Abstract
Neospora caninum is a protozoan parasite that is closely related to Toxoplasma gondii. Dogs are a definitive host. Prior to its discovery in 1988, N. caninum infection in animals was often mistakenly diagnosed as toxoplasmosis. Neosporosis in animals is characterized by encephalitis, abortion, and other conditions that clinically and pathologically resemble toxoplasmosis. The potential of N. caninum to infect humans is unknown. Therefore, evidence of human exposure to this parasite was sought by screening for antibodies in blood donors by indirect fluorescent antibody (IFA) tests and immunoblotting. Of 1,029 samples screened, 69 (6.7%) had titers of 1:100 by IFA testing. Fifty of the 69 (72%) sera that were positive for N. caninum were also negative for a closely related protozoan pathogen of humans, T. gondii. Immunoblot analysis confirmed the specificity of the positive sera for N. caninum antigens, with several sera recognizing multiple Neospora antigens with molecular masses similar to those of antigens recognized by monkey anti-N. caninum serum. An immunodominant antigen of approximately 35 kDa was observed with 12 sera. These data provide evidence of human exposure to N. caninum, although the antibody titers in healthy donors were low. The significance of human exposure to, and possible infection with, this parasite is unknown and warrants further study.
There are no reports of human infection with the protozoal parasite Neospora caninum, but it is possible that cases of neosporosis have been misdiagnosed as toxoplasmosis. Inoculation of pregnant monkeys with N. caninum results in transplacental transmission of the parasite and the induction of fetal encephalitis (3). N. caninum (Apicomplexa; Sarcocystidae; Toxoplasmatinae) was described and named in 1988 (6). It is closely related to Toxoplasma gondii as determined by ultrastructural and genetic comparisons (7), but its oocysts are shed by dogs instead of cats (10, 11). Infections are common in cattle, dogs, and a variety of other domestic and wild animals (7). Neosporosis in animals is characterized by encephalitis, abortion, and other conditions that resemble toxoplasmosis both clinically and pathologically (7).
Humans infected with T. gondii are usually asymptomatic or suffer a flu-like illness, but the pathogen is clinically important in immunocompromised individuals and the fetuses of infected mothers (5). Infection in the immunocompromised host most commonly leads to encephalitis. Women who are first infected during pregnancy may miscarry or give birth to infants with encephalitis or hydrocephalus. Many prenatal infections are subclinical at birth but can lead to impaired vision or hearing, mental retardation, or convulsions (5).
The goal of this study was to determine if there is evidence of human exposure to N. caninum. Sera were collected from blood donors in central California, where N. caninum is recognized as a major cause of abortion in dairy cattle (1). The sera were screened for antibodies against N. caninum by an indirect fluorescent-antibody (IFA) test and immunoblotting. To confirm the specificity for N. caninum, the sera were similarly screened for antibodies against T. gondii.
Sera.
A total of 1,029 serum samples were obtained from the Central California Blood Center (Fresno, Calif.). As a positive control, immune serum against N. caninum was generated by immunizing a rhesus macaque with formalin-killed N. caninum (NC-Liverpool strain) tachyzoites. This serum was negative by IFA testing for T. gondii antibodies. A human serum sample that was positive for T. gondii antibodies by IFA testing, but negative for N. caninum antibodies, was used as a positive control for the T. gondii screening.
IFA test.
Human sera were screened for antibodies against both N. caninum and T. gondii by an IFA test. Slides spotted with whole N. caninum (NC-1 strain) tachyzoites were acquired from a commercial supplier (VMRD, Inc., Pullman, Wash.). For T. gondii testing, slides were spotted with whole tachyzoites prepared by scraping infected African green monkey kidney (Vero) epithelial cell (CCL 81; American Type Culture Collection) cultures and filtering the tachyzoites through a 3-μm-pore-size filter to remove Vero cell debris. Tachyzoites were then washed three times in phosphate-buffered saline (PBS; 25 mM NaPO4–150 mM NaCl [pH 7.2]) and diluted to 106 per ml. One drop of the solution was placed in each of 12 wells per slide and allowed to dry at 37°C. The cells were fixed with 80% acetone–20% methanol, rinsed with distilled water, and stored at −20°C until use.
Sera used in the IFA test were diluted 1:100 in PBS, and 25 μl of each sample was added to a well containing tachyzoites and incubated in a humidified chamber at 37°C for 30 min. The sera were then removed, and each well was rinsed and washed for 10 min with rinse buffer (25 mM Na2CO3, 100 mM NaHCO3, 36 mM NaCl [pH 7.4]). Fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (IgG) plus IgA plus IgM (Accurate Chemical & Scientific Corp., Westbury, N.Y.), diluted 1:100 in PBS, was then placed in each well. The slides were incubated and washed as described above, overlaid with mounting medium (50% glycerol–50% rinse buffer) and a coverslip, and viewed at a ×400 magnification by fluorescence microscopy. As controls, one well on each slide was tested with N. caninum-positive control serum and another well was tested with T. gondii-positive control serum. Monkey immune serum had a titer of 1:3,200 to N. caninum by this procedure and did not react with T. gondii.
Immunoblotting.
N. caninum (NC-1 strain) and T. gondii tachyzoites harvested from infected Vero cells were used as antigens in immunoblots. Infected monolayers were removed by scraping, and cells were forced through a 20-gauge needle to release the tachyzoites. The organisms were washed twice with PBS, and the tachyzoites were purified from the Vero cell material by pelleting through a 30% Renografin (E. R. Squibb & Sons, Inc., Princeton, N.J.) pad at 58,424 × g for 30 min. Pellets were washed with PBS and suspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer at approximately 108 organisms per ml. Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis of samples through a 10% gel, proteins were electrophoretically transferred to Immobilon-P membrane (Millipore Corp., Bedford, Mass.), and the blots were blocked overnight in PBST (PBS plus 0.1% Tween 20) plus 2% bovine serum albumin (BSA-PBST). The blots were then incubated with human sera diluted 1:50 in BSA-PBST for 1 to 2 h at room temperature and washed three times with PBST for 10 min. Immunoblots were then incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated protein A (Pierce, Rockford, Ill.) for 1 h followed by three washes with PBST for 10 min. Horseradish peroxidase-conjugated protein A was detected by chemiluminescence with the SuperSignal CL substrate system (Pierce).
Results.
Of the 1,029 human serum samples screened, 69 (6.7%) had titers of 1:100 in the N. caninum IFA test. The positive sera showed uniform peripheral membrane staining (Fig. 1A) or enhanced apical staining in addition to peripheral staining (Fig. 1B). Sera were considered negative for N. caninum antibodies if no staining of tachyzoites was observed or if peripheral staining was incomplete at a dilution of 1:100 (Fig. 1C). Incomplete peripheral staining is likely due to the reaction of antibodies not specific for N. caninum (13). All human sera were negative at a dilution of 1:200. To eliminate the possibility that a positive IFA test for N. caninum was the result of previous exposure to T. gondii, we tested the 69 N. caninum-positive sera against T. gondii. Fifty (72.5%) of the 69 samples positive for N. caninum antibodies by IFA testing were negative for T. gondii antibodies.
FIG. 1.
IFA testing of human sera for reactivity against N. caninum. Slides were prepared with N. caninum tachyzoites as described in the text and treated with a 1:100 dilution of human sera. (A) Positive reactivity showing uniform peripheral staining of tachyzoites; (B) positive reactivity showing enhanced apical staining in addition to uniform peripheral staining of tachyzoites; (C) negative reactivity showing strong apical staining but no peripheral staining of tachyzoites.
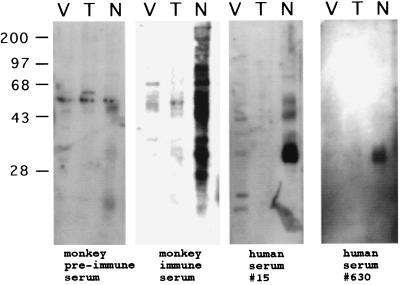
To confirm the seroreactivity to N. caninum observed by IFA testing, all 50 sera displaying N. caninum-positive and T. gondii-negative titers were screened by immunoblotting. Sixteen of the human serum samples strongly recognized distinct antigenic bands that comigrated with antigens recognized by monkey anti-N. caninum serum. These same bands were absent in lanes containing T. gondii or Vero cell lysates (Fig. 2 and data not shown). Twelve sera, including samples 15 and 630, recognized N. caninum antigens of approximately 35 kDa (Fig. 2). This diffuse band of reactivity appears to be the result of two closely migrating antigens, with the lower-molecular-weight antigen showing greater reactivity. Antigens of similar molecular weight were also recognized by monkey immune serum. A direct comparison of the spectra of antigens recognized by human serum sample 15 and those recognized by the monkey immune serum revealed several additional comigrating bands. These included reactive species of approximately 30, 43, 55, and 75 kDa. To further confirm that the 35-kDa N. caninum antigens were specifically recognized by N. caninum antibodies, 50 T. gondii-positive serum samples that were negative for N. caninum by IFA testing were screened against N. caninum antigen. Only one serum sample positive for T. gondii reacted with an antigen of similar molecular mass (data not shown). Thus, the 35-kDa antigen appears to be a major immunogen that is specifically recognized in the context of some human exposure to N. caninum.
FIG. 2.
Immunoblot analysis of reactivity of human sera against lysates of N. caninum (N), T. gondii (T), and Vero cells (V). The serum used to probe each blot is shown at the bottom. Strong bands of reactivity in the 35-kDa range are apparent with N. caninum lysates treated with either human serum sample. This reactivity is absent in lanes containing Vero cell or T. gondii lysates. Molecular masses are indicated in kilodaltons.
Discussion.
In this report, we provide evidence for human exposure to, and possible infection with, N. caninum by using two different but complementary methods of serological testing. By IFA testing, 50 (4.9%) of 1,029 human sera were positive for N. caninum antibodies and negative for T. gondii antibodies, with titers of 1:100. Sixteen of these sera (1.6%) also reacted strongly with N. caninum antigens by immunoblotting. The reactive bands comigrated with those recognized by monkey anti-N. caninum serum. Moreover, antigenic bands of similar molecular mass were not observed when these sera were tested against T. gondii and Vero cell lysates.
A clearly established titer that would serve as a positive indicator of animal infection with N. caninum has been difficult to determine. Titers of >1:200 have been found for all confirmed cases of neosporosis in dogs (7). Titers of >1:320 were found in the sera of infected cows at the time of abortion or calving, while cattle without evidence of infection with N. caninum had titers of 1:320 or less (4). However, retesting of infected cows during the 5 months following abortion indicates that titers can fall to 1:160 and then rise again during a subsequent pregnancy (4). In the present study of human sera, perhaps the 1:100 titers attest to past infections that have been overcome or are inactive, or perhaps the systemic humoral response remained low due to restriction of infection to the gastrointestinal mucosa. Alternatively, some low titers may be the result of ingestion of nonviable Neospora.
The majority of positive N. caninum IFA test results reported in this study were not due to a cross-reaction of antibodies to T. gondii, as 50 (72.5%) of the 69 N. caninum-positive samples were negative for T. gondii. These findings are in keeping with those of Dubey et al. (8), which described minimal or no cross-reactivity between N. caninum and T. gondii by IFA testing. Although our data strongly suggest human exposure to N. caninum, we have not eliminated the possibility of some false-positive results in our IFA testing. False-positive N. caninum IFA test results with animal sera have been shown to result from infection with Babesia spp. (14). False-positive T. gondii IFA test results due to cross-reaction with antinuclear antibodies have also been reported (2), although a similar cross-reaction for N. caninum has not yet been demonstrated. Immunoblot analysis used in this study confirmed positive IFA test results by demonstrating specific reactions of serum antibodies to N. caninum antigens in at least 16 individuals. In some specimens, multiple antigenic bands that comigrated with bands recognized by monkey anti-N. caninum control serum were clearly observed. A strong band of reactivity in the 35-kDa range was observed with a number of sera. Interestingly, a recent report by Howe et al. (9) indicates that a 35-kDa surface protein, designated Ncp35, is one of two major antigens recognized by antisera from Neospora-infected mice, dogs, and cattle. This finding, in conjunction with our observation that staining by Neospora-positive human sera was primarily localized to the tachyzoite surface, leads to speculation that the 35-kDa protein observed by immunoblotting may be Ncp35. Some sera that were positive for N. caninum by IFA testing were negative by immunoblotting. However, this may be due to the limits of detection of the immunoblot procedure in conjunction with low serum titers, or it could indicate false-positive IFA test results for some specimens.
The results of this serosurvey suggest human exposure to N. caninum, but further study is needed to determine the extent and significance of exposure. The potential for human exposure to N. caninum is great, because dogs are a definitive host and excrete oocysts in their feces (10). This study used sera collected from presumably healthy individuals in an area in which N. caninum infection of dairy cattle is known to be endemic. The infection of healthy individuals by N. caninum may follow a course similar to that of T. gondii, where the vast majority of infections are asymptomatic (12). Testing of tissues and fluids from immunocompromised individuals and fetuses with suspected toxoplasmosis for N. caninum may reveal that a subpopulation of these patients are infected with N. caninum.
Acknowledgments
We thank Dan Rockey and Shelly Robertson for critical review of the manuscript and Rebecca A. Wills for technical assistance.
This work was supported by the University of Wyoming’s Faculty Grant-in-Aid Program and by the National Institutes of Health, grant AI-39454 (to L.M.W.).
REFERENCES
- 1.Anderson M L, Blanchard P C, Barr B C, Dubey J P, Hoffman R L, Conrad P A. Neospora-like protozoan infection as a major cause of abortion in California dairy cattle. J Am Vet Med Assoc. 1991;198:241–244. [PubMed] [Google Scholar]
- 2.Araujo F G, Barnett E V, Gentry L O, Remington J S. False-positive anti-Toxoplasma fluorescent-antibody tests in patients with antinuclear antibodies. Appl Microbiol. 1971;22:270–275. doi: 10.1128/am.22.3.270-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr B C, Conrad P A, Sverlow K W, Tarantal A F, Hendrickx A G. Experimental fetal and transplacental Neospora infection in the nonhuman primate. Lab Investig. 1994;71:236–242. [PubMed] [Google Scholar]
- 4.Conrad P A, Sverlow K, Anderson M, Rowe J, BonDurant R, Tuter G, Breitmeyer R, Palmer C, Thurmond M, Ardans A. Detection of serum antibody responses in cattle with natural or experimental Neospora infections. J Vet Diagn Investig. 1993;5:572–578. doi: 10.1177/104063879300500412. [DOI] [PubMed] [Google Scholar]
- 5.Dubey J P, Beattie C P. Toxoplasmosis of animals and man. Boca Raton, Fla: CRC Press; 1988. [Google Scholar]
- 6.Dubey J P, Carpenter J L, Speer C A, Topper M J, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;192:1269–1285. [PubMed] [Google Scholar]
- 7.Dubey J P, Lindsay D S. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/s0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 8.Dubey J P, Lindsay D S, Adams D S, Gay J M, Baszler T V, Blagburn B L, Thulliez P. Serologic responses of cattle and other animals infected with Neospora caninum. Am J Vet Res. 1996;57:329–336. [PubMed] [Google Scholar]
- 9.Howe D K, Crawford A C, Lindsay D, Sibley L D. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect Immun. 1998;66:5322–5328. doi: 10.1128/iai.66.11.5322-5328.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAllister M M, Dubey J P, Lindsay D S, Jolley W R, Wills R A, McGuire A M. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. 1998;28:1473–1478. [PubMed] [Google Scholar]
- 11.McAllister M M, Jolley W R, Wills R A, Lindsay D S, McGuire A M, Tranas J D. Oral inoculation of cats with tissue cysts of Neospora caninum. Am J Vet Res. 1998;59:441–444. [PubMed] [Google Scholar]
- 12.McCabe R E, Remington J S. Toxoplasma gondii. In: Mandell G L, Douglas R G Jr, Bennett J E, editors. Principles and practice of infectious diseases. 2nd ed. New York, N.Y: John Wiley & Sons; 1985. pp. 1540–1549. [Google Scholar]
- 13.Pare J, Hietala S K, Thurmond M C. Interpretation of an indirect fluorescent antibody test for diagnosis of Neospora sp. infection in cattle. J Vet Diagn Investig. 1995;7:273–275. doi: 10.1177/104063879500700222. [DOI] [PubMed] [Google Scholar]
- 14.Yamane I, Thomford J W, Gardner I A, Dubey J P, Levy M, Conrad P A. Evaluation of the indirect fluorescent antibody test for diagnosis of Babesia gibsoni infections in dogs. Am J Vet Res. 1993;54:1579–1584. [PubMed] [Google Scholar]