Abstract
Background
The optimal timing of surgery following chemoradiotherapy (CRT) is controversial. This trial aimed to assess disease recurrence and survival rates between patients with locally advanced rectal adenocarcinoma (LARC) who underwent total mesorectal excision (TME) after a waiting interval of 8 weeks or less (classic interval; CI) versus more than 8 weeks (long interval; LI) following preoperative CRT.
Methods
This was a phase III, single-centre, randomized clinical trial. Patients with LARC situated within 12 cm of the anal verge (T3–T4 or N+ disease) were randomized to undergo TME within or after 8 weeks after CRT.
Results
Between January 2006 and January 2017, 350 patients were randomized, 175 to each group. As of February 2022, the median follow-up time was 80 (6–174) months. Among the 322 included patients (CI, 159; LI, 163) the cumulative incidence of locoregional recurrence at 5 years was 10.1 per cent in the CI group and 6.9 per cent in the LI group (P = 0.143). The cumulative incidence of distant metastasis at 5 years was 30.8 per cent in the CI group and 18.6 per cent in the LI group (sub-HR = 1.78; 95 per cent c.i. 1.14 to 2.78, P = 0.010). The disease-free survival (DFS) in each group was 59.7 and 69.9 per cent respectively (P = 0.157), and overall survival (OS) rates at 5 years were 73.6 versus 77.9 per cent (P = 0.476).
Conclusion
Incidence of distant metastasis decreased with an interval between CRT and surgery exceeding 8 weeks, but this did not impact on DFS or OS.
Registration number
The optimal timing of surgery following preoperative chemoradiotherapy (CRT) for rectal cancer is controversial. This trial compared oncological results obtained after an interval of 8 weeks or less versus more than 8 weeks. This study was a phase III, single-centre, randomized, parallel-group (1:1 ratio) clinical trial. In conclusion, the incidence of distant metastasis significantly decreased with an interval between CRT and surgery exceeding 8 weeks.
Introduction
In conjunction with the use of total mesorectal excision (TME), chemoradiotherapy has reduced local recurrence and improved long-term survival in patients with rectal cancer1. Randomized trials have demonstrated superior local control, lower toxicity, and better compliance with neoadjuvant radiotherapy (RT) or chemoradiotherapy (CRT) compared with adjuvant treatment2–4. The two main RT approaches for rectal cancer are short-course RT and long-course chemoradiotherapy (preoperative CRT); the latter is used more commonly for locally advanced rectal cancer (LARC). Randomized studies comparing these approaches have reported no significant differences in local control and survival5–7; however, preoperative CRT yields a higher tumour regression rate and the possibility of a pathological complete response (pCR) rate. The delayed waiting intervals were 4–8 weeks after preoperative CRT in all the randomized studies5–8.
Oncological outcomes are better in patients with pCR. Several studies with different treatment protocols have been conducted, comparing9–15 waiting intervals after preoperative CRT to increase pCR. In the Lyon R 90-01 study, the rates of clinical tumour response and pathological stage regression were higher with waiting intervals of 6–8 weeks compared with 2 weeks16. Although no significant difference in locoregional recurrence (LR) or overall survival (OS) on long-term follow-up was found between the groups17,18, the waiting interval of 6–8 weeks gained wide acceptance. Recently, studies have demonstrated higher rates of pCR, stage regression, and especially higher disease-free survival (DFS) with longer (more than 8 weeks) waiting intervals19,20. In the randomized study GRECCAR-6 (comparing 7 and 11 weeks), no significant difference in pCR rates was found, whereas a significant increase in morbidity and decrease in TME quality were observed in the 11-week interval group21. There were no significant differences in 3-year OS, DFS, and recurrence rates between the groups22. The randomized study by Terzi et al.23 reported extending the interval between CRT and surgery from 8 to 12 weeks resulted in a two-fold increase in pCR rate without any difference in mortality and morbidity. The oncological results of the trial are not yet known. There remains ongoing discussion regarding the optimal timing of surgery following preoperative CRT.
The aim of this study was to present the long-term oncological outcomes (disease recurrence, DFS, and OS rates) in patients with LARC who underwent TME after a waiting interval of 8 weeks or less (classic interval (CI), 28–56 days) and those who underwent curative TME after a waiting interval of more than 8 weeks (long interval (LI), 57–84 days) following preoperative CRT24.
Methods
Study design and patients
This RCT involved patients treated at the Ege University Faculty of Medicine Hospital (Izmir, Turkey) between January 2006 and January 2017.
This study was a phase III, single-centre, randomized, parallel-group (1:1 ratio), clinical trial, and details of the study have been described previously24. The study consisted of patients for whom preoperative CRT was indicated after diagnosis with LARC (T3–4 or/and N (+) disease)25 using a multidisciplinary approach. Patients with a histologically confirmed diagnosis of stage II–III rectal adenocarcinoma situated within 12 cm of the anal verge were included in this study. The patients were scheduled to undergo TME surgery with curative intent (Fig. 1).
Fig. 1.
CONSORT flow diagram
Classic interval group (28–56 days), long interval group (57–84 days). CI, classic interval; LI, long interval; TME, total mesorectal excision.
Exclusion criteria
Patients with stage I, stage IV, or recurrent disease; patients younger than 18 years; patients with a malignancy other than adenocarcinoma; patients who underwent palliative (R2) resection or emergency surgery; patients with poor general condition (ASA grade less than 3); patients for whom CRT was contraindicated; and patients with previous/concurrent cancer were excluded.
Randomization
Eligible patients were randomly divided into two groups to receive curative TME after a waiting interval of less than 8 weeks (CI group; range 4–8 weeks; 28–56 days) or longer than 8 weeks (LI group; range 8–12 weeks; 57–84 days) after preoperative CRT. The treatment interval was calculated from the end of neoadjuvant therapy. Randomization was performed using an adaptive biased coin technique by two surgeons on the first day of RT26.
This study was approved by the local ethics committee (Ege University Ethics Committee approval number 17-4.1/13). Written informed consent was obtained from each patient by the surgeon. The study was conducted in accordance with the principles of the Declaration of Helsinki and was registered at Clinicaltrials.gov (NCT03287843).
Procedures
The details of the CRT protocol have been previously reported24,27. Briefly, the total dose was 50.4 Gy with a 1.8 Gy/fraction to the gross tumour and 45 Gy to the pelvic lymph nodes. As concomitant chemotherapeutic agents, 5-fluorouracil (380 mg/m2) and leucovorin (20 mg/m2) were administered every 28 days (days 1–4) for two cycles. The patients received adjuvant chemotherapy 4 weeks after surgery. Four cycles of 5-fluorouracil (425 mg/m2) and leucovorin (20 mg/m2) were administered every 28 days (days 1–5).
All patients underwent surgery by two experienced colorectal surgeons using a standardized open TME technique. The double-staple technique was used for all anastomoses, except for very low-lying rectal tumours. A routine protective stoma was used in patients with an anastomosis level of 5 cm or less from the anal verge. Bowel preparation and prophylactic antibiotics were routinely administered.
Patients were followed every 3 months for 2 years, followed by once every 6 months until 5 years after surgery, then annually thereafter. The routine evaluation consisted of physical examination, blood tests, abdominal ultrasonography and/or CT, colonoscopy (annually), and chest X-ray.
Outcomes
The present study assessed the long-term secondary outcome oncological results (LR, distant metastasis, DFS, and OS rates) of the CI and LI groups from the previously published study24.
LR refers to pelvic recurrence only (isolated local recurrence) or to both pelvic recurrence and distant recurrence (occurring simultaneously or in various intervals, whichever occurred first). The recurrence of disease outside the pelvis was considered a distant metastasis. Disease recurrence (relapse) refers LR or/and distant metastasis.
DFS was defined as the time from randomization to confirmed local recurrence, distant metastasis, or death from any cause, whichever occurred first. OS was defined as the time from randomization to death from any cause.
Pathological examination
All pathological examinations were performed by two experienced gastrointestinal pathologists blinded to the group assignment. The resected specimens were macroscopically examined and classified according to the College of American Pathologists protocols. The initial examination was performed on a fresh sample for completeness of mesorectal dissection, which was graded according to the criteria of Quirke et al.28. The histological typing and grading of differentiation were performed according to the WHO 2010 criteria. Tumour staging was performed using the TNM staging system, which classifies the depth of tumour invasion (T), the presence of regional lymph node metastasis (N), and the presence of distant metastasis (M).
Treatment response was evaluated by a five-tiered system following Mandard et al.29. The resection was defined as R1 if the circumferential resection margin was 1 mm or less or the distal margin was 1 cm or less from the tumour.
Statistical analysis
The original trial24 was powered to detect a difference in the primary endpoint (pCR rates). Based on an expected pCR rate of 13 per cent in the CI group and 26 per cent in the LI group, the trial was designed to have 80 per cent power to detect a difference in the primary endpoint using a two-sided Fisher’s exact test at the 5 per cent significance level. According to this hypothesis, approximately 316 patients were required (without dropout) for this analysis. Patients were randomly assigned to the study arms in a 1:1 ratio using an adaptive biased coin technique26 by two surgeons (E.A. and C.C.) on the first day of RT.
In this part of the trial, groups were compared in terms of LR, distant recurrence, OS, and DFS rates (secondary endpoints). Factors that were predicted to effect secondary endpoints were sex, age, tumour localization, type of surgery, necessity of multivisceral resection, cT category, cN category, c stage, tumour differentiation, pT category, pN category, p stage, perineural invasion, lymphovascular invasion, satellite tumour, R resection, Mandard grade, TME quality, and concomitant adjuvant CT.
The CI and LI groups were compared for OS/DFS using the Kaplan–Meier method, and the differences were evaluated using the log rank test. The Cox proportional hazards model was used to calculate HRs and 95 per cent confidence intervals (95 per cent c.i.). After analysing the effect of each prognostic factor on OS and DFS, multiple Cox regression analysis (according to the enter method, no selection method was used) was applied again in the groups.
In the competing risk analysis, groups were compared in terms of LR and distant recurrence. The effect of each prognostic factor was investigated using univariable competing risk regression (Fine and Gray method30, using the sub-HR (SHR)) separately in the groups. After these analyses, the multiple effects of prognostic factors were investigated using the same approach. The competing risk groups for LR and distant recurrence included all deaths.
A two-sided P value less than or equal to 0.05 was considered statistically significant. No interim analyses of the efficacy of secondary endpoints were performed.
Results
In total, 380 patients were evaluated for eligibility. Thirty patients did not meet the inclusion criteria for this study. The remaining 350 patients were randomly assigned to either the CI (175 patients) or the LI (175 patients) group. Figure 1 shows the flowchart of the randomization process. All patients underwent a complete course of RT. There was no statistically significant difference between the groups regarding concurrent CT with RT (P = 0.960) or adjuvant CT use (P = 0.696).
After preoperative and perioperative evaluations of the 350 patients who underwent CRT after randomization, 16 patients in the CI group were excluded from the study (withdrew consent, 8; refused surgery, 7; and postoperative mortality, 1) and 12 patients were excluded in the LI group (withdrew consent, 4; refused surgery, 4; and postoperative mortality, 4) (Fig. 1). Overall, 322 patients (159 in the CI group and 163 in the LI group) were included in the analysis. The baseline characteristics are summarized in Table 1. The details of the histopathological results, postoperative morbidity, and mortality have been reported elsewhere24. In brief, the findings suggested that the rates of pCR (10.0 versus 18.6 per cent; P = 0.027) and disease stage regression were significantly increased in patients who waited for more than 8 weeks to undergo TME. A waiting interval of 8 weeks or more had no detrimental effect on surgery quality, complications, or death (Table 2).
Table 1.
Baseline characteristics and type of surgery of 322 eligible patients
| Classic interval group (n=159) | Long interval group (n=163) | P | |
|---|---|---|---|
| Age (years), mean/median/range | 60.42/61.00/22–85 | 61.74/64.00/32–85 | 0.318 |
| Sex ratio M:F | 94:65 | 95:68 | 0.729 |
| cT category | |||
| T2 | 5 (3.1) | 7 (4.3) | 0.438 |
| T3 | 103 (65.0) | 101 (62.1) | |
| T4 | 51 (31.9) | 55 (33.6) | |
| cN category | |||
| N0 | 66 (41.6) | 73 (45.5) | 0.284 |
| N1 | 61 (38.3) | 55 (33.7) | |
| N2 | 32 (20.1) | 35 (20.8) | |
| Clinical stage | |||
| Stage II | 66 (41.5) | 73 (44.8) | 0.652 |
| Stage III | 93 (58.5) | 90 (55.2) | |
| Tumour localization* | |||
| Low (0–5 cm) | 101 (63.5) | 93 (57.1) | 0.298 |
| Middle (5–12 cm) | 58 (36.5) | 70 (42.9) | |
| Operation type | |||
| LAR | 94 (59.0) | 101 (62.0) | 0.729 |
| APR | 62 (39.1) | 57 (34.9) | |
| Others | 3 (1.9) | 5 (3.1) | |
| Multivisceral resection | |||
| None | 148 (93.1) | 156 (95.7) | 0.593 |
| Applied | 11 (6.9) | 7 (4.3) | |
Values are n (%) unless otherwise indicated. LAR, low anterior resection (also includes intersphincteric resection and hand-sewn coloanal anastomosis); APR, abdominoperineal resection; Others, includes total proctocolectomy (with or without pouch) and Hartmann's procedure. *Tumour distance from anal verge measured by rigid rectoscope.
Table 2.
Postoperative histopathological results and morbidities of patients
| Classic interval | Long interval | P | |
|---|---|---|---|
| Stage* | 0.004 | ||
| 0 (pCR) | 16 (10) | 31 (18.6) | |
| I | 26 (16.3) | 38 (22.8) | |
| IIA/IIB/IIC | 51 (31.9)/2 (1.3)/4 (2.5) | 46 (27.5)/5 (3.0)/3 (1.8) | |
| IIIA/IIIB/IIIC | 7 (4.4)/47 (29.4)/7 (4.4) | 6 (3.6)/31 (18.6)/7 (4.2) | |
| Differentiation | 0.011 | ||
| Well | 17 (10.6) | 4 (2.4) | |
| Moderate | 124 (77.5) | 141 (84.4) | |
| Poor/mucinous | 7 (4.4)/12 (7.5) | 13 (7.8)/9 (5.4) | |
| T category | 0.001 | ||
| T0 | 17 (10.6) | 31 (18.6) | |
| T1 | 4 (2.5) | 13 (7.8) | |
| T2 | 30 (18.8) | 31 (18.6) | |
| T3 | 101 (63.1) | 74 (44.3) | |
| T4a/T4b | 3 (1.9)/5 (3.1) | 14 (8.4)/4 (2.4) | |
| N category* | 0.048 | ||
| N0 | 100 (62.5) | 122 (73.1) | |
| N1A/1B/1C | 22 (13.8)/13 (8.1)/3 (1.9) | 13 (7.8)/15 (9.0)/4 (2.4) | |
| N2A/2B | 16 (10.0)/6 (3.8) | 9 (5.4)/4 (2.4) | |
| PNI (+) | 16 (10) | 15 (9.0) | 0.753 |
| LVI (+) | 6 (3.8) | 8 (4.8) | 0.642 |
| Satellite tumour (+) | 10 (6.3) | 7 (4.2) | 0.402 |
| R1 resection | 12 (7.5) | 15 (9.0) | 0.626 |
| pCR(+) Mandard 1 | 16 (10) | 31 (18.6) | 0.027 |
| Mandard score | 0.161 | ||
| 2 | 37 (23.1) | 36 (21.6) | |
| 3 | 73 (45.6) | 67 (40.1) | |
| 4 | 31 (19.4) | 29 (17.4) | |
| 5 | 3 (1.9) | 4 (2.4) | |
| TME quality | 0.713 | ||
| Good | 144 (90.0) | 149 (89.2) | |
| Moderate | 11 (6.9) | 10 (6.0) | |
| Bad | 5 (3.1) | 8 (4.8) | |
| Overall morbidity | 36 (22.5) | 33 (19.8) | |
| (Clavien–Dindo classification) | 0.307 | ||
| 1 | 4 (2.5) | 7 (4.2) | |
| 2 | 15 (9.4) | 14 (8.4) | |
| 3a/3b | 4 (2.5)/8 (5.0) | 3 (1.8)/2 (1.2) | |
| 4a/4b | 4 (2.5)/0 (0.0) | 2 (1.2)/1 (0.6) | |
| 5 | 1 (0.6) | 4 (2.4) |
Values are n (%). pCR, pathological complete response; PNI, perineural invasion; LVI, lymphovascular invasion; TME, total mesorectal excision. *P is calculated by Mann–Whitney U test for stage and N category; otherwise, values are calculated by chi-squared test.
Events during follow-up
As of February 2022, the median follow-up time was 80 months (mean 82.59). The ranges of the follow-up times were 7–164 months for the CI group and 6–148 months for the LI group. The mean(s.d.) waiting time was 46.1(7.3) days in the CI group and 71.1(9.3) days in the LI group. Overall, 193 surviving patients were followed up for at least 5 years (range, 61–164 months). With the exception of five patients with postoperative mortality, 129 deaths occurred during follow-up. Seventy-three deaths were related to rectal cancer (disease progression, n = 69; treatment-related, n = 4), and 56 to other causes.
LR occurred in 30 patients: 7 had local recurrence alone, and 23 had local recurrence with distant recurrences. Sixty patients had only (isolated) distant recurrences.
Locoregional recurrence
During follow-up, 19 patients from the CI group (3 patients with isolated pelvic recurrence only) and 11 patients from the LI group (4 patients with isolated pelvic recurrence only) had LR. The cumulative incidence of LR at 5 years was 10.1 per cent in the CI group and 6.9 per cent in the LI group (P = 0.143; Fig. 2). The SHR for LR in the CI group, compared with the LI group, was 1.73 (95 per cent c.i. 0.83 to 3.63).
Fig. 2.
Cumulative incidences of locoregional recurrence and distant metastasis among 322 patients randomly assigned to classic interval (CI) and long interval (LI) groups using competing risk regression analysis for events
a Locoregional recurrence. b Distant metastasis.
In the CI group, univariable analyses revealed that distal tumour location (P = 0.017), development of postoperative complications (P = 0.013), and R1 resection (P = 0.001) were significant prognostic factors for LR. In the LI group; clinical T4 tumour (P = 0.025); abdominoperineal resection (P = 0.038); pN2 tumour (P = 0.013), pT4 tumour (P = 0.024); R1 resection (P = 0.033); Mandard score (P = 0.025); and incomplete TME (P = 0.015) were significant prognostic factors for the development of LR.
Multivariable analyses of the prognostic factors for LR in the CI and LI groups are presented in Table 3.
Table 3.
Statistically significant prognostic factors affecting locoregional and distant recurrence (multivariable analyses)
| CI group Mult | LI group Mult | |||||
|---|---|---|---|---|---|---|
| P | SHR | 95% c.i. | P | SHR | 95% c.i. | |
| Locoregional recurrence | ||||||
| Tumour localization | ||||||
| Middle (ref) | – | – | – | – | – | – |
| Low | 0.037 | 11.79 | 1.16–119.93 | – | – | – |
| Mandard score | ||||||
| 1–2 (ref) | – | – | – | – | – | – |
| 3 | – | – | – | 0.003 | 29.70 | 3.17–278.18 |
| 4–5 | – | – | – | 0.012 | 16.79 | 1.88–150.16 |
| TME quality | ||||||
| Good (ref) | – | – | – | – | – | – |
| Moderate | – | – | – | 0.004 | 54.83 | 3.62–830.90 |
| Bad | – | – | – | 0.002 | 132.8 | 5.58–3162.13 |
| R1 resection | 0.007 | 3.87 | 1.45–10.28 | – | – | – |
| Postoperative complication | 0.022 | 3.00 | 1.17–7.69 | – | – | – |
| Distant recurrence | ||||||
| Tumour localization | ||||||
| Middle (ref) | – | – | – | – | – | – |
| Low | 0.039 | 1.98 | 1.04–3.79 | – | – | – |
| Clinically T4 | 0.038 | 2.15 | 1.04–4.42 | – | – | – |
| pN category | ||||||
| N0 (ref) | – | – | – | – | – | – |
| N2 | 0.015 | 2.85 | 1.22–6.66 | 0.045 | 4.02 | 1.03–15.63 |
| TME quality | 0.044 | 2.97 | 1.03–8.61 | – | – | – |
CI, classic interval; LI, long interval; Mult, multivariable analysis; SHR, sub-distribution HR; TME, total mesorectal excision; ref, reference.
Distant metastasis
During follow-up, 52 patients from the CI group (36 patients with isolated distant recurrence only) and 31 patients from LI group (24 patients with isolated distant recurrence only) had a distant recurrence. The cumulative incidence of distant metastasis at 5 years was 30.8 per cent in the CI group and 18.6 per cent in the LI group (P = 0.010; Fig. 2). The SHR for distant metastasis in the CI group compared with the LI group was 1.78 (95 per cent c.i. 1.14 to 2.78).
In the CI group, univariable analyses revealed that distal rectum tumour (P = 0.033); clinical T4 tumour (P = 0.005); pN2 tumour (P = 0.001); and incomplete TME were significant prognostic factors for the development of distant recurrence. In the LI group; clinical T4 (P = 0.025); histopathological stage III (P= 0.013); pN2 (P = 0.001); pT4 (P = 0.009); Mandard score (P = 0.003); R1 resection (P = 0.002); and incomplete TME (P = 0.003) were significant prognostic factors for the development of distant recurrence.
Results of multivariable analyses for both groups are presented in Table 3.
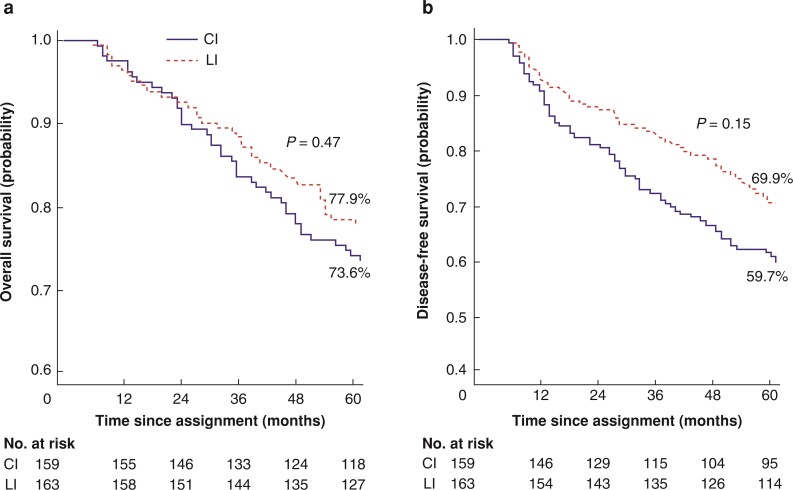
Overall survival
During the follow-up interval, 75 patients in the CI group and 54 patients in the LI group died. The OS rate at 5 years was 73.6 per cent in the CI group and 77.9 per cent in the LI group (P = 0.476; Fig. 3). The HR for death in the CI group compared with the LI group was 1.14 (95 per cent c.i. 0.80 to 1.63).
Fig. 3.
Overall survival and disease-free survival among 322 patients randomly assigned to classic interval (CI) or long interval (LI) groups using Kaplan–Meier estimation
a Overall survival. b Disease-free survival.
In the CI group, univariable analyses revealed that age (P = 0.007); clinical T (P = 0.009); histopathological stage III (P = 0.014); pN2 (P = 0.001); R1 resection (P = 0.001); and incomplete TME (P = 0.008) were significant prognostic factors for OS. In the LI group, age (P = 0.005); male sex (P = 0.029); histopathological stage III (P = 0.038); pN2 (P = 0.038); pT4 (P = 0.011); R1 resection (P = 0.038); Mandard score (P = 0.012); incomplete TME (P = 0.035); development of postoperative complications (P = 0.001); and incomplete dose of adjuvant CT (P = 0.001) were significant prognostic factors for OS. Multivariable analyses for both groups are presented in Table 4.
Table 4.
Statistically significant prognostic factors affecting disease-free and overall survival (multivariable analysis)
| CI groupMult | LI groupMult | |||||
|---|---|---|---|---|---|---|
| P | HR | 95% c.i. | P | HR | 95% c.i. | |
| Disease-free survival | ||||||
| TME quality | ||||||
| Good (ref) | – | – | – | – | – | – |
| Moderate | – | – | – | 0.004 | 7.02 | 1.89–26.05 |
| Bad | – | – | – | 0.001 | 12.85 | 2.83–58.30 |
| Age (years) | – | – | – | 0.01 | 1.043 | 1.01–1.08 |
| pN category | ||||||
| N0 (ref) | – | – | – | – | – | – |
| N1 | 0.050 | 2.35 | 0.99–5.53 | – | – | – |
| N2 | 0.016 | 3.97 | 1.29–12.25 | – | – | – |
| Male sex | – | – | – | 0.021 | 2.17 | 1.12–4.20 |
| Adjuvant chemotherapy | ||||||
| Complete dose (ref) | – | – | – | – | – | – |
| Incomplete dose | – | – | – | <0.001 | 6.82 | 2.65–17.55 |
| None | – | – | – | 0.031 | 2.71 | 1.09–6.73 |
| Mandard grade | ||||||
| 1–2 (ref) | – | – | – | – | – | – |
| 3 | – | – | – | 0.031 | 2.65 | 1.09–6.42 |
| Overall survival | ||||||
| Age (years) | 0.036 | 1.025 | 1.00–1.05 | 0.005 | 1.06 | 1.02–1.10 |
| Male sex | – | – | – | 0.006 | 2.89 | 1.36–6.15 |
| Adjuvant chemotherapy | ||||||
| Complete dose (ref) | – | – | – | – | – | – |
| Incomplete dose | – | – | – | <0.001 | 11.19 | 3.64–34.45 |
| pN category | ||||||
| N0 (ref) | – | – | – | – | – | – |
| N2 | 0.001 | 3.85 | 1.72–8.62 | – | – | – |
| TME quality | ||||||
| Good (ref) | – | – | – | – | – | – |
| Moderate | – | – | – | 0.004 | 10.85 | 2.13–55.34 |
| Bad | – | – | – | 0.001 | 12.32 | 2.70–56.23 |
| Mandard grade | ||||||
| 1–2 (ref) | – | – | – | – | – | – |
| 4–5 | – | – | – | 0.022 | 3.84 | 1.21–12.17 |
| Postoperative complication | ||||||
| None (ref) | – | – | – | – | – | – |
| Dindo–Clavien 3–4 | 0.019 | 2.62 | 1.17–5.87 | – | – | – |
CI, classic interval; LI, long interval; Mult, multivariable analysis; ref, reference; TME, total mesorectal excision.
Disease-free survival
During the follow-up, 47 patients from the CI group died due to disease progression or treatment, 28 patients died due to other reasons, and seven patients had a relapse (LR or/and distant metastasis). In the LI group, 26 patients died due to disease, 28 patients died for other reasons, and nine patients had a relapse. The DFS rates were 59.7 per cent and 69.9 per cent in the CI and LI groups respectively (P = 0.157; Fig. 3). The HR for DFS in the CI group compared with the LI group was 1.27 (95 per cent c.i. 0.91 to 1.78).
In the CI group, univariable analyses revealed that age (P = 0.043); clinical T (P = 0.005); histopathological stage of tumour (P = 0.008); pN2 (P = 0.001); pT (P = 0.031); R1 resection (P = 0.005); and incomplete TME (P = 0.043) were significant prognostic factors for DFS. In the LI group, age (P = 0.013); male sex (P = 0.043); clinical T (P = 0.017); histopathological stage III (P = 0.002); pN2 (P = 0.003); pT (P = 0.004); R1 resection (P = 0.036); Mandard score (P = 0.003); incomplete TME (P = 0.049); and incomplete dose of adjuvant CT (P = 0.001) were significant prognostic factors for DFS. Multivariable analyses for both groups are presented in Table 4.
Discussion
In this study, the cumulative incidence of LR at 5 years was 10.1 per cent in the CI group and 6.9 per cent in the LI group. The cumulative incidence of distant metastasis at 5 years was higher in the CI group (30.8 per cent) compared with the LI group (18.6). The DFS (59.7 versus 69.9 per cent) and OS rates at 5 years (73.6 versus 77.9 per cent) were not statistically significant.
Considering the similarities of the baseline characteristics and CRT protocols, this study suggests a significant differences in distant metastases related to the timing of surgery. Some hypotheses have been suggested to explain this.
First, extending the waiting interval significantly decreased the pT and pN category in the LI group. It has been demonstrated that the final TNM stage (p stage) is a good prognostic factor in predicting LR and distant recurrence and is a better predictor of DFS and OS than clinical stage (cTNM) before CRT31–35. Patients with pCR have improved oncological outcomes and this positive effect is recognized for tumours with partial response12,36,37. The findings that pCR and stage regression rates were higher in the LI group are not surprising, because prolonging the waiting interval is considered to lead to higher pCR rates given the biological effects of RT. DNA damage develops during irradiation, but cellular lysis occurs weeks after irradiation21,38. This may be accurate for the primary tumour (pT); however, lymph nodes are more complicated. In some patients with complete response in the primary tumour (pT0) after preoperative CRT, tumour cells may still be detected in the lymph nodes. Glynne-Jones et al.39 found that of 545 patients with pT0 from 47 studies, 6.6 per cent had a pN(+), with the highest rate of 17 per cent. Lymph node sterilization may occur later than in the primary tumour and it is possible to provide optimal lymph node sterilization by extending the waiting interval. In this study, detection of lymph node invasion in the 31 cases of LI group with ypT0 supports this (in the CI group, lymph node invasion was observed in 1 of 17 ypT0 cases; 5.9 per cent). The pN category was the most important prognostic factor affecting the development of distant metastasis in both groups (Table 3). These results are consistent with the medical literature40–42. Bujko et al.43 also described the association between a poor pathological response of clinically positive nodes to preoperative CRT and a high risk of distant recurrence.
Some suggest that tumour cell death occurs during treatment and not during the delay44. The effects of DNA damage on tumour cells are not detected morphologically until later, but the risk of recurrence will not be affected whether the surgery is delayed. It is suggested that the persistent effect of neoadjuvant treatment would continue to cause cell death over time, and consequently, waiting longer before surgery could yield less viable carcinoma at the time of surgery45 and it may decrease leaking of viable cancer cells thorough vascular and lymphatic channels during surgical manipulation, thereby reducing the chances of systemic recurrence10.
Tumour effects are generally thought to be due to cell death caused directly by DNA damage, although indirect effects such as reduction of tumour vascularity or enhanced immune recognition (immunological effects) may be important. Normal tissue effects may be acute due to direct cell death to the mucosal surface or late, due to indirect effects on the vasculature or on the stem cell component, reducing the capacity to repair future damage46. Extending the waiting interval may affect the clinical phase and improve oncological outcomes. There is emerging evidence that radiotherapy promotes molecular mechanisms (e.g. vascular endothelial growth factor and p53) within rectal cancer, which contribute to tumour growth, survival, and subsequent RT resistance. The extent to which this occurs may depend on the surgical interval14,47.
Rates of LR are significantly decreased after multimodal therapy and TME. Indicators of surgical quality include R resection status, TME quality, tumour perforation, spillage during surgery, and anastomotic leaks. These factors were compared in our previous study24, and no differences were detected between the groups. In the present study, even though the groups were similar regarding the rates of 5-year cumulative LR, the SHR of 1.73 may be interpreted as better local control being obtained in the LI group. The leading reason of LR was R1 resection, especially involvement of the circumferential resection margin (ICRM), which is consistent with the outcomes of the present study. Regarding LR, R1 resection was the most important prognostic factor in the CI group and was detected as a substantial factor in univariable analysis of LI group. The major prognostic factor in the LI group was TME quality. Quirke et al.48 found that both ICRM and mesorectal grading are predictors of local recurrence. Maslekar et al.49 demonstrated an association between incomplete mesorectal excision, and both local and overall recurrence. Conversely, Madbouly et al.50 claimed that in patients who received preoperative CRT and adjuvant chemotherapy, grading had no long-term prognostic value regarding recurrence unless it resulted in ICRM.
In the GRECCAR-6 study22 with a similar design to this study, 3-year oncological outcomes (LR, DM, OS, and DFS) were found to be similar between groups (7 versus 11 weeks). This may be because, contrary to the results of this study, there was no difference between the groups in terms of pTNM and pCR. The randomized clinical study of Terzi et al.23, which compared the 8 and 12-week waiting interval, demonstrated increased pCR rates in the LI group. Unfortunately, the oncological results of this study are not yet known. In conclusion, multicentred randomized clinical studies are needed to determine the optimal waiting time.
The optimal interval should facilitate maximal tumour regression, defined by maximal tumour downstaging and downsizing, with minimal risk of deterioration in the surgical outcomes, defined by low short-term morbidity (perineal and anastomotic complications) and better long-term oncological and functional outcomes51. In the first part of this RCT24, the rates of pCR and stage regression were significantly higher in the LI group than in the CI group, with similar surgical quality and postoperative morbidity. In the present part of the study, the results suggested better long-term oncological outcomes with the interval between CRT and surgery exceeding 8 weeks.
This study has limitations, including its single-centre design and the time taken to complete the study because all operations were performed by two surgeons. Another limitation is that the oncological outcomes were secondary endpoints (the study was powered to detect differences in pCR rates). The timing of surgery in the two groups (less than 8 weeks versus more than 8 weeks) was similar for some patients and could blunt the effect on the primary and secondary outcomes.
Acknowledgements
E.A. and C.C. were responsible for study design, data analysis, data interpretation, drafting and writing the article, and revision of the article. O.B. and T.Y. were responsible for data collection, data analysis, data interpretation, drafting and writing the article, and revision of the article. B.D., S.O. and B.K. were responsible for data collection, data analysis, data interpretation, drafting and writing the article. T.K. was responsible for data analysis, data interpretation, and drafting and writing the article, and revision of the article. M.H. and O.O. were responsible for data analysis, data interpretation, drafting and writing the article. Each author has given approval for the final form of the manuscript.
Contributor Information
Erhan Akgun, Department of General Surgery, Ege University School of Medicine, Izmir, Turkey.
Cemil Caliskan, Department of General Surgery, Ege University School of Medicine, Izmir, Turkey.
Osman Bozbiyik, Department of General Surgery, Ege University School of Medicine, Izmir, Turkey.
Tayfun Yoldas, Department of General Surgery, Ege University School of Medicine, Izmir, Turkey.
Basak Doganavsargil, Department of Pathology, Ege University School of Medicine, Izmir, Turkey.
Serdar Ozkok, Department of Radiation Oncology, Ege University School of Medicine, Izmir, Turkey.
Timur Kose, Department of Biostatistics, Ege University School of Medicine, Izmir, Turkey.
Bulent Karabulut, Department of Medical Oncology, Ege University School of Medicine, Izmir, Turkey.
Nevra Elmas, Department of Radiology, Ege University School of Medicine, Izmir, Turkey.
Omer Ozutemiz, Department of Gastroenterology, Ege University School of Medicine, Izmir, Turkey.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Data Availability
Data are available upon reasonable request.
References
- 1. Berho M, Narang R, Van Koughnett JAM, Wexner SD. Modern multidisciplinary perioperative management of rectal cancer. JAMA Surg 2015;150:260–266 [DOI] [PubMed] [Google Scholar]
- 2. Påhlman L, Glimelius B, Graffman S. Pre- versus postoperative radiotherapy in rectal carcinoma: an interim report from a randomized multicentre trial. Br J Surg 1985;72:961–966 [DOI] [PubMed] [Google Scholar]
- 3. Frykholm GJ, Glimelius B, Påhlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum 1993;36:564–572 [DOI] [PubMed] [Google Scholar]
- 4. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau Ret al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740 [DOI] [PubMed] [Google Scholar]
- 5. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215–1223 [DOI] [PubMed] [Google Scholar]
- 6. Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph Det al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–3833 [DOI] [PubMed] [Google Scholar]
- 7. Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu Cet al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336–346 [DOI] [PubMed] [Google Scholar]
- 8. Saglam S, Bugra D, Saglam EK, Asoglu O, Balik E, Yamaner Set al. Fourth versus eighth week surgery after neoadjuvant radiochemotherapy in T3–4/N0+ rectal cancer: Istanbul R-01 study. J Gastrointest Oncol 2014;5:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJet al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–844 [DOI] [PubMed] [Google Scholar]
- 10. Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu Aet al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 2012;19:2822–2832 [DOI] [PubMed] [Google Scholar]
- 11. Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 2012;99:918–928 [DOI] [PubMed] [Google Scholar]
- 12. Biondo S, Navarro M, Marti-Rague J, Arriola E, Pares D, Del Rio Cet al. Response to neoadjuvant therapy for rectal cancer: influence on long-term results. Colorectal Dis 2005;7:472–479 [DOI] [PubMed] [Google Scholar]
- 13. Smith FM, Waldron D, Winter DC. Rectum-conserving surgery in the era of chemoradiotherapy. Br J Surg 2010;97:1752–1764 [DOI] [PubMed] [Google Scholar]
- 14. Kerr SF, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg 2008;95:1534–1540 [DOI] [PubMed] [Google Scholar]
- 15. Kalady MF, De Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery ICet al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 2009;250:582–588 [DOI] [PubMed] [Google Scholar]
- 16. Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky Cet al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396–2402 [DOI] [PubMed] [Google Scholar]
- 17. Glehen O, Chapet O, Adham M, Nemoz JC, Gerard JP. Long-term results of the Lyons R90-01 randomized trial of preoperative radiotherapy with delayed surgery and its effect on sphincter-saving surgery in rectal cancer. Br J Surg 2003;90:996–998 [DOI] [PubMed] [Google Scholar]
- 18. Cotte E, Passot G, Decullier E, Maurice C, Glehen O, François Yet al. Pathologic response, when increased by longer interval, is a marker but not the cause of good prognosis in rectal cancer: 17-year follow-up of the Lyon R90-01 randomized trial. Int J Radiat Oncol Biol Phys 2016;94:544–553 [DOI] [PubMed] [Google Scholar]
- 19. Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661–2667 [DOI] [PubMed] [Google Scholar]
- 20. Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem Eet al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012;19:2833–2841 [DOI] [PubMed] [Google Scholar]
- 21. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, De Chaisemartin Cet al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol 2016;34:3773–3780 [DOI] [PubMed] [Google Scholar]
- 22. Lefevre JH, Mineur L, Cachanado M, Denost Q, Rouanet P, De Chaisemartin Cet al. Does a longer waiting period after neoadjuvant radio-chemotherapy improve the oncological prognosis of rectal cancer?: three years’ follow-up results of the Greccar-6 randomized multicenter trial. Ann Surg 2019;270:747–754 [DOI] [PubMed] [Google Scholar]
- 23. Terzi C, Bingul M, Arslan NC, Ozturk E, Canda AE, Isik Oet al. Randomized controlled trial of 8 weeks’ vs 12 weeks’ interval between neoadjuvant chemoradiotherapy and surgery for locally advanced rectal cancer. Colorectal Dis 2020;22:279–288 [DOI] [PubMed] [Google Scholar]
- 24. Akgun E, Caliskan C, Bozbiyik O, Yoldas T, Sezak M, Ozkok Set al. Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2018;105:1417–1425 [DOI] [PubMed] [Google Scholar]
- 25. Edge S, Byrd D, Compton C, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual (7th edn). New York: Springer, 2010 [Google Scholar]
- 26. Wei LJ. The adaptive biased coin design for sequential experiments. Ann Stat 1978;6:92–100 [Google Scholar]
- 27. Akgun E, Ozkok S, Tekin M, Yoldas T, Caliskan C, Kose Tet al. The effects of chemoradiotherapy on recurrence and survival in locally advanced rectal cancers with curative total mesorectal excision: a prospective, nonrandomized study. World J Surg Oncol 2017;15:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagtegaal ID, Van de Velde CJH, Van Der Worp E, Kapiteijn E, Quirke P, Van Krieken JHJM. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 2002;20:1729–1734 [DOI] [PubMed] [Google Scholar]
- 29. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Pefiot JFet al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer 1994;73:2680–2686 [DOI] [PubMed] [Google Scholar]
- 30. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 31. Kim CH, Lee SY, Kim HR, Kim YJ. Pathologic stage following preoperative chemoradiotherapy underestimates the risk of developing distant metastasis in rectal cancer: a comparison to staging without preoperative chemoradiotherapy. J Surg Oncol 2016;113:692–699 [DOI] [PubMed] [Google Scholar]
- 32. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess Cet al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 2014;32:1554–1562 [DOI] [PubMed] [Google Scholar]
- 33. Kuo LJ, Liu MC, Jian JJM, Horng CF, Cheng TI, Chen CMet al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol 2007;14:2766–2772 [DOI] [PubMed] [Google Scholar]
- 34. Vychnevskaia K, Dumont F, Agostini J, Julié C, Dartigues P, Lazure Tet al. Prognostic value of sterilized lymph nodes after preoperative chemoradiotherapy for patients with ypN0 rectal cancer. Ann Surg Oncol 2017;24:1304–1311 [DOI] [PubMed] [Google Scholar]
- 35. Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U Jr, Silva E Sousa AH Jret al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg 2005;9:90–101 [DOI] [PubMed] [Google Scholar]
- 36. Geva R, Davidovics H, Soyfer S, Pelles-Avraham S, Klausner JM, Inbar Met al. Does residual microscopic disease after chemoradiotherapy for locally advanced rectal cancer translate into a good clinical outcome? Colorectal Dis 2017;19:237–242 [DOI] [PubMed] [Google Scholar]
- 37. Lee YC, Hsieh CC, Chuang JP. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum 2013;56:1093–1101 [DOI] [PubMed] [Google Scholar]
- 38. Suid HD, Gallager HS. Intact tumor cells in irradiated tissue. Arch Pathol 1964;78:648–651 [PubMed] [Google Scholar]
- 39. Glynne-Jones R, Wallace M, Livingstone JIL, Meyrick-Thomas J. Complete clinical response after preoperative chemoradiation in rectal cancer: is a ‘wait and see’ policy justified? Dis Colon Rectum 2008;51:10–20 [DOI] [PubMed] [Google Scholar]
- 40. Duchalais E, Glyn Mullaney T, Spears GM, Kelley SR, Mathis K, Harmsen WSet al. Prognostic value of pathological node status after neoadjuvant radiotherapy for rectal cancer. Br J Surg 2018;105:1501–1509 [DOI] [PubMed] [Google Scholar]
- 41. Hall MD, Schultheiss TE, Smith DD, Fakih MG, Kim J, Wong JYCet al. Impact of total lymph node count on staging and survival after neoadjuvant chemoradiation therapy for rectal cancer. Ann Surg Oncol 2015;22:580–587 [DOI] [PubMed] [Google Scholar]
- 42. Huebner M, Wolff BG, Smyrk TC, Aakre J, Larson DW. Partial pathologic response and nodal status as most significant prognostic factors for advanced rectal cancer treated with preoperative chemoradiotherapy. World J Surg 2012;36:675–683 [DOI] [PubMed] [Google Scholar]
- 43. Bujko K, Michalski W, Kepka L, Nowacki MP, Nasierowska-Guttmejer A, Tokar Pet al. Association between pathologic response in metastatic lymph nodes after preoperative chemoradiotherapy and risk of distant metastases in rectal cancer: an analysis of outcomes in a randomized trial. Int J Radiat Oncol Biol Phys 2007;67:369–377 [DOI] [PubMed] [Google Scholar]
- 44. Glimelius B. On a prolonged interval between rectal cancer (chemo)radiotherapy and surgery. Ups J Med Sci 2017;122:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Campos-Lobato LF, Geisler DP, da Luz Moreira A, Stocchi L, Dietz D, Kalady MF. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg 2011;15:444–450 [DOI] [PubMed] [Google Scholar]
- 46. Gwynne S, Staffurth J. Principles of cancer treatment by radiotherapy. Surg. Elsevier 2012;30:191–193 [Google Scholar]
- 47. Cascinu S, Graziano F, Catalano V, Staccioli MP, Rossi MC, Baldelli AMet al. An analysis of p53, BAX and vascular endothelial growth factor expression in node-positive rectal cancer. Relationships with tumour recurrence and event-free survival of patients treated with adjuvant chemoradiation. Br J Cancer 2002;86:744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture Jet al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009;373:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maslekar S, Sharma A, MacDonald A, Gunn J, Monson JRT, Hartley JE. Mesorectal grades predict recurrences after curative resection for rectal cancer. Dis Colon Rectum 2007;50:168–175 [DOI] [PubMed] [Google Scholar]
- 50. Madbouly KM, Hussein AM, Abdelzaher E. Long-term prognostic value of mesorectal grading after neoadjuvant chemoradiotherapy for rectal cancer. Am J Surg 2014;208:332–341 [DOI] [PubMed] [Google Scholar]
- 51. Wasserberg N. Interval to surgery after neoadjuvant treatment for colorectal cancer. World J Gastroenterol 2014;20:4256–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.