Abstract
Introduction
Sarcoma as a cause of laryngeal cancer is rare and is even rarer to have an Ewing sarcoma out of the bone presents as laryngeal cancer. In this report, we present this extremely rare case.
Case presentation
A 41-year-old man was seen at the ENT clinic complaining of chronic hoarseness and a lump in his neck. Flexible laryngoscopy showed a large mass occupying the left side of the larynx and then a computerized tomography scan proved it. For further evaluation, the laryngoscopy was performed and the frozen section revealed a malignancy. Consequently, the surgical decision was taken and a total Laryngectomy and thyroidectomy were carried out. A final diagnosis of Ewing sarcoma was established using histological examination and immunohistochemical staining. The patient was referred for adjuvant chemo-radiotherapy as recommended by the oncology service.
Clinical discussion
laryngeal cancer is rarely diagnosed as Ewing sarcoma. The defined diagnosis should be made based on histological study and immunohistochemical staining besides the clinical presentation and other examinations. Our patient was a candidate for surgical treatment and negative surgical margins were achieved. He was referred for adjuvant chemo-radiotherapy as some studies demonstrated the efficacy of multimodal therapy in treating Ewing sarcoma.
Conclusion
Because of the lack of similar studies and documented data in the medical literature about this rare case, Ewing sarcoma should be included in the differential diagnosis in laryngeal cancer cases.
Keywords: Ewing sarcoma, Larynx cancer, Laryngeal sarcoma, Laryngectomy, Case report
Abbreviations: EES, Extraskeletal Ewing Sarcoma; PNET, Primitive Neuroectodermal Tumor; CT, Computerized Tomography; IHC, Immunohistochemisrty
Highlights
-
•
Ewing sarcoma is a bony malignancy and rarely exists in the larynx.
-
•
Laryngeal cancer itself is rarely diagnosed as sarcoma, and rarer as Ewing sarcoma.
-
•
Ewing sarcoma should be included in the differential diagnosis of laryngeal cancer.
-
•
Ewing sarcoma should be treated with surgical removal, chemotherapy and radiotherapy as many studies showed.
1. Introduction
Laryngeal cancer is a malignant cellular proliferation primarily located in the larynx. According to a 2022 estimate, there would be 12,470 new cases diagnosed with larynx cancer for about 0.7% of all new cancer cases worldwide and 3820 deaths caused by this malignancy for about 0.6% of all cancer deaths [1]. Clinically, it may present with hoarseness, breathing difficulty, dysphagia, or neck lump. Histologically, squamous cell carcinoma (SCC) is the most common type of laryngeal cancer whereas non-epithelial types form the minor portion [2]. Laryngeal sarcomas specifically comprise less than 1% of laryngeal cancers and among their subtypes, chondrosarcoma is the most common one with an extremely low presentation of the others [3].
Ewing sarcoma, which is a bony sarcoma, is even rarer as a subtyped laryngeal sarcoma and to the best of our knowledge, only a small number of cases of this rare presentation were previously reported [[4], [5], [6], [7], [8], [9], [10], [11]]. Hence, since laryngeal cancer rarely manifests as Ewing sarcoma which itself rarely manifests outside the skeleton, we present an extremely rare case of Ewing sarcoma of the larynx. This case report has been reported in line with the SCARE 2020 criteria [21].
2. Case presentation
A 41-year-old male patient with no significant past medical history presented to the otolaryngology clinic with symptoms of stable hoarseness for 3 months and breathing difficulty. In the physical examination, a level Ⅵ neck mass was found with no other abnormalities. Flexible laryngoscopy was performed and revealed a large mass occupying the left side of the larynx. No enlarged lymph nodes were palpated in the neck.
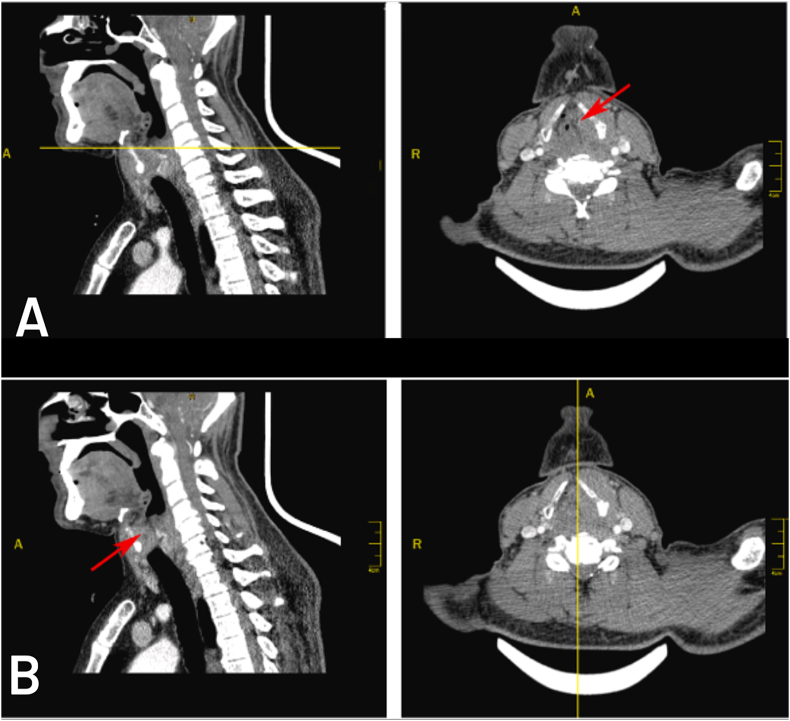
Computerized Tomography imaging of the neck showed a 3.5 × 2x3 cm soft tissue lesion at the level of the left vocal cord and anterior commissure extending upward through the left side of the larynx involving the left laryngeal ventricle, false vocal cord, and aryepiglottic fold without obvious involvement of epiglottis figure (1, A), the lesion approximately occluded the lumen figure (1, B). Other associated CT findings included deviation of the left arytenoid cartilage and aryepiglottic fold medially toward the midline (sail sign) with dilation of the pyriform sinus, which indicates left vocal cord paralysis caused by the invasion of the lesion. Additionally, sclerosing and splitting of the left plate of the thyroid cartilage with thickening of the adjacent neck's muscles were noticed.
Fig. 1.
Computerized Tomography imaging of the patient's neck. Sagittal and transverse slices.
Fig. 1-A: The transverse slice shows a soft tissue lesion occupying the left side of the larynx (red arrow on the transverse slice).
Fig. 1-B: The sagittal slice at the midline shows a lesion occluding the lumen of the larynx (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In light of these findings, the patient underwent laryngoscopy under general anesthesia which revealed a left side laryngeal mass within the structures mentioned above, supporting CT findings. A frozen section from that mass was taken to make an intraoperative diagnosis and it was positive for malignancy. Regarding these results, the medical decision was made for total Laryngectomy and bilateral neck dissection. U-shaped skin incisions and Ⅱ, Ⅲ, and Ⅳ neck levels dissection on both sides were made with the preservation of the spinal root of the accessory nerve, internal jugular vein, and sternocleidomastoid muscle. Subsequently, the larynx and thyroid were separated from the carotid sheath and other nearby structures, whereas the separation between the thyroid and the larynx was severely difficult due to tumor grossly invasion within thyroid tissue. Therefore, total resection of the thyroid gland, larynx, and both first and second tracheal rings was performed.
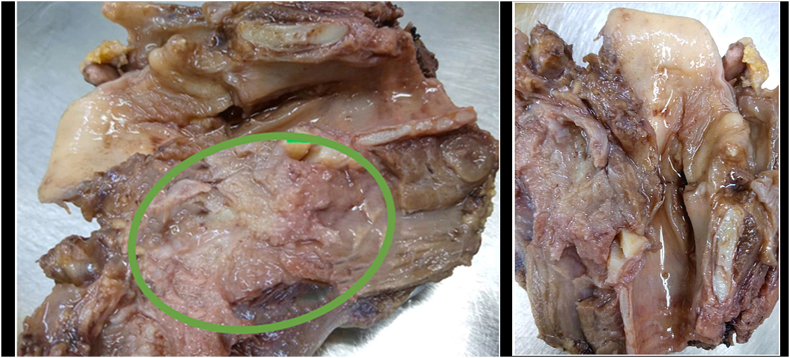
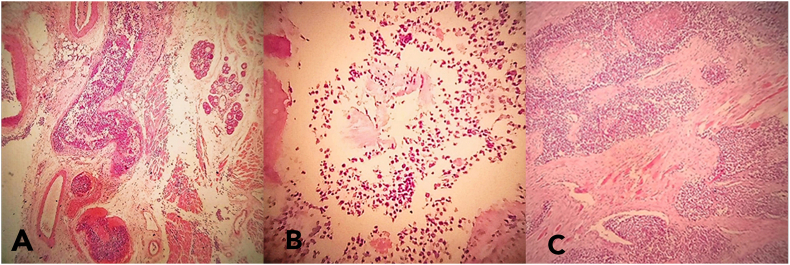
Gross examination of the total laryngectomy sample showed that it measured 10 cm with a tumor measuring 5 cm long and a 4.5 cm tumor thickness figure (2). While microscopic findings showed a malignant small round cell tumor involving the left laryngeal portion with thyroid cartilage and extra-laryngeal soft tissue infiltration, focally close to the left thyroid lobe (4mm) figure (3). In an Immunohistochemistry (IHC) study, the CD99 marker showed diffusely membranous positivity. All other immunostaining, including LCA, desmin, actin, MyoD1, S-100 protein, CD56, chromogranin, HMB-45, EMA, and CK were negative ruling out many differential diagnoses; mainly lymphoblastic lymphoma, rhabdomyosarcoma, neuroblastoma, melanoma, and undifferentiated carcinoma. This panel supports the diagnosis of PNET/Ewing's sarcoma involving the larynx. All surgical margins were free of malignancy. No lymph nodes were reactive when studying right and left neck lymph nodes (0/12 and 0/14). The patient was referred to the oncology department for adjuvant chemo- and radiotherapy after the PET scan evaluation.
Fig. 2.
The sample used for the pathology study consists of a total laryngectomy product measuring 10 cm long, showing a tumor (green circle) involving the left half of the larynx, not reaching surgical margins, and measuring 5 cm long and 4.5 cm tumor thickness with invasion into the thyroid cartilage and extralaryngeal soft tissues. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
The histological study of the tumor showed sheets of small round cells with an invasion of laryngeal tissues (vocal cord tissue in Fig. 3, A), thyroid cartilage (Fig. 3, B), and extralaryngeal tissues (skeletal muscle fibers in Fig. 3, C).
3. Discussion
In this report we presented an extremely rare case, getting its rarity from being a combination of two rare cases; larynx cancer presents as a sarcoma as we mentioned before, and Ewing sarcoma outside the bone.
Ewing sarcoma is known to be a primary bone malignancy usually presents in the pelvis, axial skeletal, and femur, mostly affecting adolescents and young adults with median ages of 10–20 years [12]. However, evidence traced back to 1969 suggested the probability of the extraosseous existence of Ewing sarcoma [13]. After several studies, the concept of Ewing sarcoma extended to include a subtype called Extraskeletal Ewing sarcoma (EES) which may present in different ages and anatomical regions [5,12,14]. In course of our discussion, head and neck took around 8.5–12% of all EES according to some studies [15,16]. Still, EES in the larynx is even rarer and only recorded as case reports.
So far, 5 cases of EES in the larynx were reported at advanced ages (between 33 and 74 years) [[5], [6], [7], [8], [9]] similar to our case, in contrast to classic Ewing sarcoma which is more common in younger ages [12]. Furthermore, 3 cases were reported at far younger ages (newborn, 9 months, 5 years) [4,10,11].
Histologically, Ewing sarcoma reveals uniform sheets of small round cells in a lobular or diffuse arrangement with scanty and pale cytoplasmic staining. However, a wide spectrum of malignancies shows those histologic characteristics so it may be confused to distinguish it from other differential diagnoses like malignant lymphoma and reticulum cell sarcoma that could be ruled out in light of the appearance of cells arrangement and presence of intracellular glycogen, as well as the lack of lymphatic node involvement [14]. In addition, the expression of the MIC2 (CD99) marker in IHC staining has been shown to be a highly specific marker to distinguish PNET/Ewing sarcoma from other small round cell tumors [17].
Ewing sarcoma and Primitive Neuroectodermal Tumor (PNET) are regarded as a single category called “PNET/Ewing Sarcoma” from a current perspective since they share similar chromosomal translocations especially t(11:22) (q24:q12), practical diagnosis, and immunohistochemical characteristics of CD99 expression, as well as therapeutic strategies [18]. This perspective was adopted in several reports that made the diagnosis of PNET/Ewing Sarcoma based on histological findings and CD99 marker besides other markers to exclude other differential diagnoses, and ours was one of them [4,5,8]. The opposing perspective tries to separate them into different entities depending on neural differentiation and markers exhibited by PNET (S-100 protein, vimentin ..) [6,18].
A few reports used microbiology of these tumors that is characterized by a reciprocal translocation leading to the formation of hybrid genes with the EWSR1-FTI1 being the most common one and the gold standard for diagnosis using Fluorescence in situ hybridization (FISH) [4,7,8,18].
Although a solid conclusion on the treatment of Ewing Sarcoma in the larynx remains to be established due to the small number of cases in medical literature, some reports mentioned applying surgical treatment with/without adjuvant chemo-radiotherapy [4,9] while others applied chemo-radiotherapy without surgery depending on the case [[6], [7], [8]]. Larger studies investigated the favorable treatment of Ewing sarcoma outside the bone and in the head and neck region [19,20]. Those studies demonstrated the efficacy of Multimodal therapy consisting of surgical resection with adjuvant chemotherapy and radiotherapy. It might be difficult to resect or radiate in the head and neck as a delicate region comprising virtual structures, however, achieving surgical negative margins is still the gold standard for oncologic resection.
Our patient was a candidate for surgical treatment since the tumor locally invaded the larynx as shown in the CT scan and a laryngectomy was decided to be made after assuring malignancy through a frozen section. The thyroid gland could not be separated from the larynx so total larynx and thyroid resection and bilateral neck dissection were performed. Histological examination showed negative surgical margins with no lymph node involvement. Additionally, the patient was referred to chemo-radiotherapy, as some evidence suggested postoperative radiotherapy with all local resection reduces the risk of local recurrence, and adjuvant chemotherapy might help eliminate early microscopic metastasis [19,20].
4. Conclusion
In this case, Ewing sarcoma involved the larynx as an unexpected region and an unusual range of age. Because of the lack of similar studies and documented data in the medical literature about this rare case, Ewing sarcoma should be included in the differential diagnosis in laryngeal cancer cases. The diagnosis should be made based on a combination of clinical presentation, laryngoscopy, radiological imagining, histological study, and most importantly, immunohistochemical study.
Ethical approval
No ethical approval was needed.
Sources of funding
There were no sources of funding for this study.
Author contributions
Dima Alhomsi and Hamzeh Al Asadi: reviewed the literature, wrote and revised the manuscript, and made grammar and spelling language editing.
Areej Alassaf: provided medical treatment, supervise the scientific and academic aspects of the manuscript, and revised it.
Registration of research studies
No registration was needed.
Guarantor
Dima Alhomsi.
Hamzeh Al Asadi.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
There were no conflicts of interest.
Abbreviations
- EES
Extraskeletal Ewing Sarcoma
- PNET
Primitive Neuroectodermal Tumor
- CT
Computerized Tomography
- IHC
Immunohistochemistry
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104575.
Contributor Information
Dima Alhomsi, Email: dimaalhomsi11@gmail.com.
Hamzeh Al Asadi, Email: Hamza.alasadi@hotmail.com.
Areej Alassaf, Email: Jalilabraik@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cancer Stat Facts: Laryngeal Cancer National Cancer Institution: Surveillance, Epidemiology, and End Results (SEER) Program. 2022. https://seer.cancer.gov/statfacts/html/laryn.html [updated April 2022. Available from: [Google Scholar]
- 2.Koroulakis A., Agarwal M. 2022. Laryngeal Cancer. StatPearls. Treasure. Island (FL) [PubMed] [Google Scholar]
- 3.Karatayli-Ozgursoy S., Bishop J.A., Hillel A.T., Akst L.M., Best S.R. Non-epithelial tumors of the larynx: a single institution review. Am. J. Otolaryngol. 2016;37(3):279–285. doi: 10.1016/j.amjoto.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Pasha H.A., Ghaloo S.K., Wasif M., Siddiqui M.I., Din N.U. Ewing sarcoma of larynx: a rare case in a 5-year-old boy. Turk. Arch. Otolaryngol. 2020;58(1):65–68. doi: 10.5152/tao.2020.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maroun C.A., Khalifeh I., Tfayli A., Moukarbel R.V. Primary Ewing sarcoma of the larynx with distant metastasis: a case report and review of the literature. Curr. Oncol. 2019;26(4):e574–e577. doi: 10.3747/co.26.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ijichi K., Tsuzuki T., Adachi M., Murakami S. A peripheral primitive neuroectodermal tumor in the larynx: a case report and literature review. Oncol. Lett. 2016;11(2):1120–1124. doi: 10.3892/ol.2015.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch M.C., Baker A., Drabick J.J., Williams N., Goldenberg D. Extraskeletal Ewing's sarcoma arising in the larynx. Head Neck Pathol. 2014;8(2):225–228. doi: 10.1007/s12105-013-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wygoda A., Rutkowski T., Ponikiewska D., Hejduk B., Skladowski K. Ewing's sarcoma of the larynx. Effective treatment with organ preservation. Strahlenther. Onkol. 2013;189(7):586–589. doi: 10.1007/s00066-013-0356-8. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y.S., Hong K.H. Extraskeletal Ewing's sarcoma of the larynx. J. Laryngol. Otol. 2004;118(1):62–64. doi: 10.1258/002221504322731682. [DOI] [PubMed] [Google Scholar]
- 10.Abramowsky C.R., Witt W.J. Sarcoma of the larynx in a newborn. Cancer. 1983;51(9):1726–1730. doi: 10.1002/1097-0142(19830501)51:9<1726::aid-cncr2820510928>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Jones J.E., McGill T. Peripheral primitive neuroectodermal tumors of the head and neck. Arch. Otolaryngol. Head Neck Surg. 1995;121(12):1392–1395. doi: 10.1001/archotol.1995.01890120050009. [DOI] [PubMed] [Google Scholar]
- 12.Durer S., Shaikh H. 2022. Ewing Sarcoma. StatPearls. Treasure. Island (FL) [PubMed] [Google Scholar]
- 13.Tefft M., Vawter G.F., Mitus A. Paravertebral "round cell" tumors in children. Radiology. 1969;92(7):1501–1509. doi: 10.1148/92.7.1501. [DOI] [PubMed] [Google Scholar]
- 14.Angervall L., Enzinger F.M. Extraskeletal neoplasm resembling Ewing's sarcoma. Cancer. 1975;36(1):240–251. doi: 10.1002/1097-0142(197507)36:1<240::aid-cncr2820360127>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Huh J., Kim K.W., Park S.J., Kim H.J., Lee J.S., Ha H.K., et al. Imaging features of primary tumors and metastatic patterns of the extraskeletal ewing sarcoma family of tumors in adults: a 17-year experience at a single institution. Korean J. Radiol. 2015;16(4):783–790. doi: 10.3348/kjr.2015.16.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Weshi A., Allam A., Ajarim D., Al Dayel F., Pant R., Bazarbashi S., et al. Extraskeletal Ewing's sarcoma family of tumours in adults: analysis of 57 patients from a single institution. Clin. Oncol. 2010;22(5):374–381. doi: 10.1016/j.clon.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Ambros I.M., Ambros P.F., Strehl S., Kovar H., Gadner H., Salzer-Kuntschik M. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67(7):1886–1893. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Batsakis J.G., Mackay B., el-Naggar A.K. Ewing's sarcoma and peripheral primitive neuroectodermal tumor: an interim report. Ann. Otol. Rhinol. Laryngol. 1996;105(10):838–843. doi: 10.1177/000348949610501014. [DOI] [PubMed] [Google Scholar]
- 19.Olson M.D., Van Abel K.M., Wehrs R.N., Garcia J.J., Moore E.J. Ewing sarcoma of the head and neck: the Mayo Clinic experience. Head Neck. 2018;40(9):1999–2006. doi: 10.1002/hed.25191. [DOI] [PubMed] [Google Scholar]
- 20.Rud N.P., Reiman H.M., Pritchard D.J., Frassica F.J., Smithson W.A. Extraosseous Ewing's sarcoma. A study of 42 cases. Cancer. 1989;64(7):1548–1553. doi: 10.1002/1097-0142(19891001)64:7<1548::aid-cncr2820640733>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.