Abstract
Background
This study reports early mortality and survival from colorectal cancer in relation to the pattern of treatments delivered by the multidisciplinary team (MDT) meeting at a high-volume institution in England over 14 years.
Methods
All patients diagnosed with colorectal cancer and discussed during MDT meetings from 2003 to 2016 at a single institution were reviewed. Three time intervals (2003–2007, 2008–2012, and 2013–2016) were compared regarding initial surgical management (resection, local excision, non-resection surgery, and no surgery), initial oncological therapy, 90-day mortality, and crude 2-year survival for the whole cohort. Sub-analyses were performed according to age greater or less than 80 years.
Results
The MDT managed 4617 patients over 14 years (1496 in the first interval and 1389 in the last). Over this time, there was a reduction in emergency resections from 15.5 per cent to 9.0 per cent (P < 0.0001); use of oncological therapies increased from 34.6 per cent to 41.6 per cent (P < 0.0001). The 90-day mortality after diagnosis of colorectal cancer dropped from 14.8 per cent to 10.7 per cent (P < 0.001) and 2-year survival improved from 58.6 per cent to 65 per cent (P < 0.001). Among patients aged 80 years or older (425 and 446, in the first and last intervals respectively) there was, in addition, a progressive increase in ‘no surgery’ rate from 33.6 per cent to 50.2 per cent (P < 0.0001) and a reduction in elective resections from 42.4 per cent to 33.9 per cent (P = 0.010). The 90-day mortality after elective resection fell from 10.0 per cent (18 of 180) to 3.3 per cent (5 of 151; P = 0.013).
Conclusions
Survival from colorectal cancer improved significantly over 14 years. Among patients aged ≥80 years, major changes in the type of treatment delivered were associated with a decrease in postoperative mortality.
This report highlights the possibility that, in addition to improving survival from individual treatments, a multidisciplinary team may improve its outcomes by doing more or less of particular treatments.
Introduction
Multidisciplinary teams (MDTs) manage patients with colorectal cancer1 in light of advances in staging, oncological therapies and surgery, to ensure that individual patients receive appropriate advice regarding treatment. Surgical resection offers the best chance of cure for most patients with colorectal cancer2. For the old, the frail, or those with advanced disease, best management can be more difficult to determine3,4. Patients presenting as emergencies often share these characteristics5,6. In these groups, local expertise and the evolution of therapies will strongly influence the choice of treatment advised. Many high-risk patients may be managed by local excision, radiotherapy (if rectal cancer), de-functioning stomas, or stenting4.
MDTs have the potential to increase survival from cancer by improving results from existing treatments, by developing or adopting more effective treatments and by offering more patients more appropriate management. Most reports on the treatment of colorectal cancer focus on the effects and outcomes of particular interventions for selected patients such as minimally invasive surgery, chemotherapy, and stenting7–9. There has been little consideration of the types and distribution of treatments recommended by an MDT as potentially significant determinants of clinical outcomes or of survival from the disease among its catchment population.
In this unit, changes in the type of treatment delivered, implemented to reduce post-resection mortality, were evaluated to assess this important function in the cancer pathway.
This study investigated the potential relationship between major changes in the treatments delivered by the MDT and early mortality and crude survival in a cohort of patients with colorectal cancer managed by the MDT and among patients aged 80 years or older.
Methods
Study design and setting
All NHS patients diagnosed with colorectal cancer between January 2003 and December 2016 at Portsmouth Hospitals University NHS Trust were reviewed. Patients with metachronous colorectal cancers were included and analysed in relation to management of their first cancer. Patients with features typical of colorectal cancer (such as on CT) but not biopsy proven, were also included.
All data used for this study had been approved for use by, and previously submitted to, the National Bowel Cancer Audit. Data collected prospectively included patient demographics, date of presentation, cancer stage, treatment received, and postoperative outcomes, including perioperative mortality and long-term survival. Deaths and date of death were identified from ‘patient administration systems’ records (updated regularly) up to August 2020. This study followed STROBE guidelines and has been conducted in compliance with the Declaration of Helsinki10.
Of note, Portsmouth Hospitals University NHS Trust is a large District General Hospital providing acute and elective services to a catchment population of approximately 650 000. The colorectal cancer MDT meets weekly to discuss all newly diagnosed elective patients and to recommend management. For patients admitted acutely and needing urgent management, discussions take place between relevant MDT specialists and the patient to decide the best care. Such patients are notified to the full MDT later. Ultimately, choice of treatment is decided in discussion between the responsible clinician and the patient. Deaths and complications are discussed weekly in a surgical quality assurance meeting. Portsmouth joined the National Bowel Cancer Screening Programme in 2009. Advances in the MDT’s care of patients with colorectal cancer during the course of this study included a policy that a consultant be scrubbed for all colorectal cancer resections (2001), improved surgical risk assessment (2001), better cross-sectional imaging (2003), stenting (2003), laparoscopic resection (2003), increasing use of radiological TNM staging (2004), 24-h availability of CT (2005), provision of a surgical high care unit (2008), enhanced recovery programme (2008), cardiopulmonary exercise testing (2013), robotic resection (2013), better and more personalized oncological therapy (including cetuximab and panitumumab for RAS wild-type metastatic cancers, 2017), and routine assessment of resected specimens for mismatch-repair deficiency (2019).
Treatment groups
Patients were grouped for analysis according to initial surgical treatment delivered, namely ‘resection’ (elective or emergency), ‘non-resection surgery’ (NRS) (elective or emergency), ‘no surgery’, and ‘local excision’. Whether they furthermore received oncological therapies as part of the initial treatment phase (alone, neoadjuvant, or adjuvant) was documented but not used to subcategorize treatment groups. NRS included de-functioning stomas or stent insertion without subsequent resection. Local excision included any trans-anal or trans-anal endoscopic microsurgical or advanced endoscopic techniques (such as endoscopic mucosal resection) to remove a cancer without segmental bowel resection. Treatment delivered was equated with MDT treatment recommendation except in a small number of patients for whom it was recorded that they declined surgery advised by the MDT. As the unit worked to reduce post-resection mortality, for increasing numbers of patients in whom the decision to resect was borderline, alternative management was recommended.
Outcomes of interest
The proportions of patients in the different treatment groups were compared over three time intervals (2003–2007, 2008–2012, and 2013–2016) to assess outcomes associated with the MDT’s approach to reducing postoperative mortality. Principal endpoints were 90-day mortality and crude 2-year survival of the whole colorectal cancer cohort and separately, according to age (under 80 years or 80 years or older). Secondary endpoints were 90-day mortality and 2-year survival by treatment group. Postoperative mortality was counted from the date of surgery. Survival was measured from the date of operation, or the date of diagnosis if there was no surgery. Crude survival was chosen over other indices because it was the measure used in the National Bowel Cancer Audit over this time interval11. Reasons for not operating were recorded prospectively. For patients undergoing excision or resection, Dukes’ classification is reported as TNM was not used in the first years of the study.
Statistical analysis
Categorical variables were analysed with Pearson’s chi-squared test or Fishers exact test as appropriate. Grouped continuous data (such as age at presentation) were compared with the non-parametric Mann–Whitney U Test (binary) or Kruskal–Wallis test (more than two categories). Missing data were handled as follows: CT staging was excluded because the data were not consistently entered on the database; also, patients who did not have surgery for unknown reasons were excluded from the relevant analysis. Kaplan–Meier analysis was used to plot survival curves. Survival is presented as median (95 per cent c.i.) absolute survival or as per cent survival at 2 years. Comparisons between groups were assessed with log rank tests with significance set at less than 0.05. Statistical analysis was performed with SPSS® version 26.0 (IBM, Armonk, New York, USA) and GraphPad Prism® version 7.0 (GraphPad Software, San Diego, California, USA).
Results
Overall, 4617 patients were diagnosed with colorectal cancer and discussed in the colorectal MDT meetings over 14 years, including 33 metachronous cancers and 265 detected by screening (Table 1). Although there was no change in median age over time, the proportion of patients aged 80 years or older increased from 28.4 per cent (425 of 1496) in 2003–2007 to 32.2 per cent (446 of 1389) in 2013–2016 (P = 0.031).
Table 1.
Patient demographics and patterns of treatment delivered by the multidisciplinary team over time
| Time interval | ||||
|---|---|---|---|---|
| 2003–2007 | 2008–2012 | 2013–2016 | P 2003–2007 versus 2013–2016 | |
| Number diagnosed in time interval | 1496 | 1732 | 1389 | – |
| Number diagnosed per year (mean) | 299 | 346 | 347 | – |
| Screening detected per year (mean) | 0 | 21 | 48 | – |
| Age (years), median (i.q.r) | 74 (23–102) | 74 (24–100) | 74 (27–98) | |
| Sex ratio (M:F) | 784:712 | 960:772 | 783:606 | 0.033 |
| Distribution of surgical treatments over time | ||||
| Resections | 1046 (70.2) | 1195 (69) | 849 (61.1) | <0.0001 |
| Elective | 814 (54.4) | 997 (57.6) | 724 (52.1) | <0.001 |
| Emergency | 232 (15.5) | 198 (11.4) | 125 (9.0) | <0.0001 |
| Non-resectional surgery | 93 (6.2) | 79 (4.6) | 64 (4.6) | – |
| Local excision | 40 (2.7) | 56 (3.2) | 79 (5.7) | <0.001 |
| No surgery | 317 (21.2) | 402 (23.2) | 397 (28.6) | <0.0001 |
| Distribution of oncological treatments over time | ||||
| Chemotherapy with/without radiotherapy | 517 (34.6) | 721 (41.6) | 578 (41.6) | <0.0001 |
Values are n (%) unless otherwise indicated. Comparisons between groups were conducted with a chi-squared test.
Overall cohort
Over time, there was a reduction in overall resections from 70.2 per cent to 61.1 per cent (P < 0.0001) and in emergency resections from 15.5 per cent to 9.0 per cent (P < 0.0001) (Table 1). Use of oncological therapies increased from 34.6 per cent to 41.6 per cent (P < 0.0001). The 90-day mortality improved significantly for the whole cohort (however treated) as well as for each treatment group (Table 2). Among patients undergoing elective resection, 90-day mortality fell from 4.4 per cent to 1.5 per cent, (P < 0.001). There was a relative increase in median survival of 44 per cent (3.4 (2.9–3.9) to 4.9 (4.0–5.7) years; P < 0.0001. The 2-year survival increased from 58.6 per cent to 65 per cent (P < 0.001). Most of the survival improvement occurred between the first and second time intervals (Table 2).
Table 2.
Results in the whole cohort according to surgical treatment received over time, all ages
| Surgical treatment group | Time interval | ||||
|---|---|---|---|---|---|
| 2003–2007 | 2008–2012 | P versus 2003–2007 | 2013–2016 | P versus 2003–2007 | |
| Elective resection | |||||
| 90-day mortality | 36 (4.4) | 19 (1.9) | – | 11 (1.5) | <0.001 |
| *2-year survival | 685 (84.2) | 883 (88.6) | – | 664 (91.7) | <0.001 |
| Emergency resection | |||||
| 90-day mortality | 44 (18.9) | 34 (17.2) | – | 18 (14.4) | – |
| 2-year survival | 118 (50.9) | 101 (51.0) | – | 70 (56.0) | – |
| All resections | |||||
| 90-day mortality | 80 (7.6) | 53 (4.4) | – | 29 (3.4) | <0.001 |
| 2-year survival | 803 (76.8) | 984 (82.3) | – | 734 (86.5) | <0.0001 |
| Non-resectional surgery | |||||
| 90-day mortality | 26 (28) | 17 (21.5) | – | 6 (9.4) | 0.005 |
| 2-year survival | 9 (9.7) | 14 (17.7) | – | 12 (18.8) | – |
| Local excision | |||||
| 90-day mortality | 0 (0) | 0 (0) | – | 0 (0) | – |
| 2-year survival | 38 (95) | 51 (91.1) | – | 75 (94.9) | – |
| No surgery | |||||
| 90-day mortality | 115 (36.3) | 126 (31.3) | – | 113 (28.5) | 0.026 |
| Median survival (95% c.i.) (months) | 5.3 (4.0–6.5) | 7.3 (5.9–8.6) | – | 8.0 (6.4–9.5) | <0.001 |
| 2-year survival | 26 (8.2) | 59 (14.7) | – | 84 (21.2) | <0.001 |
| All patients, all treatments | |||||
| 90-day mortality | 221 (14.8) | 196 (11.3) | – | 148 (10.7) | <0.001 |
| Median survival (95% c.i.) (years) | 3.4 (2.9–3.9) | 4.8 (4.0–5.6) | <0.0001 | 4.9 (4.0–5.7) | <0.001 |
| 2-year survival | 876 (58.6) | 1108 (64) | 0.002 | 905 (65.2) | <0.001 |
Values are n (%) unless otherwise indicated. *Survival presented as median (95 per cent c.i.) or as per cent survival at 2 years. Comparisons between groups assessed using log rank tests with significance set at less than 0.05 or chi-squared test as appropriate.
Patients aged under 80 years
Between 2009 and 2016 a mean 36 patients per annum (range 9–63) aged 60–69 years, were referred to the MDT from the Bowel Cancer Screening Programme (mean 21 per annum in 2008–2012, increasing to a mean 48 per annum in 2013–2016). Among patients undergoing elective local excision or resection, there was an increase in Dukes’ class A over time from 21.4 per cent (141 of 258) to 32.3 per cent (202 of 625) (P < 0.001), a drop in Dukes’ B and an increase in the proportion of patients with metastatic disease (4.7 per cent (31 of 658) rising to 8.0 per cent (50 of 625); P = 0.015). Table 3 shows changes in the pattern of treatments delivered, 90-day mortality, and 2-year survival according to treatment. Compared with the first time interval, there was a significant reduction in the proportion of patients undergoing emergency resection in periods two (11.9 per cent versus 16.3 per cent; P = 0.002) and three (10 per cent; P < 0.001). The elective resection rate remained unchanged overall—an increased rate in patients aged under 70 years from 57.9 per cent to 65.8 per cent (in keeping with screening), balanced by a drop among patients aged 70–80 years. There was no significant increase in patients allocated to ‘no surgery’ among those aged under 80 years. Use of chemotherapy or radiotherapy increased from 43.1 per cent to 51.2 per cent, mostly between the first and second time intervals (P < 0.001).
Table 3.
Results among patients aged under 80 years according to surgical treatment received over time
| Surgical treatment group | Time interval | ||||
|---|---|---|---|---|---|
| 2003–2007 | 2008–2012 | P versus 2003–2007 | 2013–2016 | P versus 2003–2007 | |
| Resections | 809 (75.5) | 950 (76.5) | – | 667 (70.7) | 0.015 |
| 90-day mortality | 44 (5.4) | 27 (2.8) | 0.006 | 19 (2.8) | 0.014 |
| *2-year survival | 654 (80.8) | 803 (84.5) | 0.041 | 592 (88.8) | <0.001 |
| Elective | 634 (59.2) | 802 (64.6) | 0.008 | 573 (60.8) | – |
| 90-day mortality | 18 (2.8) | 8 (1.0) | 0.009 | 6 (1.0) | <0.001 |
| 2-year survival | 557 (87.9) | 725 (90.4) | – | 539 (94.1) | <0.001 |
| Emergency | 175 (16.3) | 148 (11.9) | 0.002 | 94 (10.0) | <0.001 |
| 90-day mortality | 26 (14.9) | 19 (12.8) | – | 13 (13.8) | – |
| 2-year survival | 97 (55.4) | 78 (52.7) | – | 53 (56.4) | – |
| Non-resectional surgery | 63 (5.9) | 52 (4.2) | – | 51 (5.4) | – |
| Elective | 13 (1.2) | 11 (0.8) | – | 14 (1.5) | – |
| Emergency | 50 (4.7) | 41 (3.3) | – | 37 (3.9) | – |
| 2-year survival | 7 (11.1) | 10 (19.2) | – | 10 (19.6) | – |
| Local excision | 25 (2.3) | 40 (3.2) | – | 52 (5.5) | <0.001 |
| 2-year survival | 24 (96) | 38 (95) | – | 50 (96.2) | – |
| No surgery | 174 (16.2) | 200 (16.1) | – | 173 (18.3) | – |
| 2-year survival | 13 (7.5) | 26 (13) | – | 37 (21.4) | 0.0001 |
| Total patients in time interval | 1071 (100) | 1242 (100) | – | 943 (100) | – |
| 90-day mortality | 119 (11.1) | 100 (8.1) | 0.012 | 73 (7.7) | 0.010 |
| 2-year survival | 698 (65.2) | 877 (70.6) | <0.001 | 689 (73.1) | <0.001 |
| Median survival (95% c.i.) (years) | 5.5 (4.4–6.6) | 8.9 (7.5–10.3) | <0.001 | Insufficient deaths | – |
| Oncology | |||||
| Radiotherapy | 38 (3.6) | 45 (3.6) | – | 27 (2.9) | – |
| Chemotherapy | 335 (31.3) | 434 (34.9) | – | 351 (37.2) | 0.005 |
| Chemoradiotherapy | 89 (8.3) | 146 (11.8) | – | 105 (11.1) | 0.032 |
| Chemo/radiotherapy | 462 (43.1) | 625 (50.3) | <0.001 | 483 (51.2) | <0.001 |
Values are n (%) unless otherwise indicated. Patients were grouped according to nature of surgery or none. Use of oncological therapy is not broken down according to surgical treatment group. *Survival presented as median (95 per cent c.i.) or as per cent survival at 2 years. Comparisons between groups using log rank tests with significance set at less than 0.05 or chi-squared test as appropriate.
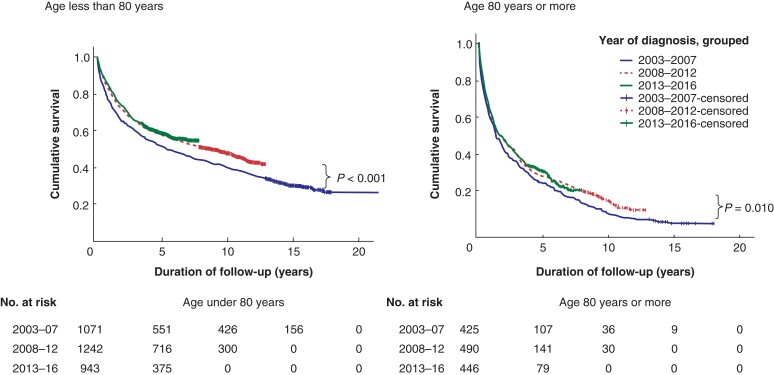
The 90-day mortality after diagnosis of colorectal cancer in patients aged under 80 years dropped steadily, with significant improvement in the second time interval (Table 3). The 90-day mortality after elective or any resection dropped significantly by the second time interval and did not drop further. Median and 2-year survival for all patients increased early (significantly by the second time interval). The relative increase in 2-year survival was 12 per cent (Table 3 and Fig. 1).
Fig. 1.
Kaplan–Meier survival curves for all patients diagnosed with colorectal cancer by age and time interval as in Tables 3 and 4
Significance values refer to survival among all patients diagnosed 2013–2016 compared with 2003–2007.
Patients aged 80 years or more
Among those undergoing elective resection or local excision in this age group, there was no significant change in resection pathology (Dukes’ classification) over time.
Table 4 shows changes in the pattern of treatments delivered, and in survival outcomes, according to treatment. As with the younger patients, there was a reduction in emergency resections and an increase in oncological treatment. In contrast with younger patients, however, there was a significant increase in ‘no surgery’ and a drop in elective resections among those aged 80 years or older. An increasing proportion of patients (41.2 per cent in second interval, P = 0.018; 50.2 per cent in the third; P < 0.0001) were not offered any surgery (Table 4). Advanced disease accounted for 13.9 per cent (59 of 425) patients not being offered surgery in the first interval and 15.2 per cent (68 of 446) in the last. Patient unfitness was the reason given for not operating in 14.4 per cent (61 of 425) in the first interval increasing to 26.9 per cent (120 of 446) (P < 0.00001) in the last. For patients not undergoing surgery in this age group, use of chemotherapy or radiotherapy decreased from 25.9 per cent (37 of 143) to 21.4 per cent (48 of 224; P < 0.001). Median survival following diagnosis increased from 16.1 to 21.2 months mostly between the first and second time intervals (P = 0.013). The relative increase in 2-year survival for all diagnosed patients was 18 per cent by the third time interval (Table 4, Fig. 1). After resections (overall or electively), the 2-year survival increased significantly by the second time interval concomitant with significant increases in ‘no surgery’ and in oncological treatment but before significant changes in overall resection rate or in elective resections (Table 4).
Table 4.
Results among patients aged 80 years or older according to surgical treatment received over time
| Surgical treatment group | Time interval | ||||
|---|---|---|---|---|---|
| 2003–2007 | 2008–2012 | P versus 2003-2007 | 2013–2016 | P versus 2003–2007 | |
| Resections | 237 (55.8) | 245 (50.0) | – | 182 (40.8) | <0.001 |
| 90-day mortality | 36 (15.2) | 26 (10.6) | 0.008 | 10 (5.5) | 0.002 |
| *2-year survival | 149 (62.9) | 181 (73.9) | 0.009 | 142 (78) | <0.001 |
| Elective | 180 (42.4) | 195 (39.8) | – | 151 (33.9) | 0.010 |
| 90-day mortality | 18 (10) | 11 (5.6) | – | 5 (3.3) | 0.017 |
| 2-year survival | 128 (71.1) | 158 (81) | – | 125 (82.8) | 0.013 |
| Emergency | 57 (13.4) | 50 (10.2) | – | 31 (7.0) | 0.002 |
| 90-day mortality | 18 (31.6) | 15 (30) | – | 5 (16.1) | – |
| 2-year survival | 21(36.8) | 23 (46) | – | 17 (54.8) | – |
| Non-resectional surgery | 30 (7.1) | 27 (5.5) | – | 13 (2.9) | 0.002 |
| Elective | 4 (1.3) | 8 (1.6) | – | 1 (0.2) | 0.048 |
| Emergency | 26 (6.1) | 19 (3.9) | – | 12 (2.7) | – |
| 2-year survival | 2 (6.7) | 4 (14.8) | – | 2(15.4) | – |
| Local excision | 15 (3.5) | 16 (3.3) | >0.05 | 27 (6.1) | 0.008 |
| 2-year survival | 14 (93.3) | 13 (81.3) | – | 25 (92.6) | |
| No Surgery | 143 (33.6) | 202 (41.2) | 224 (50.2) | <0.0001 | |
| 2-year survival | 13(9.1) | 33 (16.3) | 0.049 | 47 (21) | 0.003 |
| Total patients in time interval | 425 (100) | 490 (100) | – | 446 (100) | – |
| 90-day mortality | 102 (24.0) | 96 (19.6) | 0.038 | 75 (16.8) | 0.008 |
| 2-year survival | 178 (41.9) | 231 (47.1) | – | 216 (48.4) | 0.052 |
| Median survival (95% c.i.) (months) | 16.1 (12.7–19.5) | 20.4 (15.0–25.8) | – | 21.2 (15.8–26.6) | 0.013 |
| Oncology | |||||
| Radiotherapy | 41 (9.7) | 64 (13.1) | – | 45 (10.1) | – |
| Chemotherapy | 9 (2.1) | 27 (5.5) | – | 43 (9.7) | 0.001 |
| Chemoradiotherapy | 5 (1.2) | 5 (1.0) | – | 7 (1.6) | – |
| Chemo/radiotherapy | 55 (12.9) | 96 (19.6) | 0.004 | 95 (21.3) | <0.0001 |
Values are n (%) unless otherwise indicated. Patients were grouped according to nature of surgery or none. Use of oncological therapy is not broken down according to surgical treatment group. *Survival presented as median (95 per cent c.i.) or as per cent survival at 2 years. Comparisons between groups using log rank tests with significance set at less than 0.05 or by chi-squared test (or Fishers exact test) as appropriate.
The 90-day mortality after diagnosis of colorectal cancer or following resection (elective/any) showed no significant change in the second time interval. In the third interval, there was a significant improvement in 90-day mortality after elective resection from 10 per cent to 3.3 per cent (P = 0.017) accompanying a drop in the elective resection rate from 42.4 per cent to 33.9 per cent (P = 0.010) and increase in ‘no surgery’ to 50.2 per cent.
Discussion
Management of colorectal cancer varies across England with regard to treatment delivered and clinical outcomes12,13. At a local level, MDT meetings recommend treatment for individual patients based on the evidence presented. Team-working within MDT meetings has been extensively studied14,15. The relationship between the choice of treatment it delivers, and clinical outcomes has received less attention16. This study examined survival outcomes over a 14-year interval during which, major changes in treatment distribution had been instituted to reduce post-resection mortality among the old. The report highlights the possibility that, in addition to improving results from individual treatments, an MDT may improve its survival outcomes by doing more or less of particular treatments.
The 90-day mortality and 2-year survival by age group and for the whole colorectal cancer cohort were selected as primary endpoints for the study11,17. The 2-year rather than 5-year survival was chosen to provide earlier insights into MDT outcomes that might prompt remedial action if needed. The potential for discrepancy between management recommended versus that provided was dealt with by regarding the latter as the care for which the MDT was accountable18. Changes in outcomes for individual treatment groups over the course of the study were not primary endpoints because the treatment groups were potentially influenced by selection bias as well as by treatment improvements over time.
During the study, 2-year crude survival from colorectal cancer increased from 59 per cent to 65 per cent. Much of this improvement took place in the early part of the study and likely reflected progress in care across the UK19. Among patients aged less than 80 years, there was an early reduction in emergency resections and increase in oncological therapy but no change in the elective resection rate and no increase in ‘no surgery’. The observed improvements in overall 90-day mortality and 2-year survival in this age group are consistent with the impacts of screening, advances in surgical and perioperative care, and increased use of more effective oncological therapies reported during this time7,20–22.
Patients aged 80 years or more also benefitted from improving cancer care in the NHS: 2-year survival after resection increased early in this study, alongside increased use of oncological therapies (before any substantial change in resection rates); however, significant improvement in 90-day mortality for all diagnosed patients and for those undergoing resection in this age group followed major changes in the distribution of treatments delivered by the MDT, notably a drop in the elective (as well as emergency) resection rate and a substantial increase in the proportion of patients receiving no surgery. Reducing the elective resection rate by a quarter was associated with a two-thirds fall in 90-day mortality after resection. Contrasted with patients aged under 80 years, the proportion of those aged 80 years or more turned down for resection on account of impaired fitness almost doubled as the team raised the threshold for surgery to reduce post-resection mortality. Improved 90-day mortality for all diagnosed patients in this age group occurred despite decreasing use of resections. Improving survival was also seen among the growing group of patients aged 80 years or more allocated to ‘no surgery’. This could not be explained by increased use of oncological therapies (which decreased in the ‘no surgery’ group) and likely reflects inclusion of patients who would previously have undergone surgery but lived a little longer without it.
Survival from bowel cancer in England is poorer than in many countries, arguably due to lower resection rates23,24, especially among the old25; however, high rates of resection are only appropriate if postoperative mortality rates are acceptable. Reducing the resection rate to improve outcomes was an initial response while the MDT simultaneously worked to improve perioperative and operative care in the old and for all its patients. Recent excellent 90-day mortality following elective resection suggests that this unit should now offer resection more readily. Advances in individual risk assessment, selection, optimization, and surgical care may make resection appropriate for some patients who would previously have been turned down.
This study has several limitations. Although all data were entered prospectively, the analysis was retrospective and covered a time during which there were substantial changes in referral pathways, quality of imaging, and improvements in individual treatments, which likely contributed to the better outcomes observed and which are acknowledged (above). Discussion of detailed radiological staging, which would have been valuable in comparing groups over time, was not possible because data collection was not consistent over the study interval. The MDT did not set criteria for determining who would be offered resection (as is reflected in the gradual changes in numbers resected) and this study does not attempt to advise on this. The study focused mostly on surgical rather than oncological management because it arose from an attempt to deal with post-resection (surgical) mortality. Finally, although we had reliable data concerning urgency of operation, we did not have data on urgency of presentation. Some of the drop in emergency operating could have been attributable to reduced emergency presentations after screening and public awareness campaigns20.
Despite working to provide optimal treatment for each patient, MDT outcomes for particular subgroups may be less satisfactory. These insights are most easily gained by regular review of treatments delivered by the MDT in relation to results among the whole managed population and in patient subgroups. For some MDTs, changing the type of treatment provided to particular groups of patients has the potential to improve outcomes.
Acknowledgements
The authors are grateful to A. Roe, Southmead Hospital, Bristol, for critical review of an earlier version of this manuscript. This research was not pre-registered with an independent institutional registry nor was an analysis plan for this research pre-registered with an independent institutional registry. The lead author (D.O.L.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; and that no important aspects of the study have been omitted. There have been no discrepancies from the study as originally planned.
Contributor Information
David M Layfield, Colorectal Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
Karen G Flashman, Colorectal Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
Sara Benitez Majano, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK.
Asha Senapati, Colorectal Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
Christopher Ball, Colorectal Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
John A Conti, Colorectal Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
Jim S Khan, Colorectal Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
Daniel P O’Leary, Colorectal Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
Funding
The authors have no funding to declare.
Disclosure
All authors have completed the International Committee of Medical Journal Editors uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the work submitted; no financial relationship with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Data Availability
Data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Calman K, Hine D. A policy framework for commissioning cancer services: a report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales. Department of Health Publications, The National Archive, 1995
- 2. Liang J, Fazio V, Lavery I, Remzi F, Hull T, Strong Set al. . Primacy of surgery for colorectal cancer. Br J Surg 2015;102:847–852 [DOI] [PubMed] [Google Scholar]
- 3. Okabe H, Ohsaki T, Ogawa K, Ozaki N, Hayashi H, Akahoshi Set al. . Frailty predicts severe postoperative complications after elective colorectal surgery. Am J Surg 2019;217:677–681 [DOI] [PubMed] [Google Scholar]
- 4. Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller Det al. . Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 2015;26:463–476 [DOI] [PubMed] [Google Scholar]
- 5. Wallace D, Walker K, Kuryba A, Finan P, Scott N, van der Meulen J. Identifying patients at risk of emergency admission for colorectal cancer. Br J Cancer 2014;111:577–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simon HL, Paula T, Luz MM, Nemeth SK, Moug SJ, Keller DS. Frailty in older patients undergoing emergency colorectal surgery: USA National Surgical Quality Improvement Program analysis. Br J Surg 2020;107:1363–1371 [DOI] [PubMed] [Google Scholar]
- 7. Hemandas AK, Abdelrahman T, Flashman KG, Skull AJ, Senapati A, O'Leary DPet al. . Laparoscopic colorectal surgery produces better outcomes for high-risk cancer patients compared to open surgery. Ann Surg 2010;252:84–89 [DOI] [PubMed] [Google Scholar]
- 8. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka Det al. . ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–1422 [DOI] [PubMed] [Google Scholar]
- 9. Takahashi H, Okabayashi K, Tsuruta M, Hasegawa H, Yahagi M, Kitagawa Y. Self-expanding metallic stents versus surgical intervention as palliative therapy for obstructive colorectal cancer: a meta-analysis. World J Surg 2015;39:2037–2044 [DOI] [PubMed] [Google Scholar]
- 10. von Elm, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ (Clin Res ed.) 2007;335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Bowel Cancer Audit Annual Report 2013 . NHS Digital. https://www.nboca.org.uk/reports/annual-report-2013/
- 12. Morris EJ, Finan PJ, Spencer K, Geh I, Crellin A, Quirke Pet al. . Wide variation in the use of radiotherapy in the management of surgically treated rectal cancer across the English National health service. Clin Oncol 2016;28:522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fenton HM, Taylor JC, Lodge J, Lodge JPA, Toogood GJ, Finan PJet al. . Variation in the use of resection for colorectal cancer liver metastases. Ann Surg 2019;270:892–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soukup T, Konstantinos V, Petrides KV, Sarkar S, Arora S, Shah Set al. . The anatomy of clinical decision-making in multidisciplinary cancer meetings. A cross-sectional observational study of teams in a natural context. Medicine (Baltimore) 2016;95:e3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamb BW, Sevdalis N, Mostafid H, Vincent C, Green JS. Quality improvement in multidisciplinary cancer teams: an investigation of teamwork and clinical decision-making and cross-validation of assessments. Ann Surg Oncol 2011;18:3535–3543 [DOI] [PubMed] [Google Scholar]
- 16. Munro AJ. Multidisciplinary team meetings in cancer care: an idea whose time has gone? Clin Oncol 2015;27:728–731 [DOI] [PubMed] [Google Scholar]
- 17. Fowler H, Belot A, Njagi EN, Luque-Fernandez MA, Maringe C, Quaresma Met al. . Persistent inequalities in 90-day colon cancer mortality: an English cohort study. Br J Cancer 2017;117:1396–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blazeby JM, Wilson L, Metcalfe C, Nicklin J, English R, Donovan JL. Analysis of clinical decision-making in multi-disciplinary cancer teams. Ann Oncol 2006;17:457–460 [DOI] [PubMed] [Google Scholar]
- 19. National Bowel Cancer Annual Report 2017 . The Healthcare Quality Improvement Partnership, London, 2017. https://nboca.org.uk/content/uploads/2017/12/NBOCA-annual-report-2017-v2.pdf
- 20. Geraghty J, Shawihdi M, Devonport E, Sarkar S, Pearson MG, Bodger K. Reduced risk of emergency admission for colorectal cancer associated with the introduction of bowel cancer screening across England: a retrospective national cohort study. Colorectal Dis 2018;20:94–104 [DOI] [PubMed] [Google Scholar]
- 21. ERAS Compliance Group . The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 2015;261:1153–1159 [DOI] [PubMed] [Google Scholar]
- 22. André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand Aet al. . Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015;33:4176–4187 [DOI] [PubMed] [Google Scholar]
- 23. Maringe C, Walters S, Rachet B, Butler J, Fields T, Finan Pet al. . Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007. Acta Oncologica 2013;52:919–932 [DOI] [PubMed] [Google Scholar]
- 24. Dejardin O, Rachet B, Morris E, Bouvier V, Jooste V, Haynes Ret al. . Management of colorectal cancer explains differences in 1-year relative survival between France and England for patients diagnosed 1997–2004. Br J Cancer 2013;108:775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Majano S B, Di Girolamo C, Rachet Bet al. . Surgical treatment and survival from colorectal cancer in Denmark, England. Norway, and Sweden: a population-based study. Lancet Oncol 2019;20:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying this article will be shared on reasonable request to the corresponding author.