Abstract
Background
Head computed tomography (CT) is a guideline recommended method to predict functional outcome after cardiac arrest (CA), but standardized criteria for evaluation are lacking. To date, no prospective trial has systematically validated methods for diagnosing hypoxic-ischaemic encephalopathy (HIE) on CT after CA. We present a protocol for validation of pre-specified radiological criteria for assessment of HIE on CT for neuroprognostication after CA.
Methods/design
This is a prospective observational international multicentre substudy of the Targeted Hypothermia versus Targeted Normothermia after out-of-hospital cardiac arrest (TTM2) trial. Patients still unconscious 48 hours post-arrest at 13 participating hospitals were routinely examined with CT. Original images will be evaluated by examiners blinded to clinical data using a standardized protocol. Qualitative assessment will include evaluation of absence/presence of “severe HIE”. Radiodensities will be quantified in pre-specified regions of interest for calculation of grey-white matter ratios (GWR) at the basal ganglia level. Functional outcome will be dichotomized into good (modified Rankin Scale 0–3) and poor (modified Rankin Scale 4–6) at six months post-arrest. Prognostic accuracies for good and poor outcome will be presented as sensitivities and specificities with 95% confidence intervals (using pre-specified cut-offs for quantitative analysis), descriptive statistics (Area Under the Receiver Operating Characteristics Curve), inter- and intra-rater reliabilities according to STARD guidelines.
Conclusions
The results from this prospective trial will validate a standardized approach to radiological evaluations of HIE on CT for prediction of functional outcome in comatose CA patients.
The TTM2 trial and the TTM2 CT substudy are registered at ClinicalTrials.gov NCT02908308 and NCT03913065.
Keywords: Neuroprognostication, Computed tomography, Hypoxic-ischaemic encephalopathy (HIE), Cardiac arrest, Targeted temperature management, Outcome, GWR grey-white matter ratio
Background
Post-cardiac arrest brain injury is the result of a transient ischaemia with subsequent reperfusion which may manifest as hypoxic-ischaemic encephalopathy (HIE) on neuroimaging.1 Guidelines recommend that the presence of “diffuse and extensive anoxic injury” on head computed tomography (CT) or magnetic resonance imaging (MRI) may be used as a predictor of poor neurological outcome after cardiac arrest (CA), yet the level of evidence is low.2 Despite its widespread clinical use, meta-analyses have concluded that the majority of prognostic studies on neuroimaging are single centre studies limited by a retrospective design.2, 3, 4, 5 Furthermore, radiological evaluation of HIE on CT for prognostic purposes lacks standardized criteria.2 Its performance for outcome prediction in clinical practice is likely influenced by both interrater variability (at least partly due to lack of standardization of the CT evaluation process) and technical parameters – inter-scanner variability.6, 7, 8, 9, 10, 11 This potentially introduces a risk for a false pessimistic prediction of poor outcome.7, 8
To improve prognostic performance, various approaches to quantitative CT analysis have been investigated, but their clinical application is limited by the lack of consensus on which regions of interest (ROI) and cutoff values are most valid.4, 12, 13, 14 We previously performed studies to compare prognostic accuracies and interrater variabilities of various qualitative, semi-quantitative and quantitative methods on CT for prediction of poor functional outcome.13, 15 We have used these results to establish Standard Operating Procedures (SOP) for both qualitative and quantitative radiological evaluations of head CT images in CA patients. Here we present these SOP and the protocol for their validation in a prospective international observational trial, the CT substudy of the targeted Hypothermia versus targeted Normothermia after out-of-hospital cardiac arrest (TTM2) trial.16
Hypotheses of the TTM2 CT substudy are:
In patients unconscious more than 48 h after CA:
-
1.
A standardized qualitative assessment of head CT has a higher prognostic accuracy than non-standardized qualitative assessment (current reference standard) for poor outcome prediction
-
2.The following findings are predictive of a poor functional outcome with 0% false positive ratio (FPR):
-
a)Definite signs of “severe HIE” diagnosed using our SOP for qualitative assessment.
-
b)Grey-white matter ratio (GWR) at the basal ganglia level below pre-specified cutoffs (1.10 and 1.15) determined using our SOP for quantitative assessment.
-
c)Automated, atlas-based GWR on the basal ganglia level (auto GWR) below 1.10 as described by Kenda et al.17.
-
a)
-
3.
The prognostic accuracies of the CT analysis methods described above is independent from the type of CT scanner, previous use of CT contrast agent and targeted temperature management.
-
4.
No patient with “severe HIE” on head CT will have low blood levels of the brain injury marker Neurofilament light (NFL) (0% FPR).18
-
5
The reliability of agreement for prediction of poor outcome using qualitative and quantitative assessment of head CT will be good (Fleisś kappa > 0.7).19
Methods/design
Participants and ethical consent
The TTM2-trial (Clinicaltrials.gov NCT02908308) was an international, multicentre, parallel group, investigator-initiated trial which randomised 1900 adult patients with an out-of-hospital cardiac arrest to a target temperature of 33°C or to a strategy to maintain normothermia and early treatment of fever (≥37.8°C).16, 20
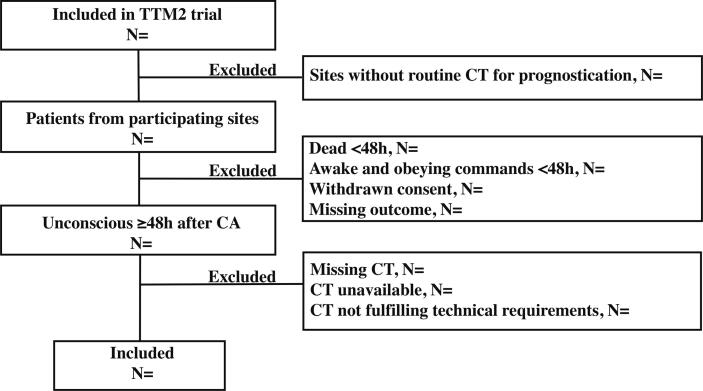
The TTM2-CT-substudy (Clinicaltrials.gov NCT039130659) is a prospective international multicentre observational study examining the prognostic accuracy of head CT for prediction of functional outcome after CA. Between 11/2017 and 01/2020, patients were recruited at 13 TTM2 sites routinely examining patients still unconscious at 48 hours post-arrest with head CT (Flowchart Fig. 1). Unconsciousness was defined as not obeying verbal commands and a response to painful stimulus < 4 on the Full-Outline of Unresponsiveness (FOUR) motor response (at best localizing pain).20, 21.
Fig. 1.
Flowchart patient inclusion for CT substudy CT, head computed tomography; N, number of patients; h, hours.
Each participating country obtained approval by the appropriate ethics committee prior to patient enrolment.16 Patients were treated according to the TTM2-trial protocols regarding inclusion and exclusion criteria, randomisation, clinical management, neurological prognostication, decisions on withdrawal of life-sustaining therapy (WLST) and follow-up.20, 22, 23
Procedure
CT images were initially evaluated by radiologists at the patient’s local hospital without any pre-specified criteria for evaluation and results were available to the treating team when predicting outcome. The results of the local radiologists’ evaluations were entered into the electronic case report form (eCRF) as a “yes”/”no” answer to the question “Are there signs of global, diffuse or bilateral multifocal ischaemic injury on CT?”. The date and time for examination and the number of CT examinations performed was also reported.
Technical requirements and data collection
For this substudy, collection of the original Digital Imaging and Communications in Medicine (DICOM) images was initiated after randomisation of the last patient in the TTM2 trial. Images were collected and stored in a digital database labelled with the patient́s trial identification number. Data will be kept according to national legislation for record keeping.
All types of scanners and software were permitted, and specifics for each scan will be registered and analysed. Technical prerequisites for including CTs in the analysis are: Axial slices of 4–5 mm slice thickness of the entire brain available with tube voltage 120 kV. We will extract the following technical metadata: time of CT examination, manufacturer, scanner and convolution kernel. Radiological evaluations will be started after this study protocol has been accepted for publication. Examiners will either be radiologists or neurologists, all with clinical experience in CT evaluation of CA patients.
Outcomes
Patient outcomes will be poor functional outcome six months after randomisation, defined as modified Rankin Scale (mRS) 4 -6.22 Serum levels of neurofilament light (NFL) at 48 h post-randomisation analysed with an Elecsys® electrochemiluminescence immunoassay (ECLIA) will be used as a surrogate marker of brain injury.24 The level of agreement between examiners evaluations will be reported using measures of inter- and intra-rater variability as described below.
Standardized operating procedure for CT evaluation
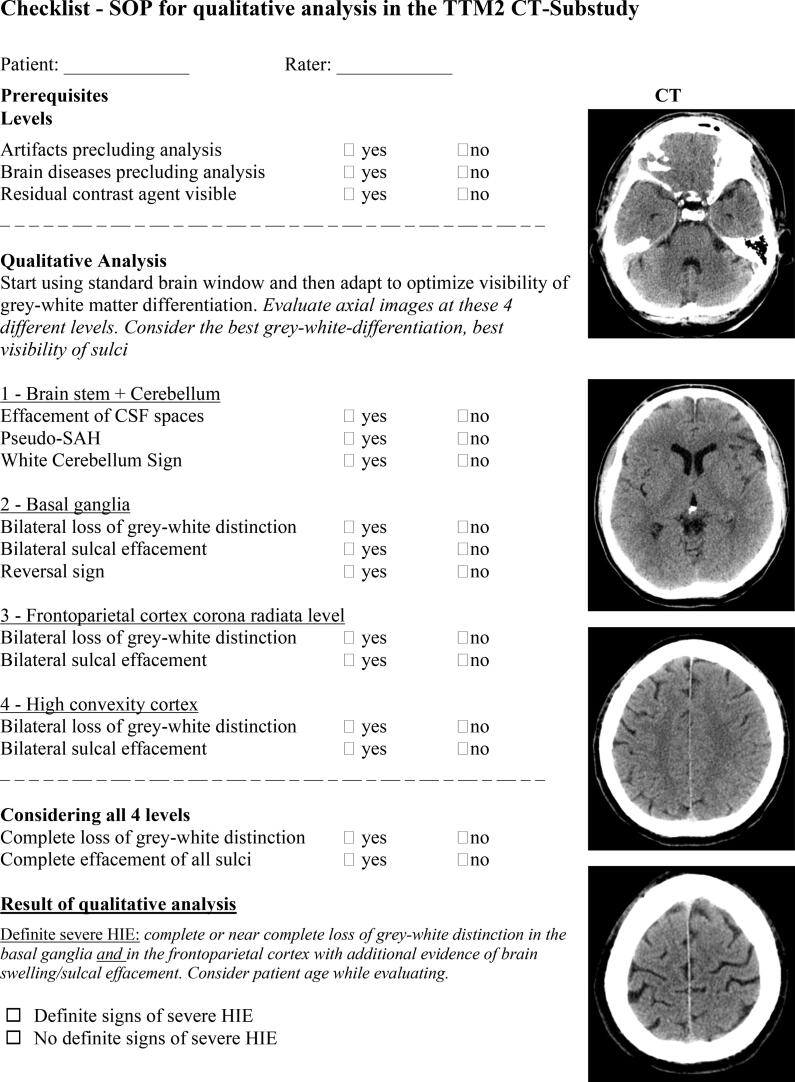
SOP qualitative analysis
Qualitative analysis will be performed according to the checklist in Fig. 2. The full SOP can be seen in S1. The examiners will first determine whether the prerequisites for qualitative analysis are fulfilled; that there are no imaging artifacts precluding analysis, and absence of significant intracranial pathologies such as haemorrhage, stroke, tumour, extensive calcification which could interfere with analysis. Importantly, CTs with moderate brain atrophy, moderate vascular leukoencephalopathy or chronic strokes not affecting analysis of grey-white matter distinction or sulcal effacement at the basal ganglia level and bilateral frontoparietal regions should be used for analysis. The examiner will also report whether residual contrast agent from for example coronary angiography is visible.
Fig. 2.
SOP checklist for qualitative analysis Standardised operating procedure checklist for qualitative radiological evaluation. CSF; cerebrospinal fluid, SAH; subarachnoid haemorrhage, HIE; hypoxic-ischaemic encephalopathy.
Image evaluation starts by applying a standard “brain window” (WW: 80, WL: 40) which is then adapted to optimize visibility of grey-white-matter differentiation.25 The focus of evaluation will be on distinction of grey and white matter and/or on sulcal effacement evaluated at the following levels: 1) brainstem + cerebellum, 2) basal ganglia, 3) cortex at corona radiata level, 4) high convexity cortex. Cerebrospinal fluid spaces will be evaluated considering the age of the patient (i.e physiologically high brain volume and small subarachnoid spaces in young patients).
Additional characteristics of severe HIE may include “Pseudo-subarachnoid haemorrhage sign” (hyperdense cerebrospinal fluid signal in basal cisterns or over the convexity), “reversal sign” (lower radiodensity of grey compared to white matter structures) and “white cerebellum sign” (higher radiodensity of cerebellum as compared to cerebral hemispheres).26, 27
The main question to be answered after evaluation of the entire CT is “Are there definite signs of severe HIE?” This question should be answered with “yes”, if complete or near-complete loss of grey-white distinction is noted both in the 1) basal ganglia and 2) in the frontoparietal lobes bilaterally with additional evidence of brain swelling/sulcal effacement. Severe HIE will also be diagnosed from complete sulcal effacement when residual grey-white distinction is present.
SOP quantitative analysis – grey-white matter ratio
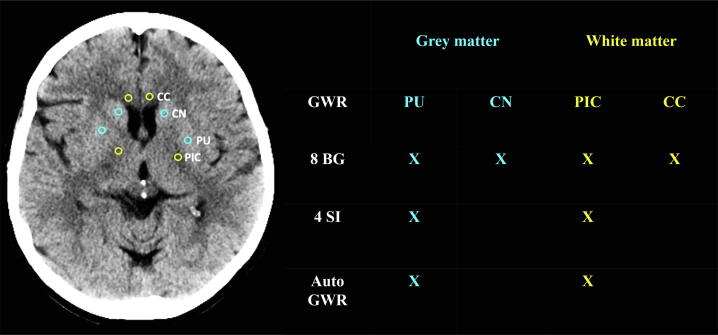
For quantitative analysis, circular ROIs (0.1 cm2) will be placed manually in pre-defined anatomical regions of grey and white matter bilaterally at the basal ganglia level (Figs. 3, S2). The examiners are instructed to check the Hounsfield Units (HU) during measurements to ensure ROI placement in an area where the radiodensity is representative of the entire target brain region. Positioning ROIs in focal hypo- or hyperdensities, e.g. resulting from small vascular lesions, calcifications or noise must be avoided. Grey-white-matter ratios (GWR) will be calculated as the average density of the grey matter ROIs divided by the average density of the white matter ROIs using either 8 ROIs or for a simplified version, 4 ROIs at the basal ganglia level (Fig. 3).
Fig. 3.
SOP for the qualitative measurement of grey-white matter ratio Placement of Regions of Interest (ROI) for determination of the grey-white matter ratio (GWR) at the basal ganglia level including 8 or 4 ROIs.12, 13 Yellow indicates white matter ROIs and blue indicates ROIs in the grey matter. All axial slices containing basal ganglia structures should be evaluated and ROIs placed bilaterally in the slice best representative of that target region. Thus, these 8 ROIs may be placed in different slices: 1) Putamen (PU); 2) Head of the caudate nucleus (CN); 3) Posterior limb of the internal capsule (PIC), and 4) Genu of the corpus callosum (CC). In case of complete loss of grey-white distinction, use ventricles and midline as landmarks. In some patients with severe HIE radiodensity is similar in grey and white matter, exact location of target regions cannot always be determined. Nonetheless, ROIs should be placed and patients should not be excluded from GWR determination. Crosses indicate the ROIs included in each grey-white matter ratio method: 8 BG (basal ganglia model), 4SI (simple model) and auto GWR (automated GWR determination).17.
Automated density measurements
In addition to our qualitative and quantitative SOPs, we will apply automated techniques for CT evaluation. First, we will validate an already published algorithm for automated GWR-assessment. Scans fulfilling technical prerequisites as stated above and without significant artifacts or acute/chronic pathologies other than HIE will be analysed. CT scans will be co-registered to an MRI-based digital standard brain using the free analysis software FSL-FMRIB Software v5.0 by Analysis Group, FMRIB, Oxford, UK) as previously described.17 Tissue probability maps derived from standard brain atlases will then be used to automatically identify anatomical regions on each CT scan. GWR will be calculated by the densities of the putamen and of the posterior limb of the internal capsule (Fig. 3).17
Plan for statistical analysis
The reporting of results will follow the recommendations of the Standards for Reporting Diagnostic Studies (STARD).28 We will present a flow-chart of included and excluded patients, and reasons for exclusion (Fig. 1). We will describe patient data as displayed in Table 1.
Table 1.
Example table for clinical characteristics and baseline variables.
|
Included N= |
Excluded N= |
|
|---|---|---|
| Demographic characteristics | ||
| Age (years) | Mean (SD) | Mean (SD) |
| Male | N (%) | N (%) |
| Time from CA to first CT performed48 h | Median (IQR) | Median (IQR) |
| Characteristics of cardiac arrest | ||
| Minutes from CA to ROSC | Median (IQR) | Median (IQR) |
| First monitored rhythm on ECG shockable | N (%) | N (%) |
| Bystander witnessed CA | N (%) | N (%) |
| Bystander CPR performed | N (%) | N (%) |
| Cardiac arrest at the place of residence | N (%) | N (%) |
| Clinical characteristics on hospital admission | ||
| Corneal reflexes bilaterally absent on hospital admission | N (%) | N (%) |
| Pupillary reflexes bilaterally absent on hospital admission | N (%) | N (%) |
| ST-segment elevation myocardial infarction | N (%) | N (%) |
| Arterial lactate level on admission mmol/L | Mean (SD) | Mean (SD) |
| Circulatory shock on admission | N (%) | N (%) |
| Randomised to hypothermia | N (%) | N (%) |
| Medical history | ||
| Hypertension | N (%) | N (%) |
| Dementia | N (%) | N (%) |
| Cerebrovascular disease | N (%) | N (%) |
| Hemiplegia | N (%) | N (%) |
| Diabetes | N (%) | N (%) |
| Myocardial infarction | N (%) | N (%) |
| Heart failure NYHA III or IV | N (%) | N (%) |
| Renal failure | N (%) | N (%) |
| Moderate or severe liver failure | N (%) | N (%) |
| Charlson comorbidity index | Median (IQR) | Median (IQR) |
| Functional outcome after 180 days | ||
| Good outcome (mRS 0–3) | N (%) | N (%) |
| Poor outcome (mRS 4–6) | N (%) | N (%) |
| Structured assessment of neurological outcome at 180 days | ||
| mRS 0 | N (%) | N (%) |
| mRS 1 | N (%) | N (%) |
| mRS 2 | N (%) | N (%) |
| mRS 3 | N (%) | N (%) |
| mRS 4 | N (%) | N (%) |
| mRS 5 | N (%) | N (%) |
| mRS 6 | N (%) | N (%) |
| Withdrawal of life-sustaining therapy (WLST) | ||
| WLST performed | N (%) | N (%) |
| WLST neurological reason only | N (%) | N (%) |
| WLST neurological based on CT | N (%) | N (%) |
Data will be presented in numbers (percentages) for categorical variables, and in mean (standard deviation) or median (interquartile range) as appropriate for continuous variables.
Prognostic accuracies
For qualitative and quantitative evaluations, we will present sensitivities and specificities for prediction of poor functional outcome (mRS 4–6 at six months) with 95% confidence intervals (CI) calculated using Wilsońs method. Results will be reported separately for each examiner to avoid errors by averaging measurements by different investigators. Both for the manual and automated GWR evaluations we will validate cutoff values 1.10 and 1.15 for prediction of poor outcome. Several prior studies, including our own, suggest certainly poor outcome at GWR threshold below 1.10, yet at a cost of low sensitivity. To increase sensitivity we also validate the threshold 1.15.3, 4, 15
The overall prognostic performance for good versus poor functional outcome will also be tested by the area under the receiver-operating characteristics curve (AUROC) with 95% confidence intervals. P-values will be calculated based on a test of difference in AUROC using the method of DeLong.
Influence of targeted temperature management
Based on previous results, we do not expect that targeted temperature management influences the prognostic accuracies of CT.29 However, we will evaluate whether the approach to temperature management may have had any effect of the false positive predictions of poor outcome in our cohort.
Serum levels of neurofilament light as a surrogate marker of brain injury
Since results from CT evaluations by local radiologists were available upon clinical decision-making, we want to gather indirect evidence whether life-sustaining therapy withdrawn for neurological reasons (in this case based on CT findings) may have been leading to self-fulfilling prophecies. We will therefore investigate whether severe HIE was diagnosed from head CT in patients with low blood levels of neurofilament light, which indicate little or no brain injury.18
Reliability of agreement and intrarater variability
Fleisś kappa will be calculated as a measure of the reliability of agreement for qualitative analysis between investigators (local radiologists and study investigators) and between study investigators.19 With evaluation of n = 200 images, at least five blinded study examiners would yield a precision of 0.044 with 95% CI for Fleiss Kappa. To assess the intrarater variability (Coheńs Kappa), each examiner will re-examine 20% of images using the qualitative and quantitative SOP in evaluations performed independently from their first assessments.
Technical issues
A subgroup analysis will examine the group-wise difference in manually determined GWR between the different CT manufacturers, scanners, and convolution kernels.
Exploratory analyses
Exploratory analyses will include prediction of outcome using quantification of regional brain water uptake, in patients with two or more CTs as well as other artificial intelligence-based methods.30
Discussion
We present the study protocol of a prospective international multicentre trial which aims to validate prognostic accuracy of standardized radiological assessments of severe HIE on CT after CA. To our knowledge, no prospective studies have examined CTs acquired in CA patients at a pre-defined time-point 48 h post-arrest. The rationale behind the 48-hour time-point is based on retrospective studies demonstrating increased sensitivity of HIE for poor outcome prediction after the first 24 hours post-arrest.11, 29, 31, 32 The increasing sensitivity of late as compared to early (within the first hours after CA) head CT is in line with the pathophysiology of post-cardiac arrest brain injury, frequently developing over the first days post-arrest.1, 33 This is also demonstrated in studies using diffusion weighted imaging sequences in patients repeatedly examined with MRI and in decreasing GWR in patients repeatedly examined with CT.31, 34
Qualitative evaluation of HIE by local radiologists demonstrated a very high specificity in the TTM-trial, but since results were available to treating physicians, the risk of a self-fulfilling prophecy influencing outcome could not be excluded.29 In a recent guideline validation study from South-Korea where therapy is rarely withdrawn, Youn et al. reported a specificity of only 86% for the finding of a “poor CT” when evaluated by radiologists blinded to clinical data.6 Similar results have been reported for diffusion-weighted sequences on MRI.35 Although combinations of imaging techniques with other prognostic methods predicted poor outcome without false positive predictions in validation studies, the lack of a standardized radiological assessment of HIE may pose a risk for patients.6, 29, 36, 37 For this reason, since decisions on WLST within the TTM2 trial could also be based on CT findings, we will evaluate how well our radiological assessments correlate with blood levels of a surrogate marker of brain injury, the serum concentration of NFL. We have previously demonstrated that low levels of NFL predicted good outcome in 95% of patients, and that by combining biomarkers with other prognostic methods, the risk of false pathological CT evaluations could be minimized.18
Guidelines suggest “using the presence of a marked reduction of the GWR on brain CT within 72 h after ROSC in combination with other predictors for prognosticating a poor neurologic outcome in patients who are comatose after cardiac arrest and who are treated with TTM” (very low level of evidence).2 GWR cutoffs between 1.1 and 1.2 have demonstrated 100% specificity for poor outcome prediction.2 Our pilot study demonstrates that the GWR cutoff for 0% FPR varies with the anatomical localization of ROIs included in the measurement and is subject to interrater variability.15 Further, a quantitative assessment is rarely part of clinical routine and its implementation would require time-efficient tools that are easy to use. Previously, the 16 ROI model demonstrated lower levels of interrater variability compared to other models. Nonetheless, this method is not very well suited for practical use due to the high number of ROIs. The 8 ROI model only measuring densities at the basal ganglia level (BG) demonstrated similar prognostic accuracies and interrater variability as the 16 ROI model.12 We also include a simplified 4 ROI version at the basal ganglia level, which is the preferred GWR model by the examiners using GWR in clinical practice.13 However, since the simplified version demonstrated larger interrater variability in our pilot study, we want to validate whether this GWR model is indeed as accurate as the 8 BG model.
We previously found that a GWR below 1.1 predicted poor outcome with 100% specificity whilst a GWR below 1.15 in combination with severe HIE on the qualitative evaluation increased sensitivity and maintained this high specificity.15 Since a radiological evaluation always includes the qualitative assessment, GWR measurements should be used as an add-on for the highest accuracy.
Based on previous results, we include technical prerequisites for evaluation. Only CTs with axial slices of 4–5 mm and a tube voltage of 120kvP will be used in this study since HU are highly dependent on these parameters. We will also investigate to which extent the type of CT scanner used or whether residual contrast agent from coronary angiography may influence the reliability of our SOP.
Strengths and limitations
Strengths include the prospective design and the blinded assessment by several examiners. As serum NFL samples were analysed after trial completion, we can compare CT evaluation with an independent marker of HIE severity which was not available during clinical decision-making.24 The TTM2 trial had a standardized and conservative approach to neurological prognostication with strict criteria for WLST.16 Evaluation of functional outcome was standardized and performed by experienced investigators blinded to clinical data.16, 22 The standardized criteria for radiological assessments presented in this manuscript were defined by radiologists and neurologists with clinical experience in radiological neuroprognostication and are based on results from pilot studies.15 We acknowledge, that these criteria partly reflect on our own clinical traditions, yet they are a step towards a standardization of head CT analysis for neuroprognostication.
Our study has several limitations: the TTM2 trial included adult patients with an out-of-hospital CA with a presumed cardiac origin, or with an unknown cause with a stable return to spontaneous circulation. Our results should be validated in other patient cohorts since results may vary. The results from local radiological evaluations were included when making decisions on level-of-care and the risk of a self-fulfilling prophecy cannot be excluded. We will therefore examine whether WLST for neurological reasons was performed in patients diagnosed with severe HIE on CT despite low serum levels of NFL as a surrogate marker of brain injury.
Conclusion
The results from this prospective trial will provide a unique opportunity to validate a standardized approach to quantitative and qualitative radiological evaluations of HIE on CT for prediction of functional outcome in comatose cardiac arrest patients. We believe that our study will deliver clinically important information on an area where level of evidence is sparse.
Funding
The TTM2 trial is supported by independent research grants from nonprofit or governmental agencies (the Swedish Research Council [Vetenskapsrådet], Swedish Heart–Lung Foundation, Stig and Ragna Gorthon Foundation, Knutsson Foundation, Laerdal Foundation, Hans-Gabriel and Alice Trolle-Wachtmeister Foundation for Medical Research, and Regional Research Support in Region Skåne) and by governmental funding of clinical research within the Swedish National Health Service. In addition, the CT substudy is supported by grants from the Bundy Academy, the Segerfalk Foundation and the Elsa Schmitz Foundation. MK is supported by the Laerdal Foundation and the Berlin Institute of Health Junior Digital Clinician Scientist Program. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Conflicts of interest
CL declares institutional fees for lectures from Bard and Zoll. ND reports past lecture and travel fees for Bard and Zoll companies outside the present work. No other conflicts of interest were reported.
Acknowledgements
The authors would like to thank Michael Borring from Skåne university hospital, Malmö for help with data collection and storage.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2022.100316.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Sandroni C., Cronberg T., Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47:1393–1414. doi: 10.1007/s00134-021-06548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nolan J.P., Sandroni C., Bottiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation. 2021;161:220–269. doi: 10.1016/j.resuscitation.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Sandroni C., D'Arrigo S., Cacciola S., et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46:1803–1851. doi: 10.1007/s00134-020-06198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keijzer H.M., Hoedemaekers C.W.E., Meijer F.J.A., Tonino B.A.R., Klijn C.J.M., Hofmeijer J. Brain imaging in comatose survivors of cardiac arrest: Pathophysiological correlates and prognostic properties. Resuscitation. 2018;133:124–136. doi: 10.1016/j.resuscitation.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Soto C., Dragoi L., Heyn C.C., et al. Imaging for Neuroprognostication After Cardiac Arrest: Systematic Review and Meta-analysis. Neurocrit Care. 2020;32:206–216. doi: 10.1007/s12028-019-00842-0. [DOI] [PubMed] [Google Scholar]
- 6.Youn C.S., Park K.N., Kim S.H., et al. External validation of the 2020 ERC/ESICM prognostication strategy algorithm after cardiac arrest. Crit Care. 2022;26:95. doi: 10.1186/s13054-022-03954-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caraganis A., Mulder M., Kempainen R.R., et al. Interobserver Variability in the Recognition of Hypoxic-Ischemic Brain Injury on Computed Tomography Soon After Out-of-Hospital Cardiac Arrest. Neurocrit Care. 2020;33:414–421. doi: 10.1007/s12028-019-00900-7. [DOI] [PubMed] [Google Scholar]
- 8.Oh J.H., Choi S.P., Wee J.H., Park J.H. Inter-scanner variability in Hounsfield unit measured by CT of the brain and effect on gray-to-white matter ratio. Am J Emergency Med. 2019;37:680–684. doi: 10.1016/j.ajem.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Oh J.H., Choi S.P., Zhu J.H., et al. Differences in the gray-to-white matter ratio according to different computed tomography scanners for outcome prediction in post-cardiac arrest patients receiving target temperature management. PLoS One. 2021;16:e0258480. doi: 10.1371/journal.pone.0258480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beekman R., Maciel C.B., Ormseth C.H., et al. Early head CT in post-cardiac arrest patients: A helpful tool or contributor to self-fulfilling prophecy? Resuscitation. 2021;165:68–76. doi: 10.1016/j.resuscitation.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch K., Heymel S., Gunther A., et al. Prognostication of neurologic outcome using gray-white-matter-ratio in comatose patients after cardiac arrest. BMC Neurol. 2021;21:456. doi: 10.1186/s12883-021-02480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metter R.B., Rittenberger J.C., Guyette F.X., Callaway C.W. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation. 2011;82:1180–1185. doi: 10.1016/j.resuscitation.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentsch A., Storm C., Leithner C., et al. Outcome prediction in patients after cardiac arrest: a simplified method for determination of gray-white matter ratio in cranial computed tomography. Clin Neuroradiol. 2015;25:49–54. doi: 10.1007/s00062-013-0281-3. [DOI] [PubMed] [Google Scholar]
- 14.Scheel M., Storm C., Gentsch A., et al. The prognostic value of gray-white-matter ratio in cardiac arrest patients treated with hypothermia. Scandinavian J Trauma, Resuscitat Emergency Med. 2013;21:23. doi: 10.1186/1757-7241-21-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang M., Nielsen N., Ullen S., et al. A pilot study of methods for prediction of poor outcome by head computed tomography after cardiac arrest. Resuscitation. 2022;179:61–70. doi: 10.1016/j.resuscitation.2022.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Dankiewicz J., Cronberg T., Lilja G., et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med. 2021;384:2283–2294. doi: 10.1056/NEJMoa2100591. [DOI] [PubMed] [Google Scholar]
- 17.Kenda M., Scheel M., Kemmling A., et al. Automated Assessment of Brain CT After Cardiac Arrest-An Observational Derivation/Validation Cohort Study. Crit Care Med. 2021;49:e1212–e1222. doi: 10.1097/CCM.0000000000005198. [DOI] [PubMed] [Google Scholar]
- 18.Moseby-Knappe M., Mattsson-Carlgren N., Stammet P., et al. Serum markers of brain injury can predict good neurological outcome after out-of-hospital cardiac arrest. Intensive Care Med. 2021;47:984–994. doi: 10.1007/s00134-021-06481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schober P., Mascha E.J., Vetter T.R. Statistics From A (Agreement) to Z (z Score): A Guide to Interpreting Common Measures of Association, Agreement, Diagnostic Accuracy, Effect Size, Heterogeneity, and Reliability in Medical Research. Anesth Analg. 2021;133:1633–1641. doi: 10.1213/ANE.0000000000005773. [DOI] [PubMed] [Google Scholar]
- 20.Dankiewicz J., Cronberg T., Lilja G., et al. Targeted hypothermia versus targeted Normothermia after out-of-hospital cardiac arrest (TTM2): A randomized clinical trial-Rationale and design. Am Heart J. 2019;217:23–31. doi: 10.1016/j.ahj.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Wijdicks E.F., Bamlet W.R., Maramattom B.V., Manno E.M., McClelland R.L. Validation of a new coma scale: The FOUR score. Annals of neurology. 2005;58:585–593. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 22.Lilja G., Nielsen N., Ullen S., et al. Protocol for outcome reporting and follow-up in the Targeted Hypothermia versus Targeted Normothermia after Out-of-Hospital Cardiac Arrest trial (TTM2) Resuscitation. 2020;150:104–112. doi: 10.1016/j.resuscitation.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Blennow Nordstrom E., Lilja G., Vestberg S., et al. Neuropsychological outcome after cardiac arrest: a prospective case control sub-study of the Targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest trial (TTM2) BMC Cardiovasc Disord. 2020;20:439. doi: 10.1186/s12872-020-01721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moseby-Knappe M., Levin H., Blennow K., et al. Biomarkers of brain injury after cardiac arrest; a statistical analysis plan from the TTM2 trial biobank investigators. Resusc Plus. 2022;10 doi: 10.1016/j.resplu.2022.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zatz, LM. In: T.H. Newton, D.G. Potts, (Eds.), Technical Aspects of Computed Tomography. (Mosby, 1981). p. 3853–76.
- 26.Yuzawa H., Higano S., Mugikura S., et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol. 2008;29:1544–1549. doi: 10.3174/ajnr.A1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalela J.A., Rothlisberger J., West B., Hays A. The white cerebellum sign: an under recognized sign of increased intracranial pressure. Neurocrit Care. 2013;18:398–399. doi: 10.1007/s12028-013-9836-z. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J.F., Korevaar D.A., Altman D.G., et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799. doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moseby-Knappe M., Pellis T., Dragancea I., et al. Head computed tomography for prognostication of poor outcome in comatose patients after cardiac arrest and targeted temperature management. Resuscitation. 2017;119:89–94. doi: 10.1016/j.resuscitation.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Broocks G., Flottmann F., Ernst M., et al. Computed Tomography-Based Imaging of Voxel-Wise Lesion Water Uptake in Ischemic Brain: Relationship Between Density and Direct Volumetry. Invest Radiol. 2018;53:207–213. doi: 10.1097/RLI.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 31.Streitberger K.J., Endisch C., Ploner C.J., et al. Timing of brain computed tomography and accuracy of outcome prediction after cardiac arrest. Resuscitation. 2019;145:8–14. doi: 10.1016/j.resuscitation.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 32.In Y.N., Lee I.H., Park J.S., et al. Delayed head CT in out-of-hospital cardiac arrest survivors: Does this improve predictive performance of neurological outcome? Resuscitation. 2022;172:1–8. doi: 10.1016/j.resuscitation.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Sekhon M.S., Ainslie P.N., Griesdale D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21:90. doi: 10.1186/s13054-017-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heradstveit B.E., Larsson E.M., Skeidsvoll H., et al. Repeated magnetic resonance imaging and cerebral performance after cardiac arrest–a pilot study. Resuscitation. 2011;82:549–555. doi: 10.1016/j.resuscitation.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Ryoo S.M., Jeon S.B., Sohn C.H., et al. Predicting Outcome With Diffusion-Weighted Imaging in Cardiac Arrest Patients Receiving Hypothermia Therapy: Multicenter Retrospective Cohort Study. Crit Care Med. 2015;43:2370–2377. doi: 10.1097/CCM.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 36.Bongiovanni F., Romagnosi F., Barbella G., et al. Standardized EEG analysis to reduce the uncertainty of outcome prognostication after cardiac arrest. Intensive Care Med. 2020;46:963–972. doi: 10.1007/s00134-019-05921-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S.E., Maciel C.B., Ormseth C.H., Beekman R., Gilmore E.J., Greer D.M. Distinct predictive values of current neuroprognostic guidelines in post-cardiac arrest patients. Resuscitation. 2019;139:343–350. doi: 10.1016/j.resuscitation.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.