Abstract
Adverse reactions, including the onset of diabetes, after coronavirus disease 2019 (COVID‐19) vaccination have been reported. Herein, we report a case of a man who developed anti‐glutamic acid decarboxylase (GAD) antibody‐positive fulminant type 1 diabetes 15 weeks after COVID‐19 vaccination, atypical of the previously reported anti‐GAD antibody‐negative cases.
Keywords: anti‐glutamic acid decarboxylase (GAD) antibody, COVID‐19 vaccination, diabetic ketoacidosis, fulminant type 1 diabetes
1. INTRODUCTION
Fulminant type 1 diabetes is a subtype of type 1 diabetes characterized by severe progression of hyperglycemia to ketoacidosis without a significant increase in glycated hemoglobin (HbA1c) levels, typically in a short period (within a week). Additionally, depletion of insulin secretion has been confirmed in patients with fulminant type 1 diabetes. Interestingly, islet‐related autoantibodies are absent in most patients with fulminant type 1 diabetes. 1
Vaccination is one of the most important global responses to the coronavirus disease 2019 (COVID‐19) pandemic. However, adverse reactions to the vaccine have been reported, including the onset and worsening of diabetes symptoms. 2 , 3 Autoantibody abnormalities and onset of type 1 diabetes after COVID‐19 vaccination have been reported recently. Sasaki et al. reported a case of a patient diagnosed with type 1 diabetes at 8 weeks after vaccination, in whom anti‐glutamic acid decarboxylase (GAD) antibodies were strongly positive (≥2000 IU/L), insulin secretion was reduced but not completely depleted, and HbA1c level was at 9.3%. Therefore, the patient was diagnosed with non‐fulminant type 1 diabetes. 4 Meanwhile, Yano et al. 5 reported a case of a patient who was diagnosed with acute‐onset type 1 diabetes (anti‐GAD antibody‐negative, anti‐insulinoma‐associated protein‐2 [IA‐2] antibodies‐negative) 6 weeks after vaccination.
A few cases of fulminant type 1 diabetes have been reported to date. In particular, three studies have reported separate cases of developing fulminant type 1 diabetes (anti‐GAD antibodies‐negative, anti‐IA‐2 antibodies‐negative) a few days after vaccination. 6 , 7 , 8 In typical fulminant type 1 diabetes, anti‐GAD antibodies are negative. 1 According to a previous study, the prevalence of anti‐GAD antibody‐positive patients is only 4.8%. 6
The causal relationship between the onset of type 1 diabetes and COVID‐19 vaccination has not been elucidated. However, the immune response to vaccination has been associated with the development of type 1 diabetes. In this study, we report a case of a patient who developed anti‐GAD antibodies‐positive new‐onset fulminant type 1 diabetes after the COVID‐19 vaccination.
2. CASE HISTORY
The patient was a 59‐year‐old man who showed no signs of diabetes in his annual health checks and had no history of obesity. A blood test from the previous year's health check also did not indicate diabetes (fasting blood glucose and HbA1c were 88 mg/dl and 5.6%, respectively.)
Moreover, he had no history of alcohol consumption or social drinking and no family history of diabetes or autoimmune diseases. Additionally, he had no history of excessive soft drink consumption during the course. He was admitted to our hospital due to general fatigue and difficulty in body movement 15 weeks after receiving the second dose of the BNT162b2 messenger ribonucleic acid (mRNA) (Pfizer‐BioNTech) vaccine.
No fatigue or fever was observed after vaccination. He visited a nearby hospital with a chief complaint of fever of 37.7°C 4 days before the onset of prodromal symptoms. He was diagnosed with upper respiratory inflammation and was prescribed antibiotics and antipyretics. He reported general fatigue from the morning of the day of the onset of premonitory symptoms and had a few episodes of vomiting. From the morning of the following day, he had complications including weakness, worsening of general fatigue, and difficulty in body movements. Therefore, he was transported to the emergency department of a nearby hospital. In addition to hyperglycemia (serum glucose levels, 1454 mg/dl), arterial blood gas analysis showed severe acidosis (pH, 6.95), and he received an emergency transfer to our hospital.
3. DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT
Upon admission, he showed signs of dehydration (i.e., dry tongue). Blood test findings showed significant hyperglycemia (serum glucose levels, 1514 mg/dl), dehydration, and prerenal failure (blood urea nitrogen, 74.1 mg/dl; and creatinine, 2.97 mg/dl). HbA1c level was 7.8% (Table 1). Arterial blood gas analysis showed significant metabolic acidosis (pH, 7.004; HCO3‐, 3.5 mmol/L; Anion gap, 35.5 mmol/L). Blood ketone body fractions were remarkably higher (3‐hydroxybutyric acid, 13,555 μmol/L), and he was diagnosed with diabetic ketoacidosis.
TABLE 1.
Laboratory findings
| Assessment | Results | Reference range |
|---|---|---|
| Arterial blood gas (room air) | ||
| pH | 7.004 | 7.35–7.45 |
| pCO2, mmHg | 14.3 | 35–45 |
| pO2, mmHg | 113.3 | 80–100 |
| HCO3−, mmol/L | 3.5 | 22–26 |
| Base excess, mmol/L | −25.9 | 0 ± 2 |
| Anion gap, mmol/L | 35.5 | 10–14 |
| Peripheral blood | ||
| White blood cell/mm3 | 22,800 | 3300–8600 |
| Red blood cell, ×104/mm3 | 387 | 386–492 |
| Hemoglobin, g/dl | 11.7 | 11.6–14.8 |
| Hematocrit, % | 40 | 35.1–44.4 |
| Platelet, ×104/mm3 | 42.0 | 15.8–34.8 |
| Biochemistry | ||
| Blood urea nitrogen, mg/dl | 74.1 | 8–20 |
| Creatinine, mg/dl | 2.97 | 0.65–1.09 |
| Sodium, mmol/L | 120 | 135–145 |
| Potassium, mmol/L | 6.9 | 3.6–4.8 |
| Chlorine, mmol/L | 81 | 98–108 |
| Plasma glucose, mg/dl | 1514 | 73–109 |
| Hemoglobin A1c, % | 7.8 | 4.6–6.2 |
| Fasting C‐peptide, ng/ml | <0.03 | 0.80–2.50 |
| Glucagon‐stimulated C‐peptide, ng/ml | <0.03 | 0.80–2.50 |
| β‐hydroxybutyrate, μmol/L | 13,555 | 0–76 |
| Acetoacetate, μmol/L | 1669 | 13–69 |
| Free T4, ng/ml | 1.13 | 0.90–1.70 |
| Thyroid stimulating hormone, μ IU/ml | 3.33 | 0.50–5.00 |
| Urine C‐peptide (μg/day) | <2.6 | 50–100 |
| Immunological tests | ||
| Anti‐GAD a antibody, U/ml | 44.8 | <5.0 |
| Anti‐IA‐2 b antibody, U/ml | <0.6 | <0.6 |
| Anti‐ZnT8 c antibody, U/ml | <10.0 | <10.0 |
| Anti‐thyroglobulin antibody | 11.13 | <28 |
| Anti‐thyroid peroxidase antibody | 10.81 | <16 |
| HLA d ‐DNA typing | ||
| DRB1*04:05:01/09:01:02 | ||
| DQB1*03:03:02/04:01:01 | ||
GAD: glutamic acid decarboxylase.
IA‐2: insulinoma‐associated protein‐2.
ZnT8: zinc transporter 8.
HLA: human leukocyte antigen.
After admission, he received an intravenous infusion of normal saline (4000 ml/day) and a continuous intravenous infusion of insulin. Metabolic status improved with the improvement of blood glucose levels. On disease day four, oral food intake was initiated, and the route of insulin administration was changed from intravenous to subcutaneous. Glucagon stimulation test on disease day eight showed complete depletion of insulin secretion (fasting C‐peptide <0.3 ng/ml; C‐peptide levels 6 min after glucagon stimulation <0.3 ng/ml; and C‐peptide levels during a urinary continence assessment <2.3 μg/day).
The laboratory tests on disease day two revealed normal amylase levels (76 U/L) and significantly elevated pancreatic exocrine enzyme levels (trypsin, 8970 ng/ml; elastase‐1, 2010 ng/dl; and pancreatic phospholipase A2 [PLA2], 7390 ng/dl) (Table 2). The results of tests during hospitalization were as follows: anti‐GAD antibody‐positive (44.8 U/ml), anti‐IA‐2 antibody‐negative, and ZnT8 antibody‐negative.
TABLE 2.
The transition of pancreatic exocrine enzyme
| Assessment | Day 1 | Day 7 | Day 17 | Day 42 | Day 82 | Reference range |
|---|---|---|---|---|---|---|
| Pancreas amylase, U/L | 38 | 80 | 60 | 43 | 39 | 21–64 |
| Elastase1, ng/dl | 2010 | 1600 | 425 | 123 | <80 | <300 |
| Trypsin, ng/ml | 8970 | 1300 | 938 | 783 | 618 | 210–570 |
| PLA2, ng/dl a | 7390 | 871 | 667 | 555 | 447 | 130–400 |
PLA, pancreatic phospholipase A.
Glycemic control improved after frequent insulin therapy comprising insulin lispro (morning, 15 units; daytime, 8 units; and evening, 12 units) and insulin degludec (11 units). He was discharged on disease Day 16.
A blood test during outpatient examination 45 days after the onset showed a negative conversion of anti‐GAD antibodies. A test of pancreatic exocrine function showed a slow reduction in trypsin, elastase, and pancreatic PLA2. Results of magnetic resonance imaging (MRI) 8 days after the onset showed no enlargement of the pancreas. In addition, re‐evaluation 50 days after the onset showed no morphologic changes in the size of the pancreas (Figure 1). However, 50 days after the onset, MRI showed more increased apparent diffusion coefficient (ADC) values relative to that in 8 days (Figure 2).
FIGURE 1.
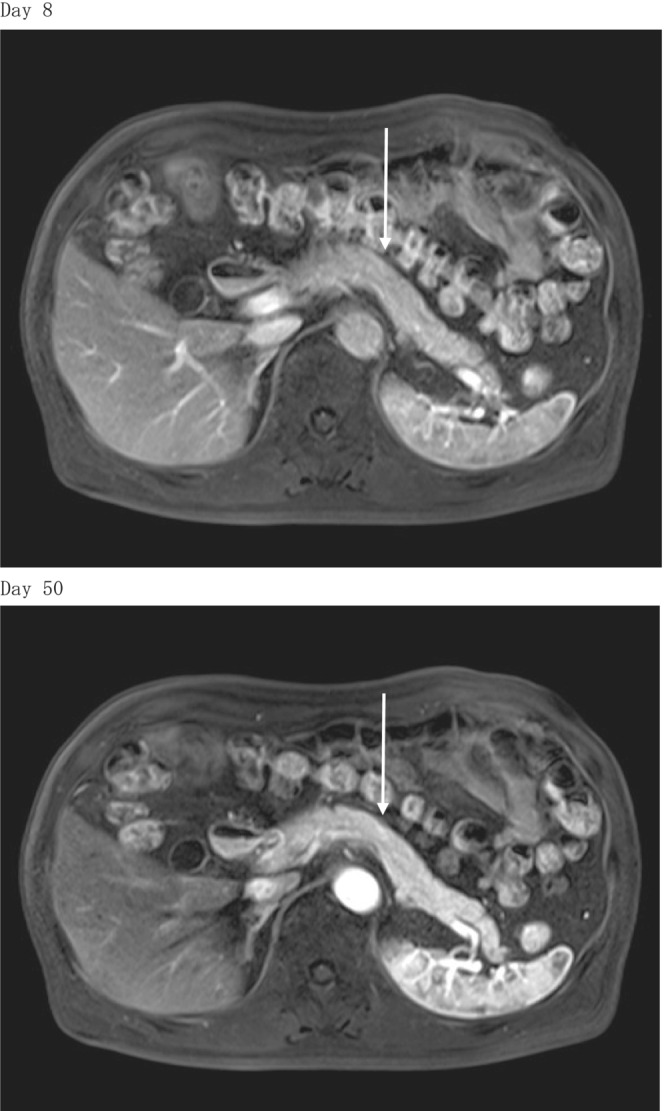
T1‐weighted magnetic resonance imaging (MRI). MRI revealed that the size of the pancreas did not change between Days 8 and 50 after the onset.
FIGURE 2.
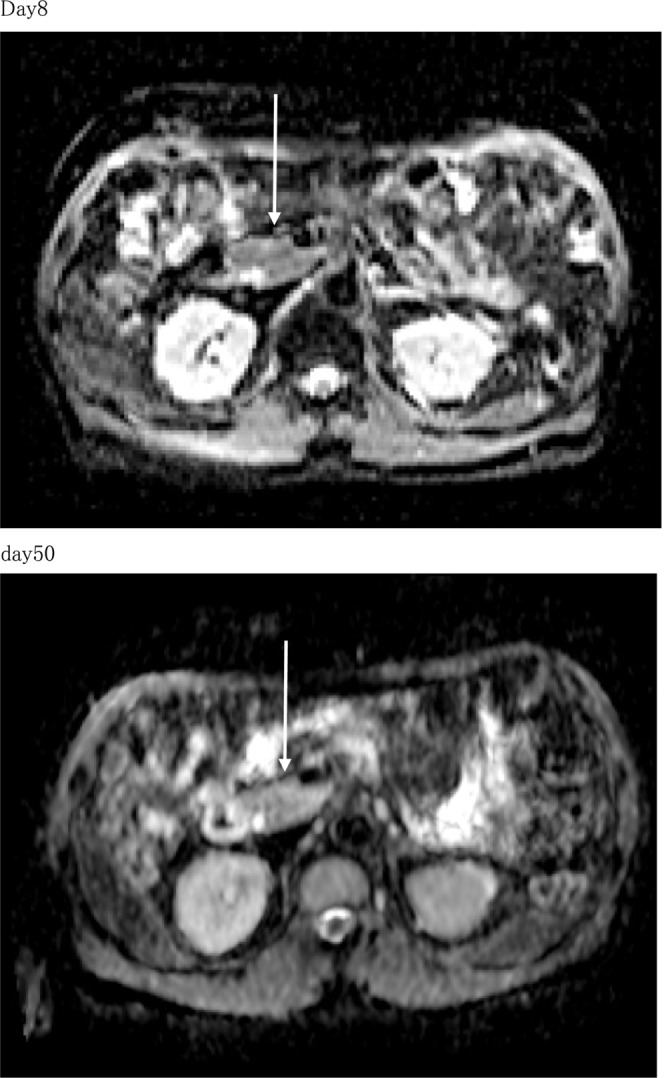
Apparent diffusion coefficient (ADC) map: pancreas head. MRI 50 days after the onset showed more increased apparent diffusion coefficient (ADC) values than 8 days.
4. OUTCOME AND FOLLOW‐UP
Blood glucose control after discharge is properly maintained with similar insulin treatment as at the time of discharge. The patient has already returned to his profession without disability.
5. DISCUSSION
We report an atypical case of GAD antibody‐positive new‐onset fulminant type 1 diabetes following COVID‐19 vaccination. This is the first case report on GAD antibody‐positive new‐onset fulminant type 1 diabetes after the COVID‐19 vaccination. The symptoms of the patient progressed rapidly from reported general fatigue (i.e., prodromal symptoms) within approximately 24 h. He had shown no signs of diabetes in previous annual health checks and had no history of obesity. In addition, during the course up to hospital admission, he had no history of excessive soft drink consumption. Diabetic retinopathy was not observed in ophthalmofundoscopy. On his first visit to our hospital, he was positive for urine ketone bodies, blood ketone body levels were elevated, blood glucose was ≥288 mg/dl, HbA1c was <8.7%, fasting C‐peptide was <0.3 ng/ml, C‐peptide levels 6 min after glucagon stimulation were <0.3 ng/ml, and C‐peptide level during a urinary continence assessment was <10 μg/day, fulfilling the diagnostic criteria for fulminant type 1 diabetes. 7 Therefore, he was diagnosed with diabetic ketoacidosis due to fulminant type 1 diabetes.
The rapid depletion of insulin secretion was typical of fulminant type 1 diabetes. However, anti‐GAD antibodies at the onset of fulminant type 1 diabetes were moderately positive (44.8 IU/L) and atypical. In addition, outpatient examination 6 weeks after the onset showed negative conversion of anti‐GAD antibodies that were previously positive. This suggested the loss of immune response to anti‐GAD antibodies due to the depletion of insulin secretion caused by the rapid destruction of pancreatic beta cells and resultant loss of beta cells. In human leukocyte antigen (HLA) analysis, the haplotypes DRB1*04:05‐DQB1*04:01 and DRB1*09:01‐DQB1*03:03 in fulminant type 1 diabetes are reported to be susceptible to disease. 8 In the present study, HLA analysis showed genotypes with susceptibility to fulminant type 1 diabetes (DRB1*04:05:01/09:01:02‐ DQB1*03:03:02/04:01:01).
There are several case reports of the development of acute‐onset type 1 diabetes after the COVID‐19 vaccination. Previous case reports of non‐fulminant acute‐onset type 1 diabetes reported a relatively long period for the progression of symptoms from onset after vaccination (Table 3). 4 , 5 By contrast, the time for progression of symptoms from onset after vaccination was relatively short (approximately 1 week) in fulminant type 1 diabetes (Table 4). 9 , 10 , 11 Moreover, anti‐GAD antibodies in patients in previous reports were negative in all cases. In the present study, the patient's anti‐GAD antibodies were moderately positive.
TABLE 3.
Comparison of the cases of new‐onset acute type 1 diabetes after COVID‐19 vaccination
| Authors | Sasaki et al. | Yano et al. | Present case |
|---|---|---|---|
| Age and sex | 73 years, male | 51 years, female | 59 years, male |
| Period from vaccination to onset | 8 weeks | 6 weeks | 15 weeks |
| Onset patterns of type 1 diabetes | Acute‐onset | Acute‐onset | Fulminant |
| HbA1c on admission | 9.3% | 10.3% | 7.8% |
| Islet autoantibodies |
IA‐2 Ab (−) d IAA (+) e |
GAD Ab (−) IA‐2 Ab (−) IAA (+) |
GAD Ab (+) IA‐2 Ab (−) IAA (−) |
| Thyroid autoantibodies |
TgAb (−) f TPOAb (−) g TRAb (−) h |
TgAb (+) TPOAb (+) |
TgAb (−) TPOAb (−) TRAb (−) |
|
ClassII HLA c genotype |
DRB1*04:05/04:05 DQB1*04:01/04:01 |
DRB1*09:01/09:01 DQB1*03:03/03:03 |
DRB1*04:05/09:01 DQB1*03:03/04:01 |
Ab, antibody.
GAD, glutamic acid decarboxylase.
HLA, human leukocyte antigen.
IA‐2, insulinoma‐associated protein‐2.
IAA, insulin antibody.
Tg, thyroglobulin.
TPO, thyroid peroxidase.
TR, thyrotropin receptor.
TABLE 4.
Comparison of the cases of new‐onset fulminant type 1 diabetes after COVID‐19 vaccination
| Authors | Sasaki et al. | Tang et al. | Sakurai et al. | Present case |
|---|---|---|---|---|
| Age and sex | 45 years, female | 50 years, male | 36 years, female | 58 years, male |
| Period from vaccination to onset | 8 days | 6 days | 10 days | 15 weeks |
| Onset patterns of type1 diabetes | Fulminant | Fulminant | Possibility of fulminant | Fulminant |
| HbA1c on admission | 7.6% | No data | 7.0% | 7.8% |
| Islet autoantibodies |
IA‐2 Ab (−) d |
GAD Ab (−) IA‐2 Ab (−) |
GAD Ab (−) IA‐2 Ab (−) |
GAD Ab (+) IA‐2 Ab (−) |
| Thyroid autoantibodies |
TgAb (−) e TPOAb (−) f |
No data | No data |
TgAb (−) TPOAb (−) |
|
ClassII HLA genotype c |
DRB1*04:05 /13:02 DQB1*04:01 /06:04 |
DRB1*09:01 /09:01 DQB1*02:03 /03:03 |
DRB1*04;05 /08:03 DQB1*04:01 /06:01 |
DRB1*04:05 /09:01 DQB1*03:03 /04:01 |
Ab, antibody.
GAD, glutamic acid decarboxylase.
HLA, human leukocyte antigen.
IA‐2; insulinoma‐associated protein‐2.
Tg, thyroglobulin.
TPO, thyroid peroxidase.
Oikawa et al. showed a negative correlation between time from the onset of premonitory symptoms to diagnosis and anti‐GAD antibody levels in GAD‐positive fulminant type 1 diabetes. 13 The patient in the present study showed more rapid onset of GAD‐positive fulminant type 1 diabetes (4.4 days earlier than that in the national survey results) 6 and was hospitalized approximately 24 h after the onset of premonitory symptoms. Nevertheless, GAD antibodies remained moderately positive (44.6 IU/L), and the results were not consistent with those of previous studies.
More than 70% of patients with fulminant type 1 diabetes have upper respiratory and gastrointestinal symptoms, and an increase in pancreatic exocrine enzymes. 1 , 6 In this study, a minimal increase in pancreatic amylase levels was observed in the patient during the course. In addition, a marked increase in pancreatic PLA2, trypsin, and elastase‐1 levels was observed. The analysis of 45 days after the onset showed persistent elevation in these values, suggesting rapid progression of pancreatic inflammation at the onset of fulminant type 1 diabetes.
Tokunaga et al. reported that ADC values, which are calculated from diffusion‐weighted MRI (DWI), are low in all parts of the pancreas early in fulminant type 1 diabetes and increase over time, making ADC values a useful additional tool for diagnosing fulminant type 1 diabetes. 14 MRI revealed that the size of the pancreas did not change between Days 8 and 50 after the onset. However, MRI showed higher ADC values at Day 50 than at Day 8, which corroborated with the results of previous studies on fulminant type 1 diabetes. 14
Considering the fever of the patient 4 days before the onset, he might have had a preceding infection. This is consistent with the clinical course after the onset of typical fulminant type 1 diabetes.
It has been reported that both specific IgG antibodies and neutralizing antibodies peak at 4–30 days after administering the BNT16262 mRNA vaccine and decrease thereafter. 15
Considering the relatively long period after vaccination (15 weeks after vaccination), this may not be due to the direct effects of vaccination as in previously reported cases of fulminant type 1 diabetes. 9 , 10 , 11 The results may be due to multiple coincidences during the relatively long period (15 weeks) after vaccination. HLA analysis showed genotypes associated with fulminant type 1 diabetes risk.
Therefore, the production of anti‐GAD antibody production during the clinical course of typical fulminant type 1 diabetes may be the only factor associated with vaccination.
In a previous study, Yasuda et al. reported a case of fulminant type 1 diabetes with thrombocytopenia following influenza vaccination in which anti‐GAD and anti‐IA‐2 antibodies were negative. 16
Vera‐Lastra et al. 17 reported two cases of Graves' disease following COVID‐19 vaccination, detailing an autoimmune/inflammatory syndrome induced by adjuvants in the vaccines. Our patient did not demonstrate thyroid dysfunction or abnormalities in thyroid autoantibodies.
Discussions on autoimmune diseases that occur with COVID‐19 vaccination are limited, although it is difficult to prove the extent to which vaccination contributes to autoimmune disease onset. Therefore, the results must be interpreted cautiously.
In conclusion, we reported a case of a patient who developed fulminant type 1 diabetes, 15 weeks after the COVID‐19 vaccination. There have been a few reported cases of fulminant type 1 diabetes after vaccination (anti‐GAD antibodies were negative in all cases). The patient in this study was diagnosed with anti‐GAD antibody‐positive fulminant type 1 diabetes.
The association between COVID‐19 vaccination and type 1 diabetes onset is yet to be fully elucidated. Physicians should be aware of the possibility of the onset of type 1 diabetes in patients with haplotypes associated with type 1 diabetes several months after COVID‐19 vaccination.
AUTHOR CONTRIBUTIONS
T.K. and F.Y. designed the project. T.K., F.Y., and M.S. obtained written informed consent from the patient. T.K., F.Y., and M.S. clinically characterized the patient and collected clinical information. T.K. and F.Y. wrote the manuscript. M.S., A.H., H.A., Y.H., D.Y., N.A., A.O., and T.M. were major contributors to the editing of the manuscript. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with this journal's patient consent policy.
ACKNOWLEDGMENTS
We would like to the thank Editage for English language editing.
Kobayashi T, Yakou F, Saburi M, et al. New‐onset atypical fulminant type 1 diabetes after COVID‐19 vaccination: A case report. Clin Case Rep. 2022;10:e06473. doi: 10.1002/ccr3.6473
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
REFERENCES
- 1. Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes‐related antibodies. Osaka IDDM study group. N Engl J Med. 2000;342(5):301‐307. doi: 10.1056/NEJM200002033420501 [DOI] [PubMed] [Google Scholar]
- 2. Abu‐Rumaileh MA, Gharaibeh AM, Gharaibeh NE. COVID‐19 vaccine and hyperosmolar hyperglycemic state. Cureus. 2021;13(3):e14125. doi: 10.7759/cureus.14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of Graves' disease and type 1 diabetes mellitus following SARS‐CoV‐2 vaccination. J Autoimmun. 2021;125:102738. doi: 10.1016/j.jaut.2021.102738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sasaki H, Itoh A, Watanabe Y, et al. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: a case report. J Diabetes Investig. 2022;13(6):1105‐1108. doi: 10.1111/jdi.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yano M, Morioka T, Natsuki Y, et al. A case of new‐onset type 1 diabetes after Covid‐19 mRNA vaccination. Intern Med. 2022;61(8):1197‐1200. doi: 10.2169/internalmedicine.9004-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26(8):2345‐2352. doi: 10.2337/diacare.26.8.2345 [DOI] [PubMed] [Google Scholar]
- 7. Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute‐onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig. 2012;3(6):536‐539. doi: 10.1111/jdi.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsutsumi C, Imagawa A, Ikegami H, Makino H, Kobayashi T, Hanafusa T. Class II HLA genotype in fulminant type 1 diabetes: a nationwide survey with reference to glutamic acid decarboxylase antibodies. J Diabetes Investig. 2012;3(1):62‐69. doi: 10.1111/j.2040-1124.2011.00139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasaki K, Morioka T, Okada N, et al. New‐onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. J Diabetes Investig. 2022;13(7):1286‐1289. doi: 10.1111/jdi.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang X, He B, Liu Z, Zhou Z, Li X. Fulminant type 1 diabetes after COVID‐19 vaccination. Diabetes Metab. 2022;48(2):101324. doi: 10.1016/j.diabet.2022.101324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakurai K, Narita D, Saito N, et al. Type 1 diabetes mellitus following COVID‐19 RNA‐based vaccine. J Diabetes Investig. 2022;13(7):1290‐1292. doi: 10.1111/jdi.13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oikawa Y, Shimada A. Possible involvement of autoimmunity in fulminant type 1 diabetes. Diabetol Int. 2020;11(4):329‐335. doi: 10.1007/s13340-020-00460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tokunaga A, Imagawa A, Nishio H, et al. Diffusion‐weighted magnetic resonance imaging in the pancreas of fulminant type 1 diabetes. Diabetol Int. 2018;9(4):257‐265. doi: 10.1007/s13340-018-0355-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yasuda H, Nagata M, Moriyama H, et al. Development of fulminant type 1 diabetes with thrombocytopenia after influenza vaccination: a case report. Diabet Med. 2012;29(1):88‐89. doi: 10.1111/j.1464-5491.2011.03391.x [DOI] [PubMed] [Google Scholar]
- 17. Vera‐Lastra O, Navarro OA, Domiguez CMP, Medina G, Valadez STI, Jara LJ. Two cases of Graves' disease following SARS‐CoV‐2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31(9):1436‐1439. doi: 10.1089/thy.2021.0142 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.


