because L-DOS47 was administered alone on days 8 and 15 of each treatment cycle, and on all days for cycle 5 onward, a more granular determination of its potential to cause such reactions could be made and no AE associated with hypersensitivity reactions was judged related to L-DOS47. Analysis of biomarkers was limited to circulating CEACAM6 levels. CEACAM6 overexpression is generally associated with worse OS and DFS in a variety of solid tumors,18 and serum CEACAM6 has been reported as a potential biomarker in pancreatic adenocarcinoma and plasma cell disorders.19, 20, 21 In addition, Cao et al.22 evaluated differentially expressed genes including CEACAM6 in peripheral blood exosomes as potential biomarkers for lung cancer; although the study was not quantitative, CEACAM6 exosome expression was higher in lung adenocarcinoma than in squamous cell carcinoma. In the L-DOS47 monotherapy study, although plasma CEACAM6 levels varied between less than the lower limit of quantitation (LLOQ, 20 ng/mL) to 276 ng/mL with no apparent relationship between concentration and ORR or number of treatment cycles received, in the current study, plasma CEACAM6 level was below LLOQ in all patient samples.17 Although these seem to be the first reports of plasma CEACAM6 in patients with NSCLC, its potential role as a prognostic biomarker is unclear.
Although the use of a binding ADA assay rather than both a binding and a neutralizing ADA assay is typical for this stage of clinical development, given the intriguing apparent association between ADA titer and BORR, future efforts will focus on development and implementation of a neutralizing antibody assay.
This multiple ascending dose study provides preliminary evidence that L-DOS47 administered in combination with standard pemetrexed and carboplatin doublet chemotherapy is well tolerated with encouraging antitumor activity in patients with recurrent or metastatic nonsquamous NSCLC at doses up to 9.0 μg/kg.
CRediT Authorship Contribution Statement
Sarina Piha-Paul: Investigation, Methodology, Writing—review and editing.
Chandra Belani: Investigation, Methodology, Writing—review and editing.
George Simon: Investigation, Writing—review and editing.
Heman Chao: Conceptualization, Methodology, Writing—review and editing.
Brenda Lee: Formal analysis, Project administration, Visualization, Writing—review and editing.
Kim Gaspar: Formal analysis, Sponsor QA for bioanalytical, Visualization, Writing—review and editing.
Afshin Dowlati: Investigation, Methodology, Writing—review and editing.
Acknowledgments
This study was sponsored by Helix BioPharma Corp. The authors thank all the participating patients and their families and the network of investigators, research nurses, study coordinators, and operation staff. Medical writing and editorial assistance were provided by John Howell, PhD (Toronto, Ontario, Canada), supported by Helix BioPharma Corp. All authors contributed to the interpretation of the results, development of the article, and approval of the final version.
Footnotes
Disclosure: Dr. Piha-Paul reports receiving grants from the National Cancer Institute/National Institutes of HealthP30CA016672—Core Grant (CCSG Shared Resources); receiving other fees from AbbVie, Inc., ABM Therapeutics, Inc., Acepodia, Inc., Alkermes, Aminex Therapeutics, Amphivena Therapeutics, Inc., BioMarin Pharmaceutical, Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Cerulean Pharma, Inc., Chugai Pharmaceutical Co., Ltd., Curis, Inc., Cyclacel Pharmaceuticals, Daiichi Sankyo, Inc., Eli Lilly, ENB Therapeutics, Five Prime Therapeutics, F-Star Beta Limited, F-Star Therapeutics, Limited, Gene Quantum, Genmab A/S, Gilead Sciences, Inc., GlaxoSmithKline, Helix BioPharma Corp., HiberCell, Inc., Incyte Corp., Jacobio Pharmaceuticals Co., Ltd., Lytix Biopharma AS, Medimmune, LLC, Medivation, Inc., Merck Sharp & Dohme Corp., Novartis Pharmaceuticals, Pieris Pharmaceuticals, Inc., Pfizer, Phanes Therapeutics, Principia Biopharma, Inc., Puma Biotechnology, Inc., Purinomia Biotech, Inc., Rapt Therapeutics, Inc., Seattle Genetics, Silverback Therapeutics, Synlogic Therapeutics, Taiho Oncology, Tesaro, Inc., TransThera Bio., and ZielBio, Inc., outside of the submitted work; and having worked as a consultant for CRC Oncology. Dr. Chao was formerly a paid employee, was formerly a paid consultant, and owns stock options from Helix BioPharma Corp. Dr. Gaspar and Ms. Lee are paid employees of Helix BioPharma Corp. Dr. Dowlati reports receiving other fees from Helix BioPharma Corp., during the conduct of the study; and personal fees from AstraZeneca, Ipsen, and Merck, outside of the submitted work. Drs. Simon and Belani declare no conflict of interest.
Cite this article as: Piha-Paul S, Simon G, Belani CP, et al. A phase 1, open-label, dose-escalation study of L-DOS47 in combination with pemetrexed/carboplatin in patients with stage IV recurrent or metastatic nonsquamous NSCLC. JTO Clin Res Rep. 2022;3:100408.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100408.
Supplementary Data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N.H., Robinson A.G., Temin S., et al. Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39:1040–1091. doi: 10.1200/JCO.20.03570. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N.H., Schneider B.J., Temin S., et al. Therapy for stage IV non–small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38:1608–1632. doi: 10.1200/JCO.19.03022. [DOI] [PubMed] [Google Scholar]
- 4.Terry S., Faouzi Zaarour R., Hassan Venkatesh G., et al. Role of hypoxic stress in regulating tumor immunogenicity, resistance and plasticity. Int J Mol Sci. 2018;19:3044. doi: 10.3390/ijms19103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.San-Millán I., Brooks G.A. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2016;38:119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchemin N., Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013;32:643–671. doi: 10.1007/s10555-013-9444-6. [DOI] [PubMed] [Google Scholar]
- 7.Wong W.Y., DeLuca C.I., Tian B., et al. Urease-induced alkalinization of extracellular pH and its antitumor activity in human breast and lung cancers. J Exp Ther Oncol. 2005;5:93–99. [PubMed] [Google Scholar]
- 8.Helix BioPharma Corp. Data on file: combination treatment of carboplatin plus pemetrexed and L-DOS47 on A549 and HCC-1954 cells (study number: 30BC11402); 2014.
- 9.Le Tourneau C., Lee J.J., Siu L.L. Dose escalation methods in phase I cancer clinical trials. JNCI J Natl Cancer Inst. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gervais R., Robinet G., Clément-Duchêne C., et al. Pemetrexed and carboplatin, an active option in first-line treatment of elderly patients with advanced non-small cell lung cancer (NSCLC): a phase II trial. Lung Cancer. 2013;80:185–190. doi: 10.1016/j.lungcan.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Zukin M., Barrios C.H., Rodrigues Pereira J., et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non–small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013;31:2849–2853. doi: 10.1200/JCO.2012.48.1911. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Abreu D., Powell S.F., Hochmair M.J., et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Kanazawa K., Yokouchi H., Wang X., et al. Phase II trial of carboplatin and pemetrexed as first-line chemotherapy for non-squamous non-small cell lung cancer, and correlation between the efficacy/toxicity and genetic polymorphisms associated with pemetrexed metabolism: Hokkaido Lung Cancer Clinical Study Group Trial (HOT) 0902. Cancer Chemother Pharmacol. 2014;74:1149–1157. doi: 10.1007/s00280-014-2589-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y.H., Hirabayashi M., Togashi Y., et al. Phase II study of carboplatin and pemetrexed in advanced non-squamous, non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0902. Cancer Chemother Pharmacol. 2012;70:271–276. doi: 10.1007/s00280-012-1910-2. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues-Pereira J., Kim J.H., Magallanes M., et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:1907–1914. doi: 10.1097/JTO.0b013e318226b5fa. [DOI] [PubMed] [Google Scholar]
- 16.Wong W.Y., Tian B., Kumar P., et al. Abstract A144: urease-mediated alkalization of tumor microenvironment and its effects on T cell proliferation, cytokine release, and PD-1/PD-L1 interactions. Cancer Immunol Res. 2016;4 A144–A144. [Google Scholar]
- 17.Helix BioPharma Corp. Data on file: a phase I open-label, non-randomized dose escalation study of immunoconjugate L-DOS47 as a monotherapy in non-squamous non-small cell lung cancer patients (study number LDOS002); 2017.
- 18.Burgos M., Cavero-Redondo I., Alvarez-Bueno C., et al. Prognostic value of the immune target CEACAM6 in cancer: a meta-analysis. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359211072621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebauer F., Wicklein D., Horst J., et al. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5, and 6 as biomarkers in pancreatic cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurlinkus B., Ger M., Kaupinis A., Jasiunas E., Valius M., Sileikis A. CEACAM6’s role as a chemoresistance and prognostic biomarker for pancreatic cancer: a comparison of CEACAM6’s diagnostic and prognostic capabilities with those of CA19-9 and CEA. Life (Basel) 2021;11:542. doi: 10.3390/life11060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner N., Hajek R., Nachbaur D., et al. Levels of CEACAM6 in peripheral blood are elevated in patients with plasma cell disorders: a potential new diagnostic marker and a new therapeutic target? Dis Markers. 2019;2019 doi: 10.1155/2019/1806034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao B., Wang P., Gu L., Liu J. Use of four genes in exosomes as biomarkers for the identification of lung adenocarcinoma and lung squamous cell carcinoma. Oncol Lett. 2021;21:249. doi: 10.3892/ol.2021.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



