Abstract
Digital workflow transformation continues to sweep throughout a diversity of pathology departments spanning the globe following catalyzation of whole slide imaging (WSI) adoption by the SARS-CoV-2 (COVID-19) pandemic. The utility of WSI for a litany of use cases including primary diagnosis has been emphasized during this period, with WSI scanning devices gaining the approval of healthcare regulatory bodies and practitioners alike for clinical applications following extensive validatory efforts. As successful validation for WSI is predicated upon pathologist diagnostic interpretability of digital images with high glass slide concordance, departmental adoption of WSI is tantamount to the reliability of such images often predicated upon quality assessment notwithstanding image interpretability but extending to quality of practice following WSI adoption. Metrics of importance within this context include failure rates inclusive of different scanning errors that result in poor image quality and the potential such errors may incur upon departmental turnaround time (TAT). We sought to evaluate the impact of WSI implementation through retrospective evaluation of scan failure frequency in archival versus newly prepared slides, types of scanning error, and impact upon TAT following commencement of live WSI operation in May 2017 until the present period within a fully digitized high-volume academic institution. A 1.19% scan failure incidence rate was recorded during this period, with re-scanning requested and successfully executed for 1.19% of cases during the reported period of January 2019 until present. No significant impact upon TAT was deduced, suggesting an outcome which may be encouraging for departments considering digital workflow adoption.
Keywords: Whole slide imaging (WSI), Digital pathology (DP), Laboratory workflow, WSI scan failure types, WSI scan frequency, WSI turnaround time (TAT), Quality assurance
Introduction
Advances wrought from the past 20 years of exponential technological growth have propelled prospects for the digitization of glass slides into reality for that of entire anatomical pathology laboratory workloads.1 Improvements in digital imaging speed, quality, useability, and utility have progressively encouraged the adoption of digitized workflow elements in laboratories spanning the globe, with some departments now documenting encouraging results following full workflow digitization.2, 3, 4 Whole slide imaging (WSI) solutions have evolved in tandem with challenges prevailing throughout the modern pathology landscape, e.g., shorter turnaround times, increased report complexity, and diminishing specialist numbers confronted by aging patient demographics with progressively increasing incidences of disease.5
Such issues have steadfastly persisted as a silent undercurrent plaguing many departments, though were fervently exacerbated and exhumed as problems demanding solutions upon inception of the COVID-19 pandemic.6,7 As the pathogen terraformed the global topography of clinical practice, shifting practitioner attitudes favoring WSI adoption for a litany of use cases lay in its wake.5,7, 8, 9, 10 A concurrently dynamic regulatory environment, e.g., US-government sanctioned “enforcement discretion” of CLIA regulations, allowed a restrictive laxity for remote sign out accelerating WSI adoption for primary diagnosis.5,6,11 The US Food and Drug Administration (USFDA) granted clearance for medical marketing of the second WSI scanning system for primary diagnoses in surgical pathology following the Philips IntelliSite Pathology Solution™ in 2017 after affirming diagnostic concordance with glass slides and scanning reproducibility.1,5,12,13 WSI adoption for clinical diagnostics continues to increase,5 primarily for routine surgical pathology with focus in teleconsultation.14, 15, 16, 17, 18, 19, 20, 21, 22, 23
Large-scale adoptions catalyzed by the pandemic have spearheaded numerous concordance studies validating WSI for surgical pathology, amassing significant data demonstrating the capacity for WSI to meet and exceed the capabilities of traditional light microscopy. An increasing number of WSI scanner models now undergo evaluation for USFDA 510(k) clearance and European Union Conformité Européenne (EU CE) mark approval.5,6 Yet, USFDA approval for primary clinical diagnostics in other pathology subspecialties remains to be achieved.1 Despite substantial documentation of WSI employment for a variety of use cases within a diversity of departments, clinical deployment still straggles behind that of digital imaging for radiology, stymied primarily from concerns including WSI quality and its potential impact upon pathology image analysis.24, 25, 26
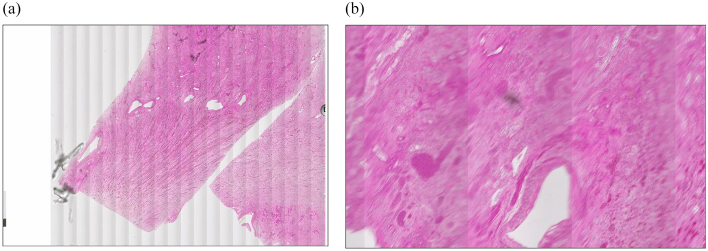
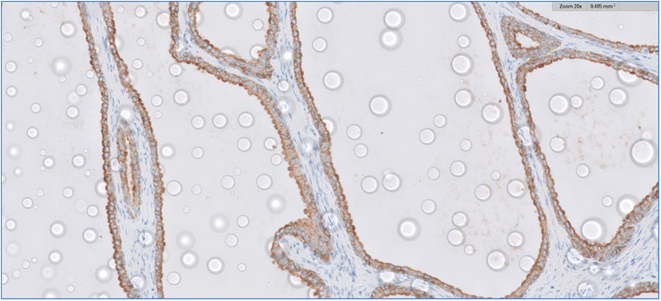
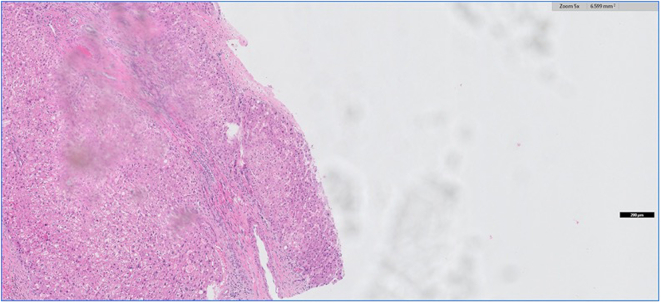
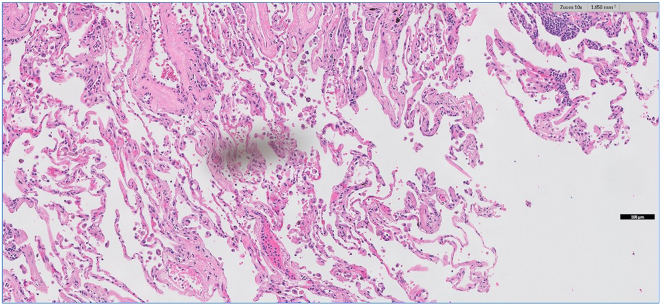
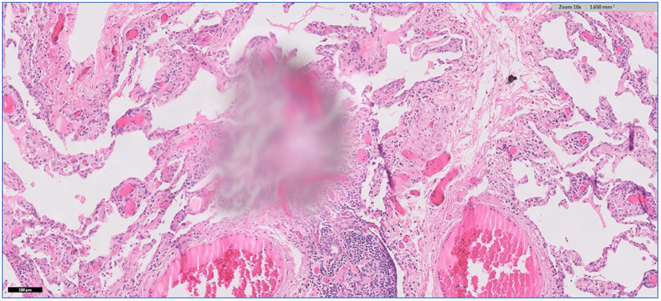
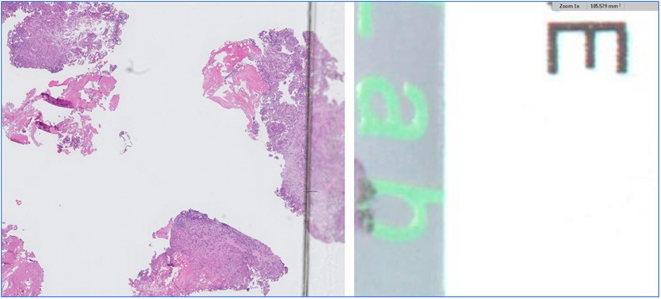
Common image quality errors stemming from digital acquisition of glass slide specimens during the process of WSI scanning include venetian blinding from contaminated objective lens (Figs 1a, 1b), “champagne” bubbling from coverslip errors on frothy mounting media (Fig. 2), slides with dirt (Fig. 3), dust (Fig. 4), mounting media (Fig. 5), and markings (Fig. 6) that have not been appropriately cleaned prior to scanning, clipping from WSI devices (Fig. 7), tissue beyond the coverslip (Fig. 8), and image stitching errors (Fig. 9).
Fig. 1.
(a) Venetian blinding. Captured at 0.25x. (b) Venetian blinding. Captured at 2x; contaminated scanner must be taken offline for inspection while previously scanned slides undergo review for rescanning in a different WSI device.
Fig. 2.
Champagne bubbling. Captured at 20x; requires reapplication of coverslip.
Fig. 3.
Dirty slide. Captured at 5x; clean slide and rescan.
Fig. 4.
Dust on slide. Captured at 10x; clean slide and rescan.
Fig. 5.
Mounting media on slide. Captured at 10x; clean slide and rescan.
Fig. 6.
Markings on slide. Slide markings causing focus issues.
Fig. 7.
Image clipping. Rescan in a different machine or place 3–5 red dots around tissue on rear of slide before rescanning.
Fig. 8.
Tissue beyond the coverslip. Captured at 1x; histology error.
Fig. 9.
Image stitching error. Machine error requiring technologist consulting.
Whole slide images must replicate glass slides with complete accuracy before ubiquitous utilization for routine pathologic diagnosis is realized. As evaluation of WSI device output acts as the singularity from which further workflow transformation may find genesis or remain pendant, a litany of departmental concerns may precipitate from prospective analysis of WSI device acquisition including financial cost benefit, diagnostic utility, and practitioner satisfaction. A diverse array of WSI devices introduced to the market within this recent period have demonstrated the capacity to handle rigors of low-to-high volume departments for research, clinical, and educational settings. Features such as continuous or random-access processing facilitate glass slide uploading during the image capture and digitization of others, with improvements in batch-scanning capabilities functioning in tandem to improve laboratory efficiency.1,27 Many WSI devices are now equipped to navigate a slew of slide mediums cast on slides of varying dimensions. WSI scanning cameras and image sensors deliver greater sensitivity, resolution, field-of-view, and frame rates than ever before for optimal capture and digitization of glass slide specimens.
A prospective evaluation of digital deployment as a value driver for departments with specific needs and objectives often prioritizes return-on-investment (ROI) and quality of practice, the latter of which may be analyzed as a function of turnaround time (TAT).
WSI scan failures resulting in image errors and subsequent rescanning efforts are of importance during preassessment of WSI device robustness. Many WSI devices require coverslips for glass slides which may introduce debris, either on the coverslip itself or lodged within the slide upon coverslip casing, interfering with tissue finding and image analysis.5,28 Some glass slides may fail automated scanning and may require reloading for manual scanning. These errors may arise from slide dimensions incompatible with a specific WSI device or broken/damaged glass slides that are loaded into a device yet are not suitable for scanning, e.g., human-errors unrelated to the function of the WSI device itself.29 Although WSI scan failures resulting in image errors and subsequent rescanning efforts are relatively uncommon, failure-to-scan and rescan rates are of importance during purchasing preassessment of devices from which all other components of laboratory digitization ultimately emerge. Though there is limited literature providing true WSI scan failure rate data documented during daily operations within fully digitized departments, such metrics have notably piqued the interest of WSI vendors. High rates of successful first-time scans coupled with low rescan rates for WSI devices are occasionally touted in commercial literature geared toward a consumer-base of pathologists and laboratory managers.5,29
Whole slide imaging (WSI) for primary diagnosis is crucial towards achieving uninterrupted histopathology diagnosis and maintaining revenue regardless of monumentally disruptive forces, as observed during the COVID-19 pandemic.6 Our institution, among the largest of clinical DP scanning facilities in operation worldwide, routinely monitors scan failure data as a part of quality control and quality assurance. Our study aims to address issues related to scan failure and scan failure impact on TATs, with objective in providing data beneficial to healthcare providers considering transition to a complete digital workflow.
Design
2017 marked our transition from the scanning of archival slides to primarily new slides for primary diagnosis. 13 Philips UFS scanners were used, scanning a total of 2,289,266 slides representing nearly 233,864 cases. Scan failure data was collected from 3 resources: (1) Errors detected by machine, (2) retrospective quality control review, and (3) errors reported by pathologists. Every slide image was appraised by each WSI scanner for defects including failed region of interest (ROI) detections, slides skipped, slides dropped, tissue not detected, and other faults. Each image was also checked by scan technician to determine if the ROI was correctly captured or not. 1.5% of the daily scans were inspected by senior staff for quality assurance. Slides are scored on a scale of 1 to 10 using different parameters. Scans scoring <8 are designated as failed scans and are typically rescanned. Total scan failure rates and rescan (since 2019) rates were recorded and monitored.
Results
Table 1 summarizes WSI scan failure data at our facility. Our overall scan failure rate was only 1.19% with most failures attributable to machine error followed by failures due to slide preparation features. The most common machine error was failed ROI followed by skipped tissue error.
Table 1.
Summary of whole slide imaging scan failure types and frequency.
| Archival slides | Primary diagnosis slides | Total life of project | |
|---|---|---|---|
| Tissue skipped errors | 4,392 | 1,841 | 6,233 |
| ROIA errors | 8,030 | 9,512 | 17,542 |
| Slides dropped | 1,233 | 271 | 1,504 |
| Other errorsB | 894 | 670 | 1,564 |
| Errors identified on QCC review | 283 | 203 | 486 |
| Total errors | 14,832 | 12,497 | 27,329 |
| Total slides scanned | 1,244,763 | 1,044,503 | 2,289,266 |
| % Total errors | 1.19% | 1.20% | 1.19% |
| Rescan requests | Data unavailable | 327 | 327 |
Key: A – region of interest; B – errors stemming from out of focus, staining faintness, tissue thickness, tissue size, broken slides, and other inciting events; C – quality control.
Conclusion
WSI scan failure is exceedingly uncommon (1.19%) in a facility with experienced slide scanning staff and optimal slide preparations. Rescanning was requested for only 1.19% cases and was feasible in 100% cases. Scanning of archival versus newly prepared slides did not have an impact on scan failure rates. Recorded scan failures at our institution were not encountered with enough frequency to significantly impact TATs and therefore our results may be of encouragement for similar departments considering transition to digital workflow.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ankush U. Patel, Email: ankushpatel@rcsi.ie, ankushpatel@alumnircsi.com.
Nada Shaker, Email: nada.shaker@osumc.edu.
Savannah Erck, Email: savannah.erck@osumc.edu.
David A. Kellough, Email: david.kellough@osumc.edu.
Erin Palermini, Email: erin.palermini@osumc.edu.
Zaibo Li, Email: zaibo.li@osumc.edu.
Giovanni Lujan, Email: Giovanni.lujan@osumc.edu.
Swati Satturwar, Email: Swati.satturwar@osumc.edu.
Anil V. Parwani, Email: Anil.parwani@osumc.edu.
References
- 1.Kumar N., Gupta R., Gupta S. Whole slide imaging (WSI) in pathology: current perspectives and future directions. J Digit Imaging. Aug 2020;33(4):1034–1040. doi: 10.1007/s10278-020-00351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraggetta F., Garozzo S., Zannoni G.F., Pantanowitz L., Rossi E.D. Routine digital pathology workflow: the catania experience. J Pathol Inform. 2017;8:51. doi: 10.4103/jpi.jpi_58_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraggetta F., Caputo A., Guglielmino R., Pellegrino M.G., Runza G., L’Imperio V. Diagnostics (Basel) Oct 16 2021;11(10) doi: 10.3390/diagnostics11101916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Retamero J.A., Aneiros-Fernandez J., Del Moral R.G. Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch Pathol Lab Med. Feb 2020;144(2):221–228. doi: 10.5858/arpa.2018-0541-OA. [DOI] [PubMed] [Google Scholar]
- 5.Patel A., Balis U.G.J., Cheng J., Zaibo L., Lujan G., McClintock D.S., et al. Contemporary whole slide imaging devices and their applications within the modern pathology department: a selected hardware review. J Pathol Inform. 2021;12:50. doi: 10.4103/jpi.jpi_66_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelson M.I., Chen S.J., Boukhar S.A., Schnieders E.M., Walhof M.L., Bellizzi A.M., et al. Rapid validation of whole-slide imaging for primary histopathology diagnosis. Am J Clin Pathol. Apr 26 2021;155(5):638–648. doi: 10.1093/ajcp/aqaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassell L.A., Peterson J., Pantanowitz L. Pushed across the digital divide: COVID-19 accelerated pathology training onto a new digital learning curve. Acad Pathol. 2021/01/01. 2021;8 doi: 10.1177/2374289521994240. 2374289521994240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giaretto S., Renne S.L., Rahal D., Bossi P., Colombo P., Spaggiari P., et al. Digital pathology during the COVID-19 outbreak in Italy: survey study. J Med Internet Res. 2021;23(2):e24266. doi: 10.2196/24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koelzer V.H., Grobholz R., Zlobec I., Janowczyk A. Update on the current opinion, status and future development of digital pathology in Switzerland in light of COVID-19. J Clin Pathol. Sep 13 2021 doi: 10.1136/jclinpath-2021-207768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans A.J., Depeiza N., Allen S.-G., Fraser K., Shirley S., Chetty R. Use of whole slide imaging (WSI) for distance teaching. J Clin Pathol. 2021;74(7):425. doi: 10.1136/jclinpath-2020-206763. [DOI] [PubMed] [Google Scholar]
- 11.Rao V., Kumar R., Rajaganesan S., Rane S., Deshpande G., Yadav S., et al. Remote reporting from home for primary diagnosis in surgical pathology: a tertiary oncology center experience during the COVID-19 pandemic. J Pathol Inform. 2021/01/01. 2021;12(1):3. doi: 10.4103/jpi.jpi_72_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration FDA allows marketing of first whole slide imaging system for digital pathology. Updated April 12. 2022. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology Accessed February 23.
- 13.Mukhopadhyay S., Feldman M.D., Abels E., Ashfaq R., Beltaifa S., Cacciabeve N.G., et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study) Am J Surg Pathol. Jan 2018;42(1):39–52. doi: 10.1097/PAS.0000000000000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohanty S.K., Parwani A.V. In: Whole Slide Imaging: Current Applications and Future Directions. Parwani A.V., editor. Springer International Publishing; 2022. Whole slide imaging: applications; pp. 57–79. [Google Scholar]
- 15.Isaacs M., Lennerz J.K., Yates S., Clermont W., Rossi J., Pfeifer J.D. Implementation of whole slide imaging in surgical pathology: a value added approach. J Pathol Inform. 2011;2:39. doi: 10.4103/2153-3539.84232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero Lauro G., Cable W., Lesniak A., Tseytlin E., McHugh J., Parwani A., et al. Digital pathology consultations-a new era in digital imaging, challenges and practical applications. J Digit Imaging. Aug 2013;26(4):668–677. doi: 10.1007/s10278-013-9572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montalto M.C. Pathology RE-imagined: the history of digital radiology and the future of anatomic pathology. Arch Pathol Lab Med. May 2008;132(5):764–765. doi: 10.5858/2008-132-764-PRTHOD. [DOI] [PubMed] [Google Scholar]
- 18.Montalto M.C. An industry perspective: An update on the adoption of whole slide imaging. J Pathol Inform. 2016;7:18. doi: 10.4103/2153-3539.180014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein R.S., Graham A.R., Richter L.C., Barker G.P., Krupinski E.A., Lopez A.M., et al. Overview of telepathology, virtual microscopy, and whole slide imaging: prospects for the future. Hum Pathol. Aug 2009;40(8):1057–1069. doi: 10.1016/j.humpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein R.S., Descour M.R., Liang C., Bhattacharyya A.K., Graham A.R., Davis J.R., et al. Telepathology overview: from concept to implementation. Hum Pathol. Dec 2001;32(12):1283–1299. doi: 10.1053/hupa.2001.29643. [DOI] [PubMed] [Google Scholar]
- 21.Lopez A.M., Graham A.R., Barker G.P., Richter L.C., Krupinski E.A., Lian F., et al. Virtual slide telepathology enables an innovative telehealth rapid breast care clinic. Hum Pathol. Aug 2009;40(8):1082–1091. doi: 10.1016/j.humpath.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Feldman M.D. Beyond morphology: whole slide imaging, computer-aided detection, and other techniques. Arch Pathol Lab Med. May 2008;132(5):758–763. doi: 10.5858/2008-132-758-BMWSIC. [DOI] [PubMed] [Google Scholar]
- 23.Aeffner F., Zarella M.D., Buchbinder N., Bui M.M., Goodman M.R., Hartman D.J., et al. Introduction to digital image analysis in whole-slide imaging: a white paper from the digital pathology association. J Pathol Inform. 2019;10:9. doi: 10.4103/jpi.jpi_82_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright A.I., Dunn C.M., Hale M., Hutchins G.G.A., Treanor D.E. The effect of quality control on accuracy of digital pathology image analysis. IEEE J Biomed Health Inform. 2021;25(2):307–314. doi: 10.1109/JBHI.2020.3046094. [DOI] [PubMed] [Google Scholar]
- 25.Hipp J.D., Fernandez A., Compton C.C., Balis U.J. Why a pathology image should not be considered as a radiology image. J Pathol Inform. 2011;2:26. doi: 10.4103/2153-3539.82051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atallah N.M., Toss M.S., Verrill C., Salto-Tellez M., Snead D., Rakha E.A. Potential quality pitfalls of digitalized whole slide image of breast pathology in routine practice. Mod Pathol. 2021/12/27. 2021 doi: 10.1038/s41379-021-01000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarella M.D., Bowman D., Aeffner F., Farahani N., Xthona A., Absar S.F., et al. A practical guide to whole slide imaging: a white paper from the digital pathology association. Arch Pathol Lab Med. Feb 2019;143(2):222–234. doi: 10.5858/arpa.2018-0343-RA. [DOI] [PubMed] [Google Scholar]
- 28.Kohlberger T., Liu Y., Moran M., Chen P. -H.C., Brown T., Hipp J.D., et al. Whole-slide image focus quality: automatic assessment and impact on ai cancer detection. J Pathol Inform. 2019;10:39. doi: 10.4103/jpi.jpi_11_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantanowitz L., Farahani N., Parwani A. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives. Pathol Lab Med Int. 2015 doi: 10.2147/plmi.S59826. [DOI] [Google Scholar]