Abstract
Background
Of late, numerous randomised controlled trials report platelet-rich plasma (PRP) to be ineffective with preparation protocols of low platelet yield despite using expensive commercial PRP kits.
Objective
This study aims to identify and standardize a preparation protocol for PRP with maximum platelets yield and concentration to obtain favourable results without the use of commercial preparation kits.
Materials & methods
Blood samples were collected from 40 healthy volunteers who signed informed consent for participation in the study. The double spin protocol of PRP preparation was analyzed for variables such as centrifugal acceleration, time, and volume of blood processed and final product utilized. The final PRP prepared was investigated for platelet recovery, concentration, integrity, and viability. Each protocol investigated with technical and biological duplicates to avoid reporting and sampling bias.
Results
We noted maximum platelet recovery (86–99%) with a consistent 6.4 ± 0.8 times the baseline concentration of platelets with first centrifugation at 100g for 15 min followed by second centrifugation at 1600g for 20 min. We did not note a loss of integrity or viability of the platelets in the final product from the above-said protocol. We also validated the protocol among all the study participants demonstrating consistency.
Conclusion
The preparation of PRP by the double-spin protocol using 10 ml of blood at 100 g followed by 1600 g for 15 and 20 min respectively in a 15 ml tube and using the lower 1/3rd of the final product demonstrated consistent high platelet recovery (86–99%) and concentration (6x) without disturbing the platelet integrity or viability.
Keywords: Platelet-rich plasms, PRP, Preparation protocol, Double-spin centrifugation, Standardization
Highlights
-
•
Standardized protocol is necessary for comparable results across studies using PRP.
-
•
The preparation of PRP by the double-spin protocol is recommended.
-
•
Double-spin protocol at 100 g followed by 1600 g for 15 and 20 min is validated.
-
•
Lower 1/3rd of the final product demonstrated high platelet recovery (86–99%).
-
•
Consistent concentration (6x) is obtained without disturbing the platelet viability.
1. Introduction
Platelet-rich plasma (PRP) is an ultra-concentrate of plasma containing a supraphysiological dose of platelets [1]. They are rich in growth factors necessary for tissue regeneration and healing. There is a growing list of indications of its use across disciplines ranging from orthopedics [2], rheumatology [3] to plastic surgery [4,5], dentistry [6], and oral maxilla facial surgery [7]. Although various meta-analyses have proven their effectiveness across various indications [[8], [9], [10]], we did also find some studies reporting their ineffectiveness despite the robust study design [11].
Of late, numerous randomised controlled trials report platelet-rich plasma (PRP) to lack a treatment benefit compared to placebo when used in various inflammatory and degenerative disorders [11,12]. Upon critically analysing, we could note that the preparation protocol utilized in these trials had low yield with insufficient platelet concentration in the final product despite using expensive commercial PRP kits in them. On further probing into the cause for the heterogeneity among the study results it was noted that there was no uniformity in the PRP preparation protocols and no uniformity in the dose of the platelet delivered at the site of action [8].
All the studies usually followed a generic sequence of blood collection, first centrifugation to separate the red blood cells (RBCs) and second centrifugation to sediment the platelets to the bottom of the plasma. Here the variables that come into play include the centrifugal acceleration used for each of the steps along with their duration. Following these steps, some studies employed exogenous activation of the PRP before injection while some relied on endogenous tissue activation [13]. Sometimes the variables in the process itself may affect the integrity of the platelets affecting the effectiveness of the final product [14].
There is an ongoing debate on the presence or absence of white blood cells (WBCs) in the PRP. Proponents of WBC inclusion voice it for their protection against infection or allergic response [15,16] while those who do not recommend its presence owing to the release of nonselective and toxic reactive oxygen species at high levels at the site of action. Even some studies have shown a catabolic gene expression in leucocyte-rich PRP relating to impaired tissue healing in in-vitro studies [17,18]. However, various randomised controlled trials and meta-analyses of studies [[19], [20], [21]] that analyzed this variable did not find any clinical difference in either of the groups.
Various protocols are existing in the literature that was considered optimal for centrifugation to obtain maximum yield of platelets [22]. However, the protocols used in them were optimized based on the individual variables involved in the preparation of PRP such as volume of blood processed, the total number of spins utilized, and time and duration of centrifugation. To achieve consistent results with an autologous product such as PRP which has inherent subjective variability in its effectiveness, the preparation protocol must be standardized to ensure quality control [23]. Hence, the main purpose of this study is to arrive at an ideal PRP preparation protocol considering all the variables at play to obtain consistent results without the use of expensive commercial PRP preparation kits.
2. Materials & Methods
This study was conducted in accordance with the Declaration of Helsinki after being approved by the Institutional Ethical Committee (IEC-2022-R5) and registered in Research Registry with UIN8174. The research has been reported in line with the STROCSS criteria [24].
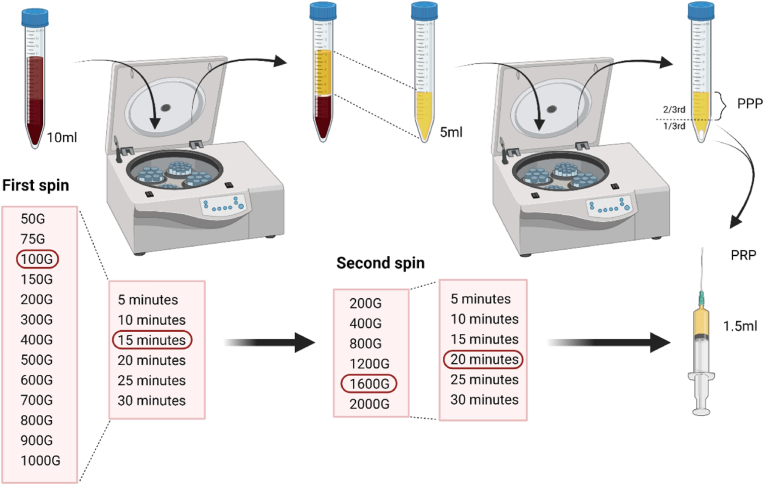
Blood Collection: We collected blood from 40 healthy volunteers who signed informed consent for participation in the study without any comorbid illness or history of any medications in the past 3 months. We collected 300 ml peripheral venous blood from the donors following sterile aseptic precautions and used Citrate-Phosphate-Dextrose-Adenine (CPD1) anticoagulant solution at the ratio of 1.5 ml per 10 ml of blood. We used 20 samples as the standardization cohort to arrive at a protocol and 20 samples as the validation cohort of the standardized protocol. We used 15 ml centrifugation tubes Spinwin ™ (Tarsons, India) or 5 ml blood collection tubes BioPro (Alchem Diagnostics, India) based on the protocol analyzed. Each protocol investigated with technical and biological duplicates to avoid reporting and sampling bias. Average of the values were taken for analysis.
First spin: To standardize the protocol for the first spin 10 ml of whole blood from 20 standardization cohort samples were centrifuged at incremental acceleration rates ranging from 50 to 1000×g (50, 75, 100, 150, 200, 300, 400, 500, 600, 700, 800, 900, and 1000×g) for 10 min in a laboratory centrifuge R–8C (REMI, India) with swing-out 16-well R-81 rotor head (REMI, India) with rotor radius of 150 mm. To make the protocol applicable to clinical practice we performed the centrifugation at room temperature (22 °C) and the centrifuges were not refrigerated. Following the first spin, the whole blood is separated into the RBC layer at the bottom, buffy coat in the middle, and plasma layer at the top. We collected the upper plasma layer without disturbing the middle/bottom layers using a pipette. Depending on the centrifugal force utilized the volume of the upper layer ranged from 2 to 6 ml. The sample was then thoroughly mixed and blood cell counting was performed using cell counter XP-300™ (Sysmex America, Inc.). With selected the minimal centrifugal acceleration giving maximum platelet recovery, and titrated the time ranging from 5 to 30 min (5, 10, 15, 20, 25, and 30 min) to investigate the effect of time on the recovery efficacy of centrifugation. Based on the results the final first spin centrifugation protocol was finalized.
Second Spin: The whole blood was initially centrifuged as per the identified ideal centrifugation protocol for the first spin to give 5 ml of the plasma. We performed second centrifugation in them at various centrifugal accelerations ranging from 200 to 2000×g (200, 400, 800, 1200, 1600, and 2000×g) for 10 min. The level of plasma to be discarded as platelet-poor plasma (PPP) was identified by performing blood cell counting of the upper, middle, and lower 1/3rd of the final plasma obtained. The PRP thus obtained by discarding the PPP was analyzed for its cellular constituents. With the selected minimal centrifugal acceleration giving maximum platelet recovery, we titrated the time ranging from 5 to 30 min (5, 10, 15, 20, 25, and 30 min) to investigate the effect of time of centrifugation in the second spin. To investigate the effect of the volume of the whole blood utilized we ran the protocol with minimal centrifugal acceleration giving maximum platelet recovery in both the first and second spin for 5, 10, and 15 ml of whole blood volume.
PRP Characterisation: The final PRP obtained was characterized by measuring the platelet concentration gradient at lower, middle and upper 1/3rd of the final product obtained after the second spin, its cellular composition, and the integrity of the platelets.
First Spin Analysis: After the first spin, the concentration of platelets and WBC was measured to calculate the recovery efficiencies of the plasma (E pl), platelets (E pt), and WBC (E WBC) along with the platelet concentration factor (Fc pt). These values were calculated using the following formulae:
Second Spin Analysis: After the second spin, the cellular concentration of the upper, middle, and lower 1/3rd of the plasma is analyzed to identify the concentration gradient. The recovery efficiencies and the concentration factor of the platelets in the layer of the plasma were calculated using the following formulae:
Platelet Concentration Gradient: After the second spin, the volume of plasma was divided into upper, middle, and lower 1/3rd and analyzed for cellular components to estimate the demarcation of PPP and PRP in the final plasma product obtained. Before cell counting the separated plasma layer was mixed by inversion manually for 30 s to minimize the gradient effect upon cell counting.
Platelet Integrity: The effect of the centrifugal acceleration on the integrity of the platelets was assessed in the final PRP obtained after the second spin by measuring the lactate dehydrogenase (LDH) levels and comparing it to the serum baseline levels. This indirectly measures the integrity of the platelet membranes and any damage to the platelet would result in a rise of the LDH levels significantly compared to the serum baseline measured beforehand.
Protocol Validation: The final protocol arrived was validated from the samples of 20 validation cohort. In all the cases, the analysis was repeated twice to prevent the reporting bias and the final protocol arrived was also run on common bench-top laboratory centrifugation machines such as Medico Plus (REMI, India) and R–4C (REMI, India) to establish the external validity of the PRP preparation protocol ascertained.
We used descriptive statistics to describe the results of the parameters analyzed. We presented the continuous variables with mean and standard deviation. Variables were tested for distribution normalcy using the Kolmogorov-Smirnov test and we found a normal distribution of all the variables analyzed (p > 0.30 for all variables). We used paired t-test to analyze the significance of the increase in the levels of LDH compared to the baseline. We used IBM SPSS Version 25 (Chicago, IL, USA) for statistical analysis. We considered a p value less than 0.05 as significant.
3. Results
We did not note any significant difference across the baseline cellular counts among the 40 volunteers involved in the study.
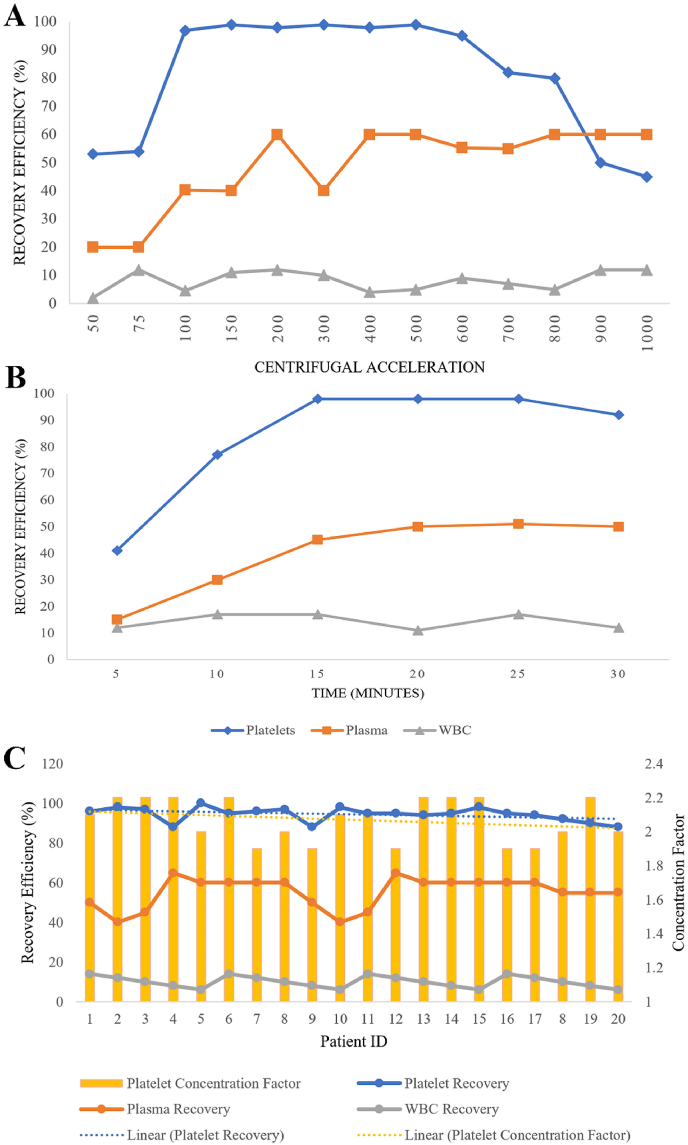
First Spin Analysis: The cellular composition and the recovery efficiency at various centrifugal accelerations were shown in Fig. 1A. The mean platelet count of all the donors was 1.795 × 10 [5] ± 0.54 cells/μl. The recovery efficiency of the platelets increased as the centrifugal acceleration increased from 50 to 75×g and peaked at 100 and began to decline from 600×g as shown in Fig. 1A. The recovery efficiency of the plasma also increased with the centrifugal acceleration. The recovery of WBC remained between 2 and 12% regardless of the centrifugal accelerations. On analyzing the effect of time, we noted that the recovery efficiency of platelets increased with increasing time from 5 to 10 min and peaked at 15–20 min, and began to decline at 25–30 min as shown in Fig. 1B.
Fig. 1.
A: The first spin mean recovery efficiency of the plasma, WBC, and platelets at varying centrifugal acceleration for 10 min; B: First spin mean recovery efficiency of the plasma, WBC, and platelets at 100 g for varying time duration; C: First spin mean recovery efficiency of the plasma, WBC and platelets at 100 g for 15 min in the validation cohort and their platelet concentration factor.
We found the centrifugal acceleration of 100×g and time of 15 min to yield maximum recovery of platelets. The protocol was repeated in 20 donors for data validation and the mean recovery rates of platelets, plasma, and WBC were approximately 98%, 60%, and 12% respectively as shown in Fig. 1C.
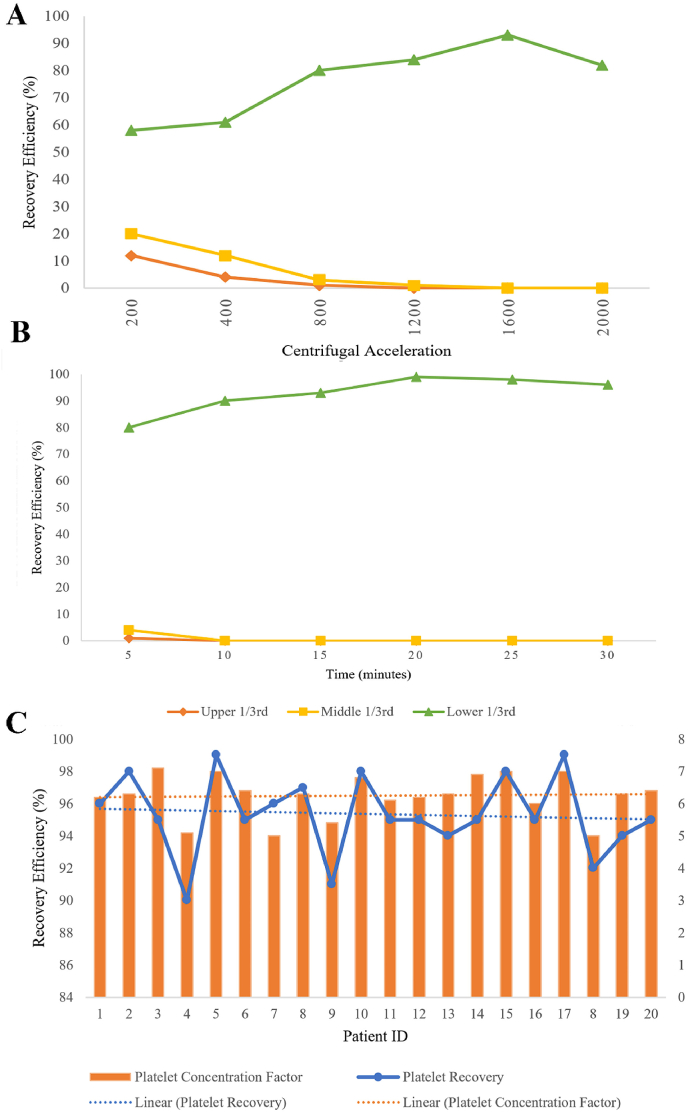
Second Spin Analysis: The platelet recovery efficiency at various centrifugal accelerations were shown in Fig. 2. The recovery efficiency of the platelets at the bottom 1/3rd of the final plasma obtained increased with increasing centrifugal acceleration from 200 to 1200×g and peaked at 1600×g and began to decline at 2000×g as shown in Fig. 2A. On analyzing the effect of time, we noted that the recovery efficiency of platelet increased with increasing time from 5 to 15 min and peaked at 20 min, and declined at 25–30 min as shown in Fig. 2B. We found the centrifugal acceleration 1600×g and a time of 20 min to yield maximum recovery of platelets. The protocol was repeated in the first-spun samples of 20 donors for data validation and the mean recovery efficiency and concentration factor of platelets noted were approximately 97% and 6.2 respectively as shown in Fig. 2C.
Fig. 2.
A: The second spin mean recovery efficiency of the platelets at varying centrifugal acceleration for 10 min in different sections of plasma analyzed; B: The second spin mean recovery efficiency of the platelets at 1600 g for varying time duration in different sections of plasma analyzed; C: Second spin mean recovery efficiency of the platelets at 1600 g for 20 min in the validation cohort and their platelet concentration factor.
Platelet Concentration Gradient: We also noted that with increasing centrifugal acceleration the number of platelets decreased in the upper and middle 1/3rd of the final plasma obtained and increased in the lower 1/3rd of the plasma. With the above-said finalized protocol we did not find any platelet in either the upper or middle 1/3rd of the plasma and the entire platelet mass settled in the lower 1/3rd as a platelet pellet which could be reconstituted with the desired volume of plasma as shown in Fig. 3. We noted a mean concentration factor of 6.4 ± 0.8 with the protocol developed from the study.
Fig. 3.
Schematic representation of standardization of preparation protocol of platelet-rich plasma.
Platelet Integrity: The mean initial plasma concentration of the LDH of the donors was 213 ± 24 units/L. The mean concentration of the LDH in the PRP was 237 ± 38 units/L which was not statistically significant (p = 0.432). Both the values were in the normal range for human plasma.
Effect of Sample Volume: We analyzed the effect of the volume of the sample processed following the finalized protocol in 5, 10, and 15 ml of whole blood. Having standardized the protocol for 10 ml of sample, we noted the loss of recovery efficiency with both higher and lower volumes of blood (5, 15 ml) as shown in Table 1. For every 10 ml of the whole blood processed we obtained 1.5 ml of PRP. In situations where 5, 15 ml of whole blood needs to be processed, the centrifugal acceleration and the time should be titrated following similar methods to achieve comparable recovery efficiency.
Table 1.
Effect of sample volume on the recovery efficiency of platelets.
| Blood Volume | Container | First Spin | Second Spin | Mean Platelet Recovery (%) (±SD) | Mean Factor Concentration of Platelets Fc (Pt) |
|---|---|---|---|---|---|
| 5 ml | 5 ml tube | 100×g x 15 min | 1600×g x 20 min | 63 (5.2) | 4.2 (1.2) |
| 10 ml | 15 ml tube | 100×g x 15 min | 1600×g x 20 min | 98 (1.4) | 6.9 (0.8) |
| 15 ml | 15 ml tube | 100×g x 15 min | 1600×g x 20 min | 82 (4.5) | 5.8 (0.4) |
SD Standard Deviation.
External Validation: Apart from validating the protocol in 20 donor samples, we also validated the protocol with 20 donor samples in 2 different centrifugation machines and we noted comparable recovery efficiencies thereby establishing the external validity of the protocol.
4. Discussion
PRP in general can be prepared using commercial kits or by conventional methods. Due to the high cost involved in the utilization of the commercial kits, we developed this standardized protocol for conventional use with consistent results. Previously, the quality of the PRP was classified based on the platelets, activation method, and WBC counts (PAW Classification) [25]. Later, a more detailed system of classification accounting for absolute platelet count, WBC count along with their neutrophil percentage, RBCs along with exogenous activation methods was developed [13]. Finally, the current DEPA classification is based on the dose of the platelet injection, efficiency, purity of production, and activation methods [26]. Hence the production variables play a major role in determining the quality of the PRP derived [27].
With the increasing number of studies on the effectiveness of PRP, there is also an increase in the number of protocols that are being followed thereby making their studies non-comparable for practical purposes [22]. Hence in this study we tried to arrive at a standardized preparation protocol for universal usage with consistent results. Moreover, the recent studies the demonstrated no significant effect with the use of PRP either did not quantify the concentration of the platelets in the their final injectate or used commercial PRP preparation kits that delivered PRP with 2–3 times the baseline concentration [11,12]. It is already a general consensus that the quality of PRP delivered largely depends on the concentration of the platelets in the final injectate and for favourable results a very high concentration of >5 billion platelets need to be delivered at the site of interest [26].
Our current protocol arrived at the first spin centrifugation speed and duration of 100 g for 15 min which was in line with various previous analyses of preparation protocols for PRP. However, our second spin centrifugation speed and duration were 1600 g for 20 min. Mazzocca et al. [28] utilized similar centrifugation speed but as a single spin protocol. The high-speed centrifugation protocol was preferred since it significantly increased the growth factor delivery. Eren et al. [29] studied the effect of centrifugation duration on the growth factor release kinetics and found that PRP produced with an increase in centrifugation time resulted in significantly higher TGF-β and VEGF release. Hence by using a higher duration protocol in the second spin, we aim at achieving higher growth factor release without any activation methods employed.
Another concern with the high speed is to maintain the integrity and efficacy of the platelet at high speed since they are fragile cells responsive to centrifugal acceleration. Hence, we made an indirect analysis of the platelet integrity and found that at the given protocol the platelet integrity and effectiveness were maintained and studies analyzing the integrity of the platelets at given centrifugal acceleration had similar results [30]. While the quest for standardization is being addressed with methods using allogenic platelet lysates with predefined growth factors per mg of the product [23], the current study gives a conventional reliable protocol for clinical use at the bedside.
The present study also has limitations. Although we measured the absolute platelet count and the concentration factor, we did not measure the changes in the concentration of the growth factors in the final PRP with each protocol analyzed. However, we validated our final protocol in the clinical setting and arrived at consistent results as found in the formulation process.
5. Conclusion
Preparation of PRP by the double spin protocol of 10 ml of blood at 100 g and 1600 g for 15 and 20 min respectively in a 15 ml tube and using the lower 1/3rd of the final product demonstrated consistent high platelet recovery (86–99%) and concentration (6x) without disturbing the platelet integrity or viability.
Ethical approval
All the authors declare the appropriate ethical committee approval has been sought before the commencement of the study. Name of the ethics committee: Institutional Ethics Committee, Government Medical College and Hospital, Dindigul, Tamil Nadu, India. Reference number: IEC-2022-R5.
Sources of funding
All the authors declare no funding being utilized for this study.
Contributorship
Conceptualization – Dr SM & Dr AK; Methodology – Dr SM & Dr KR; Software – Dr SM; Validation – Dr SM & Dr AK; Formal analysis – Dr SM & Dr AK; Investigation – Dr SM; Resources – Dr SM & Dr KR; Data Curation – Dr SM & Dr KR; Writing - Original Draft – Dr SM; Writing - Review & Editing – All authors; Visualization – Dr SM; Supervision – Dr SM & Dr KR; Project administration – Dr SM & Dr KR.
Registration of research studies
-
1.
Name of the registry: researchregistry
-
2.
Unique Identifying number or registration ID: 8174
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browse-the-registry#home/registrationdetails/62ef0e56ed6b770021d8b8c3/
Guarantor
Dr Sathish Muthu.
Provenance and peer review
Not commissioned, externally peer reviewed.
Consent
All the authors state that they obtained fully informed and written consent from the volunteers who participated in the study and no patient identifying details have been utilized in the manuscript submitted.
Declaration of competing interest
All the authors declare no conflicts of interest.
References
- 1.Arnoczky S.P., Sheibani-Rad S., Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med. Arthrosc. Rev. 2013;21:180–185. doi: 10.1097/JSA.0b013e3182999712. [DOI] [PubMed] [Google Scholar]
- 2.Oeding JF, Lansdown DA, Leucht P, et al. Influential studies in orthopaedic platelet-rich plasma research are recent and consist of high levels of evidence: a review of the top 50 most cited publications. J. Knee Surg.. Epub ahead of print 10 March 2022. DOI: 10.1055/s-0042-1744223. [DOI] [PubMed]
- 3.Chellamuthu G, Muthu S, Khanna M, et al. ‘Platelet-rich plasma holds promise in management of rheumatoid arthritis’-systematic review. Rheumatol Int. Epub ahead of print 8 April 2021. DOI: 10.1007/s00296-021-04849-9. [DOI] [PubMed]
- 4.Anudeep T.C., Jeyaraman M., Muthu S., et al. Advancing regenerative cellular therapies in non-scarring alopecia. Pharmaceutics. 2022;14:612. doi: 10.3390/pharmaceutics14030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raposio E., Bertozzi N., Bonomini S., et al. Adipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapy. Wounds. 2016;28:126–131. [PubMed] [Google Scholar]
- 6.Farshidfar N., Amiri M.A., Firoozi P., et al. The adjunctive effect of autologous platelet concentrates on orthodontic tooth movement: a systematic review and meta-analysis of current randomized controlled trials. Int. Orthod. 2022;20 doi: 10.1016/j.ortho.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Moraissi E.A., Wolford L.M., Ellis E., et al. The hierarchy of different treatments for arthrogenous temporomandibular disorders: a network meta-analysis of randomized clinical trials. J. Cranio-Maxillo-Fac. Surg. 2020;48:9–23. doi: 10.1016/j.jcms.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Muthu S., Patel S., Gobbur A., et al. Platelet-rich plasma therapy ensures pain reduction in the management of lateral epicondylitis - a PRISMA-compliant network meta-analysis of randomized controlled trials. Expet Opin. Biol. Ther. 2022;22:535–546. doi: 10.1080/14712598.2022.2032638. [DOI] [PubMed] [Google Scholar]
- 9.Muthu S. Role of intradiscal injection of platelet rich plasma in the management of lumbar disc disease systematic review & meta-analysis. IP International Journal of Orthopaedic Rheumatology. 2021;7:1. [Google Scholar]
- 10.Muthu S., Chellamuthu G. Platelets and platelet-rich plasma in rheumatoid arthritis - a systematic review of literature. IP International Journal of Orthopaedic Rheumatology. 2021;7:1–2. doi: 10.1007/s00296-021-04849-9. [DOI] [PubMed] [Google Scholar]
- 11.Bennell K.L., Paterson K.L., Metcalf B.R., et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021;326:2021–2030. doi: 10.1001/jama.2021.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis E., Merghani K., Robertson I., et al. The effectiveness of leucocyte-poor platelet-rich plasma injections on symptomatic early osteoarthritis of the knee: the PEAK randomized controlled trial. Bone Joint Lett. J. 2022;104-B:663–671. doi: 10.1302/0301-620X.104B6.BJJ-2021-1109.R2. [DOI] [PubMed] [Google Scholar]
- 13.Mautner K., Malanga G.A., Smith J., et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM&R. 2015;7:S53–S59. doi: 10.1016/j.pmrj.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Perez A.G.M., Lana J.F.S.D., Rodrigues A.A., et al. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematology. 2014;2014 doi: 10.1155/2014/176060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everts P.A., Overdevest E.P., Jakimowicz J.J., et al. The use of autologous platelet-leukocyte gels to enhance the healing process in surgery, a review. Surgical endoscopy and other interventional techniques. 2007;21:2063–2068. doi: 10.1007/s00464-007-9293-x. [DOI] [PubMed] [Google Scholar]
- 16.Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Sundman E.A., Cole B.J., Fortier L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011;39:2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 18.Scott A., Khan K.M., Roberts C.R., et al. What do we mean by the term “inflammation”? A contemporary basic science update for sports medicine. Br. J. Sports Med. 2004;38:372–380. doi: 10.1136/bjsm.2004.011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthu S., Patel S., Selvaraj P., et al. Comparative analysis of leucocyte poor vs leucocyte rich platelet-rich plasma in the management of lateral epicondylitis: systematic review & meta-analysis of randomised controlled trials. Journal of Clinical Orthopaedics & Trauma. 2021;19:96–107. doi: 10.1016/j.jcot.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palakuri S., Muthu S. Does leucocyte concentration have a role in the outcome of PRP therapy for lateral epicondylitis – a randomized controlled trial. IP International Journal of Orthopaedic Rheumatology. 2021;6:84–87. [Google Scholar]
- 21.Di Martino A., Boffa A., Andriolo L., et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am. J. Sports Med. 2022;50:609–617. doi: 10.1177/03635465211064303. [DOI] [PubMed] [Google Scholar]
- 22.Croisé B., Paré A., Joly A., et al. Optimized centrifugation preparation of the platelet rich plasma: literature review. Journal of Stomatology, Oral and Maxillofacial Surgery. 2020;121:150–154. doi: 10.1016/j.jormas.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Andia I., Perez-Valle A., Del Amo C., et al. Freeze-drying of platelet-rich plasma: the quest for standardization. Int. J. Mol. Sci. 2020;21:E6904. doi: 10.3390/ijms21186904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew G., Agha R., Albrecht J., et al. Strocss 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 25.DeLong J.M., Russell R.P., Mazzocca A.D. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 26.Magalon J., Chateau A.L., Bertrand B., et al. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport & Exercise Medicine. 2016;2 doi: 10.1136/bmjsem-2015-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lana J.F.S.D., Purita J., Paulus C., et al. Contributions for classification of platelet rich plasma – proposal of a new classification: MARSPILL. Regen. Med. 2017;12:565–574. doi: 10.2217/rme-2017-0042. [DOI] [PubMed] [Google Scholar]
- 28.Mazzocca A.D., McCarthy M.B.R., Chowaniec D.M., et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308–316. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 29.Arora S., Doda V., Kotwal U., et al. Quantification of platelets and platelet derived growth factors from platelet-rich-plasma (PRP) prepared at different centrifugal force (g) and time. Transfus. Apher. Sci. 2016;54:103–110. doi: 10.1016/j.transci.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Sabarish R., Lavu V., Rao S.R. A comparison of platelet count and enrichment percentages in the platelet rich plasma (PRP) obtained following preparation by three different methods. J. Clin. Diagn. Res. 2015;9:ZC10–ZC12. doi: 10.7860/JCDR/2015/11011.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]