Abstract
Unintentional overdose deaths, most involving opioids, have eclipsed all other causes of US deaths for individuals less than 50 years of age. An estimated 2.4 to 5 million individuals have opioid use disorder (OUD) yet a minority receive treatment in a given year. Medications for OUD (MOUD) are the gold standard treatment for OUD however early dropout remains a major challenge for improving clinical outcomes. A Cascade of Care (CoC) framework, first popularized as a public health accountability strategy to stem the spread of HIV, has been adapted specifically for OUD. The CoC framework has been promoted by the NIH and several states and jurisdictions for organizing quality improvement efforts through clinical, policy, and administrative levers to improve OUD treatment initiation and retention. This roadmap details CoC design domains based on available data and potential linkages as individual state agencies and health systems typically rely on limited datasets subject to diverse legal and regulatory requirements constraining options for evaluations. Both graphical decision trees and catalogued studies are provided to help guide efforts by state agencies and health systems to improve data collection and monitoring efforts under the OUD CoC framework.
Keywords: Opioid use disorder, cascade of care, medication for opioid use disorder (MOUD), opioid crisis
Introduction
Unintentional overdose deaths, with over 70% involving opioids,1 have eclipsed all other causes of US deaths for individuals less than 50 years of age. Opioid-involved overdose death rates in the US have further surged alongside disruptions and stressors of the COVID-19 pandemic in 2020, leading to the largest year over year increase in overdose mortality.2 An estimated 2.4 to 5 million individuals have opioid use disorder (OUD) attributed to heroin, opioid analgesics, or other synthetic opioids, including fentanyl and analogues, yet only ∼20% to 25% receive addiction treatment in a given year.3
Medications for OUD (MOUD) are the gold standard treatment for OUD. Importantly, long-term MOUD use can reduce overdose death risk upwards of 66% to 80% while patients are receiving treatment.4,5 The National Academies of Science, Engineering, and Medicine and the NIH also stress the importance of access to these medication treatments—even in the absence of traditional counseling—given their lifesaving effects.6,7 Yet MOUD is greatly under-utilized with fewer than 35% of publicly funded OUD care episodes including MOUD.8 Following medically treated non-fatal overdose events, provision of MOUD to individuals barely increased, from 29.5% before to 33.0% afterwards.9 Early dropout also remains a major challenge in treating OUD with roughly half of individuals receiving buprenorphine, the most commonly used MOUD, leaving treatment within a few months.10,11 The great majority (80–90%) subsequently relapse and face increased risks of death.4,12–14
A Cascade of Care framework, first popularized as a public health accountability strategy to stem the spread of HIV, has been adapted for substance use disorders (SUDs)15,16 and applied specifically to OUD.17–19 This framework has been promoted by NIDA20 and several states and jurisdictions (e.g., New York State, Denver, and Philadelphia) for organizing quality improvement efforts through clinical, policy, and administrative levers to improve OUD treatment initiation and retention. The Veterans Health Administration (VHA) has also implemented a simplified CoC model, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative, to improve access to MOUD.21 Although the OUD CoC encompasses a key set of interlocking clinical and organizational challenges in the delivery of OUD treatment, it is too narrow to encompass critical interventions for reducing population-wide overdose risk, especially for individuals who are not appropriate for OUD treatment.
Coordinating and standardizing measurement frameworks is especially timely as the Substance Abuse Mental Health Services Administration (SAMHSA) now expects greater measurement and reporting requirements of states (c.f. the HEAL Initiative and the Opioid Response Network (ORN)).22 Much as we have seen with the response to COVID-19 and HIV, improving data collection and reporting systems for OUD is central to federal, state and health system efforts to address the opioid epidemic. To date, however, there has been insufficient federal guidance and few incentives for data collection and reporting to ensure that patients with OUD receive quality care in a timely manner.17,23 While the OUD CoC conceptually organizes measurement and quality improvement efforts, given current limitations with existing data, analytic design decisions are needed to customize the cascade for each application.
Overview of OUD Cascade of care
Measures for the performance of addiction treatment services were first proposed in 1998.24 Since 2004, two measures, initiation and engagement in treatment, have been tracked in the Health Effectiveness Data and Information Set (HEDIS).25 These measures are used throughout the healthcare landscape by insurance plans to capture quality of care and patient outcomes. However, the HEDIS measures were designed for substance use disorders generally rather than specifically for OUD.18 Quality measures specifically for OUD are necessary for monitoring, quality assurance, improving care, reporting requirements, and guiding payment contracts. While addiction care has historically been provided in specialized and siloed treatment settings apart from general healthcare settings, there is increasing urgency to integrate screening and treatment for OUD into mainstream practice. The aforementioned SCOUTT initiative at the VHA is one example building on longstanding VHA OUD quality measurement efforts.21 With a plethora of measures now developed for the behavioral health field generally,26,27 there is a need to harmonize coordinated measure sets that can track patients with OUD across treatment settings.
The CoC tracks individual patients as well as populations through sequential stages associated with improving clinical- and system-level outcomes. Cascades measure patient flow through care systems and help identify process breakdowns missed by single outcome measures such as access or retention. In another context, the benefits of the CoC framework have been demonstrated by the 90-90-90 goals of the international HIV eradication effort.28 In this context, ambitious goals involve diagnosing 90% of people with HIV infection, initiating antiretroviral therapy for 90% of those diagnosed, and achieving viral suppression in at least 90% of those on medication was meant to optimize patient health while preventing further HIV transmission in the community. As a specific example of recent success, the CDC was able to monitor and improve effectiveness at each stage of the HIV CoC by benchmarking to these goals.29 Within a five year span this led to more than a two-fold increase in sustained viral suppression, the last stage in the HIV CoC, among persons with HIV in the US, increasing from 20% in 2011 to 51% in 2016.29
A large body of research indicates that individuals with OUD benefit from treatment that includes MOUD initiation and long-term retention under a chronic care model.30,31 Whether in specialty settings or general practice settings, individuals with OUD must first link to care providers who have the capacity to offer MOUD before patients can initiate medication. While this may sound redundant, some patients may not immediately prefer to begin MOUD and yet would nevertheless benefit from linkage to services. Work has been conducted to develop OUD CoCs to track progress in individual U.S. states32–34 and in British Columbia, Canada.35,36 Although individual studies and individual evaluations vary in detail, they consistently demonstrate a great deal of room for improvement along the CoC. The following seven core stages are often included in OUD CoCs to monitor outcomes. Each of stage is dependent on success at achieving prior stages by definition:
Estimation of the affected population
Prevention of OUD among those at risk
Diagnosis among those affected with OUD
Linkage to OUD care among those diagnosed
Medication (MOUD) initiation among those entering care
Retention (e.g. for at least six months37) among those initiating MOUD
Remission or recovery among those retained on MOUD
While the intended use of a CoC framework varies by setting and reporting requirements, developing a common understanding of CoC domains and design decisions will facilitate comparative analyses to identify disparities in care for different populations17 and help to identify the stage at which patients are most at risk of treatment discontinuation or poor outcomes. For instance Figure 1 depicts national estimates largely from survey data modeled on 90-90-90 results across an 8-stage CoC that demonstrates the greatest drop offs occur in earlier stages of the CoC. CoC frameworks can also be used to differentiate performance of specific services and MOUD modalities, provider variation, outcomes across varying levels of care or concurrent services for patients with different sociodemographic characteristics, comorbidities, geography, or addiction severity. The CoC framework can also be used for developing benchmarks and for performing comparisons across populations at core stages.17,23 Many of these applications, however, are dependent on available databases for linkage and analysis that will impact measurement design decisions.19 The following sections explicate key measurement design considerations for applying the OUD CoC.
Figure 1.
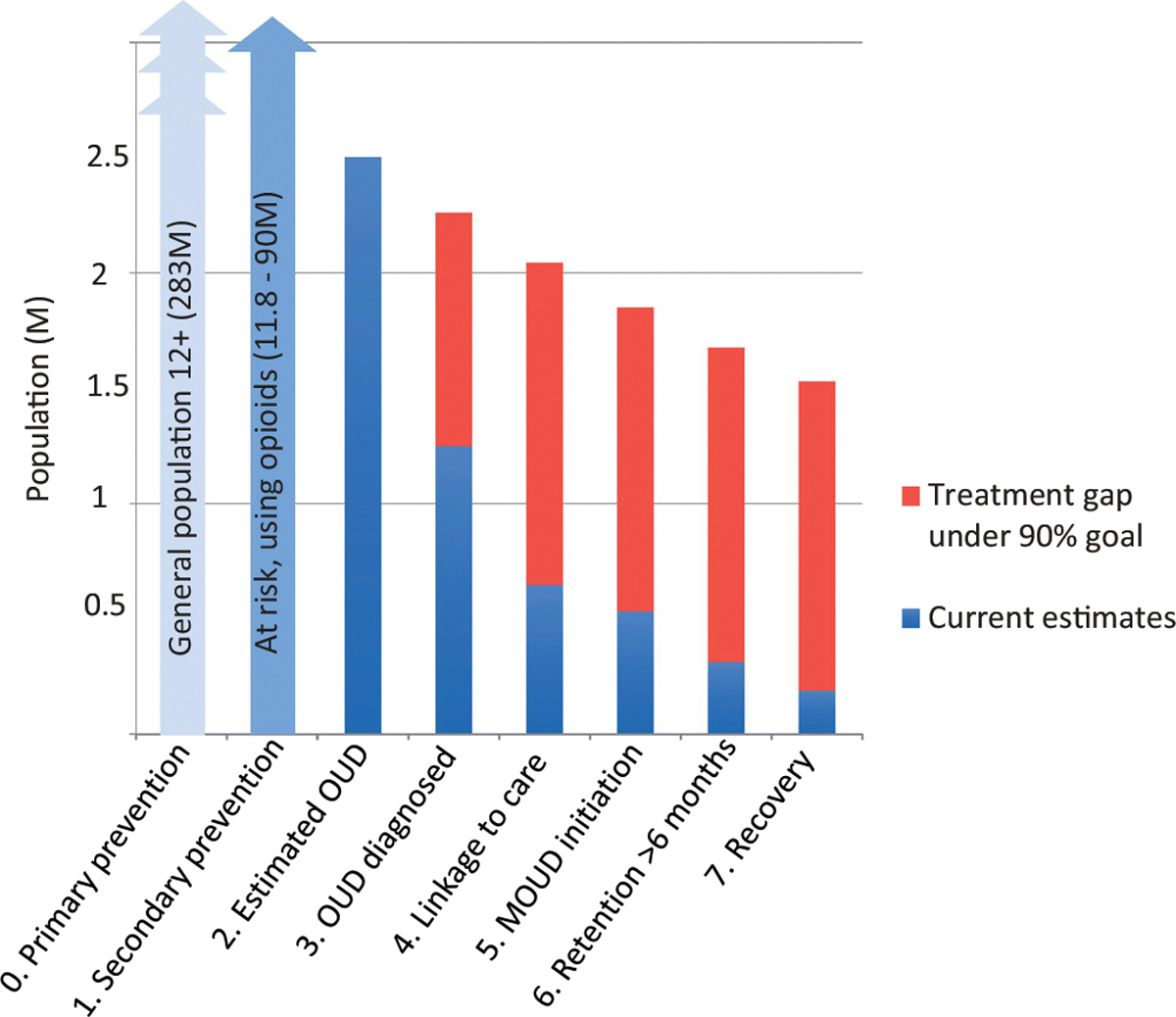
Assessing the Scope (Breadth and Depth) of an OUD Cascade of Care in the US. In this figure, adapted from Williams et al AJDAA 2019,17 the breadth of the CoC ranges from primary prevention to recovery. The CoC depicted has a fair amount of depth as it contains stages 0–7. If the authors added two additional retention stages for “Retained >3 months,” and “Retained >12 months,” the depth would increase by 2 so that the stages would be renumbered 0–9. CoC = Opioid Use Disorder Cascade of Care.
Design decisions for OUD Cascade of care framework construction
Individual agencies typically rely on various datasets that are subject to diverse legal and regulatory requirements for privacy protections. Analysis of even a single data source can be time consuming, and linkages between sources are often deemed futile given regulatory, privacy, data quality and liability considerations. We followed the Haber et al. approach38 to define a framework for the OUD CoC design and analysis based on practical limitations with which health officials may be grappling. Haber et al. describe two overarching design domains for CoC development in HIV, which are outlined and applied here. The first domain refers to the scope of staging (breadth and depth), and the second to measurement design, each of which affords different opportunities and challenges for CoC applications (Table 1). While ideally CoC design decisions should be based on what is most effective for answering specific policy-relevant questions—such as which factors drive disparities or which interventions best improve outcomes for vulnerable populations—in reality these decisions are typically constrained by the availability of data sources for linkage and analysis. Additionally, software and personnel resources and health agency budget and mission priorities also frequently constrain the scope of intended CoC uses.
Table 1.
Design decisions for OUD Cascade of care framework construction.
| Design element | Recommended when feasible | Complicates interpretation | Implications for intended use |
|---|---|---|---|
|
| |||
| Scope | Choose a breadth and depth that is feasible based on data availability and window of observation and yet tailored to satisfy specific reporting requirements or answer critical questions | Using all data in an exploratory fashion with no hypotheses or questions in mind. | Greater breadth/width may require more clinical services data across additional care settings. Depth may additionally allow for the inclusion of more patient characteristics to study demographics and comorbidity among vulnerable populations and associations with progression through stages. |
| Window of Observation | Longitudinal | Cross-Sectional | Longitudinal CoCs*, whether prospective or retrospective, avoid the synthetic cohort assumption by following the same individuals over time as they go through the CoC, whereas cross-sectional designs require more assumptions and are thus more prone to biases |
| Population | Single | Multiple | Data limitations or shortages of staff effort for more rigorous analyses may lead to multiple population studies but limitations in their interpretation should be made explicit for readers. |
| Denominator-Numerator Linkage | Linked | Unlinked (typically a multiple populations design) | Unlinked designs may be helpful for monitoring broad trends in service delivery at the population level but cannot reflect the longitudinal outcomes of the same sample and may obscure barriers for successful progression across stages |
| Denominator-Denominator Linkage | Linked (by definition a single population design) | Unlinked | Especially if unlinked, researchers and policymakers must be very clear in publishing and describing methodology and results to avoid misinterpretation of findings |
CoC = Cascade of Care; OUD = opioid use disorder.
Scope
Scope refers to two elements of CoCs: breadth and depth. Breadth denotes the span of the cascade stages, from the event defining entry into the CoC to the last defined stage (i.e., the range in stages from the first to final event). The broadest OUD CoC possible would range from the time an individual becomes at risk for developing OUD (i.e., earlier, up-stream primary prevention efforts on the left-hand side) through recovery on the right-hand, down-stream side (Figure 1). Similar to the HIV CoC, a common limitation to achieving broader OUD CoCs is the restriction to clinical data, which by definition means that individuals have already been diagnosed and linked to care, resulting in missing information on earlier stages on the left hand side of the CoC. This data constraint limits opportunities to inform interventions for the primary prevention of OUD. To overcome this limitation, clinical studies, which often rely on electronic health records (EHR) or administrative claims data, may need to draw from other data sources such as population-based surveys and smaller scale community evaluations to estimate the prevalence of individuals in pre-clinical stages. High quality data and linkage feasibility often limit the breadth of CoCs. Missing information on early stages preceding clinical interactions may result in biased analyses that exclude undetected populations particularly when the greatest attrition along the CoC often occurs between the OUD identification and linkage to care stages.17 To summarize, in Figure 1, stages 0–2 would typically be derived from population-level epidemiological or survey data whereas stages 3–7 would be derived from patient-level administrative claims or EHR data.19
The second scope element refers to the depth of the CoC. Depth is defined as the number of stages between the first and the final stage, conferring granularity or “depth.” A deeper CoC, for instance, may disaggregate retention into sub-stages denoting achievement of different durations of retention (e.g., 1 month, 3 months, 12 months etc.) and may differentiate between periods of MOUD-based care versus medication-free psychosocial care. As an example of a deeper CoC, Krebs et al.39 reported sub-stages for duration of care anywhere from 1 month to over 24 months. Greater depth could also allow for accounting for relapse in the recovery stage and adding a category for return to care following relapse or treatment disruptions. While most literature has investigated single episodes of care, OUD is a persistent condition and patients typically cycle through multiple care episodes over the lifespan. State agencies, for instance, might want to monitor care with a shallower CoC for population-wide analyses, while treatment systems with greater proximity to richer data systems might use deeper CoCs to examine details of care to identify patient characteristics that are associated with difficulty progressing successfully through sequential stages, especially for those patients who reenter care and for whom additional historic data are available.
Measurement design
Building on the Haber et al. framework,38 the measurement design domain for the OUD CoC comprises the same four primary dimensions as the HIV CoC, including: (1) window of observation (longitudinal vs. cross-sectional); (2) single or multiple populations; (3) denominator-numerator linkage; or 4. denominator-denominator linkage (See Figure 2 and Table 1).
Figure 2.
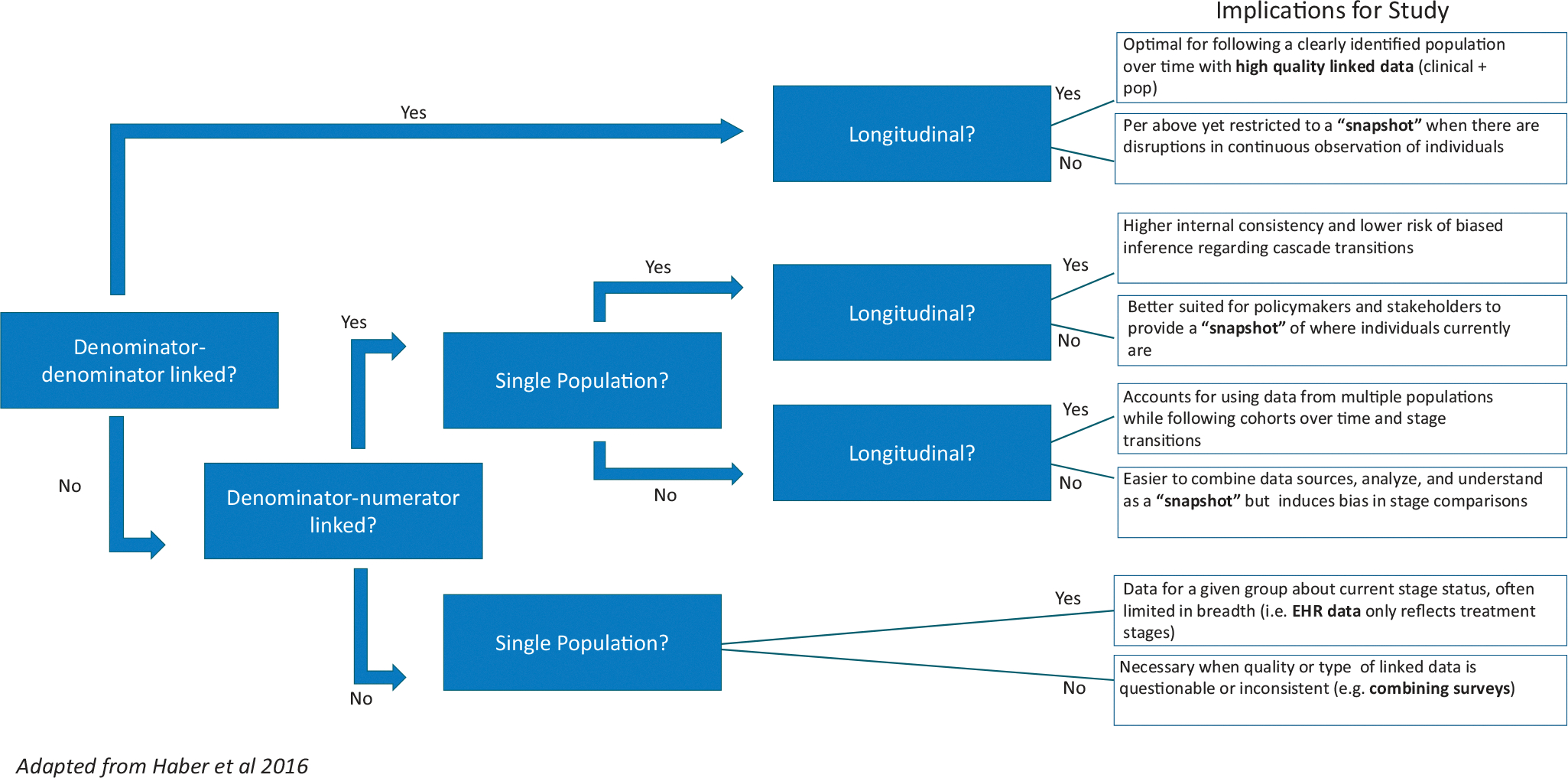
OUD Cascade of Care Measurement Designs*. *Measurement design domains for the OUD CoC comprises four dimensions related to the use of linked or unlinked data including the (1). Window of observation (longitudinal vs. cross-sectional) as well as the use of (2). Single or multiple populations and whether studies have (3). Denominator-numerator linkage or (4). Denominator-denominator linkage
CoCs based on cross-sectional analyses provide a snapshot at a given point in time of the status of the people living with OUD. Advantages of cross-sectional designs include that they are usually easier to construct as they can rely on population-level data that may be readily available through health administrative data or surveys. However, using a cross-sectional design to build a CoC requires a synthetic cohort assumption, wherein transition probabilities at a given stage are the same for individuals currently at that stage as well as for individuals who will reach this stage in the future, which is typically inaccurate. For instance, individuals with histories of more severe substance use disorders may enter care later, there may be changes over time in factors facilitating or serving as barriers to accessing care overall for specific populations, such as changes in insurance state of coverage of services, availability of treatment providers, or changes in demand for treatment. Additionally, over time new evidence-based treatments may be developed and adopted that affect retention in care. This concern may be less relevant for descriptive evaluations at a moment in time than for attempting to design inferential studies.
Longitudinal CoCs avoid the synthetic cohort assumption by following the same individuals over time as they go through the CoC. While longitudinal CoCs are preferable for some purposes, they are also more resource intensive, especially if data from pre-clinical stages (c.f. stored in separate data warehouses than the EHR or insurance claims) are required. It is important to note that longitudinal CoCs can serve key descriptive functions. Additionally, there is an element of time (i.e., time to stage transition) that cannot be captured in cross-sectional analyses. Even so, longitudinal CoCs must incorporate additional data elements to track not only stage progression, but latency between stage transitions to identify bottlenecks hampering successful progression.40
Single population cascades are those in which all data for CoC construction are drawn from the same defined population, such as Medicaid patients with an index OUD diagnosis in a single state in a given year. In contrast, data for multiple population CoCs come from different sources, possibly reflecting different calendar-times, surveys samples, or clinical settings, complicating interpretation due to the potential for variations in socio-demographic or clinical characteristics. Data limitations or shortages of staff effort for more rigorous analyses may lead to multiple population evaluations but limitations in their interpretation and potential biases should be made explicit for the data consumers.
Denominator-numerator linked cascades refer to cascades in which individuals in the numerator at a given stage are drawn from the same population that comprises the denominator, typically relying on linked, individual-level data. On the other hand, denominator-numerator unlinked cascades use aggregated data at the population level, and thus individuals in the numerator may not necessarily match those in the denominator, thereby complicating interpretation. Typically these denominator-numerator unlinked evaluations draw from multiple populations who may come in and out of care in overlapping intervals. An example of a denominator-numerator linked study was recently presented by Kimberly Johnson et al.’ 2020 study investigating outcomes among Medicaid beneficiaries in Florida, which was able to follow individuals across settings due to the availability of complete and longitudinal Medicaid data. Another example of a denominator-numerator unlinked analysis is the work by the Medicaid Outcomes Distributed Research Network (MODRN), a consortium of more than a dozen states linking longitudinal Medicaid data for quality efforts related to the opioid crisis.41
A denominator-denominator linked CoC uses the same population for all the CoC stages usually with longitudinal data drawn from administrative claims or EHR, where information on each stage is updated over time. By definition, this type of CoC is also a single population and denominator-numerator linked CoC and has the important benefit of generally having high internal validity. Researchers and policymakers should clarify whether CoC evaluations rely on linked or unlinked data so that end users are aware of whether the percent reflected in a given stage is derived from a parent population for all stages or reflects only the percent of those from the prior stage who advanced.
A common impediment to denominator-denominator linked analyses spanning the full CoC breadth is that data are often of two types and difficult to link at the individual level: (1) clinical data such as EHR data from addiction treatment programs, general practice settings, and hospitals (i.e., right side of the CoC) and administrative health data reflecting service receipt (e.g., insurance claims) or (2) population level data including epidemiological surveillance and survey data (i.e., left side of the CoC).
In order to illustrate design decisions referenced in Figure 2, we have selected recently published studies by design that reflect CoC constructions varying by data source and measurement decisions (Table 2).
Table 2.
Classification of OUD Cascade of care publications.
| Article | Setting | Data source(s) | Outcomes | Measurement design |
|---|---|---|---|---|
|
| ||||
| Belenko et al., 201742 | Juveniles in the criminal justice system | No data reported, but describes linking juvenile justice and behavioral health system data | Hypothetical framework spanning 8 stages related to screening and treatment | • Denominator-denominator linked • Denominator-numerator linked • Single population |
| Socías et al., 201835 | Individuals with opioid use in Vancouver (enrolled for longitudinal follow up) 2006–2016 | Vancouver survey data: Vancouver Injection Drug Users Study and the AIDS Care Cohort to Evaluate exposure to Survival Services | CoC stage completion, 1–4 | • Denominator-denominator linked • Single population per year |
| Yedinak et al., 201933 | Statewide population in Rhode Island 2018/2019 | NSDUH, State datasets, National Recovery Survey | CoC stages 0–4 prevalence/completion | • Cross section snapshot |
| Prieto et al., 201934 | Denver population 2017 | NSDUH, Electronic health records, data from a behavioral health registry | CoC stage completion, 0–4 | • Denominator-numerator linked • Single population |
| Johnson et al., 202132 | Florida Medicaid beneficiaries 2017/2018 | Medicaid claims | Stage completion and associated mortality, CoC stage completion, 1–4 | • Denominator- Denominator linked • Denominator-Numerator linked • Single population • Longitudinal |
| Scott et al., 202043 | Chicago population, in communities at risk | Data reported to outreach program | Stage completion CoC 0–4 | • Denominator- numerator linked • Single population |
| Piske et al., 202036 | Individuals with OUD in British Columbia from January 1, 1996 to November 30, 2017 | Provincial level linkage of four health administrative databases. | The eight-stage CoC included diagnosed OUD, ever on opioid agonist treatment (OAT), recently on OAT, currently on OAT and retained on OAT: ≥1, ≥3, ≥12 and ≥24 months). | • Denominator-denominator linked |
| Upadhyaya et al., 202144 | Patients admitted with OUD and SIRI to Wash U hospital, medical records data 2016–2019 | Hospital medical records review | All-cause readmission rate; intervention: referral to peer-led care | • Denominator-denominator linked • Single population |
| Boyd et al., 202145 | Minnesota tribal community 2018 | Minnesota Drug and Alcohol Abuse Normative Evaluation System (DAANES) health administrative data | Stage completion for stages CoC 2,3,4 | • Denominator - denominator linked • Single population |
| Krebs et al., 202139 | Adolescents (ages 12–18), young adults (19–24), Older adults (≥25), all with OUD (a subset of Piske et al. 2020) | Provincial-level linkage of six health administrative databases | The eight-stage CoC included diagnosed with OUD, ever engaged in MOUD, recently in MOUD, currently in MOUD, and retained in MOUD for ≥1, ≥3, ≥12, and ≥24 months. | • Denominator-denominator linked |
| Tiako et al., 202146 | Patients in treatment for OUD among pregnant women, national, 2013–2017 | Treatment Episode Dataset (TEDS) data from SAMHSA | Stage prevalence/completion: MOUD and retention | • Cross section snapshot |
CoC = Cascade of Care; MOUD = Medication for OUD; NSDUH = National Survey on Drug Use and Health; OUD = opioid use disorder; PWOUD = Persons with OUD.
Challenges and future directions
Agencies such as state Medicaid offices or single state substance abuse service agencies overseeing addiction prevention, treatment, and recovery services, and offices of mental health typically have access to a variety of data sources spanning administrative claims, treatment episode data, and hospital and vital records data. Typically analysis from even a single data source is resource intensive, and linkages between sources are often burdened by concerns over legal and regulatory requirements for confidentiality. Leadership, such as senior state officials, is often necessary to dedicate sufficient resources and staff effort to facilitate data management and linkages.
While federal guidance and assurances to state and local officials regarding privacy and liability concerns for expanding data collection, surveillance and linkage initiatives might reassure state efforts these agencies may already have more leeway than they are currently allowing themselves. Academic and public-private partnerships (i.e., the MODRN network),41 state initiatives such as Massachusetts’ Chapter 55 project, and statewide data ecosystems in Rhode Island and Maryland, among others, have built out pathways for successful linkages despite contemporary privacy concerns.
Beyond feasibility, design decisions and transparent reporting of methods regarding population sampling, period of observation, and limitations of any analysis- whether in peer-reviewed or gray literature- can be critical in helping end users understand inherent biases in CoC evaluations. For instance, data are often most readily available for patients who are receiving clinical care, rather than those that are persistently or even intermittently disconnected from mainstream healthcare settings and unreachable via traditional population based surveys. These difficult to measure individuals may be at the highest risk for poor outcomes. Increasing rigor and transparency in OUD CoC-based evaluations will likely yield evidence that there is even greater unmet need than is typically reflected in the existing literature. Future research could contribute by conducting a systematic review of OUD CoC studies to help determine which methodological approaches best address specific aspects of unmet need.
As data systems and linkages evolve and improve over time, it will afford greater capacity to CoC analyses. For instance, even rigorous longitudinal single population evaluations with denominator-denominator linkage at this point typically reflect stage progression at the cohort-level, but do not incorporate adjusted analyses to allow for inferential findings or use time-based analyses to assess service bottlenecks hampering successful progression through stages over time. Additionally, CoCs often do not reflect care provision in multiple settings and the integration of addiction specialty services. The integration of MOUD into primary care settings is critical to improving patient outcomes. Incorporating medical and psychiatric comorbidity into patient risk profiles may also require innovative data linkages across settings.
Finally, patient perspectives are often lacking in academic research and policy settings. There has been growing interest in patient reported outcomes across the healthcare landscape under the mantel of person-centered care. However, these data are particularly difficult to access for patients with OUD as few validated tools exist to meaningfully track and reflect recovery longitudinally and there is much debate about how to define “recovery” in the field. While access to MOUD and long-term treatment retention on medication have empirically demonstrated the greatest protection against overdose events and mortality, more research is needed to determine which aspects of care are most acceptable and meaningful to patients. Some possible examples might include patient preferences for injectable versus oral medications, possibilities for directly observed therapy, and remote treatment options, that may facilitate more successful progression through the CoC. Additionally, work is needed to identify which outcomes are most important to patients, such as markers of recovery capital tailored to individual patients. Ultimately success along any CoC, however deep or broad, linked or unlinked, is in the service of this final stage, pointed in the direction of health and well-being.
Funding
Financial support for this work was provided by grants from the National Institute on Drug Abuse (NIDA) [K23 DA044342, Williams PI], and the Substance Abuse Mental Health Services Opioid Response Network [TI-18-004 Subaward, Williams PI]. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Disclosure statement
Dr. Williams receives equity, consulting fees, and travel expenses from Ophelia Health, Inc., a telehealth provider for opioid use disorder. Dr. Socías has received support from Indivior for an investigator-initiated study, not related to the present work. Dr. Fishman receives consulting fees from Alkermes and Drug Delivery LLC, and is an investigator on a grant funded by Alkermes.
References
- [1].Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):1207–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahmad LB, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. Hyattsville: National Center for Health Statistics; 2021. [Google Scholar]
- [3].2019 National Survey of Drug Use and Health (NSDUH) Releases | CBHSQ Data. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases. Accessed October 2021.
- [4].Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. Bmj. 2017;357:j1550–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Santo T, Jr., Clark B, Hickman M, et al. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(9): 979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].National Academies of Sciences, Engineering, and Medicine (NASEM). Medications for Opioid Use Disorders save Lives. Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]
- [7].Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies-tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. [DOI] [PubMed] [Google Scholar]
- [8].SAMHSA Substance Abuse and Mental Health Services Administration, National Survey of Substance Abuse Treatment Services (N-SSATS): 2015. Data on Substance Abuse Treatment Facilities. BHSIS Series S-88, HHS Publication No. (SMA) 17–5031. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2017. [Google Scholar]
- [9].Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3): 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Samples H, Williams AR, Olfson M, Crystal S. Risk factors for premature discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of medicaid enrollees. J Subst Abus Treat. 2018;95:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis. 2016;35(1): 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. [DOI] [PubMed] [Google Scholar]
- [13].Tkacz J, Severt J, Cacciola J, Ruetsch C. Compliance with buprenorphine medication-assisted treatment and relapse to opioid use. Am J Addict. 2012;21(1):55–62. [DOI] [PubMed] [Google Scholar]
- [14].Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat. 2015;52:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Socías ME, Volkow N, Wood E. Adopting the ‘cascade of care’ framework: an opportunity to close the implementation gap in addiction care? Addiction. 2016;111(12):2079–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Knight DK, Belenko S, Wiley T, et al. Juvenile Justice-Translational Research on Interventions for Adolescents in the Legal System (JJ-TRIALS): a cluster randomized trial targeting system-wide improvement in substance use services. Implement Sci. 2016;11(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Williams AR, Nunes EV, Bisaga A, Levin FR, Olfson M. Development of a Cascade of care for responding to the opioid epidemic. Am J Drug Alcohol Abuse. 2019;45(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Williams AR, Nunes EV, Bisaga A, et al. Developing an opioid use disorder treatment Cascade: a review of quality measures. J Subst Abus Treat. 2018;91:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johnson K, Williams AR, Chalk M. Making the opioid use disorder cascade of care a reality, despite data limitations. Health Affairs Blog. 2018. [Google Scholar]
- [20].Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. The Lancet. 2019; 393(10182):1760–1772. [DOI] [PubMed] [Google Scholar]
- [21].Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped care for opioid use disorder train the trainer (SCOUTT) initiative: expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bipartisan Policy Center. Tracking Federal Funding to Combat the Opioid Crisis, 2019: p. 61. [Google Scholar]
- [23].Williams AR, Olfson M, Nunes EV. To battle the opioid epidemic, deploy the ‘Cascade of Care’ model. Health Affairs, Health Policy Lab. 2017. [Google Scholar]
- [24].McCorry F, Garnick DW, Bartlett J, Cotter F, Chalk M. Developing performance measures for alcohol and other drug services in managed care plans. Washington Circle Group. Jt Comm J Qual Improv. 2000;26(11):633–643. [DOI] [PubMed] [Google Scholar]
- [25].HEDIS® Technical Specifications for Health Plans Vol. 2. Washington, CA: National Committee for Quality Assurance; 2018. http://store.ncqa.org/index.php/catalog/product/view/id/2871/s/hedis-2018-volume-2-epub/. Accessed August 16, 2019. [Google Scholar]
- [26].NQF core measure set for Mcaid: https://www.medicaid.gov/medicaid/quality-of-care/downloads/2021-bh-core-set.pdf
- [27].National Quality Forum. Opioids and opioid use disorder: an environmental scan of quality measures; Final Report September 12, 2019; https://www.qualityforum.org/Publications/2019/09/Opioid_and_Opioid_Use_Disorder_Final_Environmental_Scan.aspx; Accessed October, 2021.
- [28].Jamieson D, Kellerman SE. The 90-90-90 strategy to end the HIV Pandemic by 2030: can the supply chain handle it? J Int AIDS Soc. 2016;19(1):20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Farnham P, Chen YH, Gopalappa C. Vital signs: HIV transmission along the continuum of care—United States, 2016. https://stacks.cdc.gov/view/cdc/76585. Accessed July 5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dematteis M, Auriacombe M, D’Agnone O, et al. Recommendations for buprenorphine and methadone therapy in opioid use disorder: a European consensus. Expert Opin Pharmacother. 2017;18(18):1987–1999. [DOI] [PubMed] [Google Scholar]
- [31].Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Johnson K, Hills H, Ma J, Brown CH, McGovern M. Treatment for opioid use disorder in the Florida medicaid population: using a cascade of care model to evaluate quality. Am J Drug Alcohol Abuse. 2021;47(2):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yedinak JL, Goedel WC, Paull K, et al. Defining a recovery-oriented cascade of care for opioid use disorder: a community-driven, statewide cross-sectional assessment. PLoS Med. 2019; 16(11):e1002963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Prieto JT, McEwen D, Davidson AJ, et al. Monitoring opioid addiction and treatment: do you know if your population is engaged? Drug Alcohol Depend. 2019;202:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Socías ME, Wood E, Kerr T, et al. Trends in engagement in the cascade of care for opioid use disorder, Vancouver, Canada, 2006–2016. Drug and Alc Depend. 2018;189:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Piske M, Zhou H, Min JE, et al. The cascade of care for opioid use disorder: a retrospective study in British Columbia, Canada. Addiction. 2020;115(8):1482–1493. [DOI] [PubMed] [Google Scholar]
- [37].National Quality Forum. #3175 Continuity of Pharmacotherapy for Opioid Use Disorder. NQF; 2018. [Google Scholar]
- [38].Haber N, Pillay D, Porter K, Barnighausen T. Constructing the cascade of HIV care: methods for measurement. Curr Opin HIV Aids. 2016;11(1):102–108. [DOI] [PubMed] [Google Scholar]
- [39].Krebs E, Min JE, Zhou H, Davison C, McGowan G, Nosyk B. The cascade of care for opioid use disorder among youth in British Columbia, 2018. J Subst Abuse Treat. 2021;130:108404. [DOI] [PubMed] [Google Scholar]
- [40].Gonsalves GS, Paltiel AD, Cleary PD, et al. A flow-based model of the HIV care continuum in the United States. J Acquir Immune Defic Syndr. 2017;75(5):548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Donohue JM, Jarlenski MP, Kim JY, et al. Use of medications for treatment of opioid use disorder among US Medicaid Enrollees in 11 States, 2014–2018. JAMA. 2021;326(2):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Belenko S, Knight D, Wasserman GA, et al. Sales J, The Juvenile Justice Behavioral Health Services Cascade: a new framework for measuring unmet substance use treatment services needs among adolescent offenders. J Subst Abuse Treat. 2017;74:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Scott CK, Dennis ML, Grella CE, et al. A community outreach intervention to link individuals with opioid use disorders to medication-assisted treatment. J Subst Abuse Treat. 2020;108: 75–81. [DOI] [PubMed] [Google Scholar]
- [44].Upadhyaya A, Marks LR, Schwarz ES, Liang SY, Durkin MJ, Liss DB. Care cascade for patients with opioid use disorder and serious injection related infections. Toxicol Commun. 2021;5(1): 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Boyd T, Stipek J, Kraft A, et al. Quantifying opioid use disorder Cascade of Care outcomes in an American Indian tribal nation in Minnesota. Drug Alcohol Depend. 2021;222:108661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tiako MJN, Friedman A, Culhane J, South E, Meisel ZF. Predictors of initiation of medication for opioid use disorder and retention in treatment among U.S. Pregnant Women, 2013–2017. Obstet Gynecol. 2021;137(4):687–694. [DOI] [PubMed] [Google Scholar]


