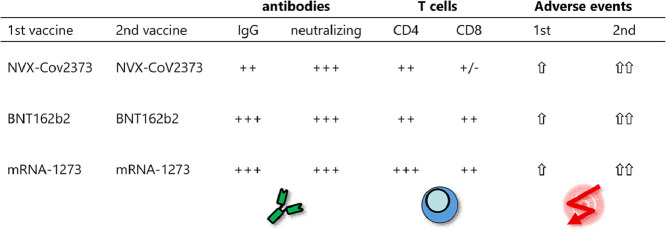
Abstract
Background
The NVX-CoV2373-vaccine has recently been licensed, although knowledge on vaccine-induced humoral and cellular immunity towards the parental strain and variants of concern (VOCs) in comparison to mRNA-regimens is limited.
Methods
In this observational study, 66 individuals were recruited to compare immunogenicity and reactogenicity of NVX-CoV2373 with BNT162b2 or mRNA-1273. Vaccine-induced antibodies were analyzed using ELISA and neutralization assays, specific CD4 and CD8 T-cells were characterized based on intracellular cytokine staining using flow-cytometry after antigen-specific stimulation with parental spike or VOCs.
Results
Two doses of NVX-CoV2373 strongly induced anti-spike IgG, although IgG-levels were lower than after vaccination with BNT162b2 or mRNA-1273 (p = 0.006). Regardless of the vaccine and despite different IgG-levels, neutralizing activity towards VOCs was highest for Delta, followed by BA.2 and BA.1. The protein-based vaccine failed to induce any spike-specific CD8 T-cells which were detectable in 3/22 (14%) individuals only. In contrast, spike-specific CD4 T-cells were induced in 18/22 (82%) individuals, although their levels were lower (p<0.001), had lower CTLA-4 expression (p<0.0001) and comprised less multifunctional cells co-expressing IFNγ, TNFα and IL-2 (p = 0.0007). Unlike neutralizing antibodies, NVX-CoV2373-induced CD4 T-cells equally recognized all tested VOCs from Alpha to Omicron. In individuals with a history of infection, one dose of NVX-CoV2373 had similar immunogenicity as two doses in non-infected individuals. The vaccine was overall well tolerated.
Conclusion
NVX-CoV2373 strongly induced spike-specific antibodies and CD4 T-cells, albeit at lower levels as mRNA-regimens. Cross-reactivity of CD4 T-cells towards the parental strain and all tested VOCs may hold promise to protect from severe disease.
Keywords: T-cells, Antibodies, Vaccination, SARS-CoV-2, COVID-19, Coronavirus, Neutralization, Variant of concern
Abbreviations: BNT, BNT162b2; CTLA-4, Cytotoxic T-lymphocyte associated protein 4; IQR, interquartile range; IFNγ, interferon γ; IL-2, interleukin 2; NVX, NVX-CoV2373; SEB, Staphylococcus aureus Enterotoxin B; TNFα, tumor necrosis factor α; VOC, variant of concern
Graphical abstract
1. Introduction
Among the COVID-19-vaccines, the NVX-CoV2373-vaccine was the first protein-based vaccine that was licensed as a homologous dual dose regimen in the European Union and in the United States [1], [2], [3]. NVX-CoV2373 is a prefusion-stabilized recombinant spike protein subunit vaccine with a saponin-based matrix M adjuvant that conferred a 89.7–90.4% protection towards parental SARS-CoV-2 infection, with a good safety profile and high efficacy against the B.1.1.7 variant [4,5]. The efficacy exceeded those of the vector-based vaccines and was in a similar range as the two mRNA-based vaccines BNT162b2 and mRNA-1273, which were shown to be 95.0% and 94.1% efficacious, respectively, at preventing severe COVID-19 illness from the parental viral strains [6,7].
The immunogenicity of the two mRNA-vaccines has been extensively characterized in real world settings, and both vaccines induce strong antibody and T-cell responses [8,9]. Likewise, the phase 1, 2 trial of the NVX-CoV2373-vaccine or a recent phase III trial has shown induction of high antibody titers [10,11], and first data on neutralizing activity towards SARS-CoV-2 variants of concern (VOCs) from participants of the pivotal trial 3 to 4 months after the second dose became available recently [12], [13], [14]. However, real world data on the immunogenicity of the NVX-CoV2373-vaccine during the induction phase, especially regarding its ability to induce CD4 and CD8 T-cells and in comparison to other licensed vaccines are currently lacking. Given the fact that the vaccine was licensed during the surge of the Delta and the Omicron wave with its subvariants that are known to escape neutralizing antibody activity, knowledge of cellular immunity and its reactivity towards VOCs is of particular importance to inform on the ability to confer protection from severe disease.
We therefore carried out an observational study on the immunogenicity and reactogenicity of the NVX-CoV2373-vaccine in immunocompetent individuals who were vaccinated when the vaccine became approved and recommended in Germany [2]. Apart from vaccine-related adverse events, we characterized the induction of spike-specific IgG as well as CD4 and CD8 T-cells including humoral and cellular reactivity towards the parental SARS-CoV-2 and variants of concern. Moreover, immune-responses after two doses of the vaccine were compared with respective data from matched cohorts of mRNA-vaccinated individuals.
2. Results
2.1. Study population
This observational study included 22 healthy immunocompetent individuals, who received the NVX-CoV2373-vaccine as a standard two-dose regimen as per German recommendations [2]. Individuals after dual vaccination with BNT162b2 (n = 22) and mRNA-1273 (n = 22) matched for age and sex served as controls and were derived in part from convenience cohorts as described before [8,9] (Table 1 , main study groups). All individuals had no known history of SARS-CoV-2 infection and were negative for nucleocapsid-specific IgG. The mean time interval between the two vaccinations was 21.5 ± 1.5 days for NVX-CoV2373, 31.2 ± 9.5 days for BNT162b2, and 40.2 ± 4.0 days for mRNA-1273, in accordance with German recommendations [2]. Blood sampling was carried out at a median of 15 (interquartile range (IQR) 2) days after the second vaccination. In addition, four individuals with a history of one prior SARS-CoV-2 infection episode were analyzed before and after one single dose of the NVX-CoV2373-vaccine (Table S1). Except for granulocytes, which were lower in individuals after BNT162b2 vaccination, the main leukocyte subpopulations did not differ between the main study groups (Table 1). This also held true for numbers of monocytes, lymphocytes and lymphocyte subpopulations such as B-cells, CD4 and CD8 T-cells. Among B-cells, plasmablast numbers, identified as CD38 positive cells among IgD−CD27+ CD19 positive switched-memory B-cells were also similar in all groups (Table 1).
Table 1.
Demographic and basic characteristics of the study population.
| 1st vaccine | NVX-CoV2373 | BNT162b2 | mRNA-1273 | |
|---|---|---|---|---|
| 2nd vaccine | NVX-CoV2373 | BNT162b2 | mRNA-1273 | |
| n = 22 | n = 22 | n = 22 | p-value | |
| Years of age (mean±SD) | 47.3 ± 14.1 | 47.0 ± 14.6 | 47.8 ± 14.7 | 0.988 |
| Female sex, n (%) | 13 (59.1) | 14 (63.6) | 14 (63.6) | 0.938 |
| Weeks between 1st and 2nd vaccination, (mean±SD) | 21.5 ± 1.5 | 31.2 ± 9.5 | 40.2 ± 4.0 | n.a. |
| Analysis time [days after 2nd vaccination], median (IQR) | 15 (2) | 14 (1) | 15 (2) | p = 0.712 |
| Differential blood cell counts | n = 22 | n = 21 | n = 22 | |
| Leukocytes (cells/µl), median (IQR) | 7100 (1625) | 6400 (2350) | 7900 (2150) | p = 0.057 |
| Granulocytes (cells/µl), median (IQR) | 4459 (1777) | 3549 (2108) | 5033 (1830) | p = 0.023 |
| Monocytes (cells/µl), median (IQR) | 559 (289) | 529 (230) | 632 (176) | p = 0.109 |
| Lymphocytes (cells/µl), median (IQR) | 2087 (455) | 2215 (792) | 2124 (1128) | p = 0.916 |
| Lymphocyte subpopulations | n = 21 | n = 16 | n = 21 | |
| CD3 T-cells (cells/µl), median (IQR) | 1556 (567) | 1572 (884) | 1636 (1068) | p = 0.846 |
| CD4 T-cells (cells/µl), median (IQR)# | 971 (378) | 933 (518)1 | 1073 (595) | p = 0.970 |
| CD8 T-cells (cells/µl), median (IQR)# | 327 (193) | 348 (365) | 402 (177) | p = 0.653 |
| CD19 B-cells (cells/µl), median (IQR)# | 152 (125) | 199 (176) | 169 (160) | p = 0.291 |
| Plasmablasts (cells/µl), median (IQR)# | 0.49 (0.455) | 0.369 (0.544) | 0.678 (0.655) | p = 0.128 |
Counts of CD4 and CD8 T-cells were calculated on 10 BNT162b2-vaccinated and on 19 mRNA-1273-vaccinated individuals, respectively.
2.2. Evolution of antibodies and T-cells after NVX-CoV2373 vaccination in individuals with and without prior infection
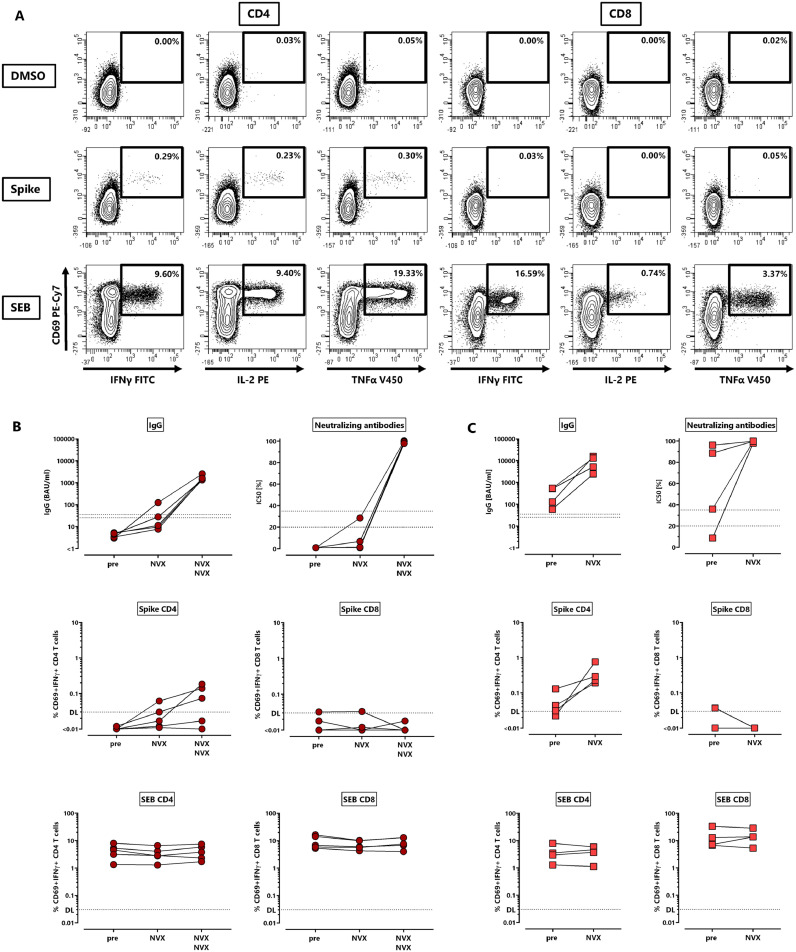
To first characterize the evolution of NVX-CoV2373-induced anti-spike IgG, neutralizing antibodies and CD4 and CD8 T-cells upon first and second vaccination, five individuals without prior SARS-CoV-2 infection were studied before vaccination, as well as after the first and the second dose. In addition, the four individuals with a history of SARS-CoV-2 infection were analyzed before and after a single dose of NVX-CoV2373 (Table S1). Spike-specific IgG were quantified using ELISA and their neutralizing activity was determined using a surrogate assay. Spike-specific CD4 and CD8 T-cells were quantified directly from whole blood samples after stimulation with overlapping peptides derived from the parental SARS-CoV-2 spike protein with diluent and Staphylococcus aureus Enterotoxin B (SEB) as negative and positive controls, respectively. Specifically stimulated CD4 and CD8 T-cells were quantified using flow-cytometry based on the induction of the activation marker CD69 and the cytokines IFNγ, TNFα or IL-2. Dotplots of a representative example of spike-specific CD4 and CD8 T-cell analyses of a 37-year-old female after the second vaccination with respective control stimulations are shown in Fig. 1 A. As shown in Fig. 1B, no specific immunity was detectable prior to vaccination in infection-naïve individuals, and the first vaccine dose only led to detectable specific antibodies, neutralizing activity and T-cells in a subset of individuals. In contrast, anti-spike IgG were induced in all individuals after the second dose with a significant increase in IgG-levels and neutralizing activity. As shown for CD69+IFNγ+ T-cells, the second vaccine-dose induced spike-specific CD4 T-cells to a variable extent, whereas CD8 T-cells were largely absent (Fig. 1B). In all individuals with prior infection, one dose of the vaccine readily induced IgG, strong neutralizing activity and CD4 T-cells, whereas spike-specific CD8 T-cells remained below detection limit (Fig. 1C). Polyclonally stimulated CD4 and CD8 T-cell levels remained constant throughout the observation period (Fig. 1B and C, bottom panels).
Fig. 1.
Evolution of antibodies and T-cells against the SARS-CoV-2 spike protein after NVX-CoV2373-vaccination. (A) Representative contour plots of CD4 and CD8 T-cells after stimulation of a whole blood sample from a 37-years old female 14 days after the second dose of the NVX-CoV2373-vaccine (NVX). Percentages of activated (CD69+) cells producing IFNγ, TNFα or IL-2 among CD4 or CD8 T-cells are shown after stimulation with DMSO (negative control), overlapping peptides of SARS-CoV-2 spike protein or SEB (positive control). Spike-specific IgG, neutralizing activity, spike-specific CD4 and CD8 T-cells, and SEB-reactive CD4 and CD8 T-cells were determined (B) in non-infected individuals (n = 5) prior to vaccination as well as after the first and the second vaccination, or (C) in individuals with history of one prior infection (n = 4) before and after a single dose of NVX-CoV2373-vaccine. Specific T-cells in panels B and C show CD4 or CD8 T-cells co-expressing CD69 and IFNγ with respective reactivity after control stimulation subtracted. Bars represent medians with interquartile ranges. Dotted lines represent detection limits for antibodies indicating negative, intermediate and positive levels or levels of inhibition, respectively as per manufacturer's instructions, and detection limits for specific T-cells. IFN, interferon; IL, interleukin; SEB, Staphylococcus aureus enterotoxin B; TNF, tumor necrosis factor.
2.3. Lower levels of spike-specific antibodies after vaccination with NVX-CoV2373 compared to dual-dose mRNA-vaccines
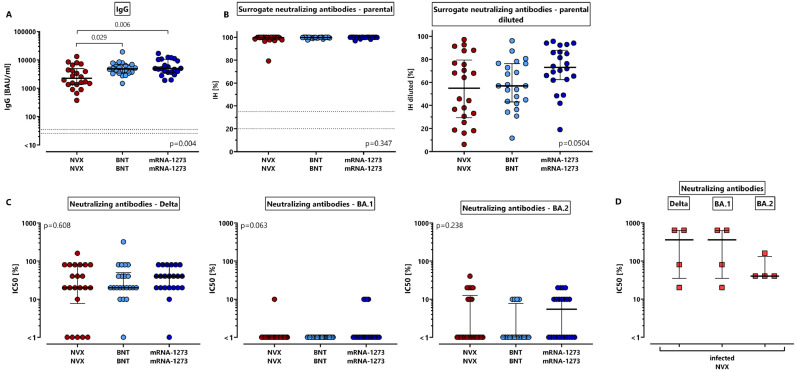
We next characterized NVX-CoV2373-induced immune responses with those after dual dose vaccination with BNT162b2 or mRNA-1273. In the whole cohort of 22 individuals, two doses of the NVX-CoV2373-vaccine induced spike-specific IgG in all individuals (Table 2 ), although median IgG-levels were significantly lower (2633 (IQR 3566) BAU/ml) as compared to individuals after homologous BNT162b2 or mRNA-1273 vaccination (4870 (IQR 3414) BAU/ml and 4932 (IQR 6686) BAU/ml, respectively, Fig. 2 A, p = 0.006). In contrast, neutralizing antibody activity against the parental spike protein which was determined by a surrogate assay was high, and reached the maximum activity in the majority of samples in all vaccine groups (Fig. 2B). A further dilution of samples revealed slightly lower neutralizing activity of NVX-CoV2373- and BNT162b2-induced antibodies, although the difference did not reach statistical significance (Fig. 2B, p = 0.0504, right panel).
Table 2.
Qualitative results of antibody and T-cell induction after the second vaccination.
| 1° vaccine | NVX-CoV2373 | BNT162b2 | mRNA-1273 | |
|---|---|---|---|---|
| 2° vaccine | NVX-CoV2373 | BNT162b2 | mRNA-1273 | |
| n (% positive) | n = 22 | n = 22 | n = 22 | p-value |
| IgG (≥35.2 BAU/ml) | 22 (100%) | 22 (100%) | 22 (100%) | p = 1.00 |
| Neutralizing activity | ||||
| Parental (IH≥35%)1 | 22 (100%) | 22 (100%) | 22 (100%) | p = 1.00 |
| Delta (>0%)2 | 17 (77.3%) | 21 (95.5%) | 21 (95.5%) | p = 0.07 |
| BA.1 (>0%)2 | 1 (4.5%) | 0 (0%) | 4 (18.2%) | p = 0.06 |
| BA.2 (>0%)2,3 | 8 (36.4%) | 4 (25%)1 | 11 (50.0%) | p = 0.29 |
| CD4 T-cells (≥0.03%) | 18 (81.8%) | 22 (100%) | 22 (100%) | p = 0.01 |
| CD8 T-cells (≥0.03%) | 3 (13.6%) | 14 (63.6%) | 17 (77.3%) | p<0.0001 |
determined by a surrogate assay.
determined by a micro-neutralization assay.
BA.2 testing only available for n = 16 individuals. Shown is the number (percentage) of individuals with specific immune responses above the respective detection limit.
Fig. 2.
Lower levels of spike-specific IgG after NVX-CoV2373-vaccination with similar neutralizing activity as mRNA-vaccinated individuals. Spike-specific antibodies were characterized among 66 individuals 13–18 days after the second vaccination with NVX-CoV2373 (NVX), BNT162b2 (BNT) or mRNA-1273 (n = 22 each). (A) ELISA and (B) a surrogate neutralization assay were performed to quantify levels of spike-specific IgG and neutralizing antibodies towards parental SARS-CoV-2 (expressed as percentage of inhibition (IH), left panel). In addition, samples were further diluted 1:16 to provide a higher resolution (right panel, expressed as percentage of inhibition of diluted samples). (C) A micro-neutralization assay was used to determine antibody-mediated neutralization of authentic SARS-CoV-2 variants of concern Delta and Omicron BA.1 and BA.2 among the three vaccine groups and (D) in the four individuals with history of infection after the first NVX-CoV2373-vaccine dose. Bars represent medians with interquartile ranges (expressed as IC50). Differences between the groups were calculated using two-sided Kruskal-Wallis test with Dunn´s multiple comparisons post-test. Dotted lines represent detection limits indicating negative, intermediate and positive IgG-levels or levels of inhibition, respectively as per manufacturer's instructions.
To further characterize neutralizing activity of vaccine-induced antibodies towards VOCs, a micro-neutralization assay was performed using the authentic SARS-CoV-2 VOCs Delta and Omicron BA.1 and BA.2. As shown in Table 2, the percentage of individuals with neutralizing activity towards Delta was highest (17/22 after NVX-CoV2373-vaccination, and 21/22 after BNT162b2- and mRNA-1273 vaccination), followed by BA.2 (25–50% of individuals), whereas activity towards BA.1 was largely absent. However, despite significant differences in anti-spike IgG-levels, median IC50-titers did not differ between the vaccine groups (Fig. 2C). Despite the small sample size of four individuals with prior infection only, it was remarkable that one dose of NVX-CoV2373-induced neutralizing activity towards all VOCs with in part markedly higher IC50-levels (Fig. 2D).
2.4. Lower levels of spike-specific T-cells after vaccination with NVX-CoV2373 compared to dual-dose mRNA-vaccines
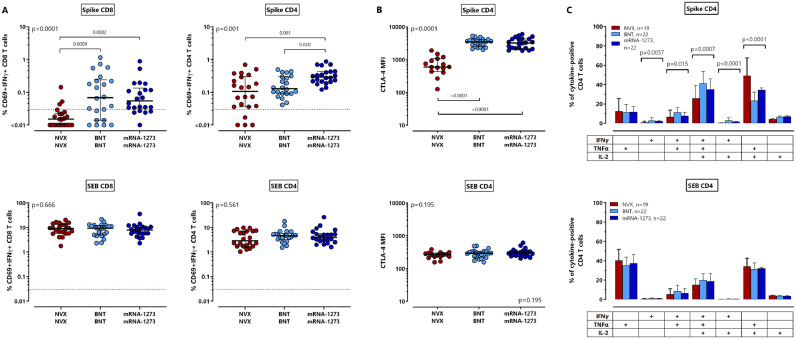
Apart from anti-spike IgG-levels, we also compared spike-specific CD4 and CD8 T-cells after NVX-CoV2373-vaccination with respective T-cells induced after mRNA-vaccination. T-cells were identified based on intracellular induction of IFNγ, TNFα and IL-2 after stimulation with overlapping peptides derived from the parental SARS-CoV-2 spike protein (contour plots from one individual each shown in Figs. 1A and S1). As exemplified for CD69+IFNγ+ T-cells, median percentages of spike-specific CD4 and CD8 T-cells were significantly lower after NVX-CoV2373-vaccination (Fig. 3 A). The difference was most pronounced for vaccine-induced CD8 T-cell levels (Fig. 3A, p<0.0001), where only 3/22 (13.6%) individuals showed detectable CD8 T-cells towards spike after NVX-CoV2373-vaccination. This contrasts with mRNA-regimens which induced specific CD8 T-cells in most individuals (Table 2, p<0.0001). Spike-specific CD4 T-cells were induced in 18/22 (81.8%) NVX-CoV2373-vaccinated individuals, although their median levels were significantly lower (0.11% (IQR 0.27%)) as compared to individuals after vaccination with BNT162b2 or mRNA-1273 (0.13% (IQR 0.21%) and 0.29% (IQR 0.22%), respectively, p = 0.001, Fig. 3A), where all had detectable CD4 T-cells (Table 2). Vaccine-induced CD4 T-cells were further characterized for phenotypical and functional properties. Contour plots of CTLA-4 expression among spike-specific and SEB-reactive T-cells are shown in supplementary Fig. S2. In line with a less pronounced induction of vaccine-induced T-cell levels, spike-specific CD4 T-cells after NVX-CoV2373-vaccination showed a significantly lower expression level of CTLA-4 as compared to both mRNA-groups, which may result from a lower extent of antigen encounter during the induction phase (Fig. 3B, p<0.0001). When spike-specific CD69 positive cells were quantified based on induction of other Th1 cytokines such as TNFα or IL-2, results were largely similar as with IFNγ producing cells (Fig. S3). In addition, Boolean gating was used to analyze cytokine-expression profiles of spike-specific CD4 T-cells producing IFNγ, TNFα and IL-2 alone or in combination. As shown in Fig. 3C, specific CD4 T-cells after NVX-CoV2373-vaccination comprised the lowest percentage of polyfunctional T-cells simultaneously expressing IFNγ, TNFα and IL-2, and a concomitant higher percentage of cells producing TNFα and IL-2. All effects were spike-specific, as SEB-reactive CD4 or CD8 T-cell levels including their CTLA-4 expression and cytokine-expression profiles were similar in all groups (Fig. 3, bottom panels).
Fig. 3.
Lower levels of spike-specific T-cells, lower CTLA-4 expression and lower percentages of polyfunctional CD4 T-cells after NVX-CoV2373-vaccination. Spike-specific CD4 and CD8 T-cells were quantified and characterized by intracellular cytokine staining after antigen-specific stimulation of whole blood samples of 66 individuals 13–18 days after the second vaccination with NVX-CoV2373 (NVX), BNT162b2 (BNT) or mRNA-1273 (n = 22 each). (A) Spike-specific and SEB-reactive CD4 and CD8 T-cells were determined based on co-expression of CD69 and IFNγ with respective reactivity after control stimulation subtracted. Bars represent medians with interquartile ranges. Dotted lines indicate detection limits for specific T-cells. (B) CTLA-4 expression of spike-specific and SEB-reactive CD4 T-cells was determined in all samples with at least 20 CD69+ IFNγ-positive CD4 T-cells (n = 15 for NVX CoV2373, n = 22 for mRNA-vaccinated individuals). Bars in panels A and B represent medians with interquartile ranges. Differences between the groups were calculated using two-sided Kruskal-Wallis test with Dunn´s multiple comparisons post-test. (C) After spike-specific or polyclonal stimulation, cytokine expressing CD4 T-cells were subclassified into 7 subpopulations according to single or combined expression of IFNγ, TNFα and IL-2. Blood samples from all individuals were analyzed. To ensure robust statistics, only samples with at least 30 cytokine-expressing CD4 T-cells after normalization to the negative control stimulation were considered (with sample size in each vaccine group indicated in the figures). Bars represent means and standard deviations, and ordinary one-way ANOVA tests were performed. Analyses in panels B and C were restricted to CD4 T-cells due to the lack of spike-specific CD8 T-cells in most NVX-CoV2373-vaccinated individuals. CTLA-4, cytotoxic T-lymphocyte associated protein 4; IFN, interferon; IL, interleukin; MFI, median fluorescence intensity; SEB, Staphylococcus aureus enterotoxin B; TNF, tumor necrosis factor.
2.5. NVX-CoV2373-induced T-cells equally recognize parental SARS-CoV-2 and variants of concern
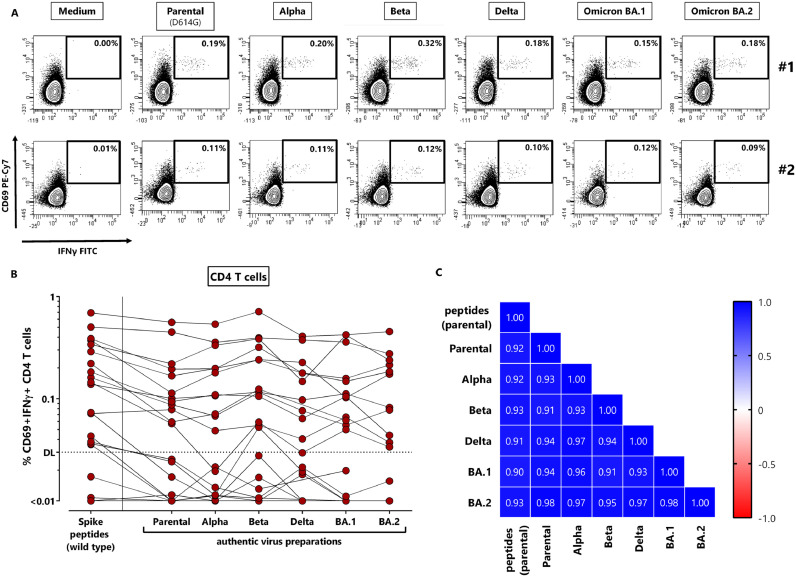
To analyze whether NVX-CoV2373-induced CD4 T-cells were able to recognize antigens from variants of concern, we used UV-inactivated authentic viruses including the parental strain (D614G, FFM7) and the VOCs Alpha, Beta, Delta, and Omicron BA.1 and BA.2 to stimulate vaccine-induced T-cells in vitro. As shown for two representative individuals with detectable spike-specific CD4 T-cell immunity, all VOCs elicited a similar percentage of specific CD4 T-cells as the parental strain (Fig. 4 A). Moreover, the percentage of CD4 T-cells after stimulation with overlapping peptides derived from parental spike showed a significant correlation with T-cell levels obtained after stimulation with the UV-inactivated parental strain (r = 0.92, p<0.0001), which indicates that the viral preparations were suitable for stimulation. Data from all individuals show that the T-cell frequencies reacting towards the parental strain within one individual largely correspond to the respective T-cell levels after stimulation with the variants (Fig. 4B). As shown in the heatmap in Fig. 4C, all pair-wise comparisons of specifically stimulated CD4 T-cell levels showed a strong, highly significant correlation.
Fig. 4.
NVX-CoV2373-induced T-cells equally recognize parental SARS-CoV-2 and variants of concern. Whole blood samples of 22 NVX-CoV2373-vaccinated individuals 13–18 days after the second vaccination were stimulated with overlapping peptides of parental SARS-CoV-2 spike or UV-inactivated parental SARS-CoV-2 or variants of concern. (A) Dotplots of two individuals with detectable spike-specific CD4 T-cells are shown (#1: 56 year old female, #2: 32 year old male). Percentages of activated (CD69+) cells producing IFNγ among CD4 T-cells are shown after stimulation with medium (negative control), parental SARS-CoV-2 or variants Alpha, Beta, Delta, Omicron BA.1 and BA.2. (B) Specific CD4 T-cells were quantified for all individuals based on co-expression of CD69 and IFNγ with respective reactivity after control stimulation subtracted. Indeterminate results of one person had to be excluded due to excessive background reactivity in the medium control; Delta and BA.2 stimulations were available from 19 individuals only. (C) Correlation matrix of specific T-cell levels determined after stimulation with spike-peptides and the different UV-inactivated virus preparations. Correlation coefficients were calculated according to two-tailed Spearman and displayed using a color code. IFN, Interferon.
2.6. The NVX-CoV2373-vaccine was well tolerated with no striking differences in adverse events between the vaccine groups
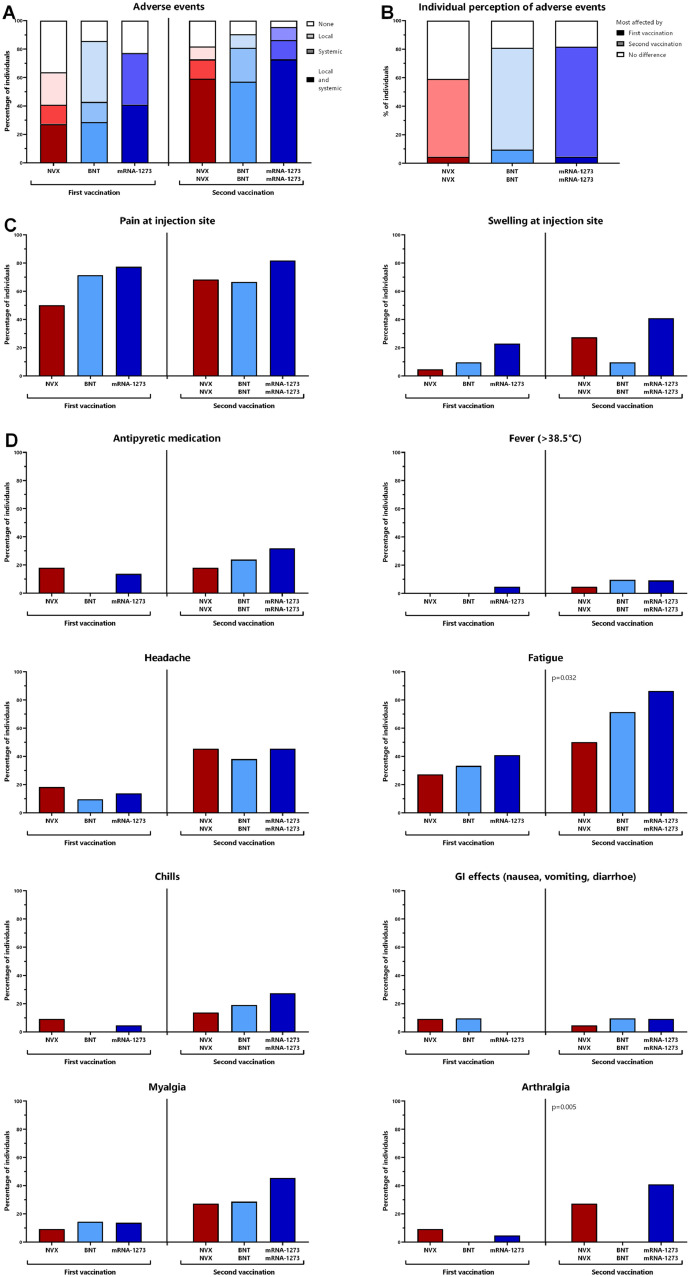
Vaccine-related adverse events after the first and the second vaccination were self-reported using a questionnaire. The percentage of individuals reporting local or systemic adverse events after the first and the second vaccination was similar for all three vaccine groups (Fig. 5 A). Regardless of the vaccine, the largest fraction of individuals reported of having been more affected by the second vaccination than by the first (Fig. 5B). Among local adverse events, pain at the injection site was more often reported than swelling (Fig. 5C) with no significant difference between the groups. Systemic adverse events including fever, headache, chills, gastrointestinal manifestations, and myalgia were similar for all vaccine groups. Arthralgia was significantly less frequently reported after the second BNT162B2-vaccination (p = 0.005), whereas fatigue was least frequent after the second NVX-COV2373-vaccination (p = 0.032). Overall, the need for antipyretic medication was similarly low in all groups. The notion that most adverse events were numerically least frequent after NVX-COV2373-vaccination indicates that this vaccine was at least equally well or better tolerated as the mRNA-vaccines.
Fig. 5.
Reactogenicity after primary and secondary vaccination with NVX-CoV2373, BNT162b2 or mRNA-1273. Self-reported reactogenicity within the first week after the first and the second vaccine dose was assessed using a standardized questionnaire. (A) The presence of local or systemic adverse events or both in general, (B) individual perception of which of the two vaccinations affected more, (C) local adverse events, or (D) systemic adverse events are shown. Statistical analysis was performed using X² test. BNT, BNT162b2-vaccine; NVX, NVX-CoV2373-vaccine.
3. Discussion
NVX-CoV2373 was recently licensed in Europe and in the United States as a dual dose regimen [1,3], but immunogenicity data regarding cellular immunity and reactivity towards variants of concern are scarce. Based on a convenience cohort in a real-world setting we show that the vaccine was well tolerated and all individuals mounted a strong anti-spike IgG-response after two doses. Despite lower IgG-levels as compared to two doses of the mRNA-vaccines BNT152b2 or mRNA-1273, IgG-levels were far higher than those induced by the single- or dual-dose vector regimens [8]. Moreover, neutralizing activity towards the SARS-CoV-2 VOCs Delta and Omicron BA.1 and BA.2 was similar as after mRNA-vaccination. Of note, in individuals with prior history of infection, one dose of NVX-CoV2373 was sufficient to induce similar anti-spike IgG-levels with high neutralizing activity towards all VOCs as two doses in infection naïve individuals. In contrast to mRNA-vaccines, the protein-based NVX-COV2373-vaccine poorly induced specific CD8 T-cells, whereas spike-specific CD4 T-cells were mounted in most individuals. Unlike poor neutralizing ability of antibodies towards VOCs, NVX-CoV2373-induced CD4 T-cells cross-reacted to all tested VOCs from Alpha to Omicron. As the vaccine was licensed only recently with use of NVX-CoV2373 coinciding with the circulation of VOCs including Delta and Omicron, our results on the neutralizing activity of antibodies and of T-cell reactivity towards VOCs is of particular importance in providing information on the ability to confer protection against infection and severe disease.
In line with results from our study, recent data including seven different vaccine regimens have shown a severe dampening of neutralizing antibody activity towards VOCs as compared to the parental virus in all tested vaccine groups [12], [13], [14]. These studies also included ten individuals after NVX-CoV2373-vaccination who participated in the randomized pivotal trial. However, the mean intervals between the last vaccination and analysis varied from 13 days in the two mRNA-vaccine groups to up to 82 days in individuals after NVX-CoV2373-vaccination [14]. Given the known dynamics in antibody titers over time, samples in some groups including NVX-CoV2373 were not captured at peak titers. Thus, although the neutralizing activity towards VOCs was consistently lower within one vaccine group, comparisons between vaccine groups were difficult. In our study, sample size was larger and analysis time was standardized in all tested groups, which allows for better head-to-head comparisons of antibody titers and neutralizing function between vaccine regimens.
Up to now, evidence for induction of NVX-CoV2373-induced memory T-cells exist from 12 individuals tested 3–4 months after the second vaccination [12], and from 27 volunteers from the phase I/II clinical trial [15]. However, no real-world data were available on NVX-CoV2373-induced T-cells during the induction phase in comparison with other vaccine regimens. In line with data from the phase I/II substudy [15], we now show that spike-specific CD4 T-cells were readily induced in most individuals, albeit at lower levels as after mRNA-1273-vaccination. Lower CD4 T-cell levels were associated with a significantly lower expression of CTLA-4 and a lower percentage of multifunctional cells. We have previously shown that the phenotypical and functional profile of antigen-specific T-cells correlated with antigen-load during active infections [16,17] or vaccination [8,9,18] to counteract excessive T-cell proliferation or immunopathology. Therefore, the phenotypical and functional differences may indicate a lower extent of antigen encounter during the induction phase in NVX-CoV2373-vaccinated individuals. However, the most striking difference between NVX-CoV2373- and mRNA-vaccinated individuals was the poor induction of spike-specific CD8 T-cells with detectable cells in only 3/22 individuals, in keeping with recent observations of 5/27 CD8 T-cell responders after NVX-CoV2373-vaccination [15]. This moderate response may result from the fact that proteins taken up by antigen-presenting cells during the induction phase of the immune response are less efficiently processed for presentation in MHC class I molecules. This contrasts with mRNA vaccines that rely on translation of the spike protein within the host cells [19]. This enables presentation of spike-derived peptides in both MHC class I and class II molecules which may explain the differences in the ability to induce CD4 and CD8 T-cells by the different vaccine platforms.
While neutralizing antibodies have been associated with protection from infection [20], vaccine-induced T-cells seem to play a major role in protecting from severe disease. Unlike neutralizing antibody activity, NVX-CoV2373-induced CD4 T-cells analyzed two weeks after the second vaccination show considerable cross-reactivity towards all tested VOCs. This is consistent with findings from individuals after BNT162b2 or Ad26.CoV2.S vaccination during the induction phase [21] or memory T-cells from eight NVX-CoV2373-vaccinated individuals tested 3, 4 months after vaccination [13]. Overall, this indicates that mutant spike-derived MHC-bound peptides recognized by T-cells are less susceptible towards immune evasion than mutated spike protein epitopes recognized by antibodies. Thus, despite the current surge in the incidence of Omicron infections due to impaired neutralizing activity, the induction of T-cells with robust cross-reactivity towards VOCs across several vaccine regimens likely plays a major role in retaining protection from severe disease. In this regard, the Th1 polarized phenotype of NVX-CoV2373-induced CD4 T-cells with the ability to produce IFNγ, TNFα and IL-2 may be instrumental in cell-mediated elimination of infected cells [22,23]. Future effectiveness studies should determine whether the differences in CD4 T-cell levels and functionality and the relative lack of vaccine-induced CD8 T-cells may reveal differences between vaccine regimens regarding disease severity upon breakthrough infection. Moreover, it will be interesting to study whether heterologous boosting with mRNA-vaccines will induce specific CD8 T-cells as was shown for heterologous mRNA-boosting after vector-vaccination [8,9,[24], [25], [26], [27]]. Regarding neutralizing activity, a third booster dose after NVX-CoV2373 may seem equally valuable to improve protection as has been shown for other primary vaccine regimens [28], [29], [30]. This is supported by promising data from a small study showing that neutralizing antibody titers towards VOCs increased in all seven NVX-CoV2373-vaccinated individuals who either received mRNA-1273, BNT162b2 or Ad26.COV.2 as a booster dose [31].
A strength of our study is the head-to-head analysis of immunogenicity and reactogenicity of the NVX-CoV2373-regimen in a real world setting in comparison with the two mRNA-regimens BNT162b2 and mRNA-1273, which are most widely used in European countries and many parts of the world [32]. Our study is limited by convenience sampling in a non-randomized study design, where the study participants on the various vaccine regimens were not enrolled in the same time frame. However, as individuals with evidence for prior infection were excluded from the main analysis, our results are not confounded by differences in circulating viral strains over time. Moreover, participants were enrolled according to national recommendations, where some vaccine-specific differences in the intervals between the first and the second vaccination may have an influence on the level of specific immunity. However, recent evidence suggests that a short interval in the range of 3–6 weeks as in our study does not appear to have an impact on immunogenicity [33]. Finally, information on the neutralizing activity or T-cell reactivity on the BA.4 and BA.5 variant is not available. However, neutralizing activity towards BA.4 and BA.5 in individuals 3 to 4 months after NVX-CoV2373-vaccination has recently been shown to be markedly lower than for BA.1 or BA.2, which has been equally observed with other vaccine regimens [31].
Occurrence of serious adverse events such as thrombosis [34,35] or myocarditis [36] after vector- or mRNA-based vaccination has resulted in vaccine hesitancy that may be overcome by the availability of traditional vaccine formulations based on recombinant proteins. No such adverse events were described for NVX-CoV2373 so far and the vaccine was well tolerated by all participants of our study. Together with the ability to induce a potent antibody and CD4 T-cell response and practical advantages such as vaccine stability during refrigeration, NVX-CoV2373 may have potential for widespread use as either primary series or as booster vaccine pending further regulatory approval.
4. Methods
4.1. Study design and subjects
Study participants for NVX-CoV2373 vaccination were enrolled prospectively prior to the start of a vaccination cycle or no longer than 13–18 days after the second vaccination as described before [9]. In addition, individuals after dual dose vaccination with BNT162b2 and mRNA-1273 matched for age and sex were chosen as control groups. Their data and frozen plasma samples were in part derived from previous observational studies [8,9]. The time interval between the first and the second vaccination (3 weeks for NVX-CoV2373, 3–6 weeks for mRNA regimens) was based on recommendations [2] and not determined by the study. Study participants completed a questionnaire for self-reporting of local and systemic adverse events occurring within the first week after the first and second vaccination, respectively. Blood samples were collected during an interval of 13–18 days after secondary vaccination, or prior to vaccination or after the first vaccination in subgroups before and after NVX-CoV2373-vaccination. The study was approved by the ethics committee of the Ärztekammer des Saarlandes (reference 76/20), and all individuals gave written informed consent.
4.2. Flow-cytometric analyses
T-cells, B-cells and plasmablasts were quantified from 100 µl heparinized whole blood as described before [9] with details specified in the supplementary methods.
4.3. Preparation of UV-inactivated viral strains
The following SARS-CoV-2 isolates were used in this study: Parental strain (SARS-CoV-2 B1 FFM7/2020, GenBank ID MT358643), Alpha (SARS-CoV-2 B1.1.7 FFM-UK7931/2021, GenBank ID MZ427280.1), Beta (SARS-CoV-2 B1.1.7 FFM-ZAF/2021, GenBank ID MW822592), Delta (SARS-CoV-2 B1.617.2 FFM-IND8424/2021, GenBank ID MZ315141), BA.1 (SARS-CoV-2 B1.1.529 FFM-SIM0550/2021 (EPI_ISL_6,959,871), GenBank ID OL800702), BA.2 (SARS-CoV-2 BA.2 FFM-BA.2–3833/2022, GenBank ID OM617939) [37], [38], [39], [40]. Preparation of UV-inactivated viral strains was based on previously established methods [41] and is described in detail in the supplementary methods.
4.4. Quantification of vaccine-induced spike-specific T-cells
SARS-CoV-2 specific T-cells were determined from heparinized whole blood after stimulation exactly as described before [9,16]. Stimulations were carried out with overlapping peptides of the parental SARS-CoV-2 strain (Swiss-Prot ID: P0DTC2, JPT, Berlin, Germany) or UV-inactivated viral strains (see above). Further details are outlined in the supplementary methods.
4.5. Determination of SARS-CoV-2 specific antibodies and neutralization capacity
Spike-specific IgG and neutralizing capacity were determined using ELISA, a surrogate neutralization assay, and a micro-neutralization assay in cell culture using infectious SARS-CoV-2 as described before [9,40] with details outlined in the supplementary methods.
4.6. Statistical analysis
Kruskal-Wallis test, followed by Dunn's multiple comparisons test, was performed to compare unpaired non-parametric data such as lymphocyte subpopulations, T-cell and antibody levels, neutralizing activities, and CTLA-4 expression. Data with normal distribution such as age or the cytokine-expression profiles were analyzed using ordinary one-way ANOVA. Categorial analyses on sex, vaccine responses, and adverse events were performed using X2 test. Correlations between levels of T-cells towards overlapping peptides and UV-inactivated viral strains were analyzed by correlation matrix according to Spearman. A p-value <0.05 was considered statistically significant. Analysis was carried out using GraphPad Prism 9.4.1 software (GraphPad, San Diego, CA, USA) using two-tailed tests.
4.7. Study approval
The study was approved by the ethics committee of the Ärztekammer des Saarlandes (reference 76/20), and all individuals gave written informed consent.
Author contributions
T.S., U.S., and M.S. designed the study; F.H., V.K., T.S., U.S., M.W. and M.S. designed the experiments, F.H., V.K., S.M., A.A.-O., L.Z., C.G., R.U., A.W., M.W. and T.S. performed experiments; F.H. and U.S., and M.S. contributed to study design, patient recruitment, and clinical data acquisition. F.H. T.S., U.S. and M.S. performed statistical analysis. T.S., U.S., and M.S. supervised all parts of the study; F.H., T.S., M.W. and M.S. wrote the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by a grant of the State chancellery of the Saarland to M.S. Support for F.H. was given by “HOMFOR exzellent” of the medical faculty at Saarland University. Moreover, the work was in part supported by the Clusterproject ENABLE and The Innovation Center TheraNova funded by the Hessian Ministry for Science and the Arts and the German Federal Ministry of Education and Research (BMBF) funding to M.W. (COVIDready, grant number 02WRS1621C).
Data availability
All figures and tables have associated raw data. The data that support the findings of this study are available from the corresponding author upon request.
Declaration of Competing Interest
M.S. has received grant support from Astellas and Biotest to the organization Saarland University outside the submitted work, and honoraria for lectures from Biotest and Novartis. M.W. has received speaker fees from Astra Zeneca and grant support from Roche Molecular Diagnostics to the organization Goethe University Frankfurt outside the submitted work. All other authors of this manuscript have no conflicts of interest to disclose.
Acknowledgments
The authors thank Dr. Christina Baum from the occupational health care center at Saarland University Medical Center, and Dr. Wilhelm Walch and Dr. Dirk Jesinghaus from the vaccine center in Neunkirchen for their support in enrolling participants. Furthermore, we would like to thank Christiane Pallas for excellent technical assistance. The authors also thank all participants to this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105321.
Appendix. Supplementary materials
References
- 1.European Medicine Agency (EMA). EMA recommends nuvaxovid for authorisation in the EU. 2021 20th december 2021 [cited; Available from: https://www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu.
- 2.Ständige Impfkommission Aktualisierung der COVID-19-impfempfehlung. Epid. Bull. 2022;7:3–18. [Google Scholar]
- 3.U.S. Food & Drug Administration (FDA). Coronavirus (COVID-19) update: FDA authorizes emergency use of novavax COVID-19 vaccine, adjuvanted. 2022 13th july [cited; Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-authorizes-emergency-use-novavax-COVID-19-vaccine-adjuvanted.
- 4.Dunkle L.M., Kotloff K.L., Gay C.L., Anez G., Adelglass J.M., Barrat Hernandez A.Q., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N. Engl. J. Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemis V., Schmidt T., Schub D., Mihm J., Marx S., Abu-Omar A., et al. Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or mRNA-1273-boosting with homologous COVID-19 vaccine regimens. Nat. Commun. 2022;13:4710. doi: 10.1038/s41467-022-32321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toback S., Galiza E., Cosgrove C., Galloway J., Goodman A.L., Swift P.A., et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet North Am. Ed. 2022;10:167–179. doi: 10.1016/S2213-2600(21)00409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z., Mateus J., Coelho C.H., Dan J.M., Moderbacher C.R., Galvez R.I., et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185:2434–2451. doi: 10.1016/j.cell.2022.05.022. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859. doi: 10.1016/j.cell.2022.01.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowen J.E., Addetia A., Dang H., Stewart C., Brown J.T., Sharkey W.K., et al. Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines. Science. 2022 doi: 10.1126/science.abq0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydyznski Moderbacher C., Kim C., Mateus J., Plested J., Zhu M., Cloney-Clark S., et al. NVX-CoV2373 vaccination induces functional SARS-CoV-2-specific CD4+ and CD8+ T cell responses. J. Clin. Invest. 2022:132. doi: 10.1172/JCI160898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schub D., Klemis V., Schneitler S., Mihm J., Lepper P.M., Wilkens H., et al. High levels of SARS-CoV-2-specific T cells with restricted functionality in severe courses of COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.142167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schub D., Fousse M., Fassbender K., Gartner B.C., Sester U., Sester M., et al. CTLA-4-expression on VZV-specific T cells in CSF and blood is specifically increased in patients with VZV related central nervous system infections. Eur. J. Immunol. 2018;48:151–160. doi: 10.1002/eji.201747079. [DOI] [PubMed] [Google Scholar]
- 18.Ledo A., Schub D., Ziller C., Enders M., Stenger T., Gartner B.C., et al. Elite athletes on regular training show more pronounced induction of vaccine-specific T-cells and antibodies after tetravalent influenza vaccination than controls. Brain Behav. Immun. 2020;83:135–145. doi: 10.1016/j.bbi.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary N., Weissman D., Whitehead K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021 doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Chandrashekar A., Sellers D., Barrett J., Jacob-Dolan C., Lifton M., et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw R.H., Liu X., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: exploratory analyses of Com-COV, a randomised control trial. Lancet North Am. Ed. 2022 doi: 10.1016/S2213-2600(22)00163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet North Am. Ed. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Morillas Ramos G., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021 doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borobia A.M., Carcas A.J., Perez-Olmeda M., Castano L., Bertran M.J., Garcia-Perez J., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanshylla K., Tober-Lau P., Gruell H., Munn F., Eggeling R., Pfeifer N., et al. Durability of omicron-neutralising serum activity after mRNA booster immunisation in older adults. Lancet Infect. Dis. 2022;22:445–446. doi: 10.1016/S1473-3099(22)00135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruell H., Vanshylla K., Tober-Lau P., Hillus D., Schommers P., Lehmann C., et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022;28:477–480. doi: 10.1038/s41591-021-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowen J.E., Addetia A., Dang H.V., Stewart C., Brown J.T., Sharkey W.K., et al. Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines. Science. 2022 doi: 10.1126/science.abq0203. eabq0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 33.Hall V.G., Ferreira V.H., Wood H., Ierullo M., Majchrzak-Kita B., Manguiat K., et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat. Immunol. 2022 doi: 10.1038/s41590-021-01126-6. [DOI] [PubMed] [Google Scholar]
- 34.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toptan T., Hoehl S., Westhaus S., Bojkova D., Berger A., Rotter B., et al. Optimized qRT-PCR approach for the detection of intra- and extra-cellular SARS-CoV-2 RNAs. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm A., Toptan T., Pallas C., Wolf T., Goetsch U., Gottschalk R., et al. Antibody-mediated neutralization of authentic SARS-CoV-2 B1.617 variants harboring L452R and T478K/E484Q. Viruses. 2021;13 doi: 10.3390/v13091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widera M., Wilhelm A., Hoehl S., Pallas C., Kohmer N., Wolf T., et al. Limited neutralization of authentic severe acute respiratory syndrome coronavirus 2 variants carrying E484K in vitro. J. Infect. Dis. 2021;224:1109–1114. doi: 10.1093/infdis/jiab355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widera M., Westhaus S., Rabenau H.F., Hoehl S., Bojkova D., Cinatl J., Jr., et al. Evaluation of stability and inactivation methods of SARS-CoV-2 in context of laboratory settings. Med. Microbiol. Immunol. 2021;210:235–244. doi: 10.1007/s00430-021-00716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All figures and tables have associated raw data. The data that support the findings of this study are available from the corresponding author upon request.