Graphical abstract
Keywords: Ultrasound, Sugarcane juice, Novel technologies, Microbial inactivation
Highlights
-
•
The role of thermal and non-thermal techniques for sugarcane juice processing were reviewed.
-
•
HHP is the most utilized non-thermal technique for sugarcane juice processing.
-
•
In novel thermal techniques, ohmic heating (OH) results are preferable.
Abstract
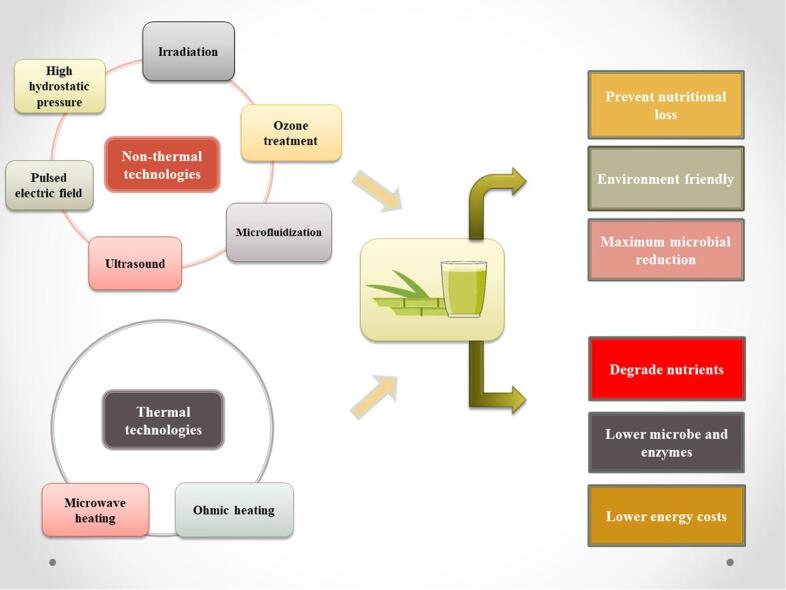
Sugarcane juice (Saccharum officinarum) is a proven nutritious beverage with high levels of antioxidants, polyphenols, and other beneficial nutrients. It has recently gained consumer interest due to its high nutritional profile and alkaline nature. Still, high polyphenolic and sugar content start the fermentation in juice, resulting in dark coloration. Lately, some novel techniques have been introduced to extend shelf life and improve the nutritional value of sugarcane juice. The introduction of such processing technologies is beneficial over conventional processes and essential for producing chemical-free, high-quality, fresh juices. The synergistic impact of these novel technologies is also advantageous for preserving sugarcane juice. In literature, novel thermal, non-thermal and hurdle technologies have been executed to preserve sugarcane juice. These technologies include high hydrostatic pressure (HHP), ultrasound (US), pulsed electric field (PEF), ultraviolet irradiation (UV), ohmic heating (OH), microwave (MW), microfludization and ozone treatment. This review manifests the impact of novel thermal, non-thermal, and synergistic technologies on sugarcane juice processing and preservation characteristics. Non-thermal techniques have been successfully proved effective and showed better results than novel thermal treatments. Because they reduced microbial load and retained nutritional content, while thermal treatments degraded nutrients and flavor of sugarcane juice. Among non-thermal treatments, HHP is the most efficient technique for the preservation of sugarcane juice while OH is preferable in thermal techniques due to less nutritional loss.
1. Introduction
Sugarcane, a grass plant that may be found in 36 forms, contains no fats and is a 100 % natural drink. Sugarcane juice (SCJ) is extracted from the sugarcane culms with the help of mechanical compression, and it is consumed in the local market with few sanitary precautions. A single 8-ounce SCJ serving has 250 calories and 30 g of natural sugars without additives. It is fat-free, cholesterol-free, fiber-free, and protein-free, including sodium, potassium, calcium, magnesium, and iron. This sugary drink is a popular summer drink that keeps hydrated and provides many health advantages, as shown in Fig. 1. It provides anti-allergic, hepatoprotective, anti-inflammatory, and cardioprotective effects to our bodies [1]. Calcium-rich advantages promote the development of the skeletal system, bones, and teeth. In addition, as a natural low-cholesterol, low-sodium diet with no saturated fats, SCJ aids the kidneys. However, diabetics might not want to drink SCJ because it has a lot of sugar. Since natural sugar has a low glycemic index, it does not cause blood glucose levels to jump as quickly as refined sugar. Hence, in moderation, SCJ may be beneficial to people with diabetes. Additionally, sugarcane juice’s potassium regulates the pH levels in the stomach, aids in releasing digestive fluids, and keeps the system running smoothly. Therefore, it is very beneficial for persons who have digestive problems. SCJ is naturally alkaline due to its high content of calcium, magnesium, potassium, iron, and manganese. The presence of flavonoids helps the body fight against malignant cells, notably prostate and breast cancer. Alkaline nature helps maintain electrolyte balance in the body.
Fig. 1.
Health advantages of sugarcane juice.
Regarding its benefits, SCJ has some limitations that affect its consumption rate. The main problems with fresh SCJ are its shorter shelf life (turns brown after some time) and sensitivity to flavors [2]. In SCJ, polyphenol oxidase (PPO), peroxidase (POD) enzymes acidity level (pH 5.0–5.5), and high amounts of phenolic and sugars cause enzymatic degradation and microbial fermentation [3]. Moreover, SCJ contains a large amount of sucrose, providing a medium for microbial growth. Degradation leads to the loss of sucrose with the formation of organic acid and ethanol caused by microbes. They convert sucrose into polysaccharides such as dextrins. Due to these microorganisms, fermentation occurs by interaction with carbohydrates that make juice unfavorable for human consumption [4]. SCJ cannot be sold as a fresh juice due to its high polyphenolic, sugar content and pH that turn it brown after some time. It is sold in bottles as a pasteurized beverage [2]. Therefore, it is important to ensure its quality and safety to meet the high demand.
The maturity of sugarcane affects its quality and reduces PPO activity. Qudsieh et al. [5] studied the effects of maturation of SCJ on the chlorophyll, tannin, PPO, and color modification of SCJ. The results showed that chlorophyll, tannin, PPO, and color decreased with maturity. In early development, the color was dark brown and then became yellowish green in maturation. The green color is decreased due to the low level of chlorophyll at maturity. Moreover, the quality of SCJ is also affected by storage temperature and extends shelf life with the help of a suitable temperature for the storage of SCJ. Krishnakumar & Chellamuthu [6] demonstrated the quality of freshly prepared SCJ in stored cans. Results elaborated that cans stored at low temperatures (10 °C) maintained the quality of SCJ for 10 days, while low temperatures (5 °C) could maintain for only 4 days. Deterioration of SCJ stored at 30 °C occurred faster than that stored at 10 °C. Fresh SCJ spoiled within a day when stored at 30 °C. Microbial count especially lactic acid bacteria count was increased, during storage. According to Eissa et al. [7], the spoilage of fresh SCJ occurred due to the rapid increase of PPO enzymes. As a solution, different non-thermal and thermal techniques as well as a combination were studied for longer shelf life and preservation of flavors and nutrients in SCJ [8]. Thermal methods can reduce the action of oxidative enzymes, but due to high heat and energy, the product’s nutritional value is affected [9]. So, the industries require processing techniques to retain the product’s organoleptic attributes. Non-thermal techniques are not only applied to juices but also to many foods. Various thermal and non-thermal techniques that focus on the quality and safety of SCJ are present in literature such as pasteurization [10], [11], ozonation [12], ohmic heating (OH) [13], gamma irradiation [14], thermosonication [15], pulsed electric field (PEF) [16], high hydrostatic pressure (HHP) [17], and microfluidization (MF) [18].
This review illustrates the overall impact of novel thermal, non-thermal, and combination of techniques on SCJ. It can be valuable for researchers and industry to perceive the commercial implementation of these technologies from the perspective of SCJ processing. Moreover, the drawbacks of these techniques have also been explored to evaluate future needs in the industry.
2. Novel thermal technologies
2.1. Ohmic heating (OH)
OH is a novel thermal technique where the alternating current passes through the food sample and heat passes without any transfer medium. This technique is preferable to conventional heating because it positively affects the quality and maintains the nutritional content of food. The important factors of this treatment are the electrical conductivity of the product, applied voltage, frequency, treatment time, and temperature [19]. OH reduced microbial load and inactivated deteriorating enzymes in juices [20], [21]. For instance, OH was applied up to 80 ˚C to SCJ without affecting its phenolic antioxidant profile [13]. The 32 V/cm electric field strength and time duration of 1 min proved to be optimum conditions for SCJ preservation. The browning reaction was stopped due to enzyme inactivation. The total phenolic content (TPC) reduction was 4.2 folds, no Yeast & Mold were detected, and PPO activity (10.07 ± 0.32 %) was reduced due to the combined effect of heat as well as electric current. Moreover, the vitamin C content decreased instantly after treatment by about 33.3 %. Organic acid reduction was associated with factors like oxygen, heat, light, storage temperature and time.
In addition, titrable acidity (TA) and reducing sugar were slightly changed. Another research by Abhilasha & Pal [22] showed that OH of SCJ (at 70 ˚C for 3 min with 48 V/cm) caused no change in reducing sugar (0.462 ± 0.004) and in TA (0.136 ± 0.004) while total soluble solids (TSS) (19.7 ± 0.3 ˚Brix) were increased. The slight change in reducing sugar and TA was associated with biochemical processes and TSS increased due to treatment temperature which evaporated the water. Besides, PPO level and total plate counts were significantly reduced (4.70 ± 0.009 log CFU/mL) and shelf life was extended to 10 days at refrigeration temperature. OH (up to 80 ˚C) was applied to SCJ which inactivated thermolabile enzymes such as PPO (62.8 to 72.6 %) and reduced TPC and total flavonoid content (TFC) by 23 and 39 % [23]. Similarly, OH (at 75 ˚C for 25 min) inactivated 75 % POD which showed promising effects of this technique for the preservation of SCJ [24].
2.2. Microwave heating (MW)
MW processing involves the non-ionizing radiation with frequency 300 MHz to 300 GHz and wavelength changes 1 mm to 1 m according to frequency [25]. It is the best substitute for conventional heating with less compromise on nutritional and sensory properties. It also lowers the microbial and enzymatic content in food samples [26]. It successfully enhances the safety of juices without affecting nutritional content. For instance, Pradhan et al. [27] reported a reduction in total plate counts (2 × 105 CFU/mL) and yeast and mold (1.5 × 105 CFU/mL) at 3 min; however, MW-treated SCJ (MW for 1–4 min, pasteurized for 25 min) showed reduced TSS levels (from 20.5 to 16.5) during storage. Furthermore, MW treatment enhanced the shelf life of SCJ up to 56 days under refrigeration temperature. Similarly, MW (at 120–700 W for 5 min) showed a significant increase in TSS (106.5 %) and TA (40.62 %) at 700 W, and decreased pH (0.036 %) in SCJ [28]. The increase in TSS was caused by evaporation while TA and pH were most likely affected by the loss of temperature. The MW treatment illustrated a decrease in the L* value by 33.11 % as compared to untreated (71.99), the a* value showed an increasing pattern (118 %), while insignificant changes were recorded for the b* parameter. After MW application, there was no detection of total plate count and Y & M. This might be associated with the generation of heating and irradiation which disorganized the DNA of microbes. Moreover, the TPC (3.53) and antioxidant activity (21.58) of SCJ remained unchanged. Similarly, MW (at 490 W for 10 min) enhanced juice extraction with increased ⁰Brix (68 %), TSS (58 %), Pol (39 %), and juice purity (7 %) while a 58 % reduction was shown in diffusion time [29].
2.3. Thermal pasteurization (TP)
Thermal pasteurization is a thermal process in which heat is applied at 80˚C for<30 s, most commonly utilized to treat juices and beverages [30]. In this treatment, heat is produced outside and transferred inside the food matrix by conduction and convection [31]. TP is considered a good technique for a microbial reduction but causes nutrient degradation [32]. The effect of TP was evaluated at different temperatures (85, 90, and 95 ˚C for 30 s) on SCJ samples. Results reported an increase in pH (3.96 to 4.19), TSS (19.7 to 20.1 °Brix) and TA (0.163 to 0.175 g/100 g citric acid); however, PPO and POD were completely inactivated at 95 ˚C/30 s. Moreover, the acceptance rate by sensory panelists was above 60 %. The shelf life of 30, 40, and 50 days were obtained at 85, 90, and 95 °C/30 s [33]. In another study, milk whey blended SCJ was prepared by the addition of whey, ginger, and lemon juice. Fresh SCJ was preserved by pasteurization at 70 °C for 10 min. This treatment enhanced the shelf life of SCJ to 55 days. However, ginger and lemon juice addition lowered the pH (to 4.1) and microbial growth [34]. In research by Karmakar et al. [35], SCJ samples were subjected to TP at various temperatures of 80, 85, 90 and 95 °C for 2 min. After that SCJ juice was stored at 4 °C. Vitamin C and microbial loads were 4.7 mg/mL and 50/1 mL, respectively. According to the authors, TP at 90 °C and 2 min showed optimum conditions for the preservation of SCJ with high nutritional content and sample with biologically safe with improved quality. Table 1 explains about the impact of thermal techniques on SCJ.
Table 1.
The impact of thermal techniques on SCJ.
| Methods | Conditions | Physiochemical | Enzymatic | Microbial | Key findings | Reference |
|---|---|---|---|---|---|---|
| TP | 95 °C, 30 s | – | – | Y & M counts were 2.63 log CFU/mL | Reduced Y & M | [36] |
| TP | 90 °C, 5 min | Vitamin C content was 4.7 mg/mL | – | Microbial level reduced to 50/1 mL | Improved nutrient value and reduced microbes | [35] |
| TP | 90 °C, 40 s | Improved TSS and TA but no change in pH | Decreased PPO (25.6 and 89.5 %) and POD level (62.5 to 93.7 %) | Mesophiles, Y & M count reductions were about 2.9 to 3.2 logs and 2.9 to 4.6 logs | Showed positive impact on the physiochemical value and negative impact on microbes + enzymes | [37] |
| TP | 90–110 °C, 10–30 s | Decreased sugar level and antioxidant activities | Reduced PPO value (60–70 %) | – | Reduced sugar, antioxidant, and enzymatic level | [38] |
| TP | 85–95 °C, 30 s | Enhanced pH (3.96 to 4.19), TSS (19.7 to 20.1) °Brix, TA (0.163 to 0.175) g/100 g | PPO (17.2 and 27.8) U/mL, and POD (107.9 and 163.4) U/mL were reduced | The coliform count below 10 MPN/mL, Salmonella spp absent | Improved pH, TSS, and TA inactivated enzymes and microorganisms | [33] |
| OH | 700 °C, 3 min | No change in reducing sugar, TA (0.136) and TSS (19.2 ˚Brix) were increased | Decreased PPO level (P < 0.05) | Total plate count 4.70 ± 0.009 log CFU/mL | Overall improved physicochemical properties, reduced enzymes, and microorganisms | [22] |
| OH | 75 °C, 7.8 V/cm, 25 min | – | 78 % POD reduction | – | Decreased enzymes level in the SCJ sample | [24] |
| OH | 75 °C, 25 min, 10–105 Hz, 20.5 V/cm | TPC and TFC degradations were about 23 and 39 % | PPO reduction (62.8 to 72.6 %) | – | Reduced enzymes level, minimal changes showed in phenolic concentration | [23] |
| MW | 120 W, 5 min | Retained TPC (3.53), antioxidant activity (21.58), increased TSS (22.3 ˚Brix), reduced pH 4.2–4.3 | – | Total plate count, Y & M at Refrigeration temperature after 56 days were 1.5 CFU/mL & 2.3 CFU/mL | Maintained nutrients, reduced bacterial growth | [28] |
| UHT sterilization | 140 °C, 4 s | Retained flavor, TSS 19.6 ˚Brix, decreased pH 5.0 | PPO reduced | – | Retained sensory and nutrients level, decreased PPO | [39] |
| Hydrodynamic cavitation treatment | 22 °C, 3.5 bars, 17 orifices, 40 min | Reduced pH 4.64, TSS 13 ˚Brix | – | Maximum microbial load 3.3 logs10 CFU/mL | Enhanced level of microbes and decreased nutrients | [40] |
TSS: Total soluble solids, PPO: Polyphenol oxidases, TA: Titratable acidity, POD: Peroxidase, TPC: Total phenolic content, TFC: Total flavonoid content, Y & M: Yeast & Mold, UHT: Ultrahigh temperature, MW: Microwave.
3. Non-thermal technologies
Non-thermal techniques inactivate the microbes and enzyme’s action and retain juice’s nutritional and organoleptic qualities [41], [42]. These novel techniques eliminate the limitations of conventional techniques by providing benefits like less consumption of energy, no use of chemicals, reduced waste emission, enhanced reliability, production of by-products, economic, etc. [43]. The main aim of these technologies is to reduce microbial load and enzymes without affecting nutritional parameters and enhance shelf life [44]. These technologies include HHP, US, PEF, ozone treatment, cold plasma, UV, etc. Table 2 demonstrates the impact of such non-thermal techniques on SCJ.
Table 2.
Impact of non-thermal techniques on SCJ.
| Methods | Conditions | Physiochemical | Enzymatic | Microbial | Key finding | Reference |
|---|---|---|---|---|---|---|
| HHP | 300 MPa, 2 min, and 5 min | Maintained TSS, color, and pH | Less impact on PPO activity (92.77) | The total plate count was reduced by 6.32 logs | Retained physiochemical parameters and reduced enzymes level | [45] |
| HHP | 600 MPa, 6 min, 60 °C | – | Greater reduction in POD than PPO | Microbial load reduction (P < 0.05) | Reduced enzymes and microbes | [46] |
| HHP | 523 MPa, 50 ˚C, 11 min | The total color difference (2.8), total phenols (33 mg), and antioxidant value (95 %) | 62 % inactivation occurred in PPO and 59 % in POD | 5 log reduction in microbes | Improved physicochemical properties and decreased enzymes and microbes | [47] |
| US | 5, 15 min, 40 °C, and 60 °C | Moisture contents 93.50 to 92.85 %, TA 0.16 %, pH 5.92, TSS 12.50 ˚Brix | – | The microbial load was 6.03 log CFU/10 mL | Reduced microbial load and moisture | [48] |
| US | 37 kHz, 10, 20, 60 min, 40 °C | No change in TSS, TPC, and pH | – | – | Retained physicochemical properties | [49] |
| US | 40 kHz, 240 W, 40 min | Retained pH and color, enhanced TSS, TPC (18 %), and TFC (16 %), reduced vitamin C by about 13 % | – | 1.95 log CFU/mL reduction in total aerobic mesophiles and 0.42 log CFU/mL in Y & M | Maintained physiochemical parameters and reduced microbial load | [50] |
| UV treatment | 30 min, 16.2 J/mL | Color change from 0 to 4.10 ± 0.3, slightly increased TA (0.131 ± 0.004), TSS (18.4 ± 0.2), reducing sugar (0.469 ± 0.009), TPC and acceptability score decreased about 5.73 ± 0.07 and 6.96 ± 0.29 | PPO activity was reduced by 59 % | The total plate count was 5.5 CFU/mL | Microbial and enzymatic inactivation with retaining maximum physicochemical content | [22] |
| UV irradiation | 250 W, 3 min | Reduced color 244.9 and °Brix 19.5, no impact on pH and turbidity | – | Decreased fungi, and bacterial load 5.42 logs | Reduced microbes and physicochemical parameters | [51] |
| PEF | 30 kV cm−1 150 pulses | Reduced pH (3.98 ± 0.33) and vitamin C (81.54 ± 0.15 mg), increased TA (0.5 ± 0.10 %), retained TSS | – | 2.71 log CFU/mL reduction in total aerobic mesophilic count | Reduced vitamin C, pH, and microbes | [16] |
| PEF | 110 V, 30 s | – | – | >1.42-log inactivation rate especially E. coli targeted | Significant reduction in microbes | [52] |
| Dielectric barrier discharge (DBD) plasma | 45 V, 2 min | No change in pH, color, increased TSS, TPC (25 %), TFC (21 %), vitamin C loss 6 % | – | Reduction in total aerobic mesophilic (3.6 log CFU/mL), Y & M (0.50 log CFU/mL) | Retained color, enhanced TSS, TFC, and TPC, reduced bacterial load | [50] |
| Ozone treatment | 4.23 g/L dose, 20 min, flow rate 5.6 l/min | Reduced TSS (6.3 %), TPC (13.5 %), TFC (22.5 %), ascorbic acid content (81.46 %), and antioxidant capacity (30 %) | 67.8 % reduction in PPO, 75.3 % in POD | TPC count reduction (3.72 logs), Y & M reduction (2.50 %) | Reduced all parameters | [53] |
| Ozone treatment | 30 min, 0.199 mg/L | Retained TSS, TA, | POD remained constant | The total plate count remained constant | No change in any parameter | [22] |
| Microfiltration | 35, 69, 104, 138 kPa, 0.123, 0.246 and 0.369 m/s | Reduced TSS (9 %), velocity (21 %), color (80 %) | – | 5 log reduction | Decreased physiochemical and microbial factors | [54] |
| Microfiltration | 45 °C, 0.87 m/s−2 | Reduced TSS (19.37 ± 0.01 g·100 mL−1), protein (0.18 ± 0.01 g. 100 mL−1), and carbohydrate 18.86 ± 0.01 g/100 mL−1, retained TSS 18.60 ± 0.14 °Brix and pH (4.13 ± 0.02) | – | – | Decreased macromolecules and maintained TSS | [55] |
| Ultrafiltration | 104 kPa, 30 l/h | Recovered sucrose (98 %), polyphenol (80 %) | Reduction of 3 folds in oxidative enzymes | 6 log reduction | Maximum recovery of sucrose, polyphenols, and reduced enzymes plus microbes | [56] |
| MF | 25 °C, 50–200 MPa, 1–7 cycles | Enhanced reducing sugars (1.2–1.4 mg/mL), Chlorophyll content (0.513 ± 0.016 mg/mL), and decreased total sugars (11–54 %) | Enhanced PPO level (39.4 to 64.7 %) POD level (16.4 to 75.0 %) | – | Increased enzymes and sugar level | [18] |
| MF | 100–150 MPa | Reduced phenols (30–58 %), TFC (14–54 %), ferric reducing antioxidant power (24–65 %) | – | Decreased bacterial count 6 log CFU/mL, APC range 0–2.73 log CFU/mL, Yeast & Mold count was 0–3.47 log CFU/mL | Decreased phenolic profile but positive impact on the microbial count | [4] |
| MF | 100, 120, 140 MPa | Reduced TSS (11 %), enhanced color value (8.5 ± 3.6) | – | Bacterial reduction 1.88 logs | Reduced physicochemical properties but positive impact on microbial load and color | [57] |
POD: Peroxidase, PPO: Polyphenol oxidases, TA: Titratable acidity, Y & M: Yeast & Mold, TPC: Total phenolic content, TFC: Total flavonoid content, APC: Aerobic plate count, TSS: Total soluble solids, MF: Microfluidization.
3.1. High hydrostatic pressure (HHP)
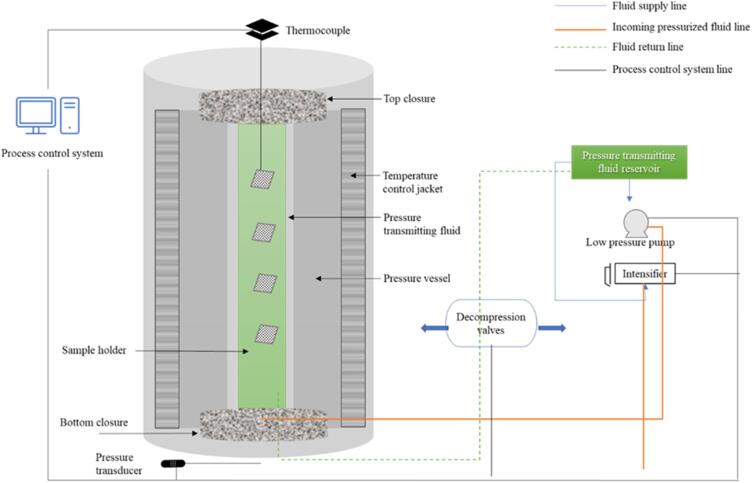
HHP is a non-thermal processing technique that operated at 100 to 800 MPa and has been widely used in food industries for different purposes like shelf-life extension [58], microbial inactivation [59], deteriorating enzymes reduction [60], preservation of food, etc. [61], [62]. According to Sehrawat, Kaur, Nema, Tiwari, & Kumar et al. [63], about 300–400 MPa pressure was effective to reduce>3 log bacterial growth such as Salmonella enteritidis, Escherichia coli and Staphylococcus aureus. In this technique, a food sample is placed in a pressure vessel and a pump is used for compression. A wide range of pressure is used to break non-covalent bonds in dense compounds like proteins and lipids. Various critical parameters are pressure level and duration (milliseconds->20 min), initial temperature (0 to 90 °C), etc. [64]. Fig. 2 shows the process layout of the juice sample by HHP.
Fig. 2.
Mechanism of HHP for juice sample.
Huang et al. [65] discussed the impact of HHP on enzymatic, nutritional, and antioxidant parameters of SCJ at 200–600 MPa for 6 min. They concluded that invertase enzyme activity on day 7 declined to 87.69 and 82.86 % The TA maintained (0.057–0.088) during storage and no significant change in pH was shown at 400 and 600 MPa. At 400 MPa, there was an increase in fructose and glucose content by about 52.8 and 46.6 %. However, a decrease of 4.5 and 4.6 log CFU/mL was observed in APC at 400 and 600 MPa, respectively, while Yeast & Mold and coliform count were observed below the detection limit (<1.0 CFU/mL). Similarly, HHP (at 600 MPa, 6 min and 60 °C) completely inactivated microbes, as well as completely stopped POD and PPO activities [47]. According to the authors, HHP damages the DNA of microbes and inactivates enzymes at a pressure that varies from 100 to 800 MPa, variations occur in their structure, and it causes the death of microbes. Microorganisms showed different behavior at various pressure and temperature resistance characteristics [66]. For instance, HHP (at 300 and 400 MPa for 10 to 25 min) reduced 4.44, 5.31 and 6.30 logs10 cycles of total bacterial count, Yeast & Mold and coliforms, respectively. Furthermore, no remarkable effect (p > 0.05) on color and pH was observed, while 57 % PPO was inactivated at 400 MPa [67]. In another study, HHP (at 300 MPa for 2 and 5 min) on red SCJ variants decreased PPO activity (2.38 ΔAbs./min) and total plate count (6.00 log CFU/mL) at 5 min. Moreover, maximum values of TPC were retained at 5 min in all variants and antioxidant capacities were also increased with a high value observed in Ragnar (37.28 %). While TSS, pH and color remained constant. In conclusion, HHP is an efficient technology for the preservation of SCJ [68].
3.2. Microfluidization (MF)
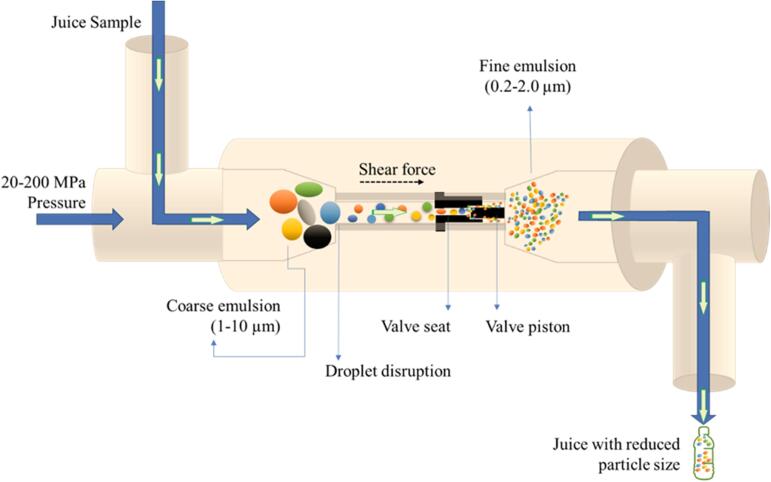
MF is another non-thermal emerging technique in the field of food technology. Recently, it has been gaining popularity in food research. It is also called high-pressure homogenization (HPH), which uses high shear force to break particles in the interaction chamber and modified their physicochemical properties [4]. In literature, the impact of microfluidization on physicochemical, microbial and enzymatic properties of various juices has been recorded [69], [70]. Fig. 3 shows the HPH set up for SCJ.
Fig. 3.
A schematic representation of HPH juice processing.
MF on SCJ at pressure (100–140 MPa) and cycle (1–2) decreased TSS (11 %) and increased color values (8.5 ± 3.6) while total plate count was recorded as 1.88 logs [57]. Similarly, MF (at 50–200 MPa with 1–7 processing cycles) decreased the PPO and POD levels by 39.4–64.7 % and 16.4–75.0 %, respectively in SCJ [17]. Moreover, reducing sugar enhanced by 1.33–6.74 mg/mL, total sugars reduced (11–54 %) while chlorophyll content changed from 0.361 ± 0.023 to 0.513 ± 0.016 mg/mL. in another study, MF (at 50, 100, 150, and 200 MPa; and cycles, 1, 3, 5, 7) reduced TSS from 18.8 °Brix to 10.15–15.7 °Brix. The pH remained unchanged while a significant reduction in TA (0.1–0.26 %) was observed. According to the authors, MF changed the electrical conductivity from 4.45 to 5.12 mS and caused degradation in Mg (42.85 %), P (38.69 %), K (18.99 %), Ca (58.83 %), Mn (39.13 %), and Pb (46.42 %). While the sensory score was 7, showing the acceptability of SCJ [71]. The effect of MF on bioactive compounds and the microbial rate of SCJ was evaluated by Tarafdar, Kumar, Kaur, & Badgujar et al. [4] at different pressures (50, 100, 150, and 200 MPa) and processing cycles (1, 3, 5, and 7). There were a significant reduction in phenols (30 %−58 %), flavonoids (14 %−54 %), ferric reducing antioxidant power (24 %−65 %), and metal ion chelating activity (13 %−64 %) was recorded. It increased hydroxyl radical scavenging activity (31 %−33 %), while radical scavenging activity of 2, 2-diphenyl-1picryl hydrazil (DPPH) remained unchanged. APC and Yeast & Mold varied from 0 − 2.73 log CFU/ml and 0–3.47 log CFU/ml, respectively. While at 7 processing cycle, no APC was detected in SCJ. According to sequential color palette analysis, 100–150 MPa pressure and 3 processing cycle were the best optimum parameters for SCJ. Hence, it results that MF is an effective technology for generating a minimum sedimentation rate in SCJ. Besides, MF is an effective processing tool to prevent sedimentation issues in fresh juices. Tarafdar & Kaur [18] subjected SCJ to 50–200 MPa pressures with 1–7 processing cycles to determine the particle size, viscosity, and its effect on the particles. At 50–150 MPa pressure and < 7 processing cycles, decreased the particle size in SCJ. It was concluded that 150 MPa pressure and 5 cycles of processing were optimum conditions to produce the smallest size and minimal sedimentation rate of particles which was recorded as ∼ 437 nm and 4.91 × 10−2 mm/day, respectively.
3.3. Pulsed electric field (PEF)
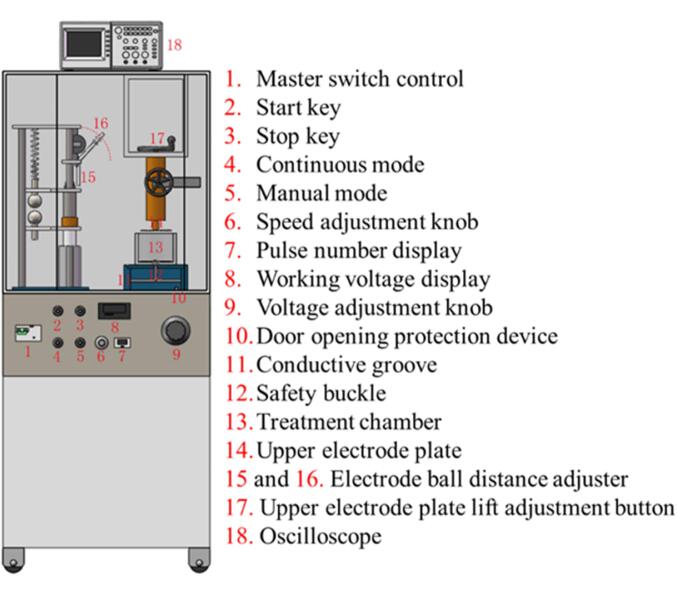
PEF is another technology in the field of juice preservation for reducing the microbial level and retaining nutritional attributes of juices [72], [73] as well as inactivating spoilage enzymes. PEF has gained the industry’s interest because it has the same effect as a thermal technique without changing the nutritional and sensory properties of food [73]. In PEF treatment, electric pulses rupture the cell membranes of microbes and they lose the possibility to perform their actions [72]. Fig. 4 demonstrates the treatment chamber of the lab-scale PEF extractor machine.
Fig. 4.
PEF extractor machine.
PEF treatment is efficient for vegetative cell inactivation but the bacteria are resistant to this treatment while yeasts are sensitive to PEF due to the thick cell wall of bacteria and the larger size of yeasts [74]. Trung et al. [52] reported a reduction in the microbial level with > 1.42-log inactivation rate, especially targeted E. coli in PEF-treated SCJ (at 110 V, 30 s). It was observed that juice treated with PEF (30 kV cm−1 and 150 pulses, 4 ˚C) was stable, and shelf life was extended up to seven days compared to the untreated sample. Moreover, there was a little decrease in pH (5.10 ± 0.30 to 4.95 ± 0.30) and TSS (17 ± 0.00 to 19.0 ± 0.00 °Brix) while TA enhanced from 0.2 ± 0.10 to 5.7 ± 0.10 %. There was no change in vitamin C (87.5 ± 0.14 mg %), while the total aerobic mesophilic count was decreased (2.71 logs CFU mL−1). There was decreasing trend in L* (25.9 ± 0.10) and b* (38.75) and increasing in a* (14.8 ± 0.18) values [16]. So, it is concluded that PEF is an effective approach for preserving nutrients and increasing the shelf life of SCJ.
3.4. Ultrasound treatment (US)
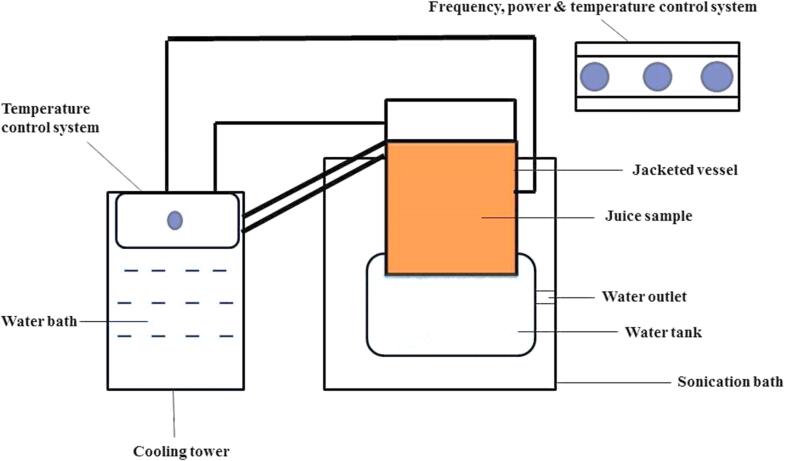
The US is being used as an alternative to thermal treatments for fruit juices, which is one of the recent developments in the food industry [75], [76]. It operated at low frequencies and high pressure. Due to cavitation microbial load reduces, functionalities increases, as well as minimum degradation, takes place [77]. Fig. 5 illustrates the general arrangement of US treatment.
Fig. 5.
A block diagram of liquid processing using US.
US treatment may produce unfavorable conditions for microbes, but this depends upon microbe type, food composition, and time duration of waves [78]. US treatment (for 15 min at 40 kHz frequency and 240 W powers) for inactivation of various types of microbes has been investigated, i.e. E. coli, Salmonella, Enterica serotypes, Listeria monocytogenes, Alicyclobacillus acidophilus, Alicyclobacillus acidoterrestris, and spoilage yeast species [79], [80]. On the other hand, US-treated SCJ showed no significant changes in pH. The US significantly reduced the L* (43.49 ± 0.04) and a* (−1.95 ± 0.04) values due to increasing treatment time while the b* (10.51 ± 0.10) value increased that indicates the color of sugarcane juice changed towards yellowish. There was an increase in TSS (16.30 ± 0.10 °brix), TPC (18 %), TFC (16 %), total sugars (16.5 ± 0.05), and reducing sugars (0.63 ± 0.02). These results have been attributed to a greater extraction ability of US treatment, an increase in sugars mainly associated with the conversion of disaccharides into monosaccharides. Ascorbic acid and aerobic microbes and Y & M were reduced to about 13 %, 1.95 log CFU/mL and 0.42 log CFU/mL respectively. Vitamin C reduction was associated with free radicle generation during sonochemical reaction while microbial load reduction associated with cavitation-induced micro bubbles [50]. In another study, US-treated SCJ samples (20 kHz and 750 W) showed a 13 % increase in TPC, and 90 % reduction in POD activity at 36 min treatment, however, a slight color change was also observed and juice became yellower (higher b* parameter) and showed a distinguishable color (ΔE = 2.36) compared with fresh juice [81]. This increase in TPC may be a result of phenolic liberation from cell wall particles that remained in the non-filtered juice. In conclusion, US treatment is an efficient way to enhance the nutritional value of SCJ.
3.5. Irradiation
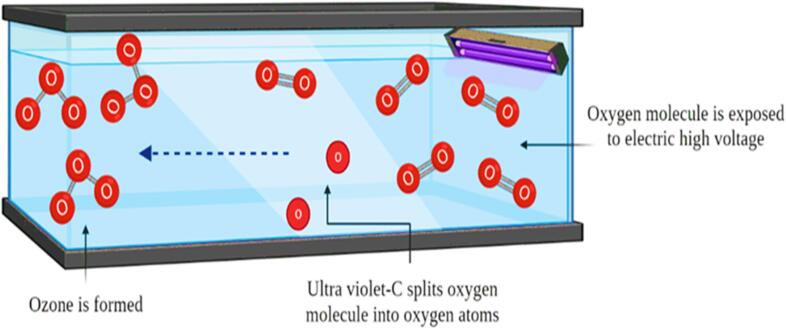
Irradiation is a non-thermal treatment used for food processing and consists of direct exposure to electromagnetic rays for enhancing shelf life through microbial inactivation. Electromagnetic rays involve specific wavelength, frequency, and energy. X-rays, γ -rays, infrared irradiation, UV rays, and radio waves are included in this treatment. Gamma (γ) and UV rays are most probably used in food processing industries for microbe inactivation in juices. Different rays are produced by various isotopes such as γ Rays produced by Co-60 and Ce-137. The international unit is Gray (Gy) which indicates the dose of rays applied to the sample. The dose of irradiation is an important parameter because preservation is achieved without compromising food quality. <1 kGy is sufficient for microbe inactivation, and shelf life enhancement of food samples requires 1 to 10 kGy [82]. About 10 to 50 kGy dose could be used for food sterilization completely but can adversely affect sensory properties. UV radiation works in the region from 200 to 280 nm, which adversely affects microbe structure and stops their action [83]. UV treated SCJ at 30 min, and 16.2 J/mL showed some modifications in color value from 0 to 4.10 ± 0.3, slightly increased TA (0.131 ± 0.004), TSS (18.4 ± 0.2), reducing sugar (0.469 ± 0.009), while TPC and acceptability score decreased about 5.73 ± 0.07 and 6.96 ± 0.29, respectively. Moreover, PPO activity (59 %) and total plate count value (5.5 log CFU/mL) were reduced. Hence, UV treatment is effective for microbial and enzymatic inactivation, retaining maximum physicochemical content [22]. Fig. 6 illustrates the process of irradiation for SCJ.
Fig. 6.
Process of irradiation for SCJ.
In another study, the impact of UV irradiation on SCJ was studied at the following conditions 250 W, and 3 min. It was concluded that color values (244.9) and TSS (19.5 °brix) reduced while no impact on pH (4.3) and turbidity (1133.5 NTU) values was shown. The bacterial count was 5.42 log CFU/mL recorded. UV treatment successfully enhanced the preservation of SCJ [51].
3.6. Membrane technology
Membrane technology is a non-thermal technique in which the nutritional and organoleptic profile of the treated sample remains unaffected. It gained great importance in the dairy and beverage industry. Major membrane processes involve electrodialysis, ultrafiltration, microfiltration, and reverse osmosis [84]. This technique involves low thermal damage, less consumption of energy, and less equipment cost is also less [85]. As a substitute to conventional preservation, membrane technology involves clarification stages, no chemical addition and retains the nutritional profile and sensory properties of the final product [86]. Fig. 7 shows the clarification of SCJ by microfiltration.
Fig. 7.
Clarification of SCJ by microfiltration.
In a study, SCJ was clarified by tubular ceramics membranes with various pore sizes. The SCJ sample could settle for one hour and the supernatant was further processed with microfiltration followed by ultrafiltration with pore diameters of 0.3 and 0.1 μm and an area of 0.005 m2. The result indicated the INCUMSA color reduction (44.8 %), high purity (2.74 units), and less turbidity (99.4 %) in the treated SCJ sample as compared to the fresh juice sample. There was a slight increase in pH (5.19 to 5.21) while Brix (13.6 %) and Pol (9.35 %) slightly decreased [87]. In another study, the effect of cross-flow microfiltration of SCJ was observed with a pore diameter of 0.4 µm and filtration area of 0.723 m2. The final permeate flux values were 7.05 to 17.84 l·h−1·m−2. There was a reduction in flux (50 %), pH (4.11), TSS (19.37), protein (0.18), vitamin C (5.32), carbohydrates (18.86) while the ash (0.32), moisture (18.64), and soluble solids (18.60) slightly changed [55]. Ultrafiltration was applied at 104 kPa, 30 l/h on SCJ to evaluate its impact on nutrient recovery, enzymes, and microbial inactivation. It successfully recovered a high value of sucrose (98 %) and polyphenol (80 %) from SCJ. Moreover, the oxidative enzymes and bacterial load reductions were 3 folds and 6 log CFU/mL, respectively [56].
3.7. Ozone treatment
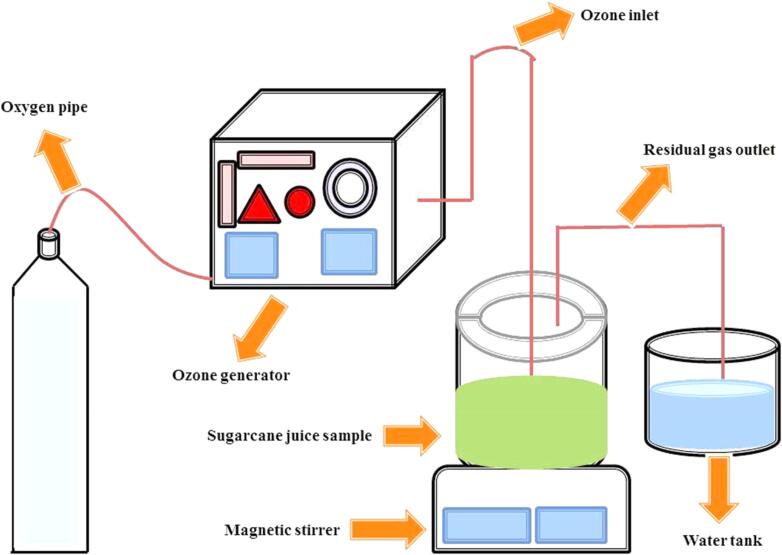
Ozone treatment gained the food industry’s interest because of oxidative agents, rapid reduction of microbes and no residue content in samples, which make it more attractive. This treatment is suggested as safe to use as antimicrobial agents in food samples [88]. It is produced by an ozone generator and oxygen concentrator [89]. Fig. 8 demonstrates the ozone treatment of SCJ.
Fig. 8.
A schematic representation of ozone treatment for SCJ.
Its main functions in the sugarcane industry are color stabilization and shelf-life improvement. The oxidation with carbonation has better results on the quality of SCJ. Lime carbonation with Fenton oxidation provides good quality juice [90]. Ozone treatment has a good ability to reduce impurities in SCJ samples [91]. Ozone (1.2 g/h for 10 min) treatment with pasteurization (85 °C for 15 min) and the preservative (lactic acid 0.5 %) resulted in a 4.3 log reduction in the microbial count and controlled 72 % activity of PPO and POD enzymes in SCJ. This treatment maintained the juice quality for 1 month [26]. In a study, the ozone-treated SCJ showed a positive result in clarification and preservation factors [11]. In another study, the ozone technique was applied to SCJ with ozone concentration (10, 20, and 30 % wt/wt), gas flow rate (3, 6.5, and 10 l/min) and time (5, 12.5, and 20 min) to evaluate nutritional, biochemical, and microbiological parameters. The PPO inactivation rate was about 67.8 % and POD about 75 % while significant reductions in total phenolic content (13.5 %), TFC (22.5 %), vitamin C (81.46 %), and antioxidant capacity (30 %) were recorded. The reduction in flavonoids and polyphenols was attributed to free radicles generation and their ability to inhibit oxidation while the ascorbic acid reduction was associated with ozone interaction with enzyme ascorbate oxidase which promoted the degradation [92]. In addition, enzyme inactivation is attributed to active site alteration of sulfhydryl groups in cysteine residue as a result of oxidation [93]. The 3.72, and 2.84 log CFU/mL log reduction in a total plate and Y & M count occurred. The mineral and sensory attributes slightly decreased with calcium (16.13 mg/100 mL), magnesium (12.1 mg/100 mL), sodium (1.65 mg/100 mL), iron (1.34 mg/100 mL), copper (0.077 mg/100 mL), zinc (0.188 mg/100 mL), cobalt (0.016 mg/100 mL), manganese (0.183 mg/100 mL), color (8.44), flavor (8.14), aroma (7.98), taste (8.26), and overall acceptability (7.82) were recorded [53].
4. Combined treatments for preservation of SCJ
The main goal of the food industry focuses on enhancing the safety and quality of products with the minimization of nutrient degradation. To fulfill the requirement researchers developed a different combination of techniques for microbial inactivation and shelf life extension [94]. Hurdle processing techniques involve synergizing benefits of two or more treatments. For example, if the cost of HHP is neglected then its combination with cold plasma is best for the quality and shelf life of juices [3]. The combination of various techniques resulted in a reduction of microbial load and enzyme inactivation as well as improved quality of juices [95], [96], [97]. Sankhla et al. [98] applied the SCJ sample following processing pasteurization (80 ˚C for 10 min) with irradiation (1.0 kGy) and sterilization (80 ˚C for 20 min). Their combinations decreased ascorbic acid content (2.31 %), and total sugar content (P˃0.05). The ascorbic acid reduction occurred due to its sensitivity to heat while the total sugar content decrease was due to the breakdown of total sugars into reducing and other sugars. The maximum reducing sugar content was detected at about 0.95 %. The increase may be due to the hydrolysis of sugars by acids or due to the degradation of disaccharides to monosaccharides. The reductions in TBC (2 × 106 CFU/mL), and Yeast & Mold count (2 × 105 CFU/mL) were recorded. There were no changes in TSS and antioxidant activity while minerals showed a slight reduction at the end of the storage period with 1.23 mg/100 mL of iron, 14.07 mg/100 mL of calcium and 6.8 mg/100 mL of phosphorus. Flavor and taste reduction were observed during the storage at room and low temperature. This decrease could be due to the loss of volatile aromatic substances responsible for taste. Overall, the shelf life of juice was increased up to 60 days at room temperature and up to 90 days at low temperature. Table 3 describes the impact of various technological combinations on SCJ.
Table 3.
Impact of combined treatments on SCJ.
| Treatments | Conditions | Physiochemical | Enzymatic | Microbial | Key findings | References | |
|---|---|---|---|---|---|---|---|
| TP + chemical preservatives | 70 °C, 10 min | Citric acid (40 mg/100 mL), ascorbic acid (150 ppm) | Reduced the pH, stopped deterioration of TSS and total sugar | – | No detection of coliforms, Decreased the Y & M counts from 2.61 to 2.04 CFU/10 mL | Improved the overall quality by decreasing microbial rate and deterioration of nutrients | [99] |
| TP + chemical preservatives | 85 °C, 10 min | Ascorbic acid 40 ppm, potassium sorbate 120 ppm, sodium metabisulphite 120 ppm | Minimal reductions in pH (4.03), TSS (18.8), browning index (13.7), antioxidant capacity, color | – | Minimum decrease in total plate count (0.6 CFU/mL), Y & M count (3.2 CFU/mL) | Showed minor changes in physicochemical parameters and reduced microbial count | [100] |
| TP + ultra-clean filling | 95 °C, 30 s | Peracetic acid solution 0.05 %,5 s, 45 °C | Reduced pH (5.55–4.35), TSS (20.7–19.4), TA (0.035–0.085) | Decreased PPO (5.6–0.7) and POD (3.4–1.6) level | <3 log reduction of aerobic mesophiles | Reduced enzymes, and microbes | [101] |
| TP + sterilization + irradiation | 80 °C,10 min | 80 ˚C, 20 min 1.0 kGY | Decreased ascorbic acid content (2.31 %), highest reducing sugar content (0.95 %), total sugar content reduction | – | TBC was (2 × 106 CFU/mL), Y & M count (2 × 105 CFU/mL) | Decreased microbial count and showed a negative impact on nutrients | [98] |
| HHP + TP | 600 MPa, 6 min | 97 °C, 60 s | No remarkable change in TA, TSS, and pH, color, antioxidant capacity value changed | Invertase enzyme’s activity reduced 87.69 and 82.86 % | Decreased coliform, Y & M count below 1.0 log CFU/mL | Improved the quality of SCJ by decreasing enzymes, microbes and retaining nutrients | [65] |
| Gamma radiation + TP | 2.5 kGy | 70 °C, 25 min | Maintained TSS (22.52 ± 1.43), color, aroma | Reduced PPO level by 50 % | 2.5 × 101FU/mL total aerobic mesophiles, <10 CFU/mL TBC | Reduced enzymes, microbes, and retained nutrients | [102] |
| Gamma radiation + preservatives | 5 kGy | Citric acid (0.3 %), sodium benzoate (0.015 %), potassium sorbate (0.025 %), sucrose (10 %) | No impact on TSS, TFC, TPC, reducing sugars, reduced reducing power about 14 %, sugar content decreased 15 % | – | Decreased TBC, and Y & M below detection limit | Reduced microbial level, minimal or no impact on physiochemical contents | [103] |
| Ozone + Lactic acid treatment | Ozone concentration: 1.2 g/h, 10 min | Lactic acid concentration: 0.5 % | No change in TSS, pH | About 60 % loss in PPO 72 % in POD level | 4.3 log reduction in TBC | Reduced enzymes and microbes without affecting the nutritional value | [104] |
| MW + chemicals | 70 °C, 1 min | 0.5 mL lime juice, 0.2 mL ginger extract, and sodium metabisulphite 125 ppm | The lowest decrease in TSS and pH was 19 ˚Brix and 4.11, TA increases from 0.04 to 0.05 | – | Reduced Y & M counts by sodium metabisulphite | Improved preservation of SCJ, degradation was reduced | [105] |
| MW + US | 2450 MHz, 120 s | 24 kHz, 20 °C, 20 min | Total phenolic compounds (from 3.04 to 4.8), and TFC (from 2.9 to 3.8) after 21 days of storage, the maximum increase in ascorbic acid (from 0.3 to 0.6), antioxidant value (84.1), increased pH and decreased TA | – | Microbes below the detection limit | Decreasing enzymatic, microbial levels and remaining nutritional content | [106] |
| OH + US | 80 °C, 85 g sample, 1250 rpm | 20 kHz, 1.2 cm, 750 W | Remained exact TFC and total phenolic content, flavones (38–49 mg/L), dilignols (22–29 mg/L), and phenolic acid derivatives (17–30 mg/L) | – | – | Maintained phenolic profile in SCJ synergistically | [81] |
| Thermosonication | 50 °C | 20 kHz, 2–10 min, 750 W | – | – | 5 log reductions in E. coli, B. cereus, and total aerobic mesophilic plate count | A clear reduction in bacterial growth was observed by combining heat with the US | [107] |
TFC: Total flavonoid content, Y & M: Yeast and mold count, PPO: Polyphenol oxidases, POD: Peroxidases, TA: Titratable acidity, TP: Thermal pasteurization, TBC: Total bacterial count, TPC: Total phenolic content.
The combined impact of US (24 kHz, 20 °C, and 20 min) and MW (2450 MHz, 120 s) on SCJ increased TPC (from 3.04 to 4.8) and TFC (from 2.9 to 3.8) after 21 days of storage, while the maximum increase in ascorbic acid (from 0.3 to 0.6), and antioxidant value (84.1) was also recorded [106]. This higher content was due to the inactivation of PPO enzymes since this enzyme utilizes these compounds as substrates and causes the degradation of these compounds during storage and due to the removal of oxygen during sonication and inactivation [108]. Moreover, MW and US combination increased the pH and decreased the TA of SCJ, as well as microbes were below the detection limit. This increase in pH and decrease in acidity was due to the acidic hydrolysis of polysaccharides where non-reducing sugars converted to reducing sugars. MW-US-processed juice samples exhibited more retention of natural color pigments, suggesting less enzymatic and non-enzymatic browning reactions took place. This color retention might be due to the inactivation of PPO and POD that cause degradation of color pigments during storage [109]. In another research, SCJ was applied to pasteurization (80 °C for 10 min) with preservatives (potassium metabisulphite 150 ppm and citric acid 0.05 %), sterilization (80 °C for 20 min), and irradiation (0.25, 0.5, 1 kGy) at various temperatures. There was a decrease in mineral values of about 1.23 mg/100 mL of iron, 14.07 mg/100 mL of calcium, and 6.8 mg/100 mL of phosphorus. The highest microbial count (4.88 × 106 CFU/mL) was observed after storage. The moisture content, ascorbic acid, and Y & M decreased significantly (P > 0.05) while reducing sugars and total sugar remained unaffected [110].
Ozone (1.2 g.h−1 for 10 min) with lactic acid (0.5 %) was applied to the SCJ samples. The result indicated that enzymes and microbes were inactivated significantly, and color reduction also occurred. Furthermore, PPO was reduced to about 60 % and POD reduction was 72 %. Hence the shelf life of SCJ was enhanced for about 1 month [104]. On the other hand, ozone with heat (17 mg/min for 20 min) caused no significant changes in color, reducing sugar and sucrose content. Thus it showed that this combination has no negative impact on the SCJ sample [111].
In a study, PEF with lemon and ginger as a preservative was applied to the SCJ sample at 20 kV/cm−1 and 150 pulse numbers. The results indicated that the shelf life of SCJ was extended to about 14 days [16]. Similarly, MF and natural polypeptides (niacin and polylysine) were compared with pasteurization and potassium metabisulfite. All the samples were stored at 5 ˚C. The results concluded that both these treatments caused a complete reduction in POD (100 %). Minimal physicochemical changes (P < 0.05) occurred in pasteurization while they remained unaffected in MF. Using non-thermal hurdles 100 % microbial reduction and shelf life extension of 56 days were observed [10]. SCJ was treated with gamma radiation (5 kGy) in combination with preservatives (sodium benzoate, potassium sorbate and sucrose) at a low storage temperature (10 ˚C). The results showed that total bacterial count and Y & M were eliminated until day 35 at 10 ◦C storage while the coliform and the Staphylococcus count were found to be below the detection limit. The flavonoids (0.20 mg CE/mL), total phenolic (0.88 mg GAE/mL), reducing sugar (68 mg/mL) DPPH scavenging activity (95 %), nitrite scavenging activity (51 %) and reducing power (0.34 %) remained unaffected due to the addition of preservatives. However, total sugar content decreased by about 15 % that was due to enzymatic and chemical oxidation reactions of sugar compounds naturally taking place during storage [103]. The sensory evaluation demonstrated that the juice was highly acceptable for consumers. Besides, the effect of ultrafiltration at 104 kPa and 30 l/h using 30 kDa hollow fiber membranes followed by ozonation at 4.58 l/min, 3.12 ppm and 8.2 min on SCJ was observed. Combined treatment caused 38.52 % reduction in phenolic degradation, 25.43 % less increase in browning and 83.5 % reduction in PPO activity. Moreover, they reduced sucrose loss by 35.7 % and decreased dextran and ethanol formation by 78 % and 95.8 %, respectively [112]. Hence it proved that the combination of techniques is the best way to reduce microbes and enzymes without impacting the nutritional value of SCJ [103].
5. Limitation of thermal and non-thermal technologies regarding SCJ
SCJ is a highly nutritious, low-cost, flavorful, and refreshing drink that provides the necessary nutrients to humans. Thus, this is the major cause of its massive consumption. Reducing sugar deterioration like sucrose is affected by the maturity of sugarcane and environmental conditions. The main challenge is controlling this deterioration and supplying good quality juice on the commercial level. In this review, thermal, non-thermal and their combination is discussed to preserve SCJ. Thermal treatments are effective methods for reducing deterioration and spoilage rate in SCJ juice but they cause undesirable changes like reduction of nutrients, loss of natural flavor, aroma and change in color, etc. [9]. Non-thermal techniques also have limitations like US treatment affected physicochemical properties such as bitter flavor, color changes, and bioactive compound destructions in SCJ [113]. Irradiation faces problems in regulation and hurdles in consumer perception of radioactivity [114]. PEF involves a high initial cost and does not impact enzymes and microbial spores [115], [116]. UV treatment is effective if the sample turbidity is high [117]. In HHP costs and expensive equipment limited its applications in the food industry [118]. In OH, monitoring and control of the process are hard [119]. Membrane processing has drawbacks of foul-smelling and low yield of juice products [120]. Ozone processing has the toxicity of ozone gas at a higher level and instability of the process [121]. More research is needed to address these issues.
6. Conclusion
Consumer requirements for fresh fruits resulted in the applications of thermal, non-thermal, and a combination of techniques to ensure the safety and quality of fresh fruit juices. Although the safety of sugarcane is a major concern due to its microbial load and high deterioration rates, there is a need arises for novel processing technologies to reduce microbial load, retain nutritional profile, and extend shelf life. The effect of thermal, non-thermal, and combination of technologies has been reviewed in this paper. Thermal techniques inactivate the enzyme but affect the nutritional profile negatively. The non-thermal technologies and their synergistic impact positively resulted in enzyme inactivation and nutritional content. There is a lack of compliance in some of these juice processing processes as they are still at the laboratory level. A basic understanding of these processes and their mode of action is essential to improve process structure and ensure desirable standard products. Most of these heat-efficient processes are currently used in the laboratory or on a testing scale, thereby reducing production costs higher than large industrial thermal equipment. Researchers should focus on the basic mechanisms of inactivity and the parameters needed to achieve the best quality juice shortly. Although the ability of heat-efficient processes to reduce foodborne pathogens, and improve nutritional value and shelf life, their high costs limit their applications in juice processing industries. The inability to handle large amounts of juice is another reason for its limited use and lack of in-depth knowledge of processes. More research is needed for improving the cost and scope of these processes according to consumer demands.
7. Data availability
Data will be made available on request.
CRediT authorship contribution statement
Kinza Mukhtar: Writing – original draft, Methodology. Brera Ghulam Nabi: Writing – original draft, Methodology. Rai Naveed Arshad: Writing – review & editing. Ume Roobab: Writing – review & editing. Muhammad Modassar Ali Nawaz Ranjha: Writing – review & editing. Rana Muhammad Aadil: Conceptualization, Writing – review & editing, Supervision. Salam A. Ibrahim: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would also like to acknowledge the support of the Agricultural Research Station at North Carolina Agricultural and Technical State University (Greensboro, NC 27411, USA). This research was funded, in part, by Grants (project Number NC.X337-5-21-170-1 and NC.X341-5-21-170-1) in addition to the 1890 capacity building project from the National Institute of Food and Agriculture (NIFA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIFA. The authors are also thankful to the University of Agriculture, Faisalabad, Pakistan for their support.
Contributor Information
Rana Muhammad Aadil, Email: muhammad.aadil@uaf.edu.pk.
Salam A. Ibrahim, Email: ibrah001@ncat.edu.
Data availability
No data was used for the research described in the article.
References
- 1.S. Arif, A. Batool, W. Nazir, R.S. Khan, N. Khalid, Physiochemical characteristics nutritional properties and health benefits of sugarcane juice, Non-Alcoholic Beverages Vol. 6. Sci. Beverages. (2019) 227–257. 10.1016/B978-0-12-815270-6.00008-6.
- 2.Eggleston G. Positive Aspects of Cane Sugar and Sugar Cane Derived Products in Food and Nutrition. J. Agric. Food Chem. 2018;66:4007–4012. doi: 10.1021/ACS.JAFC.7B05734. [DOI] [PubMed] [Google Scholar]
- 3.Panigrahi C., Shaikh A.E.Y., Bag B.B., Mishra H.N., De S. A technological review on processing of sugarcane juice: Spoilage, preservation, storage, and packaging aspects. J. Food Process Eng. 2021;44 doi: 10.1111/JFPE.13706. [DOI] [Google Scholar]
- 4.Tarafdar A., Kumar Y., Kaur B.P., Badgujar P.C. High-pressure microfluidization of sugarcane juice: Effect on total phenols, total flavonoids, antioxidant activity, and microbiological quality. J. Food Process. Preserv. 2021;45 doi: 10.1111/JFPP.15428. [DOI] [Google Scholar]
- 5.Qudsieh H.Y.M., Yusof S., Osman A., Abdul Rahman R. Effect of maturity on chlorophyll, tannin, color, and polyphenol oxidase (PPO) activity of sugarcane juice (Saccharum officinarum Var. Yellow Cane) J. Agric. Food Chem. 2002;50:1615–1618. doi: 10.1021/JF010959L. [DOI] [PubMed] [Google Scholar]
- 6.T.& D. Krishnakumar, T. & Chellamuthu, Effect of delayed extraction and storage on quality of sugarcane juice, African J. Agric. Res. 8 (2013) 930–935. 10.5897/AJAR12.1807.
- 7.H. Eissa, Hesham & Aziz, Nadir & Ramadan, Mostafa & Ali, (PDF) Preservation of Sugarcane Juice by Canning I. Effect of Thermal and Chemical Pre-treatments on the Enzymatic Browning of Sugarcane Juice., J. Am. Sci. 6. (2010) 883–888.
- 8.Jadhav H.B., Annapure U.S., Deshmukh R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021;8:248. doi: 10.3389/FNUT.2021.657090/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petruzzi L., Campaniello D., Speranza B., Corbo M.R., Sinigaglia M., Bevilacqua A. Thermal Treatments for Fruit and Vegetable Juices and Beverages: A Literature Overview. Compr. Rev. Food Sci. Food Saf. 2017;16:668–691. doi: 10.1111/1541-4337.12270. [DOI] [PubMed] [Google Scholar]
- 10.Kohli G., Jain G., Bisht A., Upadhyay A., Kumar A., Dabir S. Effect of non-thermal hurdles in shelf life enhancement of sugarcane juice. Lwt. 2019;112 doi: 10.1016/j.lwt.2019.05.131. [DOI] [Google Scholar]
- 11.G.. Chew, S.K. & Noor, N.A. & Murad, Maizura & Tan, Thuan-Chew & Rusul, (PDF) Effect of pasteurization treatment and calamansi (Fortunella japonica) juice on the physicochemical, microbiological, and sensory characteristics of black stem sugarcane juice, Int. Food Res. J. (2018) 1007–1015. https://www.researchgate.net/publication/326800382.
- 12.Rodrigues R., Sperandio L.C.C., Andrade C.M.G. Investigation of color and turbidity in the clarification of sugarcane juice by ozone. J. Food Process Eng. 2018;41 doi: 10.1111/JFPE.12661. [DOI] [Google Scholar]
- 13.Saxena J., Makroo H.A., Srivastava B. Optimization of time-electric field combination for PPO inactivation in sugarcane juice by ohmic heating and its shelf life assessment. LWT - Food Sci. Technol. 2016;71:329–338. doi: 10.1016/J.LWT.2016.04.015. [DOI] [Google Scholar]
- 14.Kapoor K., Garg N., Diwan R.K., Varshney L., Tyagi A.K. Study the effect of gamma radiation pretreatment of sugarcane bagasse on its physcio-chemical morphological and structural properties. RaPC. 2017;141:190–195. doi: 10.1016/J.RADPHYSCHEM.2017.07.010. [DOI] [Google Scholar]
- 15.Kulmann de Medeiros J., Sarkis J.R., Jaeschke D.P., Mercali G.D. Thermosonication for peroxidase inactivation in sugarcane juice. LWT. 2021;140 doi: 10.1016/J.LWT.2020.110730. [DOI] [Google Scholar]
- 16.Kayalvizhi V., Pushpa A.J.S., Sangeetha G., Antony U. Effect of pulsed electric field (PEF) treatment on sugarcane juice. J. Food Sci. Technol. 2016;53:1371–1379. doi: 10.1007/S13197-016-2172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sreedevi P., Rao P.S. Microbial destruction kinetics of high-pressure-processed sugarcane juice (Saccharum officinarum) J. Food Process Eng. 2018;41 doi: 10.1111/JFPE.12850. [DOI] [Google Scholar]
- 18.Tarafdar A., Kaur B.P., Pareek S. Effect of Microfluidization on Deteriorative Enzymes, Sugars, Chlorophyll, and Color of Sugarcane Juice. Food Bioprocess Technol. 2021;14:1375–1385. doi: 10.1007/S11947-021-02651-W. [DOI] [Google Scholar]
- 19.Varghese K.S., Pandey M.C., Radhakrishna K., Bawa A.S. Technology, applications and modelling of ohmic heating: a review. J. Food Sci. Technol. 2014;51:2304–2317. doi: 10.1007/S13197-012-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demirdöven A., Baysal T. Optimization of ohmic heating applications for pectin methylesterase inactivation in orange juice. J. Food Sci. Technol. 2014;51:1817–1826. doi: 10.1007/S13197-012-0700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.P. Abhilasha, U.S. Pal, Effect of Ohmic Heating on Quality and Storability of Sugarcane Juice, Int. J. Curr. Microbiol. Appl. Sci. 7 (2018) 2856–2868. 10.20546/IJCMAS.2018.701.340.
- 22.Brochier B., Mercali G.D., Marczak L.D.F. Effect of ohmic heating parameters on peroxidase inactivation, phenolic compounds degradation and color changes of sugarcane juice. Food Bioprod. Process. 2018;111:62–71. doi: 10.1016/j.fbp.2018.07.003. [DOI] [Google Scholar]
- 23.Brochier B., Hertz P.F., Marczak L.D.F., Mercali G.D. Influence of ohmic heating on commercial peroxidase and sugarcane juice peroxidase inactivation. J. Food Eng. 2020;284 doi: 10.1016/J.JFOODENG.2020.110066. [DOI] [Google Scholar]
- 24.Aghdase Sadeghi B.K., Hakimzadeh V. Microwave Assisted Extraction of Bioactive Compounds from Food. A Review, Int. J. Food Sci. Nutr. Eng. 2017:19–27. [Google Scholar]
- 25.Siguemoto É.S., Gut J.A.W., Martinez A., Rodrigo D. Inactivation kinetics of Escherichia coli O157:H7 and Listeria monocytogenes in apple juice by microwave and conventional thermal processing. Innov. Food Sci. Emerg. Technol. 2018;45:84–91. doi: 10.1016/J.IFSET.2017.09.021. [DOI] [Google Scholar]
- 26.N. Pradhan, D. Kumar, P. Singh, P.S. Pisalkar, Sensory and Microbial Evaluation of Microwave treated Sugarcane Juice, Int. J. Curr. Microbiol. Appl. Sci. 9 (2020) 1313–1320. 10.20546/IJCMAS.2020.903.153.
- 27.Adulvitayakorn S., Azhari S.H., Hasan H. The effects of conventional thermal, microwave heating, and thermosonication treatments on the quality of sugarcane juice. J. Food Process. Preserv. 2020;44:1–8. doi: 10.1111/jfpp.14322. [DOI] [Google Scholar]
- 28.Brodie G., Harris G., Jacob M.V., Sheehan M., Yin L. Microwave modification of sugar cane to enhance juice extraction during milling. J. Microw. Power Electromagn. Energy. 2011;45:178–187. doi: 10.1080/08327823.2011.11689812. [DOI] [PubMed] [Google Scholar]
- 29.C.L.. Miller, F.A. & Silva, Thermal treatment effects in fruit juices, (2012).
- 30.Chen Y., Yu L.J., Rupasinghe H.V. Effect of thermal and non-thermal pasteurisation on the microbial inactivation and phenolic degradation in fruit juice: A mini-review. J. Sci. Food Agric. 2013;93:981–986. doi: 10.1002/JSFA.5989. [DOI] [PubMed] [Google Scholar]
- 31.Achir N., Dhuique-Mayer C., Hadjal T., Madani K., Pain J.P., Dornier M. Pasteurization of citrus juices with ohmic heating to preserve the carotenoid profile. Innov. Food Sci. Emerg. Technol. 2016;33:397–404. doi: 10.1016/J.IFSET.2015.11.002. [DOI] [Google Scholar]
- 32.Kunitake M., Ditchfield C., Silva C., Petrus R. Effect of pasteurization temperature on stability of an acidified sugarcane juice beverage. Ciência e Agrotecnologia. 2014;38:554–561. doi: 10.1590/s1413-70542014000600004. [DOI] [Google Scholar]
- 33.Kaur G., Kumar V., Goyal A., Tanwar B., Kaur J. Optimization of nutritional beverage developed from radish, sugarcane and herbal extract using response surface methodology. Nutr. Food Sci. 2018;48:733–743. doi: 10.1108/NFS-11-2017-0247. [DOI] [Google Scholar]
- 34.Karmakar R., Kumar Ghosh A., Gangopadhyay H. Asian Journal of Food and Agro-Industry Study on the nutritional and microbiological changes of sugarcane juice and determination of optimum conditions during pasteurization, As. J. Food Ag-Ind. 2010;3:453–461. [Google Scholar]
- 35.Silva C.O., Gallo F.A. Sugarcane Juice Processing: Microbiological Monitoring. J. Food Process. Technol. 2016;7 doi: 10.4172/2157-7110.1000607. [DOI] [Google Scholar]
- 36.R. Bulegon, G. de A. Gomes, E. Rigo, Influence of the Pasteurization Conditions on Sugarcane Juice Packaged in Glass Packaging, Rev. Do Congr. Sul Bras. Eng. Aliment. 4 (2018) 12–23. 10.5965/24473650412018012.
- 37.de Lima Gomes J., da Costa Ribeiro A.S., Kushida M.M., Kamimura E.S., Maldonado R.R., Petrus R.R. Sugarcane juice pasteurization: A search for the most effective parameters. J. Food Process. Preserv. 2020;44 doi: 10.1111/jfpp.14842. [DOI] [Google Scholar]
- 38.Wang L., Deng W., Wang P., Huang W., Wu J., Zheng T., Chen J. Degradations of aroma characteristics and changes of aroma related compounds, PPO activity, and antioxidant capacity in sugarcane juice during thermal process. J. Food Sci. 2020;85:1140–1150. doi: 10.1111/1750-3841.15108. [DOI] [PubMed] [Google Scholar]
- 39.Jittanit W., Wiriyaputtipong S., Charoenpornworanam H., Songsermpong S. Effects of varieties, heat pretreatment and UHT conditions on the sugarcane juice quality. Chiang Mai J. Sci. 2011;38:116–125. [Google Scholar]
- 40.Bhukya J., Naik R., Mohapatra D., Sinha L.K., Rao K.V.R. Orifice based hydrodynamic cavitation of sugarcane juice: Changes in Physico-chemical parameters and Microbiological load. Lwt. 2021;150 doi: 10.1016/j.lwt.2021.111909. [DOI] [Google Scholar]
- 41.Oms-Oliu G., Odriozola-Serrano I., Soliva-Fortuny R., Elez-Martínez P., Martín-Belloso O. Stability of health-related compounds in plant foods through the application of non thermal processes. Trends Food Sci. Technol. 2012;23:111–123. doi: 10.1016/J.TIFS.2011.10.004. [DOI] [Google Scholar]
- 42.Shabbir M.A., Ahmed H., Maan A.A., Rehman A., Afraz M.T., Iqbal M.W., Khan I.M., Amir R.M., Ashraf W., Khan M.R., Aadil R.M. Effect of non-thermal processing techniques on pathogenic and spoilage microorganisms of milk and milk products. Food Sci. Technol. 2020;41:279–294. doi: 10.1590/FST.05820. [DOI] [Google Scholar]
- 43.Roobab U., Abida A., Chacha J.S., Athar A., Madni G.M., Ranjha M.M.A.N., Rusu A.V., Zeng X.-A., Aadil R.M., Trif M. Applications of Innovative Non-Thermal Pulsed Electric Field Technology in Developing Safer and Healthier Fruit Juices. Molecules. 2022;27:4031. doi: 10.3390/molecules27134031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.S. Zia, M.R. Khan, M.A. Shabbir, A. Aslam Maan, M.K.I. Khan, M. Nadeem, A.A. Khalil, A. Din, R.M. Aadil, An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-related Matrices, 10.1080/87559129.2020.1772283. 38 (2020) 1166–1196. 10.1080/87559129.2020.1772283.
- 45.Mansor N., Ramli S., Azhari S.H., Abd Rahim M.H. Effects of different preservation treatments on nutritional profile on juices from different sugar cane varieties. Sains Malaysiana. 2020;49:283–291. doi: 10.17576/jsm-2020-4902-06. [DOI] [Google Scholar]
- 46.Chauhan O.P., Ravi N., Roopa N., Kumar S., Raju P.S. High pressure, temperature and time-dependent effects on enzymatic and microbial properties of fresh sugarcane juice. J. Food Sci. Technol. 2017;54:4135–4138. doi: 10.1007/s13197-017-2872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sreedevi P., Jayachandran L.E., Rao P.S. Response surface optimization and quality prediction of high pressure processed sugarcane juice (Saccharum officinarum) Lwt. 2021;152 doi: 10.1016/j.lwt.2021.112190. [DOI] [Google Scholar]
- 48.Jasmi N., Mansor N., Lim E.J., Yusof N.L., Hajar-Azhari S., Rahim M.H.A. The effect of sonication and heat treatment on the physicochemical, nutritional and microbiological properties of different sugarcane variants. Food Sci. Technol. 2020;40:551–556. doi: 10.1590/fst.12619. [DOI] [Google Scholar]
- 49.Hajar-Azhari S., Shahruddin R., Rahim M.H.A. The effect of heat treatment and sonication on physicochemical and colour attributes of yellow sugarcane juice. Malaysian Appl. Biol. 2018;47:129–134. [Google Scholar]
- 50.Manzoor M.F., Ahmad N., Ahmed Z., Siddique R., Mehmood A., Usman M., Zeng X.A. Effect of dielectric barrier discharge plasma, ultra-sonication, and thermal processing on the rheological and functional properties of sugarcane juice. J. Food Sci. 2020;85:3823–3832. doi: 10.1111/1750-3841.15498. [DOI] [PubMed] [Google Scholar]
- 51.Filho W.D.S., De Sousa G.L., Henrique P., Roger G., Malpass P., Hitomiokura M. Ultraviolet Irradiation As an Alternative To Purify the Sugarcane Juice for Sugar and Ethanol Production. Int. J. Dev. Res. 2021;11:48219–48225. [Google Scholar]
- 52.Duc Trung N., Ngoc Hoang N., Minh Hieu D. Pulsed Electric Field for Pasteurization of Fresh Sugarcane Juice. ASEAN Eng. J. 2018;8:27. [Google Scholar]
- 53.Panigrahi C., Mishra H.N., De S. Effect of ozonation parameters on nutritional and microbiological quality of sugarcane juice. J. Food Process Eng. 2020;43:1–14. doi: 10.1111/jfpe.13542. [DOI] [Google Scholar]
- 54.Rai C., Rai P., Majumdar G.C., De S., DasGupta S. Mechanism of permeate flux decline during microfiltration of watermelon (Citrullus lanatus) juice. Food Bioprocess Technol. 2010;3:545–553. doi: 10.1007/S11947-008-0118-2. [DOI] [Google Scholar]
- 55.Rezzadori K., Serpa L., Penha F.M., Petrus R.R., Petrus J.C.C. Crossflow microfiltration of sugarcane juice - effects of processing conditions and juice quality. Food Sci. Technol. 2014;34:210–217. doi: 10.1590/S0101-20612014000100030. [DOI] [Google Scholar]
- 56.Panigrahi C., Mondal M., Karmakar S., Mishra H.N., De S. Shelf life extension of sugarcane juice by cross flow hollow fibre ultrafiltration. J. Food Eng. 2020;274 doi: 10.1016/j.jfoodeng.2019.109880. [DOI] [Google Scholar]
- 57.Tarafdar A., Nair S.G., Pal Kaur B. Identification of Microfluidization Processing Conditions for Quality Retention of Sugarcane Juice Using Genetic Algorithm. Food Bioprocess Technol. 2019;12:1874–1886. doi: 10.1007/s11947-019-02345-4. [DOI] [Google Scholar]
- 58.Roobab U., Fidalgo L.G., Arshad R.N., Khan A.W., Zeng X.A., Bhat Z.F., Bekhit A.E.D.A., Batool Z., Aadil R.M. High-pressure processing of fish and shellfish products: Safety, quality, and research prospects. Compr. Rev. Food Sci. Food Saf. 2022:1–29. doi: 10.1111/1541-4337.12977. [DOI] [PubMed] [Google Scholar]
- 59.Roobab U., Shabbir M.A., Khan A.W., Arshad R.N., Bekhit A.E.D., Zeng X.A., Inam-Ur-Raheem M., Aadil R.M. High-pressure treatments for better quality clean-label juices and beverages: Overview and advances. Lwt. 2021;149 [Google Scholar]
- 60.Roobab U., Abida A., Afzal R., Madni G.M., Zeng X.A., Rahaman A., Aadil R.M. Impact of high-pressure treatments on enzyme activity of fruit-based beverages: an overview. Int. J. Food Sci. Technol. 2022;57:801–815. doi: 10.1111/ijfs.15492. [DOI] [Google Scholar]
- 61.Roobab U., Inam-Ur-Raheem M., Khan A.W., Arshad R.N., Zeng X., Aadil R.M. Innovations in High-pressure Technologies for the Development of Clean Label Dairy Products: A Review. Food Rev. Int. 2021:1–22. doi: 10.1080/87559129.2021.1928690. [DOI] [Google Scholar]
- 62.Roobab U., Afzal R., Ranjha M.M.A.N., Zeng X.A., Ahmed Z., Aadil R.M. High pressure-based hurdle interventions for raw and processed meat: a clean-label prospective. Int. J. Food Sci. Technol. 2022;57:816–826. doi: 10.1111/ijfs.15499. [DOI] [Google Scholar]
- 63.Sehrawat R., Kaur B.P., Nema P.K., Tewari S., Kumar L. Microbial inactivation by high pressure processing: principle, mechanism and factors responsible. Food Sci. Biotechnol. 2021;30:19. doi: 10.1007/S10068-020-00831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georget E., Sevenich R., Reineke K., Mathys A., Heinz V., Callanan M., Rauh C., Knorr D. Inactivation of microorganisms by high isostatic pressure processing in complex matrices: A review. Innov. Food Sci. Emerg. Technol. 2015;27:1–14. doi: 10.1016/J.IFSET.2014.10.015. [DOI] [Google Scholar]
- 65.Huang H.W., Chang Y.H., Wang C.Y. High Pressure Pasteurization of Sugarcane Juice: Evaluation of Microbiological Shelf Life and Quality Evolution During Refrigerated Storage. Food Bioprocess Technol. 2015;8:2483–2494. doi: 10.1007/S11947-015-1600-2. [DOI] [Google Scholar]
- 66.Huang H.W., Lung H.M., Yang B.B., Wang C.Y. Responses of microorganisms to high hydrostatic pressure processing. Food Control. 2014;40:250–259. doi: 10.1016/J.FOODCONT.2013.12.007. [DOI] [Google Scholar]
- 67.P. Sreedevi, P. Srinivasa Rao, P. Lalitha Kameswari, Effect of High Pressure Processing on Enzyme Inactivation and Microbial Destruction of Sugarcane Juice, Int. J. Curr. Microbiol. Appl. Sci. 6 (2017) 2000–2006. 10.20546/ijcmas.2017.609.245.
- 68.Liu M., Wang R., Li J., Zhang L., Zhang J., Zong W., Mo W. Dynamic high pressure microfluidization (DHPM): Physicochemical properties, nutritional constituents and microorganisms of yam juice. Czech J. Food Sci. 2021;39(2021):217–225. doi: 10.17221/284/2020-CJFS. [DOI] [Google Scholar]
- 69.Koley T.K., Nishad J., Kaur C., Su Y., Sethi S., Saha S., Sen S., Bhatt B.P. Effect of high-pressure microfluidization on nutritional quality of carrot (Daucus carota L.) juice. J. Food Sci. Technol. 2020;57:2159–2168. doi: 10.1007/S13197-020-04251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tarafdar A., Kaur B.P. Microfluidization-Driven Changes in Some Physicochemical Characteristics, Metal/Mineral Composition, and Sensory Attributes of Sugarcane Juice. J. Food Qual. 2021;2021 doi: 10.1155/2021/3326302. [DOI] [Google Scholar]
- 71.Arshad R.N., Abdul-Malek Z., Roobab U., Munir M.A., Naderipour A., Qureshi M.I., El-Din Bekhit A., Liu Z.W., Aadil R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021;111:43–54. doi: 10.1016/j.tifs.2021.02.041. [DOI] [Google Scholar]
- 72.Arshad R.N., Abdul-Malek Z., Munir A., Buntat Z., Ahmad M.H., Jusoh Y.M.M., Bekhit A.E.D., Roobab U., Manzoor M.F., Aadil R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020;104:1–13. doi: 10.1016/j.tifs.2020.07.008. [DOI] [Google Scholar]
- 73.Arshad R.N., Abdul-Malek Z., Roobab U., Qureshi M.I., Khan N., Ahmad M.H., Liu Z.W., Aadil R.M. Effective valorization of food wastes and by-products through pulsed electric field: A systematic review. J. Food Process Eng. 2021;44:1–14. doi: 10.1111/jfpe.13629. [DOI] [Google Scholar]
- 74.Huang K., Yu L., Wang W., Gai L., Wang J. Comparing the pulsed electric field resistance of the microorganisms in grape juice: Application of the Weibull model. Food Control. 2014;35:241–251. doi: 10.1016/J.FOODCONT.2013.07.011. [DOI] [Google Scholar]
- 75.U. Roobab, R.M. Aadil, S.A. Hassan, X. Zeng, Ultrasound, in: Adv. Noninvasive Food Anal., 2020: pp. 21–26.
- 76.Mushtaq A., Roobab U., Denoya G.I., Inam-Ur-Raheem M., Gullón B., Lorenzo J.M., Barba F.J., Zeng X.A., Wali A., Aadil R.M. Advances in green processing of seed oils using ultrasound-assisted extraction: A review. J. Food Process. Preserv. 2020;44:1–14. doi: 10.1111/jfpp.14740. [DOI] [Google Scholar]
- 77.Aadil R.M., Zeng X.A., Wang M.S., Liu Z.W., Han Z., Zhang Z.H., Hong J., Jabbar S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food Sci. Technol. 2015;50:1144–1150. doi: 10.1111/IJFS.12767. [DOI] [Google Scholar]
- 78.Ranjha M.M.A.N., Irfan S., Lorenzo J.M., Shafique B., Kanwal R., Pateiro M., Arshad R.N., Wang L., Nayik G.A., Roobab U., Aadil R.M. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes. 2021;9:1–21. doi: 10.3390/pr9081406. [DOI] [Google Scholar]
- 79.Gabriel A.A. Inactivation behaviors of foodborne microorganisms in multi-frequency power ultrasound-treated orange juice. Food Control. 2014;46:189–196. doi: 10.1016/J.FOODCONT.2014.05.012. [DOI] [Google Scholar]
- 80.Guerrouj K., Sánchez-Rubio M., Taboada-Rodríguez A., Cava-Roda R.M., Marín-Iniesta F. Sonication at mild temperatures enhances bioactive compounds and microbiological quality of orange juice. Food Bioprod. Process. 2016;99:20–28. doi: 10.1016/J.FBP.2016.03.007. [DOI] [Google Scholar]
- 81.Rodrigues N.P., Brochier B., de Medeiros J.K., Marczak L.D.F., Mercali G.D. Phenolic profile of sugarcane juice: Effects of harvest season and processing by ohmic heating and ultrasound. Food Chem. 2021;347 doi: 10.1016/j.foodchem.2021.129058. [DOI] [PubMed] [Google Scholar]
- 82.Julie C.M.F., Reisz A., Bansal N., Qian J., Zhao W. - Google Search; 2014. Effects of ionizing radiation on biological molecules-mechanisms of damage and emerging methods of detection; pp. 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roobab U., Aadil R.M., Madni G.M., Bekhit A.E.D. The Impact of Nonthermal Technologies on the Microbiological Quality of Juices: A Review. Compr. Rev. Food Sci. Food Saf. 2018;17:437–457. doi: 10.1111/1541-4337.12336. [DOI] [PubMed] [Google Scholar]
- 84.A.B. Argenta, A.D.P. Scheer, Membrane Separation Processes Applied to Whey: A Review, 36 (2019) 499–528. 10.1080/87559129.2019.1649694.
- 85.Bhattacharjee C., Saxena V.K., Dutta S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017;43:136–153. doi: 10.1016/j.ifset.2017.08.002. [DOI] [Google Scholar]
- 86.Moreno R.M.C., de Oliveira R.C., de Barros S.T.D. Comparação entre microfiltração e adição de agentes coagulantes na clarificação do caldo de cana. Acta Sci. - Technol. 2012;34:413–419. doi: 10.4025/ACTASCITECHNOL.V34I4.8890. [DOI] [Google Scholar]
- 87.Gaschi P.S., Gaschi P.S., De Barros S.T.D., Pereira N.C. Efeito do pré-tratamento por microfiltração com membrana cerâmica no processo de clarificação do caldo de cana por ultrafiltração. Acta Sci. - Technol. 2014;36:303–306. doi: 10.4025/actascitechnol.v36i2.17322. [DOI] [Google Scholar]
- 88.Pandiselvam R., Chandrasekar V., Thirupathi V. Numerical simulation of ozone concentration profile and flow characteristics in paddy bulks. Pest Manag. Sci. 2017;73:1698–1702. doi: 10.1002/PS.4516. [DOI] [PubMed] [Google Scholar]
- 89.Roobab U., Chacha J.S., Abida A., Rashid S., Madni G.M., Lorenzo J.M., Zeng X., Aadil R.M. Emerging Trends for Nonthermal Decontamination of Raw and Processed Meat: Ozonation. High-Hydrostatic Pressure and Cold Plasma, Foods. 2022;11:2173. doi: 10.3390/%0Afoods11152173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gharib-Bibalan S., Keramat J., Hamdami N. Better Lime Purification of Raw Sugar Beet Juice by Advanced Fenton Oxidation Process. Ozone Sci. Eng. 2018;40:54–63. doi: 10.1080/01919512.2017.1345617. [DOI] [Google Scholar]
- 91.de Souza Sartori J.A., Angolini C.F.F., Eberlin M.N., de Aguiar C.L. Criegee mechanism as a safe pathway of color reduction in sugarcane juice by ozonation. Food Chem. 2017;225:181–187. doi: 10.1016/J.FOODCHEM.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 92.Yeoh W.K., Ali A., Forney C.F. Effects of ozone on major antioxidants and microbial populations of fresh-cut papaya. Postharvest Biol. Technol. 2014;89:56–58. doi: 10.1016/J.POSTHARVBIO.2013.11.006. [DOI] [Google Scholar]
- 93.Chakraborty S., Kaushik N., Rao P.S., Mishra H.N. High-Pressure Inactivation of Enzymes: A Review on Its Recent Applications on Fruit Purees and Juices. Compr. Rev. Food Sci. Food Saf. 2014;13:578–596. doi: 10.1111/1541-4337.12071. [DOI] [PubMed] [Google Scholar]
- 94.Li J., Suo Y., Liao X., Ahn J., Liu D., Chen S., Ye X., Ding T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017;39:101–110. doi: 10.1016/J.ULTSONCH.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 95.Samani B.H., Khoshtaghaza M.H., Lorigooini Z., Minaei S., Zareiforoush H. Analysis of the combinative effect of ultrasound and microwave power on Saccharomyces cerevisiae in orange juice processing. Innov. Food Sci. Emerg. Technol. 2015;32:110–115. doi: 10.1016/J.IFSET.2015.09.015. [DOI] [Google Scholar]
- 96.Pérez-Grijalva B., Herrera-Sotero M., Mora-Escobedo R., Zebadúa-García J.C., Silva-Hernández E., Oliart-Ros R., Pérez-Cruz C., Guzmán-Gerónimo R. Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT - Food Sci. Technol. 2018;87:47–53. doi: 10.1016/J.LWT.2017.08.059. [DOI] [Google Scholar]
- 97.Tian Y., Wang S., Yan W., Tang Y., Yang R., Zhao W. Inactivation of apple (Malus domestica Borkh) polyphenol oxidases by radio frequency combined with pulsed electric field treatment. Int. J. Food Sci. Technol. 2018;53:2054–2063. doi: 10.1111/IJFS.13781. [DOI] [Google Scholar]
- 98.Sankhla S., Chaturvedi A., Kuna A., Dhanlakshmi K. Preservation of Sugarcane Juice Using Hurdle Technology. Sugar Tech. 2012;14:26–39. doi: 10.1007/S12355-011-0127-8. [DOI] [Google Scholar]
- 99.Chauhan O.P., Singh D., Tyagi S.M., Balyan D.K. Studies on preservation of sugarcane juice. Int. J. Food Prop. 2002;5:217–229. doi: 10.1081/JFP-120015603. [DOI] [Google Scholar]
- 100.Verma A., Kumar D., Singh P., Yadav K.C. Thermal and chemical treatments for enhancing the shelf-life of sugarcane juice. Asian J. Hortic. 2016;11:373–378. doi: 10.15740/has/tajh/11.2/373-378. [DOI] [Google Scholar]
- 101.Geremias-Andrade I.M., Rocheto A.C., Gallo F.A., Petrus R.R. The shelf life of standardized sugarcane juice stored under refrigeration. Food Sci. Technol. 2020;40:95–101. doi: 10.1590/fst.33918. [DOI] [Google Scholar]
- 102.De Oliveira A.C.G., Spoto M.H.F., Canniatti-Brazaca S.G., De Sousa C.P., Gallo C.R. Efeitos do processamento térmico e da radiação gama na conservação de caldo de cana puro e adicionado de suco de frutas. Cienc. e Tecnol. Aliment. 2007;27:863–873. doi: 10.1590/S0101-20612007000400029. [DOI] [Google Scholar]
- 103.Mishra B.B., Gautam S., Sharma A. Shelf Life Extension of Sugarcane Juice Using Preservatives and Gamma Radiation Processing. J. Food Sci. 2011;76 doi: 10.1111/j.1750-3841.2011.02348.x. [DOI] [PubMed] [Google Scholar]
- 104.Garud S.R., Priyanka B.S., Rastogi N.K., Prakash M., Negi P.S. Efficacy of Ozone and Lactic Acid as Nonthermal Hurdles for Preservation of Sugarcane Juice. Ozone Sci. Eng. 2018;40:198–208. doi: 10.1080/01919512.2017.1415802. [DOI] [Google Scholar]
- 105.B. Mathew, M. V., J.M. Kuriakose, S. V.R., J. G., Effect of Thermo Chemical Processing on Storability of Sugarcane Juice, Int. J. Environ. Agric. Biotechnol. 1 (2016) 526–530. 10.22161/ijeab/1.3.32.
- 106.Zia S., Khan M.R., Zeng X.A., Sehrish M.A., Shabbir R.M.A. Combined effect of microwave and ultrasonication treatments on the quality and stability of sugarcane juice during cold storage. Int. J. Food Sci. Technol. 2019;54:2563–2569. doi: 10.1111/ijfs.14167. [DOI] [Google Scholar]
- 107.Garud S.R., Priyanka B.S., Negi P.S., Rastogi N.K. Effect of Thermosonication on Bacterial Count in Artificially Inoculated Model System and Natural Microflora of Sugarcane Juice. J. Food Process. Preserv. 2017;41 doi: 10.1111/jfpp.12813. [DOI] [Google Scholar]
- 108.C. Lagnika, J. Huang, N. Jiang, D. Li, C. Liu, J. Song, Q. Wei, M. Zhang, Ultrasound-assisted osmotic process on quality of microwave vacuum drying sweet potato, 10.1080/07373937.2017.1402786. 36 (2018) 1367–1379. 10.1080/07373937.2017.1402786.
- 109.Liu S., Zhang X., You L., Guo Z., Chang X. Changes in anthocyanin profile, color, and antioxidant capacity of hawthorn wine (Crataegus pinnatifida) during storage by pretreatments. LWT. 2018;95:179–186. doi: 10.1016/J.LWT.2018.04.093. [DOI] [Google Scholar]
- 110.K. and S.P. Rawat, Preservation of sugarcane juice using hurdle technology 2014. - Google Search, Int. J. Sci. Eng. Technol. (2014).
- 111.C. Silva, Willian & Sartori, Juliana & Aguiar, (PDF) Combination Effect of Ozone and Heat Treatment for the Color Reduction in Sugarcane Juice, Chem. Process Eng. Res. (2015).
- 112.Panigrahi C., Mishra H.N., De S. Ozone treatment of ultrafiltered sugarcane juice: Process optimization using multi-objective genetic algorithm and correlation analysis by multivariate technique. Lwt. 2022;154 doi: 10.1016/j.lwt.2021.112861. [DOI] [Google Scholar]
- 113.Majid I., Nayik G.A., Nanda V. Ultrasonication and food technology: A review. Cogent Food Agric. 2015;1 doi: 10.1080/23311932.2015.1071022. [DOI] [Google Scholar]
- 114.Galati A., Moavero P., Crescimanno M. Consumer awareness and acceptance of irradiated foods: the case of Italian consumers. Br. Food J. 2019;121:1398–1412. doi: 10.1108/BFJ-05-2018-0336/FULL/HTML. [DOI] [Google Scholar]
- 115.Q. Abbas Syed, A. Ishaq, U. Ur Rahman, S. Aslam, R. Shukat, Pulsed electric field technology in food preservation: a review, J. Nutr. Heal. Food Eng. Volume 6 (2017). 10.15406/JNHFE.2017.06.00219.
- 116.Chacha J.S., Zhang L., Ofoedu C.E., Suleiman R.A., Dotto J.M., Roobab U., Agunbiade A.O., Duguma H.T., Mkojera B.T., Hossaini S.M., Rasaq W.A., Shorstkii I., Okpala C.O.R., Korzeniowska M., Guiné R.P.F. Revisiting non-thermal food processing and preservation methods—action mechanisms, pros and cons: A technological update (2016–2021) Foods. 2021;10 doi: 10.3390/foods10061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guerrero-Beltrán J.A., Barbosa-Cánovas G.V. Review: Advantages and limitations on processing foods by UV light. Food Sci. Technol. Int. 2004;10:137–147. doi: 10.1177/1082013204044359. [DOI] [Google Scholar]
- 118.Nabi B.G., Mukhtar K., Arshad R.N., Radicetti E., Tedeschi P., Shahbaz M.U., Walayat N., Nawaz A., Inam-Ur-raheem M., Aadil R.M. High-pressure processing for sustainable food supply. Sustain. 2021;13:13908. doi: 10.3390/su132413908. [DOI] [Google Scholar]
- 119.Sakr M., Liu S. A comprehensive review on applications of ohmic heating (OH) Renew. Sustain. Energy Rev. 2014;39:262–269. doi: 10.1016/J.RSER.2014.07.061. [DOI] [Google Scholar]
- 120.A. Cassano, C. Conidi, E. Drioli, Chapter 6. Integrated membrane operations in fruit juice processing, Membr. Syst. Food Prod. (2021) 139–174. 10.1515/9783110712711-006/PDF.
- 121.Arı B., Budak N.H., Seydim A.C., Güzel-Seydim Z. Effects of Ozonation on Apple Juice Quality. Int. J. Fruit Sci. 2020;20:S1570–S1578. doi: 10.1080/15538362.2020.1822263. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
No data was used for the research described in the article.