Abstract
Diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) is a heterogenous group of aggressive lymphomas. C-MYC expression by immunohistochemical stain (IHC) is shown to be an independent prognostic factor in DLBCL. In the clinical setting, MYC stain is currently evaluated by manual quantification with a minimum positivity cut-off 40%. Manual quantification methods can be subjective and may show intra- and interobserver variability and variability between centers. Thus, stains which require definitive quantification such as MYC needs better standardized and precise methods. Here we present a simple digital algorithm for quantitative evaluation of MYC stain in DLBCL, NOS. For this, slides immunostained for C-MYC were scanned at 40X with a high-resolution, Philips Ultra Fast scanner (Koninklijke Philips N.V. Cambridge, MA). The images were manually assessed and appropriate areas with neoplastic cells were selected. For quantification, positive and negative C-MYC staining nuclei were scored using a modified Visiopharm APP Nuclei Detection, AI (Brightfield) using Visiopharm Image Analysis software (Visiopharm, Hørsholm, Denmark version 2018.09). The percentage positivity resulted by the digital method was concordant with the pathologist’s interpretation with statistical significance (rs: 0.85968; p (2-tailed) = 0). Minor disadvantages were observed including failure to detect very weak staining and inability to separate neoplastic and non-neoplastic nuclei when admixed in the same area. If combined with a quick manual evaluation, a digital method like this with precision and reproducibility will be of great use in quantitative evaluation of MYC and other similar stains in clinical setting and will reduce intra- and interobserver variability.
Keywords: C-MYC, Diffuse large B-cell lymphoma, Immunohistochemistry, Image analysis, Digital quantification
Introduction
Diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) is a heterogeneous group of aggressive B-cell lymphomas.1 Based on cell of origin (COO) determined by gene expression profiling, this group is divided into prognostic subcategories namely, germinal center origin and non-germinal center or activated B-cell origin (GCB and non-GCB or ABC) types.2,3 Gene expression by immunohistochemistry is widely used as a surrogate method for COO subtyping.4 In addition to the COO classification, there are several other prognostic markers described in DLBCL. One such example is C-MYC expression. MYC overexpression is reported in over 30% of all DLBCLs.5 MYC expression in diffuse large B cell lymphoma (DLBCL) promotes DNA replicative stress and induces DNA damage facilitating a mutated phenotype in the neoplastic B-lymphocytes.6 Double-expressor phenotype is the co-expression of C-MYC and BCL-2 by immunohistochemical (IHC) stains and these cases behave poorly compared to DLBCL negative for MYC.7 In current clinical setting, MYC expression is evaluated by manual quantification using light microscopy with a 40% lower cut-off for positivity. Manual quantification methods can be subjective and show intra- and inter-observer variability and variability between centers. Better standardization and precision are required in MYC stain scoring.
Methods
The pathology database was searched for DLBCL, NOS cases in which C-MCY stain was performed as part of the routine clinical work up. From the search results, 28 cases of DLBCL were selected including cases negative for MYC, positive for MYC with high percentage values and cases with percentage score ranging from 30 to 50%. The C-MYC stains were reviewed to confirm the quality of the stain and tissue, and to confirm the percentage score originally assigned. Slides immunostained for C-MYC were scanned at 40X with a high-resolution, Philips Ultra Fast scanner (Koninklijke Philips N.V. Cambridge, MA ) at the University’s Comparative Pathology & Digital imaging Shared Resource. For the quantification of immunoreactivity, images were imported into Visiopharm Image Analysis software (Visiopharm, Hørsholm, Denmark version 2018.09). Images were segmented into areas of tumor, and areas of necrosis, non-tumor tissue, and slide artifacts, such as folds if present, were eliminated. Positive and negative C-MYC staining cells were scored using a modified Visiopharm APP Nuclei Detection, AI (Brightfield) with an intensity threshold set to mark positive C-MYC cells from a range of 0 to 210. C-MYC positive nuclei were labeled red and C-MYC negative nuclei were labeled blue. The total nuclei per section, total positive nuclei, total negative nuclei, and a positive nuclei percentage were calculated. This was compared to the percentage positivity assigned by the pathologist who signed out the case.
Results
When the 40% positivity in neoplastic cells cut-off was applied, 25 of the 28 cases showed concordance in the positive or negative interpretation provided by the pathologist and the digital algorithm. Results are shown in Table 1. The Spearman correlation coefficient was statistically significant (rs: 0.85968; p (2-tailed) = 0). Examples of cases positive and negative for MYC are shown in Fig. 1. The 3 remaining cases were interpreted as positive by the pathologist and the algorithm interpreted them as negative. Of these 3 cases, first one had variability in tumor cell density as well as in C-MYC positivity. The pathologists interpreted the C-MYC stain in this correcting for the variation in tumor cell density (60%) and the algorithm performed the total percentage of positivity among all nuclei (25%). In this case, the pathologist’s interpretation was deemed appropriate in a retrospective review. Second case was given a value of 50% by the pathologist and the algorithm gave a value of 28%. A retrospective review showed that many of the tumor cell nuclei were staining very weakly, and the algorithm did not recognize them as positive cells, even though the intensity threshold was set at a range of 0–210. The pathologist’s interpretation was deemed appropriate in this case as well because IHC stain for MYC can show variability due to many factors. The third case was given a value of 60% by the pathologist and the algorithm gave a value of 25%. In retrospective review, the algorithm’s interpretation was found to be appropriate.
Table 1.
Comparison of C-MYC quantification by manual method and by the digital algorithm (Visiopharm APP nuclei Detection AI (Brightfield)).
| Slide number | C-MYC reported | Manual estimation C-MYC | Positive nuclei (%) |
|---|---|---|---|
| 1 | 20–30 | 20 | 10.77987003 |
| 2 | 10 | 10 | 19.41118622 |
| 3 | 10–20 | 20 | 31.35953712 |
| 4 | 20 | 10 | 27.35300064 |
| 5 | 20 | 10 | 7.786083221 |
| 6 | 60 | 60 | 24.48817635 |
| 7 | 60 | 80 | 54.73313904 |
| 8 | 50 | 50 | 43.76833725 |
| 9 | >90 | 100 | 88.59416199 |
| 10 | >90 | 100 | 90.94528198 |
| 11 | 50 | 50 | 28.37444115 |
| 12 | 100 | 100 | 98.33091736 |
| 13 | >90 | 100 | 85.14043427 |
| 14 | 75 | 75 | 55.07027435 |
| 15 | 75 | 75 | 74.24976349 |
| 16 | 50 | 50 | 43.23263931 |
| 17 | 60 | 60 | 74.38539124 |
| 18 | 40 | 40 | 44.37334824 |
| 19 | 10 | 10 | 34.01594925 |
| 20 | 90 | 90 | 84.84696198 |
| 21 | 70 | 70 | 44.7013092 |
| 22 | 70 | 60 | 48.48410797 |
| 23 | 30 | 30 | 30.68032837 |
| 24 | 70 | 60 | 25.73857307 |
| 25 | 40 | 20 | 13.01595116 |
| 26 | 90 | 80 | 65.06013489 |
| 27 | 60 | 40 | 41.49975586 |
| 28 | 40 | 20 | 21.21422005 |
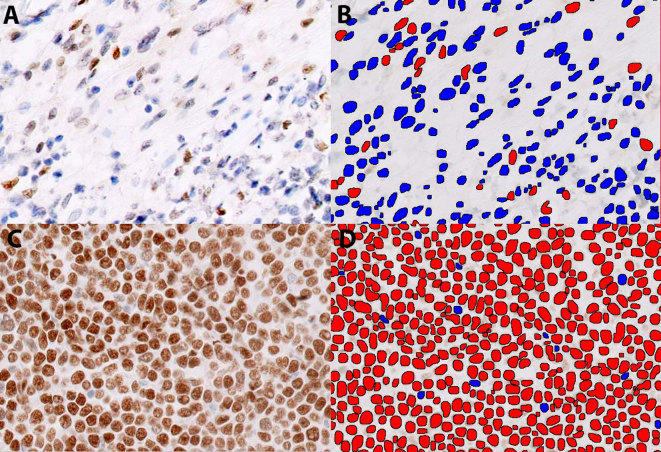
Fig. 1.
Digital quantificantion of C-MYC immunohistochemical stain using Visiopharm APP nuclei Detection AI (Brightfield): Original and labeled images of representative cases are shown. C-MYC immunostain interpretation of original image (positive nuclei - brown and negative nuclei - blue) and segmented image (positive nuclei - red and negative nuclei - blue). Top panel shows a case negative for C-MYC (A - original, IHC, C-MYC X200 and B - segmented, same field X200). Bottom panel shows a case positive for C-MYC (C - original, IHC, C-MYC X200 and D - segmented, same field X200).
Discussion
Several immunohistochemical stains require quantification and quantification is done manually in current clinical setting. Manual quantification can be subjective and variability between pathologists, same pathologist over time and between centers are common. It not possible to completely remove these differences in visual scoring. This is particularly important in case of stains in which definitive cut-off values are applied. One such example is C-MYC stain in DLBCL. The 40% positivity cut-off applied for this stain differentiates prognostic subcategories of DLBCL. Thus, simple digital algorithms are required to increase reproducibility and decrease variability.
There are several image analysis systems in use, particularly for quantification of stains with prognostic significance like Ki67. One such example is Cognition Master Professional Suite image analysis software used in Ki67 assessment in primary breast cancer.8 This digital system requires some manual intervention, including selection of fields with well-preserved tumor cells and exclusion of non-tumor areas, but is overall very quick.
A digital algorithm is not currently standardized for stains specifically used in hematopathology. Hence algorithms with precision and good concordance with visual interpretation which will assist the pathologists in interpretation of stains are required. These will reduce the variability in interpretation and reduce time taken for completion of each case. The algorithm we presented in this work utilized Visiopharm Image Analysis software and the positive nuclei were detected by modified Visiopharm APP nuclei Detection AI (Brightfield).
The results of digital C-MYC stain quantification were in concordance with the manual method with statistical significance. This method also requires manual assistance for segmentation of the sections into areas of tumor and exclusion of areas with necrosis, non-tumor tissue and staining/processing artifacts. The major advantage is the consistency in interpretation which will reduce intra- and interobserver variability. One disadvantage we found is even after setting the intensity threshold between 0 and 210, in one case, very weakly staining nuclei were not detected by the algorithm. C-MYC stain can show difference in staining intensity due to several preanalytical and analytical factors. These factors include the age of the diluted antibody, age of the tissue section, the amount of MYC antigen in the tissue, and the cause of MYC overexpression, namely gene amplification, rearrangement, or other alterations.9 Because of this, staining of any intensity is considered positive and it is important to detect the weakly staining nuclei. Another disadvantage is even though fields with tumor cells can be selected out manually, if there are non-neoplastic cells admixed with the tumor cells, the algorithm cannot be trained to separate these cells and a false low score results. This is true in many of the lymphoid neoplasms, due to the rich inflammatory background in lymphoproliferative disorders. This is not usually the case in DLBCLs, since these tumors are usually composed of diffuse proliferation of neoplastic cells. However, there are cases of DLBCL with very rich inflammatory background and in such cases, a digital system may not be as effective as manual quantification. Due to these technical limitations, a manual evaluation of the tissue and stain quality will be necessary before the algorithm does quantification, in addition to selection of appropriate fields for interpretation.
Conclusions
The digital algorithm and the pathologist’s interpretation showed statistically significant correlation. The key factors are setting the intensity threshold of the stain positivity appropriately and selecting the appropriate tumor tissue for evaluation. Similar methods may be applied for evaluation of stains, which require accurate quantification and are prognostically or therapeutically significant. These digital methods cannot be used as the exclusive method for interpretation, but as a complimentary method to assist pathologists. The major advantage is the reproducibility and consistency these methods provide. Such techniques will help in reduction of pathologist’s work time and thus reduce fatigue.
Competing interests
Authors do not have any competing interests in relation to this work.
Authors' contributions
Dr. Balakrishna contributed the idea, prepared and executed the research project, prepared the manuscript, Mr. Kulewsky performed the digital analysis of the images and Dr. Parwani provided guidance, reviewed the data and manuscript.
Contributor Information
Jayalakshmi Balakrishna, Email: Jayalakshmi.balakrishna@osumc.edu.
Jesse Kulewsky, Email: jesse.kulewsky@osumc.edu.
Anil Parwani, Email: anil.parwani@osumc.edu.
References:
- 1.Swerdlow S.H., Campo E., Harris N.L., et al. Revised 4th ed. Lyon; IARC: 2017. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 2.Yan W.H., Jiang X.N., Wang W.G., et al. Cell-of-origin subtyping of diffuse large B-cell lymphoma by using a qPCR-based gene expression assay on formalin-fixed paraffin-embedded tissues. Front Oncol. 2020;10:803. doi: 10.3389/fonc.2020.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph J., Ma J., Hennawy F., et al. Impact of cell of origin classification on survival outcomes after autologous transplantation in relapsed/refractory diffuse large B Cell lymphoma. Transplant Cell Ther. 2021;27(5):404.e401–404.e405. doi: 10.1016/j.jtct.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian S., Wang S., Major C., et al. Comparison of immunohistochemistry and gene expression profiling subtyping for diffuse large B-cell lymphoma in the phase III clinical trial of R-CHOP ± ibrutinib. Brit J Haematol. 2021;194(1):83–91. doi: 10.1111/bjh.17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn H., Ziepert M., Becher C., et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121(12):2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 6.Sangaletti S., Iannelli F., Zanardi F., et al. Intra-tumour heterogeneity of diffuse large B-cell lymphoma involves the induction of diversified stroma-tumour interfaces. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi H., Miura K., Nakagawa M., et al. Negative impact of concurrent overexpression of MYC and BCL2 in patients with advanced diffuse large B-cell lymphoma treated with dose-intensified immunochemotherapy. Leukemia Lymphoma. 2016;57(12):2784–2790. doi: 10.3109/10428194.2016.1167205. [DOI] [PubMed] [Google Scholar]
- 8.Alataki A., Zabaglo L., Tovey H., Dodson A., Dowsett M. A simple digital image analysis system for automated Ki67 assessment in primary breast cancer. Histopathology. 2021;79(2):200–209. doi: 10.1111/his.14355. [DOI] [PubMed] [Google Scholar]
- 9.Bigras G., Dong W.F., Canil S., et al. New MYC IHC classifier integrating quantitative architecture parameters to predict MYC gene translocation in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol. 2018;26(1):54–63. doi: 10.1097/PAI.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]