Abstract
Background:
Acinetobacter baumannii is one of the most common causes of ventilator-associated pneumonia (VAP) in patients hospitalized in ICU. Multiple resistance has resulted in excessive use of Colistin antibiotic, which is the latest treatment option for this bacterium. Therefore, the purpose of this study was to determine the abundance of multi-resistance and molecular characteristics of resistance to colistin among A. baumannii isolated from patients that are infected with VAP and hospitalized in ICU of “Qazvin” and “Masih Daneshvari” hospitals.
Materials and Methods:
In this study, 200 A. baumannii isolates related to VAP were collected from ICU of “Masih Daneshvari” (2012–2018) and “Qazvin” (2017–2018) hospitals, from bronchoalveolar lavage & tracheal aspirate specimens. Isolates were detected as A. baumannii by PCR with specific primers of the blaOXA-51-like gene. Antibacterial susceptibility of isolates to colistin was determined by the MIC method, and other antibiotics were examined by the disk diffusion method, according to the CLSI criteria. Multi-drug resistance (MDR) and extended–drug resistance (XDR) isolates were determined according to standard definitions of the CLSI.
Results:
All the isolates were susceptible to colistin. Moreover, they were resistant to piperacillin, piperacillin-tazobactam, ceftazidime, cefotaxime, ceftriaxone, amikacin, gentamycin, levofloxacin, co-trimoxazole, and ciprofloxacin. Antimicrobial resistance rates for tetracycline and ampicillinsulbactam were 8.5% and 20%, respectively. All isolates were MDR and XDR. All isolates were susceptible to colistin (MIC50=1 and MIC90=2 μg/ml). The sequencing results did not show any point mutation in pmr CAB genes, and mcr-1 gene was not detected in any isolates.
Conclusion:
In this study, all A. baumannii isolates collected from VAP patients were MDR and XDR. Although all isolates were susceptible to colistin, and this agent seems the most appropriate antibiotic for treatment of VAP, colistin resistance can become endemic in the world rapidly due to plasmid-mediated mobile colistin resistance mcr genes.
Keywords: Acinetobacter baumannii, MDR, XDR, Colistin, pmr CAB, mcr-1
INTRODUCTION
One of the major challenges in hospitals that challenging the treatment and prevention of infections in hospital infection. On the other hand, due to the use of some invasive methods in the ICU, the prevalence of hospital infections in these areas is high.
Commonly reported hospital infections are wound infections, urinary tract infections, lung infections, and ventilator-associated pneumonia (VAP) that are the most commonly acquired infections in the ICU. The death rate related to VAP is 20–50% (1–3).
Acinetobacter baumannii has emerged worldwide as a nosocomial pathogen, particularly in ICU. It is implicated in VAP, bacteremia, and wound infections. VAP is the most common phenomenon of aggressive therapies and long-term endotracheal intubations. Recently, multi-drug resistant (MDR) strains of A. baumannii are increasing and can become an important issue for public health. According to the last definition, MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories in A. baumannii and extended–drug resistance (XDR) was defined as nonsusceptibility to at least one agent in all but two or fewer antimicrobial categories(4,5).
Colistin and tigecycline are the latest treatment options for multiple drug A. baumannii infections. Colistin is a cationic polypeptide antibiotic. This antibiotic demonstrates its antimicrobial ac tivity with two mechanisms: primary bonding and permeability of the outer membrane by cytoplasmic membrane restoration (6–8).
Colistin resistance in Gram-negative bacteria is known to occur by several mechanisms. The main mechanism is the addition of a cationic group, such as 4-amino-4-deoxy-L-arabinose (L-Ara4N) or phosphoethanolamine (PETN) to the lipid A moiety of LPS, which results in a decrease in the net negative charge of the bacterial outer membrane regulated mainly by both PhoPQ and PmrAB, which are two-component regulatory systems. However, the phoPQ genes have not been found in the genome of Acinetobacter spp.; thus, lipid A modification in A. baumannii is mediated by mutations in PmrAB. Mutations in the pmrA or pmrB genes cause upregulation of the pmrCAB operon, leading to the synthesis and addition of PETN, which is responsible for colistin resistance in A. baumannii. Colistin-resistant mutants with no mutations in the pmrA and pmrB genes have also been identified, implying that the amino acid changes in the PmrAB two-component system are not essential for A. baumannii colistin resistance. In addition to lipid A modification of LPS, loss of LPS has been reported to be associated with colistin resistance in A. baumannii . Alterations in the lipid A biosynthesis genes (lpxA, lpxC, and lpxD) by amino acid substitutions, deletions, or insertion of ISAba1 are responsible for the loss of LPS .A recent metabolomics study revealed that an LPS-deficient, colistin-resistant A. baumannii strain showed perturbation in specific amino acid and carbohydrate metabolites, particularly pentose phosphate pathway and tricarboxylic acid (TCA) cycle intermediates (9–12).
Recently, colistin resistance has shown to be singularly due to mobilized colistin resistance MCR (mcr1-9) genes that are plasmid-mediated genes that confer resistance to colistin. Although there is no report of mcr-1 being detected in A. baumannii, its prevalence has been investigated in E. coli and K. pneumoniae. If mcr-1 gene is the most frequent among homologues genes and similar to the NDM-1 gene (the Metallo-β-lactamase gene that is carried on plasmids and is related to hydrolyzing and resistance to carbapenems), colistin resistance could become endemic in the world. The rapid dissemination of previous antibiotic resistance indicates that with the advent of transmissible colistin resistance, progression of A. baumannii from multi-drug resistance to pandrug is unavoidable. Although the levels of a maximum inhibitory concentration of colistin are not high (4–8 mg/L), an acquaintance of mcr-1 by carbapenem resistance A. baumannii isolates will make them resistant to all antibiotics. The potential of mcr-1 to become global depends upon several factors, such as the use of irrational doses of colistin, the stability of mcr-1-mediated plasmid and their ability to transfer in humans. Effective strategies that limit selection and further dissemination of plasmid-associated mcr-1 are clearly needed. It is important to prevent the dissemination of colistin by developing agents which provide effective reverse resistance strategies (13). Therefore, this study was conducted to evaluate the frequency of MDR, resistance to colistin, and molecular characteristics of it among A. baumannii isolated from patients infected with VAP and hospitalized in ICU of “Qazvin” and “Masih Daneshvari” hospitals.
MATERIALS AND METHODS
Specimens and Bacterial isolates
A total of 200 non-duplicate isolates of A. baumannii were obtained from bronchoalveolar lavage & tracheal aspirate specimens of hospitalized patients with VAP (1) in ICUs of “Masih Daneshvari” and “Qazvin” hospitals were collected during 2012–2018 and 2017–2018, respectively. Standard laboratory methods identified all isolates were identified as A. baumannii. The isolates were stored at −80 C in Trypticase soy broth (Merck Co., Germany) containing 20% glycerol.
Identification and diagnosis
Identification and confirmation of A. baumannii isolates: All bacterial isolates, isolated from clinical specimens of patients admitted to the ICU and infected with VAP, were identified as A. baumannii by standard laboratory methods. Molecular identity was determined by using the OXA-51 gene.
Antimicrobial susceptibility testing Antimicrobial susceptibility testing and detection of MDR and XDR isolates
Antimicrobial susceptibility of the isolates to antibiotics was performed by disk diffusion technique on Mueller-Hinton agar plates (Merck Co., Germany), according to the CLSI guideline (CLSI, 2018) using amikacin (30 μg), ceftazidime (30 μg), ciprofloxacin (5 μg), gentamicin(10 μg), imipenem (10 μg), piperacillin/tazobactam(100/10), piperacillin (100μg), sulfamethoxazole-trimethoprim (1.25/23.75 μg), cefepime (30 μg), cefotaxime(30), tetracycline (30 μg), ceftriaxone, and levofloxacin. Antibiotic disks were purchased from “Padtan Teb, Iran” and “Mast Co., England” Companies. E. coli ATCC: 25922, Staphylococcus aureus ATCC:29213, Pseudomonas aeruginosa ATCC:25753, and Enterococcus faecalis ATCC:29212 were used as quality control strains in antimicrobial susceptibility testing (14).
MDR and XDR strains were determined according to standard definitions of the CLSI, EAUCAST, and FDA (MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories in Acinetobacter and XDR was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories) (4,5).
Determining the Minimum inhibitory concentration (MIC) of colistin by microdilution broth methods
The Minimum inhibitory concentration (MIC) test was performed according to CLSI guidelines by microdilution broth method for Colistin (Sigma.Co., USA) by using an inoculum of 5*105 CFU/ml and Mueller Hinton broth (MHB) plates containing 2-fold dilutions of colistin (0.5–64 μg/ml) for all isolates of A. baumannii.
Detection of colistin resistance encoding genes by Polymerase chain reaction (PCR) and sequencing
The template DNA for all samples was obtained by boiling. The presence of oxa-51, pmrCAB, and mcr-1 genes was detected by PCR with a specific primer (Table 1,2). Then, the output was obtained from the PCR pmrAB gene sequencing to detect point mutations. The PCR conditions for all genes in this study were as follows: initial denaturation at 95ºC for 5 min, denaturation at 95ºC for 30s, extension at 72ºC for 45 s, and the final extension at 72ºC for 5 min.
Table 1.
Primer OXA-51
| Genes | Primers (5′–3) | Size of amplified product(bp) | References |
|---|---|---|---|
| OXA-51 | F: caccataaggcaaccaccac | 440 | In study |
| R: tgagg7ctgaacaacccatcc |
Table 2.
Thermocycler Planning Terms for PCR Reaction for the OXA-51 Gene
| Gens | Initial Denaturation | Denaturation | Annealing | Extension | Final Extension |
|---|---|---|---|---|---|
| OXA-51 | 94ºC for 5 min | 95ºC for 30s | 60ºC for 30s | 72ºC for 45s | 72ºC for 5 min |
The annealing temperature was as follows: PMR C: 65ºC for 45 s, PMR A: 57ºC for 45 s, PMR B: 65ºC for 20 s, and OXA-51: 65ºC for 30 s. Also, we used ATCC E. coli 25922 and Acinetobacter sp. strain as a negative control for PCR assays.
Data analysis
Statistical data analysis was performed for descriptive statistics, including frequencies, cross-tabulation of microbiological, clinical features, and demographic characteristics using the SPSS version 22.
RESULTS
Bacterial isolates:
All 200 isolates of A. baumannii were collected from hospitalized patients infected with VAP in ICUs of “Masih Daneshvari” and “Qazvin” hospitals. Clinical specimens included bronchoalveolar lavage (11%) and tracheal aspirate (89%).
Antimicrobial susceptibility testing and detection of MDR and XDR:
The results of antibiotics susceptibility determination by disk diffusion for the desired antibiotic are given in Table 3.
Table 3.
Antibiotic susceptibility of
| Disk | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Imipenem | - | - | 200 (%100) |
| Ciprofloxacin | - | - | 200 (%100) |
| Gentamycin | - | - | 200 (%100) |
| Co-trimoxazol | - | - | 200 (%100) |
| Amikacin | - | - | 200 (%100) |
| Piperacilin | - | - | 200 (%100) |
| Piperacilin-Tazobactam | - | - | 200 (%100) |
| cefotaxim | - | - | 200 (%100) |
| Cefatazidim | - | - | 200 (%100) |
| Cefepim | - | - | 200 (%100) |
| Tetracyclin | 183 (91.5%) | - | 17 (8.5%) |
| Ampicilin sulbactam | 160 (80%) | - | 40(20%) |
All of the A. baumannii strains were resistant to in Imipenem, Ciprofloxacin, Gentamycin, Trimetoprim – sulfometoxazol, Amikacin, Piperacillin, Piperacillin-Tazobactam, Cefotaxim, Ceftazidim, Cefepim. Resistance for the tetracyclin and ampicillin sulbactam were 8.5% and 20%respectively.
All isolates collected from Qazvin hospitals were sensitive to tetracyclin. of 160 isolates sensitive to ampicillin solbactam, 20 isolates are assigned to Qazvin hospitals.
All strains were defined as MDR & XDR
All A. baumannii strains were resistant to in imipenem, ciprofloxacin, gentamycin, trimetoprim–sulfometoxazol, amikacin, piperacillin, piperacillin-tazobactam, cefotaxim, ceftazidim, and cefepim. Resistance to the tetracyclin and ampicillin-sulbactam was 8.5% and 20%, respectively. All isolates collected from Qazvin hospitals were sensitive to tetracycline. Of 160 isolates sensitive to ampicillinsulbactam, 20 isolates were related to Qazvin hospitals. All strains were defined as MDR and XDR.
Minimum inhibitory concentration (MIC) of colistin by microdilution broth methods according to CLSI
In this study, MIC was reported at the concentration of 0.5–64.
Also, 70% of the isolates were sensitive to 1 μg / ml concentration and responded to colistin antibiotic. In addition, 30% of the isolates were sensitive to 2 μg / ml concentration. In this study, all tested isolates were sensitive to colistin. The MIC 50 and MIC 90 were 1 and 2μg/ml, respectively. All of the tested isolates were sensitive to colistin.
PCR Amplifications and sequencing of Colistin resistance encoding genes
All 200 A. baumannii isolates were positive for oxa-51 gene, and mcr-1 gene was not detected in any of the isolates. The sequencing results of PCR amplicon of pmrCAB genes did not show any point mutation in these genes; these results are in line with the antimicrobial susceptibility test results that indicated that colistin resistance was not observed in any of the isolates (Figure 1).
Figure 1.
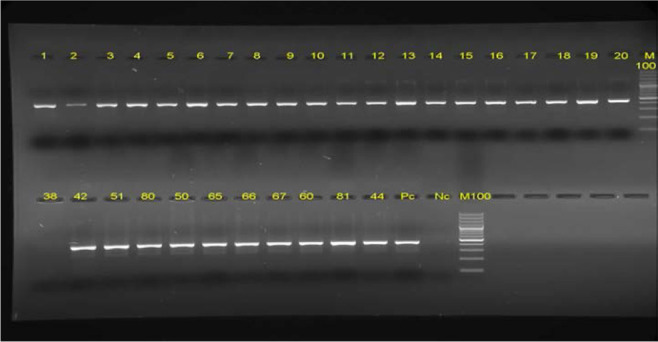
The results of Agarose gel electrophoresis of PCR products of OXA-51 isolates on 1% agarose gel. Lane M: 100bp DNA size marker, PC: positive control,NC:Negative contro
DISCUSSION
VAP is the most commonly acquired hospital infection in the ICU. One of the most important agents in VAP is A. baumannii (1). In recent years, the increased use of antibiotics has led to the emergence of resistant strains of this bacterium. In this study, All A. baumannii isolates collected from patients in ICU infected with VAP were MDR and XDR. Also, most of the samples were collected from the patients of the Masih Daneshvari Hospital (the referral hospital and national research institute of tuberculosis and lung disease) and most hospitalized patients hospitalized with serious pulmonary problems. They were using antibiotics at very high concentrations that can justify having multiple resistance. Treatment of these infections is also expensive and sometimes impossible because of its high ability to obtain antibiotic resistance genes and multi-drug resistant strains. Studies have shown that A. baumannii has a natural resistance to many antibiotics, such as beta-lactam, aminoglycosides, carbapenems, and fluoroquinolones. The treatment of this bacterium is overwhelming and costly due to its high ability to produce antibiotic-resistant genes and MDR strains (5). However, in some studies, the levels of MDR and XDR in A. baumannii are very high, such as the study by Tayebi et al. conducted on ICU patients of several hospitals in Tehran during 2018–2016. The results of this study showed that MDR and XDR rate in A. baumannii was 100% and 92.6%, respectively, indicating the high prevalence of MDR and XDR strains in Iran (15).
There are many studies in this field; for example, in a study that was conducted by Poornajaf et al. (16), 73 A. baumannii isolates were collected, and antibiotic susceptibility to ceftazidime, cefotaxime, piperacillin/tazobactam, imipenem, ciprofloxacin, tobramycin, gentamicin, piperacillin, and tetracycline trimethoprim-sulfamethoxazole was detected by similar methods to the present study(antibiotic disks diffusion); MDR and XDR rate was 92.4%and 38.3, respectively. In another study conducted by Girija and Priyadharsini in India in 2019, MDR and XDR rates were 71.23 and 39.72, respectively (17). According to the high level of resistance of A. baumannii to existing antibiotics, scientists concluded that colistin and tigecycline are the last remaining treatment options for the treatment of multiple bacterial A. baumannii infections. According to the FDA, the TG Cycline drug is in the US boxed warning and, although it exhibits less resistance to MDR strains, it should not be used in patients with VAP due to increased mortality in these patients. Colistin is the most effective antibiotic used to acupuncture-resistant Acinetobacter, especially in patients with VAP. Tracing such strains with this high resistance in clinical specimens is a warning that if colistin is used inappropriately, the emergence of resistant strains and the failure of the treatment are possible (6).
In our study, all isolates were susceptible to colistin, and 91.5% of isolates were sensitive to tetracyclines in vitro. Perhaps this is a promising topic in the treatment debate. Colistin resistance has been reported in different countries, and this kind of resistance is increasing. However, an increase in the prevalence of colistin resistance in Acinetobacter isolates has been reported worldwide. In a recent study in Turkey by Say et al., 96 isolates of A. baumannii were studied, and all isolates were sensitive to colistin, and the rate of MDR was 100%. But since colistin is used as the last line of treatment, the increased resistance to this is a concern for health systems. Many studies have been performed on colistin sensitivity in Iran. In a study on 200 A. baumannii isolates performed by Kooti et al. from different clinical specimens obtained from four Shiraz teaching hospitals, all isolates were susceptible to colistin and polymyxin B (18). In another study by Bahador et al., 100 A. baumannii isolates (from different sources) from Tehran were examined, and isolated five isolates were resistant to colistin (19). Haeili et al. reported that three isolates were resistant to colistin, and two isolates were Colistin-sensitive A. baumannii isolates (20). The result of this study showed that in resistant isolates, there was at least one point mutation in pmrB. Mutation in the pmr A gene was not observed. Analysis of RT-qPCR showed a correlation between colistin resistance and excess expression of pmrC.
During recent years, colistin-resistant clinical isolates have also been reported. Resistance to this antibiotic can be due to mutation in the pathway for enzymes involved in lipid A biosynthesis, including LpxA, LpxB, LpxC, or due to the sequence motion of pmrCAB, which results in the inactivation of genes involved in the lipid A biosynthesis. Both conditions result in the feature of bacterial lipopolysaccharide complete formation and, consequently, high resistance to colistin (11).
According to the findings of studies performed in Iran, the prevalence of colistin-resistant Acinetobacter strains is increasing. Considering past research in Iran and review of other studies confirm the accuracy of the results in Iran.
Resistance pattern of A. baumannii strains isolated from the wound of patients admitted to “Motahari” Hospital of Tehran was studied. In this case, 17 antibiotics were examined using the disk diffusion method and for five antibiotics examinations, MIC was performed. Of the strains examined 61 strains (94 %) were MDR and azetronam was the most effective antibiotic for the treatment of A. baumannii. But in this study, isolated isolates were 100% resistant to antibiotics, indicating that the frequency of MDR in this bacteria is increasing. Control and precision in the administration of antibiotics should be considered to prevent MDR (21).
From 80 isolates isolated from Korean hospitals, five species were examined for amino acid polymorphism and pmr CAB operon nucleotide. The results showed that all A. baumannii isolates had an opron sequence of pmr CAB and were resistant to colistin correlated with this opron. In this study, the correlation between a mutation in this opron and resistance to colistin was confirmed (22).
Dahdouh et al. examined five A. baumannii isolates phenotypically and genotypically. Two isolates were resistant to colistin (by E-test). The cause of resistance to colistin was detected by a mutation in the pmr CAB. These mutations caused resistance to colistin during the treatment. Their study is consistent with the present study to confirm the mutation in the gene in the situation. However, in the present study, resistance to colistin was investigated by microdilution broth. Though, according to CLSI, antibiotic colistin is not soluble in agar medium. E-test is not a suitable method for checking resistance to colistin (23). According to the results of this study, the sequencing results of PCR amplicon of pmrCAB genes did not show any point mutation in pmr CAB genes, and mcr-1 gene was not detected in any isolates. These results are in line with the antimicrobial susceptibility test results indicated that colistin resistance was not observed in the isolates (MIC 50 =1 and MIC 90 =2 μg/ml).
Regarding molecular studies on colistin-resistant clinical isolates, Moffatt et al. demonstrated that the resistance was due to mutation in the genes encoding lipopolysaccharide of these isolates. They also showed the characterization of a group of 13 colistin-resistant mutants, which each contained point mutations or deletions in one of the first three genes in the lipid A biosynthesis pathway, lpxA, lpxC, or lpxD, resulting in the loss of LPS production and high-level colistin resistance (MIC 128 g/ml). This sequence provides the expression of transposase and transposition, a phenomenon that had been described before for the ISA ba1 element in A. baumannii, and is involved in the development of resistance to a variety of antibiotics (24).
Arroyo et al. in London defined the interference of the operon pmrCAB in the resistivity of the polymyxin in the organism. Genome sequence analysis of resistant strains showed spontaneous mutations in the pmrB and PmrA genes. These mutations lead to a decrease in the sensitivity of polymixin to these strains. RT-qPCR showed a correlation between the expression of pmr C and polymixin (25).
Beceiro et al. in 2011 assessed the role of pmr CAB in A. baumannii resistance to colistin. The pmrCAB sequence was identical in all isolates with reference sequences. Resistant clinical isolates have one or two amino acid substitutions in PmrB. No mutations were found in pmr A and pmrC. Increasing the expression of pmrC, pmrA, and pmrB genes were determined by RT-PCR (26).
Ahmed et al. concluded that the emergence of mcr1 plasmid in Enterobacteriaceae MDR was a major concern, but mcr-1 was not reported in A. baumannii (13).
CONCLUSION
According to the results of this study, the prevalence of MDR and XDR in A. baumannii isolates collected from patients in ICU with VAP was very high, which is very worrying because the treatment process of infected patients with these strains will be difficult. But all of the isolates in this study were susceptible to colistin and tested colistin resistance genes and mutation were not detected in any of the isolates. Although colistin is the most appropriate antibiotic and last line antibiotic for treating MDR and XDR A. bauamannii, colistin resistance could rapidly become endemic in the world due to plasmid-mediated mobile colistin resistance mcr genes that disseminated very fast.
REFERENCES
- 1.Talebi TM, Mousavi SA, Malekpour H. Ventilator associated pneumonia: microbiology and identification of antimicrobial resistance pattern by disk-diffusion and E. test methods. Iranian Journal of Clinical Infectious Diseases 2008; 3(1); 13–8. [Google Scholar]
- 2.Japoni A, Vazin A, Davarpanah MA, Afkhami Ardakani M, Alborzi A, Japoni S, et al. Ventilator-associated pneumonia in Iranian intensive care units. J Infect Dev Ctries 2011;5(4):286–93. [DOI] [PubMed] [Google Scholar]
- 3.Khan HA, Baig FK, Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pacific Journal of Tropical Biomedicine 2017;7(5):478–82. [Google Scholar]
- 4.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 2008;46(8):1254–63. [DOI] [PubMed] [Google Scholar]
- 5.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an internat ional expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18(3):268–81. [DOI] [PubMed] [Google Scholar]
- 6.Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiari M, Mainas E, et al. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect 2009;58(4):273–84. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 2012;67(7):1607–15. [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005;40(9):1333–41. [DOI] [PubMed] [Google Scholar]
- 9.Nurtop E, Bayındır Bilman F, Menekse S, Kurt Azap O, Gönen M, Ergonul O, et al. Promoters of Colistin Resistance in Acinetobacter baumannii Infections. Microb Drug Resist 2019;25(7):997–1002. [DOI] [PubMed] [Google Scholar]
- 10.Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother 2007;60(5):1163–7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Aurosree B, Gopalakrishnan B, Balada-Llasat JM, Pancholi V, Pancholi P. The role of LpxA/C/D and pmrA/B gene systems in colistin-resistant clinical strains of Acinetobacter baumannii. Frontiers in Laboratory Medicine 2017;1(2):86–91. [Google Scholar]
- 12.Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, et al. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, baumannii and poly-β-1,6-N-acetylglucosamine. Antimicrob Agents Chemother 2012;56(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed SS, Alp E, Hopman J, Voss A. Global epidemiology of colistin resistant Acinetobacter baumannii. Journal of Infectious Disease and Therapy 2016;4:287. [Google Scholar]
- 14.Sahni RD, Veeraraghavan B, Dhiviya Prabaa MS, Jacob JJ. Will the recently reinstated clsi 2020 breakpoints of norfloxacin for urinary isolates work for India? - Tertiary care experience and evidence. Indian J Med Microbiol 2019;37(4):600–601. [DOI] [PubMed] [Google Scholar]
- 15.Tayebi Z, Doust RH, Rahimi MK, Siadat SD, Goudarzi M. Distribution of different carbapenemase genes in carbapenem-resistant Acinetobacter baumannii strains isolated from intensive care: a two year multi-center study in Tehran, Iran. Gene Reports 2019;15:100382. [Google Scholar]
- 16.Pournajaf A, Rajabnia R, Razavi S, Solgi S, Ardebili A, Yaghoubi S, et al. Molecular characterization of carbapenem-resistant Acinetobacter baumannii isolated from pediatric burns patients in an Iranian hospital. Tropical Journal of Pharmaceutical Research 2018;17(1):135–41. [Google Scholar]
- 17.Girija As S, Priyadharsini J V. CLSI based antibiogram profile and the detection of MDR and XDR strains of Acinetobacter baumannii isolated from urine samples. Med J Islam Repub Iran 2019;33:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kooti S, Motamedifar M, Sarvari J. Antibiotic resistance profile and distribution of oxacillinase genes among clinical isolates of Acinetobacter baumannii in Shiraz teaching hospitals, 2012–2013. Jundishapur Journal of Microbiology 2015;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahador A, Farshadzadeh Z, Raoofian R, Mokhtaran M, Pourakbari B, Pourhajibagher M, et al. Association of virulence gene expression with colistin-resistance in Acinetobacter baumannii: analysis of genotype, antimicrobial susceptibility, and biofilm formation. Ann Clin Microbiol Antimicrob 2018;17(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haeili M, Kafshdouz M, Feizabadi MM. Molecular Mechanisms of Colistin Resistance Among Pandrug-Resistant Isolates of Acinetobacter baumannii with High Case-Fatality Rate in Intensive Care Unit Patients. Microb Drug Resist 2018;24(9):1271–6. [DOI] [PubMed] [Google Scholar]
- 21.Ardebili A, Azimi L, Mohammadi-Barzelighi H, Beheshti M, Talebi M, Jabbari M, et al. Determination of resistance pattern of isolated Acinetobacter baumannii from hospitalized burned patients in Motahari Hospital, Tehran. J Adv Med Biomed Res 2012;20(83):112–9. [Google Scholar]
- 22.Kim DH, Ko KS. A distinct alleles and genetic recombination of pmrCAB operon in species of Acinetobacter baumannii complex isolates. Diagn Microbiol Infect Dis 2015;82(3):183–8. [DOI] [PubMed] [Google Scholar]
- 23.Dahdouh E, Gómez-Gil R, Sanz S, González-Zorn B, Daoud Z, Mingorance J, et al. A novel mutation in pmrB mediates colistin resistance during therapy of Acinetobacter baumannii. Int J Antimicrob Agents 2017;49(6):727–33. [DOI] [PubMed] [Google Scholar]
- 24.Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother 2011;55(6):3022–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 2011;55(8):3743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 2011;55(7):3370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


