Abstract
Background
congenital pouch colon (CPC) is an extremely rare Congenital gastrointestinal pathology, in which the normal colon is either partially or completely replaced by a pouch-like dilatation communicating with the urogenital tract through a fistula. That's divided into 2 types: Complete CPC and Incomplete CPC. Worldwide and middle east Arabian ethnicity except for Indians, show high scarcity regarding the incidence. Herein, we report a case of incomplete congenital pouch colon syndrome with glandular hypospadias and cardiac anomalies that are considered to be the 2nd documented case in the middle east and the first in Westbank.
Case presentation
A 1-day-old newborn boy with prenatal history of abdominal cyst in 2nd trimester US, presented hours after birth with abdominal distention (Fig- 1), in addition to the imperforate anus. The abdominal x-ray showed many dilated bowel loops and gasless soft tissue density with calcifications on the right side (Fig- 2). Exploratory laparotomy was done and showed a pouch-like colon that later on was treated via 2 stages of operation.
Clinical discussion
early identification of CPC and differentiation from colon dilatation due to anorectal malformation is essential for the patient's welfare. CPC is more common in males, usually noticed in the neonatal period with abdominal distention, absence of anus, and intestinal obstruction. CPC is managed surgically depending on its type.
Conclusion
congenital pouch colon is a rare but important differential diagnosis of abdominal distention, which should always be at the back of the surgeon's mind especially when anorectal malformation is present.
Keywords: Congenital pouch colon, Anorectal malformation, Coloplasty
Highlights
-
•
Congenital pouch colon (CPC) when colon is either completely or partially replaced by a pouch.
-
•
When there is anorectal malformation you should suspect CPC.
-
•
Partial CPC is more common but complete pouch is more dangerous.
-
•
When CPC is diagnosed, check for associated, renal, cardiac and skeletal anomalies.
1. Introduction
Congenital pouch colon syndrome (CPC) is a condition in which the colon is either partially or completely replaced by a pouch-like dilatation communicating with the urogenital tract through a fistula. This exceptionally rare condition was described as early as 1912 and categorized in 1984 into four subtypes (Types I–IV) based on the length of the normal colon proximal to the colonic pouch [1]. That was updated in 2007 to encompass the fifth type. Nowadays, a common simplified classification describes CPC as either Complete CPC or Incomplete CPC depending on the extent of involvement.
Globally, the incidence of CPC is scarce, as few cases reported worldwide, including in the Middle East. CPC is most commonly described in Northern India, which constitutes nearly 90% of reported cases, however, it is still infrequent [2,3]. CPC shows a male predominance and is mainly present in the early neonatal period with the absence of the anus and intestinal obstruction [2].
CPC syndrome is a challenging congenital disease that's hard to diagnose due to non-specific symptoms and non-definitive imaging studies. However, the presence of congenital anorectal anomalies should raise suspicion due to its high association [2,3]. Surgical management and outcome, as well as overall prognosis, mainly depend on the type of CPC, the length of the affected colon, anorectal muscle complexity, as well as other congenital associations. Although CPC is challenging to diagnose, worldwide survival rates are on the rise due to continued awareness and improved management; the mortality rate for CPC has decreased from 30–40% to 10–20%.
Herein, we report a case of incomplete congenital pouch colon syndrome with glandular hypospadias and cardiac anomalies that are considered to be the 2nd documented case in the Middle East and the first in the West Bank.
This work has been reported in line with the SCARE criteria [4].
2. Case presentation
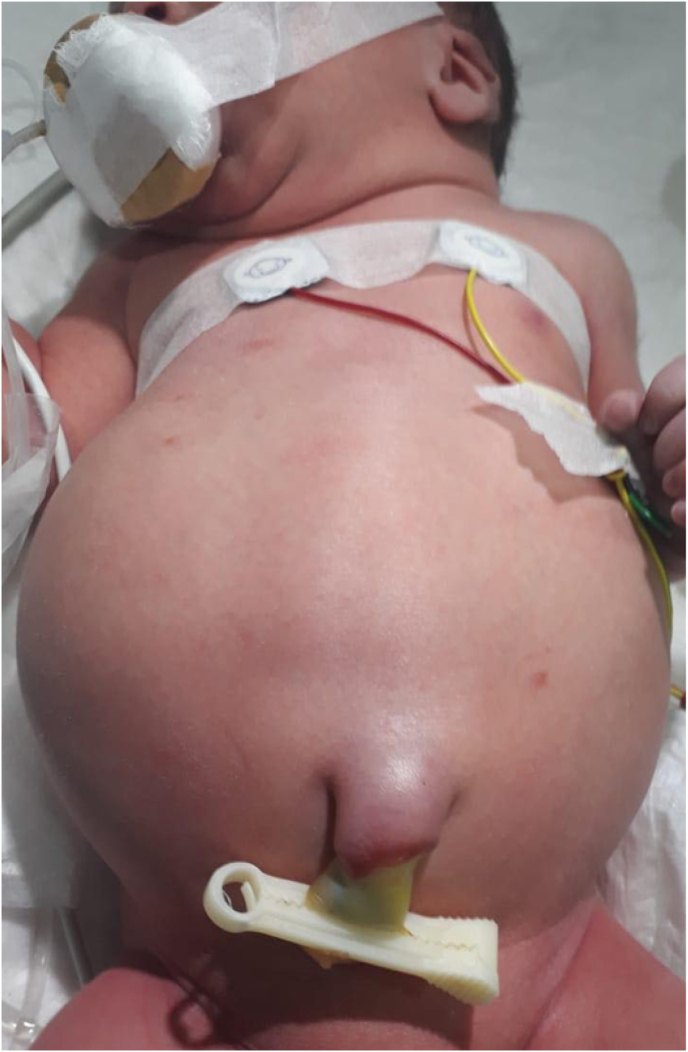
A 1-day-old male neonate was delivered by C-section at the 37th week of gestation (BW 3.8 kg, Apgar score 8/9). At birth, it was noticed that the patient had a distended abdomen (Fig-1). Physical examination revealed an imperforate anus and glandular hypospadias. Abdominal U/S was done and showed a congenital cyst that is suspected to be a giant meconium pseudocyst and right-sided ureterocele with right-sided hydroureteronephrosis. Next, the patient underwent an abdominal x-ray which revealed multiple dilated bowel loops on the left side, and gasless soft tissue density with calcifications on the right side. (Fig. 2).
Fig. 1.
1-day old baby with markedly distended abdomen with right sided abdominal bulge with ill-defined mobile mass.
Fig. 2.
Abdominal radiograph showing dilated bowel loops on left with soft tissue density occupying the right abdomen. black arrows show linear calcification laterally.
Detailed fetal U/S was done at 28th gestational weeks and showed a large hemorrhagic cyst, extending from the liver to the pelvic. otherwise, his prenatal and family history was unremarkable.
The patient was admitted to NICU for stabilization at which, Nasogastric tube was inserted to decompress the abdomen, antibiotics were given and the infant was kept at an appropriate temperature. A day after, a decision to undergo exploratory laparotomy was made. At the operation, a hugely dilated rectosigmoid full of meconium was found with no evidence of abdominal cyst or pseudocyst that is associated with imperforate anus; this made us suspect a congenital pouch colon (Fig. 3.). As a result, a left descending end colostomy with a distal mucous stoma was performed. The operation was done by a team led by 2 pediatric surgeons, 1 surgical resident, and 2 nurses.
Fig. 3.
Operative view of incomplete congenital pouch colon.
Post-operation, the patient was admitted to NICU for observation at which he remained stable with normal vital signs. Metabolic workup was normal except for a normal anion gap metabolic acidosis. Full metabolic and genetics investigations were done and failed to reveal the cause of this metabolic acidosis. He was given Sodium Bicarbonate to relieve the acidosis.
At the age of 16 days, while taking urine culture we couldn't insert an 8 French catheter through the urethral. With urine only urine dribbling and low urine output and urine coming out of the distal mucus stoma which raised the suspension of urethral hypoplasia in addition to rectourinary tract fistula. This was affirmed when spot urine for creatinine from the stoma came back positive. To further investigate that, a distal colostogram was done (Fig. 4a) and showed contrast in the urethra but failed to reveal the specific type of the fistula. This necessitated a retrograde urethrogram (Fig. 4b) which showed urethral hypoplasia in addition to a colovesical fistula at the bladder's neck.
Fig. 4a.
Colostogram showing contrast in urethra.
Fig. 4b.
Retrograde ascending urethrogram showing recto-bladder neck fistula, black arrow shows contrast in the rectum, White arrow pointing toward the fistula that connects the rectum to the bladder's neck.
With supportive measures and close observation, the patient was in good health. At the age of 51 days, through an abdominoperineal approach, the definite operation was done, the distal colon was dissected down to the fistula, a ureteric catheter was used to open the fistula, and then the fistula was ligated and transected. Fortunately, after the distal colon (megarectum) was excised, the length of the remaining distal colon was long enough to be pulled down to create an anoplasty, which was done through a limited perineal incision.
Before discharge from the hospital, MCUG was done and showed a normal bladder with no vesicoureteral reflux, and no communication between the urethra and rectum.
3. Discussion
Congenital pouch colon syndrome (CPC) is a condition in which the colon is either partially or completely replaced by a pouch-like dilatation communicating with the urogenital tract through a fistula. CPC Syndrome was described as early as 1912 by Spriggs as a specimen with an absent left half of the colon and rectum. After that, Narsimha Reo et al. suggested the condition be termed congenital colon pouch colon (CPC) syndrome [5,6]. This rare condition was first categorized in 1984 into four subtypes (Types I–IV) based on the length of the normal colon proximal to the colonic pouch, with Types I and II being the most severe ones, and Type IV CPC being the most common presentation [1]. In type I, the normal colon is absent, and the ileum opens directly into the colonic pouch. In type II, the ileum opens into a short segment of the cecum, which then opens into the colonic pouch. In type III, there is a significant amount of normal colon between the ileum and the colonic pouch. Type IV is characterized by the presence of a nearly normal colon with only the terminal portion of the colon ending into the pouch. This four-type classification was updated in 2007 (IAPS classification) to encompass a fifth type, which is the rarest variant of CPC. Nowadays, a common simplified classification describes CPC as either Complete CPC or Incomplete CPC depending on the extent of involvement.
Globally, the incidence of CPC is scarce and is most commonly described in Northern India, which constitutes nearly 90% of reported cases, though it is still uncommon [2,3]. CPC shows a male predominance as the male to female ratio ranges from 2:1 to 7:1, and is mainly present in the early neonatal period with the absence of the anus and intestinal obstruction, but could present later in female patients with the passage of the meconium from an abnormal opening in the perineum or cloaca [2].
The pathogenesis and embryology of CPC are not yet well understood. However, some theories attribute CPC to aborted development of the hindgut due to obliteration of the inferior mesenteric artery with maldevelopment of the terminal midgut early in fetal life [7]. Others propose that a vascular compromise in the anorectal fold and adjacent hindgut may account for the syndrome. Moreover, CPC is sporadic with no familial inheritance. However, some dietary and environmental factors may have a role [3].
As for the diagnosis, it is typically done by an imaging study, anteroposterior and lateral plain X-ray, which will typically but rarely show a large air-fluid level with small bowel loops displaced to the right [5], and in of case colovesical fistula, gas in the bladder or meconium ammonium hydrogen urate calcifications in the colon can be found (fistula, low colon PH, urine, stasis) [11].
Thus, CPC syndrome is challenging to diagnose due to non-specific symptoms and non-definitive imaging studies. However, neonatal intestinal obstruction (distended abdomen) and the presence of anorectal malformation (ARM) as imperforate anus, should raise suspicion due to its high association and similar presentation [2,3,8]. Other strong associations with CPC syndrome include cardiac, vertebral, and genitourinary anomalies, which are mandatory to check in suspected cases of CPC, such as echocardiography, vertebral X-ray, and MCUG [8].
Surgical management of CPC and the outcome, as well as overall prognosis, mainly depend on the type of CPC, the length of the affected colon, type of perineum, and anorectal muscle complexity, as well as other congenital associations. CPC is considered incomplete if the length of the normal colon is adequate for performing pull-through without the need for coloplasty [12]. In incomplete CPC, surgery is the preferred choice, with primary surgery excision of the colonic pouch with ileostomy; window colostomy; end colostomy; transverse colostomy, ligation of fistula, which may be combined with coloplasty, followed by definitive surgery with endorectal pull-through and coloanal anastomosis, preserving the native anal sphincter complex [9,10]. It is worth noting that the ligation of fistulas is preferred in the first stage of surgery because of the complications associated with untreated fistulas that significantly increase morbidity and mortality [8]. On the other hand, if the entire colon is in the form of a pouch or not of adequate length to accomplish pull-through; it is considered complete. In complete CPC, a coloplasty procedure is required, tabularization of pouch till about 15 cm length is essential to preserve the function of the colon, then it should be brought out as an end colostomy followed by abdominoperineal pull-through of the tabularized colon later. It is essential to take care not to make the tabularized colon longer than 15 cm as it is associated with stasis, dilation of the segment, and frequent complications in the post-pull-through period.
Finally, growing awareness and improved management of the disease led to a reduction in mortality rates from 30–40% to 10–20%. Though fecal incontinence is as high as 60% in the initial years after pull-through, urinary incontinence especially in females with wide bladder neck and sacral deformities, in addition to colonic re-dilatation due to abnormal histology can limit overall prognosis.
Our patient had anorectal agenesis, a short length of the colon, a pouch-like rectosigmoid along with a genitourinary fistula, and no transition point between the pouch colon and normal colon. This fulfilled the criteria of CPC [13] except for blood supply which wasn't evaluated. with the colon having adequate length for pulling through, this makes it incomplete CPC. Moreover, our patient had Urethral hypoplasia, but with no sign of a posterior valve, and since our patient had no signs of urine retention, no specific action was required. After the definitive surgery size 6 French urethral catheter was kept in place for 7 days, then removed and urine output was adequate. U/S was done and showed normal urine residue. MCUG was done for a better evaluation of the urinary tract, to look for hydroureteronephrosis, and to rule out vesicoureteral reflux.
Metabolic and genetics investigations failed to reveal the cause of our patient's normal anion gap metabolic acidosis, we suspect it was due to the absorption of chloride in the urine that refluxed through the colovesical fistula to the colonic mucosa. This was supported by the fact that the acidosis has relieved after the definitive surgery.
The patient's right-sided ureterocele along with right-sided ureterohydronephrosis was followed up regularly after the definitive surgery, and they resolved without intervention. Nowadays, it is recommended to do an appendectomy at the time of pull-through to prevent misdiagnosis in the event of appendicitis occurring at a later date [2]. we haven't known at the time of pull through in our case.
On follow-up, our patient development was consistent with his age, tolerating oral intake, and gaining weight appropriately. Echocardiography showed a small patent foramen ovale.
Furthermore, the patient is having recurrent UTI, which could be due to the previous fistulotomy. In addition, he has frequent loose stool, which may be due to storage problems or fecal incontinence. This will be clear once the patient gets to the age of bathroom training.
4. Conclusion
Congenital pouch syndrome is an extremely rare syndrome, but an important differential diagnosis of abdominal distention, and should be always suspected in any case of anorectal malformation to allow for early diagnosis and management to lower its morbidity and mortality.
Ethical approval
The study is exempt from ethical approval in our institution.
Sources of funding
No funding or grant support.
Author contributions
Data collection: Jihad Zalloum, Yousef S. Abuzneid.
Study concept or design: Radwan Abukarsh, Ihsan Ghazzawi.
Writing the manuscript: Qutaiba Qafisha, Razan M. Yahya, Jihad Zalloum.
Review & editing the manuscript: Abdallah Hussein, Qutaiba Qafisheh.
Registration of research studies
Not applicable.
Guarantor
Dr. Radwan AbuKarsh.
Consent
Written informed consent was obtained from the patient's parent or guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
There is no conflict of interest.
References
- 1.Chadha Rajiv, Khan Niyaz Ahmed. Congenital pouch colon. J. Indian Assoc. Pediatr. Surg. 2017;22(2):69. doi: 10.4103/jiaps.JIAPS_5_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta Devendra K., Sharma Shilpa. Congenital pouch colon-Then and now. J. Indian Assoc. Pediatr. Surg. 2007;12(1):5. doi: 10.4103/0971-9261.31081. [DOI] [Google Scholar]
- 3.Chadha Rajiv. Congenital pouch colon associated with anorectal agenesis. Pediatr. Surg. Int. 2004;20(6):393–401. doi: 10.1007/s00383-004-1162. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Wakhlu Avtar Kishan, et al. Congenital short colon. World J. Surg. 1996;20(1):107–114. doi: 10.1007/s002689900019. [DOI] [PubMed] [Google Scholar]
- 6.Chadha Rajiv, et al. The embryology and management of congenital pouch colon associated with anorectal agenesis. J. Pediatr. Surg. 1994;29(3):439–446. doi: 10.1016/0022-3468(94)90588-6. [DOI] [PubMed] [Google Scholar]
- 7.Trusler G.A., Mestel A.L., Stephens C.A. Colon malformation with imperforate anus. Surgery. 1959;45(2):328–334. doi: 10.5555/uri:pii:0039606059902636. [DOI] [PubMed] [Google Scholar]
- 8.Rao K.L.N., Menon Prema. Congenital pouch colon associated with anorectal agenesis (pouch colon syndrome) Pediatr. Surg. Int. 2005;21(2):125–126. doi: 10.1007/s00383-004-1335-z. [DOI] [PubMed] [Google Scholar]
- 9.Mathur P., Prabhu K., Jindal D. Unusual presentations of pouch colon. J. Pediatr. Surg. 2002;37(9):1351–1353. doi: 10.1053/jpsu.2002.35007. [DOI] [PubMed] [Google Scholar]
- 10.Parelkar Sandesh, et al. Congenital pouch colon with rectal atresia: a case report. J. Pediatr. Surg. 2010;45(3):639–641. doi: 10.1016/j.jpedsurg.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Shimotake Takashi, et al. Infrared spectrophotometry of intraluminal meconium calculi in a neonate with imperforate anus and rectourethral fistula. J. Pediatr. Surg. 2006;41(6):1173–1176. doi: 10.1016/j.jpedsurg.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 12.Sharma Shilpa, Gupta Devendra K. Management options of congenital pouch colon—a rare variant of anorectal malformation. Pediatr. Surg. Int. 2015;31(8):753–758. doi: 10.1007/s00383-015-3739-3. [DOI] [PubMed] [Google Scholar]
- 13.J. Indian Assoc. Pediatr. Surg. 2007;12(1):5–12. January-March. [Google Scholar]