Abstract
Excessive activation of the sympathetic nervous system is one of the pathophysiological hallmarks in hypertension and heart failure. Within the central nervous system, the paraventricular nucleus (PVN) of the hypothalamus and the rostral ventrolateral medulla in the brain stem play critical roles in the regulation of sympathetic outflow to peripheral organs. Information from the peripheral circulation, including the serum concentrations of sodium and angiotensin II, is conveyed to the PVN via adjacent structures with weak blood-brain barrier. In addition, signals from baroreceptors, chemoreceptors and cardiopulmonary receptors as well as afferent input via the renal nerves are all integrated at the level of the PVN. The brain renin-angiotensin system and the balance between nitric oxide and reactive oxygen species in these brain areas also determine the final sympathetic outflow. Additionally, brain inflammatory responses are also shown to modulate these processes. Renal denervation interrupts both the afferent inputs from the kidney to the PVN and the efferent outputs from the PVN to the kidney, resulting in suppression of sympathetic outflow and eliciting beneficial effects in both hypertension and heart failure.
Introduction
In patients with hypertension and heart failure (HF), there is increased spillover of norepinephrine in the heart and the kidney as well as augmented muscle sympathetic nerve activity (MSNA). In treatment-resistant hypertension and HF, conditions such as diabetes mellitus, chronic kidney disease (CKD), obesity, and sleep apnea syndrome (SAS) commonly exist as comorbidities and have been associated with a greater firing rate of MSNA compared to that in controlled hypertension alone1, 2. These findings suggest that the excessive activation of the sympathetic nervous system occurs as a common feature in both treatment-resistant hypertension and HF. The sympathetic nervous system is regulated by specific brain areas involved in the regulation of cardiovascular function. In cardiovascular diseases, including hypertension and HF, an imbalance between excitatory and inhibitory inputs within these specific brain areas induces an excessive activation of the sympathetic nervous system. Recently, renal denervation (RDN) has drawn a fair amount of attention as a novel innovative therapy to modify the regulation of the circulation so as to reduce the onset and severity of cardiovascular disease. The kidneys regulate electrolyte and fluid balance for optimal volume in the circulation, and their homeostatic dysfunction is directly related to the pathogenesis of hypertension and HF. RDN blocks the renal sympathetic nervous system, which is an organ-specific-related pathway between the brain and the kidney3. In the present review, we focus on the mechanisms by which the central and peripheral nervous system regulate blood pressure and fluid balance under both physiological and pathological conditions. We then consider the basic and clinical aspects of RDN as a new horizon in the treatment of hypertension and HF.
Blood pressure regulation by the central nervous system
Specific areas within the central nervous system (CNS), the paraventricular nucleus (PVN) of the hypothalamus and the rostral ventrolateral medulla (RVLM) in the brain stem govern sympathetic outflow to regulate cardiovascular function including blood pressure. Previous studies have shown that the RVLM plays a crucial role in regulating sympathetic outflow and blood pressure4, 5. RVLM neurons project to sympathetic preganglionic nuclei of the spinal cord (intermediolateral cell column: IML) to control sympathetic outflow in peripheral organs including the heart, kidneys and blood vessels. Activation of RVLM neurons causes sympathoexcitation, leading to increased blood pressure through activation of adrenoceptors in the heart, kidneys and peripheral arterioles. Activation of β1-adrenoceptor in the heart increases contractility and heart rate to augment cardiac output. Intrarenal sympathoexcitation induces β1-adrenoceptor-mediated renin secretion from the juxtaglomerular apparatus and α1-adrenoceptor-mediated sodium reabsorption in the proximal tubules. In the peripheral arterioles, activation of α1-adrenoceptor induces vasoconstriction (Figure 1). Collectively, these effects via adrenoceptors work together to increase blood pressure. The activities of RVLM neurons are modulated by the inputs from other regions in the CNS, including the PVN of the hypothalamus and the nucleus tractus solitarius (NTS) of the brainstem. The circumventricular organs, such as the subfornical organ (SFO), organum vasculosum lamina terminalis (OVLT) and median preoptic nucleus (MnPO), lack a blood-brain barrier and thus can sense the osmolarity and concentrations of sodium and angiotensin II (Ang II) in the serum and convey the information to the PVN. It is also known that the PVN receives inputs from the baroreceptors, chemoreceptors and cardiopulmonary receptors as well as afferent renal nerves6, 7. Afferent renal nerves densely innervate the pelvis of the kidney and are thought to convey its signals via baroreceptors or chemoreceptors in the pelvis8. The parvocellular neurons in the PVN projecting to the IML directly or via the RVLM integrate these inputs from the periphery, and thus this PVN-RVLM-IML pathway is considered to be important for generating sympathetic outflow (Figure 1)4, 9. The NTS is one of the main sites that receive visceral sensory afferent signals from various peripheral tissues, including baroreceptors in the aortic arch and carotid sinus. The glutamatergic neurons in the NTS project to neurons containing γ-aminobutyric acid (GABA) in the caudal ventrolateral medulla (CVLM) that inhibit RVLM neurons, resulting in suppression of sympathetic activity (Figure 1). This NTS-CVLM-RVLM pathway is the well known neural pathway utilized by the baroreflex response4. The NTS receives projections from the area postrema, which lacks a blood-brain barrier, rendering it capable of sensing humoral changes in the blood. The NTS also receives vagal afferent signals via the nodose ganglion10, 11. The NTS neurons also project to the PVN, CVLM, nucleus ambiguous and dorsal motor nucleus of the vagus to modulate the overall autonomic balance between sympathetic and parasympathetic outflows. It has been suggested that in animal models of hypertension such as spontaneously hypertensive rats (SHR) or renovascular hypertension rats, impaired functions within the NTS-CVLM-RVLM pathway contribute to the development and maintenance of hypertension1. Further investigations into central regulatory sites within the brain and their roles in human diseases are needed.
Figure 1.
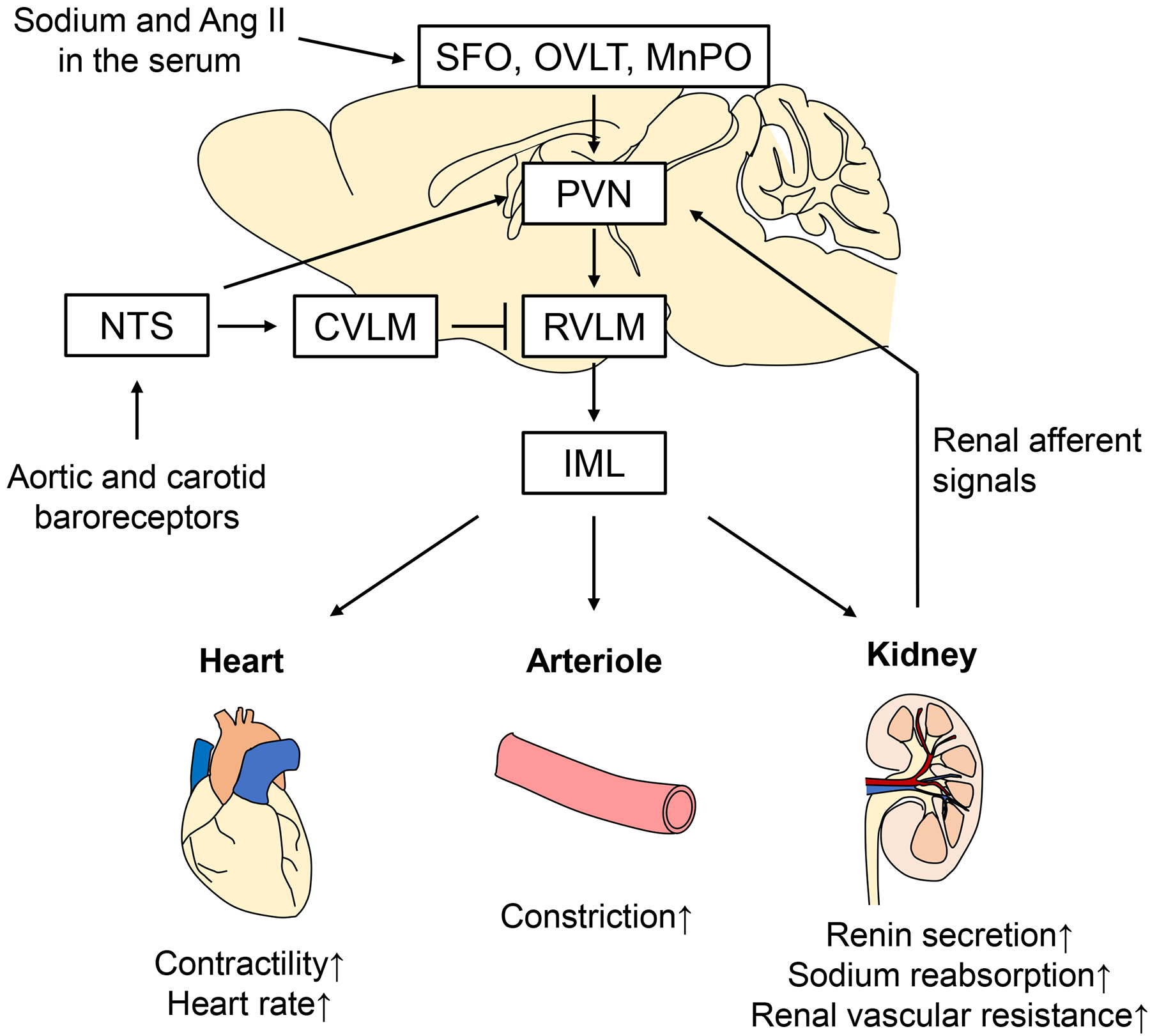
Proposed model for the integration of afferent information to the PVN and the subsequent sympathetic outflow regulating the heart, peripheral arterioles and the kidney. SFO: subfornical organ; OVLT: organum vasculosum lamina terminalis; MnPO: median preoptic nucleus; PVN: paraventricular nucleus; RVLM: rostral ventrolateral medulla; IML: intermediolateral cell column; NTS: nucleus tractus solitarius; CVLM: caudal ventrolateral medulla; Ang II: angiotensin II.
Brain renin-angiotensin system in hypertension
The brain renin-angiotensin system (RAS) contributes to regulation of blood pressure independent of the circulatory RAS5, 12. Activation of angiotensin type 1 (AT1) receptor within the hypothalamus and the brain stem existing upstream of increased reactive oxygen species (ROS) and decreased nitric oxide (NO) augments sympathetic outflow5. Activation of the (pro)renin receptor producing Ang II in the brain increases blood pressure. There are two isoforms of brain renin, namely, secreted renin and intracellular renin. It has been reported that increased secreted renin and decreased intracellular renin within the PVN and RVLM induce sympathoexitation13. Angiotensin converting enzyme 2 (ACE2) and angiotensin 1–7 (Ang (1–7)) are also key players in the brain RAS in hypertension. In transgenic mice with overexpression of brain ACE2, brain Ang (1–7) and expression of angiotensin type 2 (AT2) receptors are augmented, suppressing the development of hypertension induced by continuous infusion of Ang II14. In rats, overexpression of ACE2 specifically within the PVN attenuates Ang II-induced hypertension and increase in the expressions of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β) and IL-6 in the PVN15. In deoxycorticosterone acetate (DOCA)-salt hypertensive mice, development of hypertension is accompanied with a decrease in brain ACE2 expression and an increase in sympathetic nerve activity. In contrast, overexpression of brain ACE2 counteracts hypertension and sympathoexcitation in DOCA-salt hypertensive mice16. As possible mechanisms underlying these effects, it has been suggested that ROS and extracellular signal-regulated kinase activate A disintegrin and metalloprotease 17 (ADAM17) to inhibit expression of ACE216. The brain angiotensin III (Ang III) also has properties that lead to a capability to regulate blood pressure. Ang III is generated from Ang II and then degraded by aminopeptidase A. Both Ang II and Ang III increase blood pressure via AT1 receptors in the brain (Figure 2). A series of studies using SHR and DOCA-salt hypertensive rats have revealed a critical role for Ang III in brain RAS-mediated blood pressure regulation and demonstrated an antihypertensive effect of aminopeptidase A inhibitor17. Currently, a phase II clinical trial is ongoing to investigate the antihypertensive effect of an oral dose of firibastat, an aminopeptidase A inhibitor.
Figure 2.
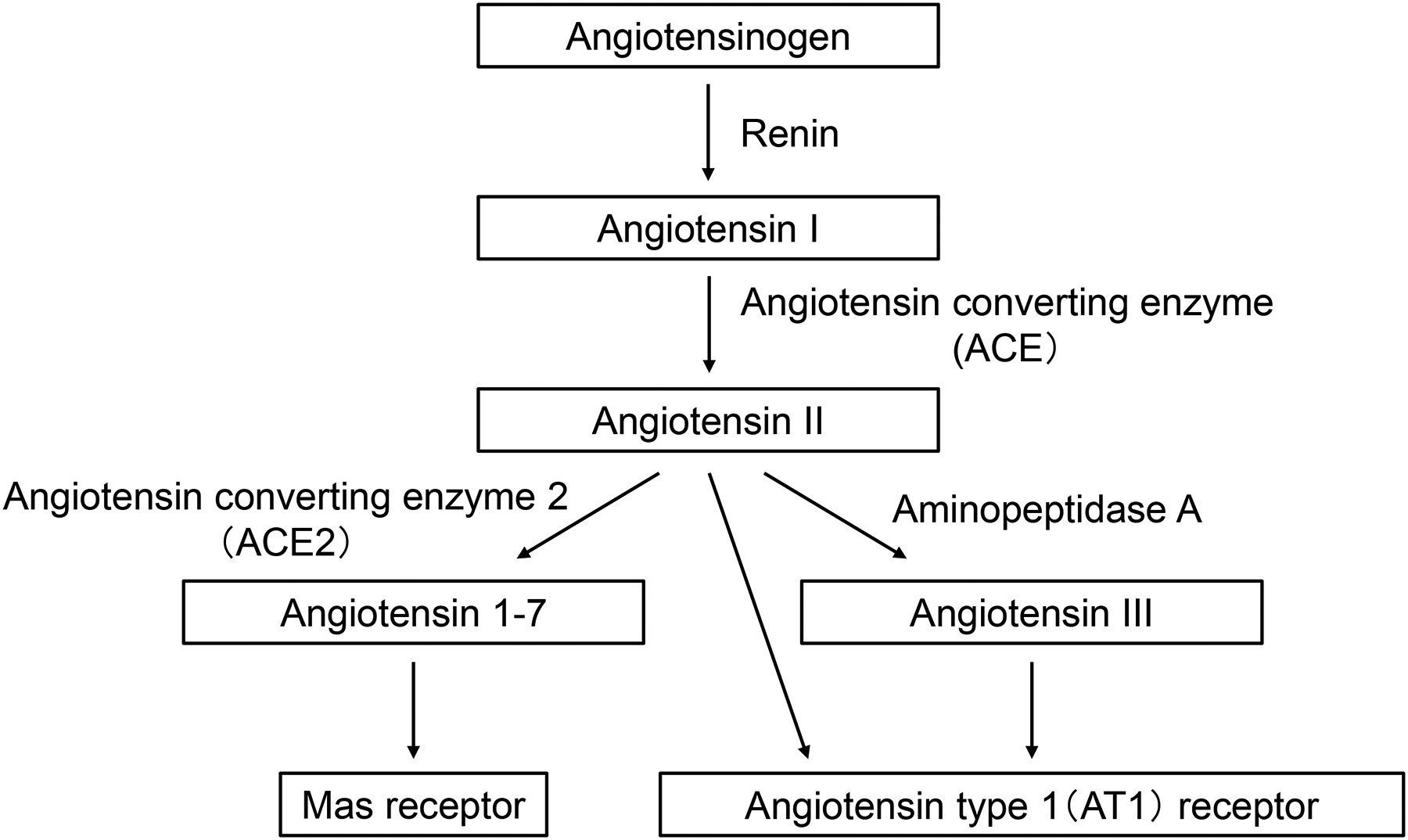
A schematic of the brain renin-angiotensin system, which affects various central nervous system sites involved in the regulation of cardiovascular function.
Brain NO and ROS in hypertension
Brain NO and ROS mechanisms are important factors in regulating sympathetic outflow5. Previous studies have shown that overexpression of endothelial NO synthase (eNOS) within the NTS18 or RVLM19 decreases blood pressure and heart rate accompanied with sympathoinhibition. Some of our previous studies showed that overexpression of neuronal NOS (nNOS) within the PVN suppresses renal sympathetic nerve activity both under normal and disease conditions such as HF20–22. It has also been reported that these brain NO-mediated sympathoinhibitory effects are impaired in various hypertensive models, including SHR and stroke-prone SHR (SHRSP)23–25. In addition, it has been shown that chronic infusion of an NOS inhibitor in normotensive rats induces development of hypertension26.
In animal models of hypertension—such as mice with Ang II infusion, SHR and SHRSP—enhanced ROS production within the hypothalamus and brain stem are thought to induce sympathoexcitation5, 27–30. In SHR, transfer of the gene of an antioxidant agent, super oxide dismutase (SOD), into the RVLM decreases blood pressure and heart rate, attenuates decreases in blood pressure and RSNA elicited by blockade of glutamine receptors, and enhances increases in blood pressure and RSNA by blockade of GABA receptors29. Overexpression of SOD in the PVN decreases heart rate and attenuates increases in blood pressure and RSNA by blockade of GABA receptors. The reduction in blood pressure and RSNA is greater following overexpression of SOD in the RVLM than following overexpression of SOD in the PVN30. These findings suggest that ROS production in the RVLM enhances excitatory glutamatergic input and attenuates inhibitory GABAergic input from the PVN, thereby increasing sympathetic outflow and leading to hypertension.
Brain inflammation in hypertension
Enhanced inflammation in specific brain areas is thought to be involved in development/maintenance of hypertension. In hypertensive rats with continuous infusion of Ang II, microglia are activated within the PVN, which induces enhanced expression of inflammatory cytokines, including IL-1β, IL-6 and TNF-α31. Intracerebroventricular injection of an anti-inflammatory agent, minocycline, inhibits activation of microglia, and then decreases the expression of inflammatory cytokines as well as blood pressure and plasma levels of norepinephrine32. Enhanced inflammation within the PVN and antihypertensive effects of anti-inflammatory treatment have also been demonstrated in other animal models of hypertension, such as rats with infusion of NOS inhibitor, SHR and SHRSP5. Additionally, another study showed that enhanced expression of leukotriene B4 (LTB4) induces inflammation in the NTS of SHR compared with normotensive Wistar Kyoto rats33. Conversely, administration of LTB4 inhibitor into the NTS decreases blood pressure and suppresses sympathetic nerve activity, as indexed by spectrum analysis for blood pressure variability33. Recently, it has also been reported that oral administration of LTB4 inhibitor to SHR decreases blood pressure accompanied with sympathoinhibition34.
RDN in experimental models of hypertension
It is now well-established that RDN decreases arterial pressure in various models of hypertension in animals as well as in humans8, 35–37. It is plausible that the antihypertensive effect of RDN is mainly mediated by sympathetic efferent denervation-induced suppression of renin secretion, sodium reabsorption and renal vascular resistance3, 8. Additionally, it has been proposed that specific afferent denervation modulates sensory signals from the kidney to the CNS to suppress sympathetic outflow3, 8. However, the role of afferent renal nerves in regulation of the cardiovascular system has not been fully elucidated. In animal models, RDN is generally performed by surgical cutting of visible renal nerves followed by phenol and/or ethanol application to destroy invisible nerves. This procedure achieves both efferent and afferent renal denervation, which is termed total RDN (T-RDN). Classically, dorsal rhizotomy has been performed to address the contribution of afferent renal nerves. This is achieved by sectioning the dorsal roots through which afferent renal nerves travel into the spinal cord. In SHR, dorsal rhizotomy does not reduce blood pressure, but T-RDN does reduce blood pressure, suggesting that afferent renal nerves do not contribute to the pathogenesis of hypertension in SHR38. However, dorsal rhizotomy has a limitation in that it does not specifically ablate renal afferent nerves. Recently, a novel method to achieve specific afferent renal denervation (A-RDN) with capsaicin application was reported39, 40. In this procedure, capsaicin is applied at a high dose (33 mM) that is known to affect only afferent nerves expressing capsaicin receptors; the applied capsaicin thus induces intracellular destruction and dysfunction of afferent nerves but not efferent nerves. By comparing the effects of T-RDN with those by A-RDN, it becomes possible to assess the different contributions of efferent and afferent renal nerves. To date, several studies have reported different effects of T-RDN or A-RDN on blood pressure in various animal models of hypertension (Table 1). In DOCA-salt hypertensive rats and renovascular hypertensive rats/mice, A-RDN reduces blood pressure, and this effect of A-RDN is comparable to that of T-RDN, suggesting that afferent renal nerves contribute to the pathology of hypertension in these animal models39, 41–43. In contrast, in Dahl salt-sensitive hypertensive rats and hypertensive mice with continuous infusion of Ang II, A-RDN does not reduce blood pressure, while T-RDN does reduce blood pressure44, 45. These observations of different effects of T-RDN or A-RDN in different animal models of hypertension suggest the potentially complicated and diversified origins of hypertension.
Table 1.
Antihypertensive effects of T-RDN or A-RDN in different animal models
| Blood pressure | |||
|---|---|---|---|
| Animal Model | T-RDN | A-RDN | References |
| SHR | ↓ | → (DR) | Can J Physiol Pharmacol 1980;58:1384–1388 |
| ↓ | Am J Physiol 1982;243:H284–8 | ||
| ↓ | Can J Physiol Pharmacol 1987;65:1548–1558 | ||
| ↓ | Clin Exp Pharmacol Physiol 1995;22:512–517 | ||
| ↓ | J Hypertension 2005;23:851–859 | ||
| ↓ | Am J Physiol Regul Integr Comp Physiol 2006;290:R341–R344 | ||
| SHR-Salt | ↓ | ? | Hypertension 1993;22:1–8 |
| SHRSP | ↓ | ? | Compr Physiol 2017; 7:263–320 |
| SHRSP-Salt | ↓ | ? | J Am Heart Assoc 2013;2:e000375 |
| SHRcp | ↓ | ? | J Am Heart Assoc 2013;2:e000197 |
| Dahl SS (Rat) | ↓ | → | Hypertension 2013;61:806–811 |
| Am J Physiol Regul Integr Comp Physiol 2016;310:R262-R267 | |||
| DOCA-Salt (Rat) | ↓ | ↓ | Am J Physiol Regul Integr Comp Physiol 2015;308:R112-R122 |
| Hypertension 2016;68:1415–1423 | |||
| AngII-Salt (Rat) | → | → | Physiol Rep 2018;6:e13602 |
| AngII-Salt (Rat) | ↓ | ? | Clin Exp Pharmacol Phys 2006;33:1225–1230 |
| AngII-Salt (Mouse) | ↓ | → | Circ Res 2015;117:547–557 |
| AngII(ICV)-Salt (Rat) | ↓ | ? | Hypertension 1997;30:331–336 |
| Obesity HT (Mouse) | ↓ | ? | Hypertension 2016;68:929–936 |
| Obesity HT (Dog) | ↓ | ? | Hypertension 2012;59:331–338 |
| CKD HT (5/6 nephrectomy Rat) | ↓ | J Hypertens 2020;38:765–773 | |
| CKD HT (2K1C Rat) | ↓ | ↓ | Auton Neurosci 2017;208:43–50 |
| Pflugers Arch 2020;472:325–334 | |||
| CKD HT (2K1C Mouse) | ↓ | ↓ | J Neurophysiol 2019;122:358–367 |
| SAS HT (Mouse) | ↓ | ? | SciRep 2018;8:17926 |
↓: depressor effect; →: no effect; ?: untested;. T-RDN: total renal denervation; A-RDN: afferent renal denervation; DR: dorsal rhizotomy; SHR: spontaneously hypertensive rat; SHRSP: stroke-prone SHR; SHRcp: SHR/NDmcr-cp(+/+) rat; Dahl SS: Dahl salt-sensitive rat; DOCA: deoxycorticosterone acetate; Ang II: angiotensin II; ICV: intracerebroventricular injection; HT: hypertension; CKD: chronic kidney disease; 2K1C: 2 kidney 1 clip model; SAS: sleep apnea syndrome.
RDN in experimental models of HF
Neuroanatomical studies suggest that afferent renal nerves project to specific areas within the CNS, such as the PVN46–49. Consistent with these observations, stimulation of afferent renal nerves has been shown to increase the neuronal discharge frequency and neuronal activity in the PVN7, 9. Recently, we have shown that basal afferent renal nerve activity is increased in rats with HF induced by coronary artery ligation (Figure 3)40, 50. An enhanced afferent renal input to the PVN may be critically involved in dictating sympathoexcitation in HF6, 7, 9. It has also been shown that there is greater activation of neurons in the PVN induced by afferent renal nerve stimulation in rats with HF7, 9, 51. It is well known that, just as in various models of hypertension, brain nNOS/NO is decreased in the PVN of various models of HF6, 40, 51–53. Both A-RDN and T-RDN restore nNOS/NO in the PVN with concomitant sympathoinhibition in rats with HF6, 40, 51.
Figure 3.
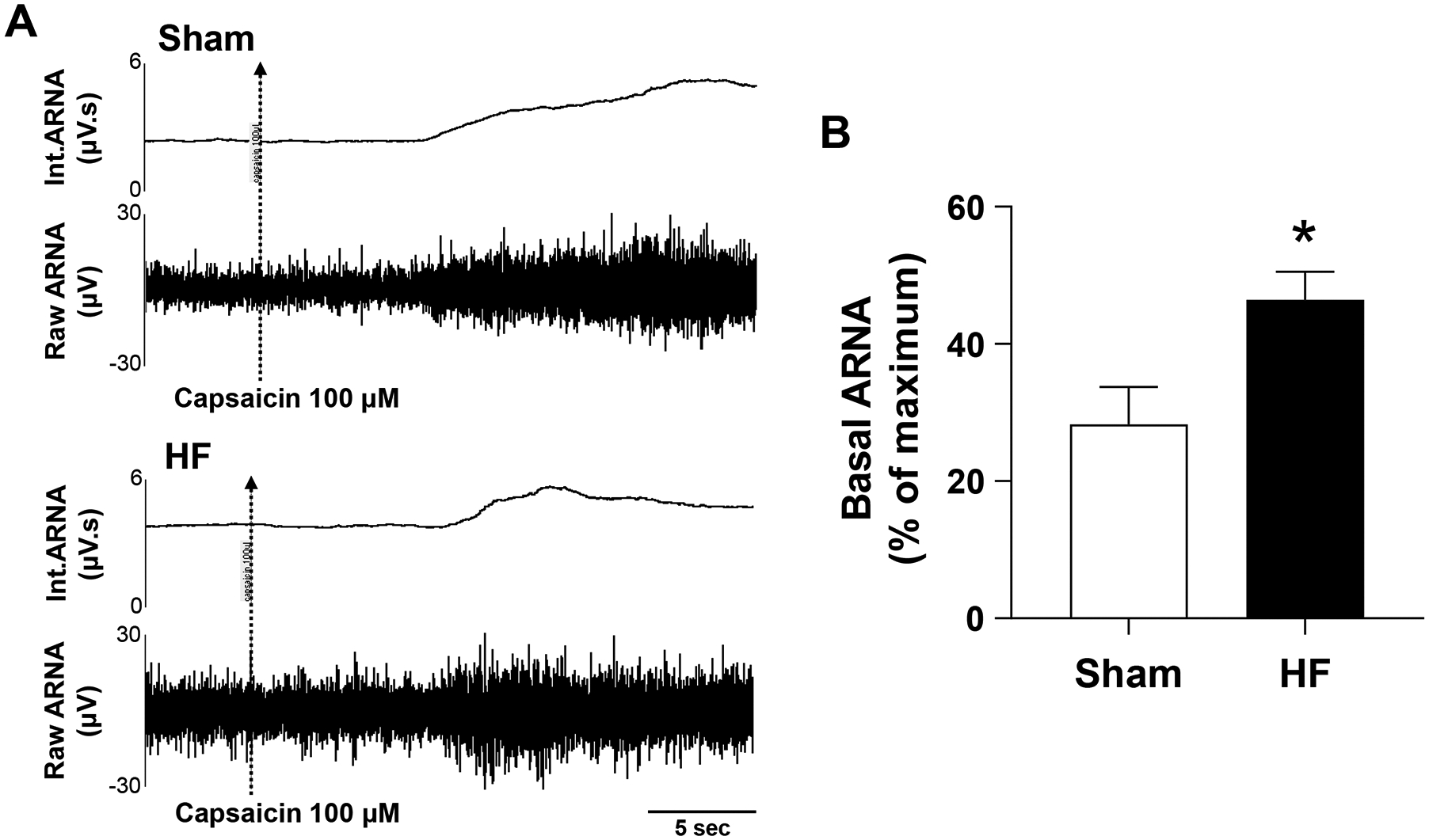
Activation of afferent renal nerve activity (ARNA) from the renal pelvis. ARNA response to intrapelvic injection of capsaicin (activating the transient receptor potential vanilloid 1, i.e., the non-selective cation channel) is shown. A: Raw tracings and integrated signals of ARNA from Sham and HF rats. The ARNA was recorded before and after intrapelvic injection of capsaicin 100 μM to determine the peak level of ARNA (ARNAmax). B: Summary data for basal ARNA expressed as a percentage of ARNAmax in the two groups of rats. Data are presented as mean ±SE. n=6. *P < 0.05 vs. Sham.
Animal studies using rats with HF have also shown that sympathoexcitation to the kidney induces enhanced expressions of renal epithelial sodium channels (ENaC) and water channel aquaporin 2 (AQP2), leading to subsequent renal sodium and water retention associated with HF. T-RDN significantly reduces the expressions of these transporters54. Another study has shown that neprilysin activity is greater in the kidneys of swine with HF induced by coronary artery occlusion. Catheter-based radiofrequency renal denervation reduces renal neprilysin activity and augments circulating levels of natriuretic peptides55. It remains to be examined whether afferent or efferent renal nerves mainly contribute to the expressions of ENaC and AQP2, and to neprilysin activity in the kidneys. In the hearts of rats with HF, sympathoexcitation induces the desensitization or downregulation of β-adrenoceptors and the fibrosis in cardiomyocytes, leading to cardiac remodeling and dysfunction associated with HF. T-RDN restores β-adrenoceptor-mediated cardiac function and attenuates cardiac fibrosis56. Moreover, the diuretic and natriuretic responses that are generated by glucagon-like peptide-1 (GLP-1) are blunted in rats with HF. A-RDN reestablishes these responses to GLP-1, indicating that A-RDN has potential therapeutic efficacy for use in HF50. Taken together, these findings demonstrate the beneficial effects of RDN during HF, and suggest that these effects are partially mediated by afferent renal denervation interrupting the signals from the kidney to the brain, and thereby ameliorating sympathetic outflow to the kidneys and the heart (Figure 4). This crosstalk between the kidney and the CNS interacting with the heart provides intriguing insight into the potential mechanisms underlying cardio-renal syndrome.
Figure 4.
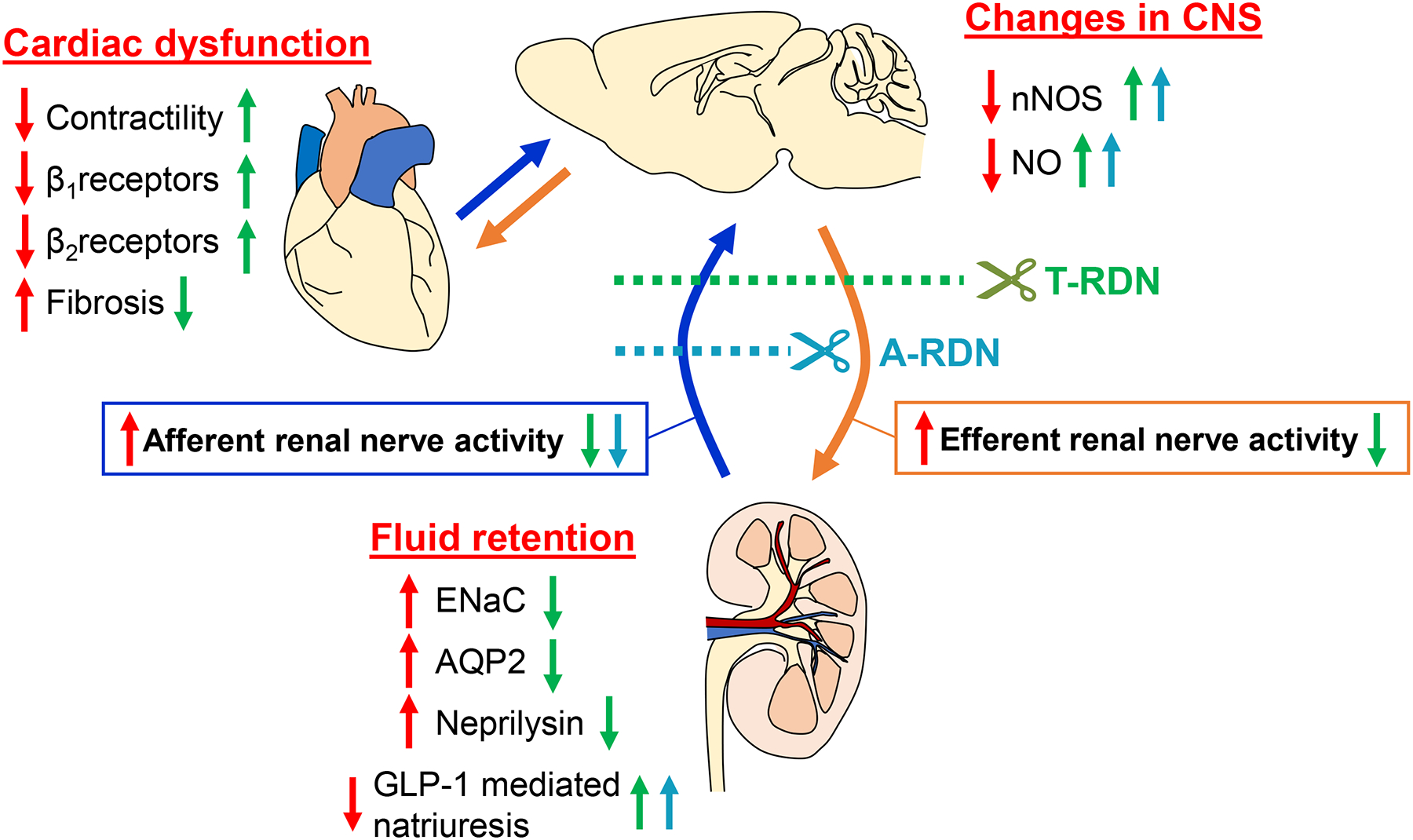
Proposed model for the potential therapeutic effects of RDN in HF on cardiac function, changes in the CNS and fluid retention. Red arrows indicate the effects of HF on these parameters. Green arrows indicate the effects of T-RDN on these parameters in HF. Blue arrows indicate the effects of A-RDN on these parameters in HF. CNS: central nervous system; NO: nitric oxide; nNOS: neuronal NO synthase; T-RDN: total renal denervation; A-RDN: afferent renal denervation; ENaC: epithelial sodium channels; AQP2: water channel aquaporin 2; GLP-1: glucagon-like peptide-1.
Clinical aspects of RDN in hypertension and HF
In drug-resistant hypertensive patients with hemodialysis, bilateral nephrectomy normalizes MSNA57 and improves control of blood pressure58, suggesting that afferent renal nerve activity could potentially elicit sympathoexcitation and consequent hypertension. Another study in patients with SAS demonstrated the relationship between enhanced MSNA and activation of the areas of the brain cortex projecting to the NTS and RVLM as assessed by functional magnetic resonance imaging59. RDN has been reported to improve SAS60, 61 and hypoxia-induced nocturnal hypertension62. As to the mechanism potentially underlying these effects, RDN is known to interrupt afferent renal inputs to the CNS to suppress the activities of specific areas in the brain, as outlined and discussed above, which could thereby inhibit sympathoexcitation and ameliorate SAS. Nighttime blood pressure and dipping status are important contributors to the risk of target organ damage and cardiovascular diseases including HF63–67. Randomized prospective sham-controlled clinical trials have shown that RDN is particularly effective in lowering nighttime blood pressure35, 36. In animal studies, it has been shown that RDN has a positive effect not only on blood pressure but also on fluid retention associated with natriuresis, renal transporters and neprilysin activity, as mentioned above50, 54, 55. Taken together, these findings suggest that RDN has potential therapeutic effects for reducing the detrimental sympathoexcitation that is commonly observed in HF as well as hypertension.
Conclusion
Blood pressure and fluid volume regulation by the sympathetic nervous system is the process that integrates visceral cardiovascular inputs from various peripheral sites, including the kidneys within the CNS, specifically within the PVN to influence sympathetic outflow innervating the kidneys, heart and systemic arterioles. Brain RAS, NO, and ROS as well as inflammation are intimately involved in these complex interactive processes within these central sites. RDN is a new approach which alleviates these pathophysiological processes by interrupting the vicious cycle of positive feedback to enhance sympathoexcitation in cardiovascular diseases such as hypertension and HF. Further investigations are needed to explore the differential roles of afferent and efferent renal nerves in the pathophysiology of hypertension and HF. In clinical terms, it would be very useful to establish serum or urinary biomarkers reflecting efferent and afferent renal nerve activity in these disease conditions in order to identify potential responders to RDN and to estimate the efficacy of RDN.
Funding
This work was supported in part by National Institutes of Health grants R56 HL124104, P01 HL62222, and R01 DK114663, by an endowed McIntyre Professorship (to K. Patel), and by Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad (to K. Katsurada).
Footnotes
Conflict of interest
Kazuomi Kario MD PhD (K.K)., received speaker fees and works as a consultant to JIMRO Co.Ltd., Medtronic Co.Inc. and Terumo Co.Inc.. The other authors declare that they have no conflict of interest.
References
- 1.Hering D and Schlaich M. The Role of Central Nervous System Mechanisms in Resistant Hypertension. Curr Hypertens Rep. 2015;17:58. [DOI] [PubMed] [Google Scholar]
- 2.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA and Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–21. [DOI] [PubMed] [Google Scholar]
- 3.Kario K. Essential manual of 24 hour blood pressure management : from morning to nocturnal hypertension. Chichester, West Sussex, UK; Malden, MA: John Wiley & Sons Inc.; 2015. [Google Scholar]
- 4.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46. [DOI] [PubMed] [Google Scholar]
- 5.Hirooka Y. Sympathetic Activation in Hypertension: Importance of the Central Nervous System. Am J Hypertens. 2020;33:914–926. [DOI] [PubMed] [Google Scholar]
- 6.Zheng H and Patel KP. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton Neurosci. 2017;204:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu B, Zheng H, Liu X and Patel KP. Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol. 2015;308:H1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborn JW and Foss JD. Renal Nerves and Long-Term Control of Arterial Pressure. Compr Physiol. 2017;7:263–320. [DOI] [PubMed] [Google Scholar]
- 9.Xu B, Zheng H and Patel KP. Enhanced activation of RVLM-projecting PVN neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton RB and Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–77. [DOI] [PubMed] [Google Scholar]
- 11.Browning KN and Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirooka Y, Kishi T, Ito K and Sunagawa K. Potential clinical application of recently discovered brain mechanisms involved in hypertension. Hypertension. 2013;62:995–1002. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara K, Liu X, Morgan DA, Davis DR, Sequeira-Lopez ML, Cassell MD, Grobe JL, Rahmouni K and Sigmund CD. Selective Deletion of the Brain-Specific Isoform of Renin Causes Neurogenic Hypertension. Hypertension. 2016;68:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD and Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sriramula S, Cardinale JP, Lazartigues E and Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukerjee S, Gao H, Xu J, Sato R, Zsombok A and Lazartigues E. ACE2 and ADAM17 Interaction Regulates the Activity of Presympathetic Neurons. Hypertension. 2019;74:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llorens-Cortes C and Touyz RM. Evolution of a New Class of Antihypertensive Drugs: Targeting the Brain Renin-Angiotensin System. Hypertension. 2020;75:6–15. [DOI] [PubMed] [Google Scholar]
- 18.Sakai K, Hirooka Y, Matsuo I, Eshima K, Shigematsu H, Shimokawa H and Takeshita A. Overexpression of eNOS in NTS causes hypotension and bradycardia in vivo. Hypertension. 2000;36:1023–8. [DOI] [PubMed] [Google Scholar]
- 19.Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H and Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901. [PubMed] [Google Scholar]
- 20.Li YF, Roy SK, Channon KM, Zucker IH and Patel KP. Effect of in vivo gene transfer of nNOS in the PVN on renal nerve discharge in rats. Am J Physiol Heart Circ Physiol. 2002;282:H594–601. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H, Liu X, Li Y, Sharma NM and Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011;58:966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBryde FD, Liu BH, Roloff EV, Kasparov S and Paton JFR. Hypothalamic paraventricular nucleus neuronal nitric oxide synthase activity is a major determinant of renal sympathetic discharge in conscious Wistar rats. Exp Physiol. 2018;103:419–428. [DOI] [PubMed] [Google Scholar]
- 23.Kishi T, Hirooka Y, Ito K, Sakai K, Shimokawa H and Takeshita A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension. 2002;39:264–8. [DOI] [PubMed] [Google Scholar]
- 24.Hirooka Y, Sakai K, Kishi T, Ito K, Shimokawa H and Takeshita A. Enhanced depressor response to endothelial nitric oxide synthase gene transfer into the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2003;26:325–31. [DOI] [PubMed] [Google Scholar]
- 25.Kishi T, Hirooka Y, Kimura Y, Sakai K, Ito K, Shimokawa H and Takeshita A. Overexpression of eNOS in RVLM improves impaired baroreflex control of heart rate in SHRSP. Rostral ventrolateral medulla. Stroke-prone spontaneously hypertensive rats. Hypertension. 2003;41:255–60. [DOI] [PubMed] [Google Scholar]
- 26.Eshima K, Hirooka Y, Shigematsu H, Matsuo I, Koike G, Sakai K and Takeshita A. Angiotensin in the nucleus tractus solitarii contributes to neurogenic hypertension caused by chronic nitric oxide synthase inhibition. Hypertension. 2000;36:259–63. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman MC, Lazartigues E, Sharma RV and Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–6. [DOI] [PubMed] [Google Scholar]
- 28.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H and Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109:2357–62. [DOI] [PubMed] [Google Scholar]
- 29.Nishihara M, Hirooka Y, Matsukawa R, Kishi T and Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. 2012;30:97–106. [DOI] [PubMed] [Google Scholar]
- 30.Nishihara M, Hirooka Y, Kishi T and Sunagawa K. Different role of oxidative stress in paraventricular nucleus and rostral ventrolateral medulla in cardiovascular regulation in awake spontaneously hypertensive rats. J Hypertens. 2012;30:1758–65. [DOI] [PubMed] [Google Scholar]
- 31.Ahmari N, Santisteban MM, Miller DR, Geis NM, Larkin R, Redler T, Denson H, Khoshbouei H, Baekey DM, Raizada MK and Zubcevic J. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am J Physiol Heart Circ Physiol. 2019;317:H279–H289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P and Shi P. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waki H, Hendy EB, Hindmarch CC, Gouraud S, Toward M, Kasparov S, Murphy D and Paton JF. Excessive leukotriene B4 in nucleus tractus solitarii is prohypertensive in spontaneously hypertensive rats. Hypertension. 2013;61:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marvar PJ, Hendy EB, Cruise TD, Walas D, DeCicco D, Vadigepalli R, Schwaber JS, Waki H, Murphy D and Paton JF. Systemic leukotriene B4 receptor antagonism lowers arterial blood pressure and improves autonomic function in the spontaneously hypertensive rat. J Physiol. 2016;594:5975–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, East C, Lee DP, Ma A, Ewen S, Cohen DL, Wilensky R, Devireddy CM, Lea J, Schmid A, Weil J, Agdirlioglu T, Reedus D, Jefferson BK, Reyes D, D’Souza R, Sharp ASP, Sharif F, Fahy M, DeBruin V, Cohen SA, Brar S, Townsend RR and Investigators SH-OMP. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. [DOI] [PubMed] [Google Scholar]
- 36.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K and Investigators SH-OMT. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 37.Kandzari DE, Mahfoud F, Bhatt DL, Bohm M, Weber MA, Townsend RR, Hettrick DA, Schmieder RE, Tsioufis K and Kario K. Confounding Factors in Renal Denervation Trials: Revisiting Old and Identifying New Challenges in Trial Design of Device Therapies for Hypertension. Hypertension. 2020;76:1410–1417. [DOI] [PubMed] [Google Scholar]
- 38.Oparil S, Sripairojthikoon W and Wyss JM. The renal afferent nerves in the pathogenesis of hypertension. Can J Physiol Pharmacol. 1987;65:1548–58. [DOI] [PubMed] [Google Scholar]
- 39.Foss JD, Wainford RD, Engeland WC, Fink GD and Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H, Katsurada K, Liu X, Knuepfer MM and Patel KP. Specific Afferent Renal Denervation Prevents Reduction in Neuronal Nitric Oxide Synthase Within the Paraventricular Nucleus in Rats With Chronic Heart Failure. Hypertension. 2018;72:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y and Osborn JW. Resting Afferent Renal Nerve Discharge and Renal Inflammation: Elucidating the Role of Afferent and Efferent Renal Nerves in Deoxycorticosterone Acetate Salt Hypertension. Hypertension. 2016;68:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veiga AC, Milanez MIO, Ferreira GR, Lopes NR, Santos CP, De Angelis K, Garcia ML, Oyama LM, Gomes GN, Nogueira FN, Carvalho PM, Campos RR, Bergamaschi CT and Nishi EE. Selective afferent renal denervation mitigates renal and splanchnic sympathetic nerve overactivity and renal function in chronic kidney disease-induced hypertension. J Hypertens. 2020;38:765–773. [DOI] [PubMed] [Google Scholar]
- 43.Lopes NR, Milanez MIO, Martins BS, Veiga AC, Ferreira GR, Gomes GN, Girardi AC, Carvalho PM, Nogueira FN, Campos RR, Bergamaschi CT and Nishi EE. Afferent innervation of the ischemic kidney contributes to renal dysfunction in renovascular hypertensive rats. Pflugers Arch. 2020;472:325–334. [DOI] [PubMed] [Google Scholar]
- 44.Foss JD, Fink GD and Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol. 2016;310:R262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd, Osborn JW, Itani HA and Harrison DG. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II-Induced Hypertension. Circ Res. 2015;117:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciriello J and Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–85. [DOI] [PubMed] [Google Scholar]
- 47.Kuo DC, Nadelhaft I, Hisamitsu T and de Groat WC. Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol. 1983;216:162–74. [DOI] [PubMed] [Google Scholar]
- 48.Wyss JM and Donovan MK. A direct projection from the kidney to the brainstem. Brain Res. 1984;298:130–4. [DOI] [PubMed] [Google Scholar]
- 49.Weiss ML, Chowdhury SI, Patel KP, Kenney MJ and Huang J. Neural circuitry of the kidney: NO-containing neurons. Brain Res. 2001;919:269–82. [DOI] [PubMed] [Google Scholar]
- 50.Katsurada K, Nandi SS, Zheng H, Liu X, Sharma NM and Patel KP. GLP-1 mediated diuresis and natriuresis are blunted in heart failure and restored by selective afferent renal denervation. Cardiovascular Diabetology. 2020;19:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel KP, Xu B, Liu X, Sharma NM and Zheng H. Renal Denervation Improves Exaggerated Sympathoexcitation in Rats With Heart Failure: A Role for Neuronal Nitric Oxide Synthase in the Paraventricular Nucleus. Hypertension. 2016;68:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramchandra R, Hood SG and May CN. Central exogenous nitric oxide decreases cardiac sympathetic drive and improves baroreflex control of heart rate in ovine heart failure. Am J Physiol Regul Integr Comp Physiol. 2014;307:R271–80. [DOI] [PubMed] [Google Scholar]
- 53.Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M and Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol. 2011;106:273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng H, Liu X, Katsurada K and Patel KP. Renal denervation improves sodium excretion in rats with chronic heart failure: effects on expression of renal ENaC and AQP2. Am J Physiol Heart Circ Physiol. 2019;317:H958–H968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharp TE 3rd, Polhemus DJ, Li Z, Spaletra P, Jenkins JS, Reilly JP, White CJ, Kapusta DR, Lefer DJ and Goodchild TT. Renal Denervation Prevents Heart Failure Progression Via Inhibition of the Renin-Angiotensin System. J Am Coll Cardiol. 2018;72:2609–2621. [DOI] [PubMed] [Google Scholar]
- 56.Zheng H, Liu X, Sharma NM and Patel KP. Renal denervation improves cardiac function in rats with chronic heart failure: Effects on expression of beta-adrenoceptors. Am J Physiol Heart Circ Physiol. 2016;311:H337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Converse RL Jr., Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F and Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–8. [DOI] [PubMed] [Google Scholar]
- 58.Zazgornik J, Biesenbach G, Janko O, Gross C, Mair R, Brucke P, Debska-Slizien A and Rutkowski B. Bilateral nephrectomy: the best, but often overlooked, treatment for refractory hypertension in hemodialysis patients. Am J Hypertens. 1998;11:1364–70. [DOI] [PubMed] [Google Scholar]
- 59.Fatouleh RH, Hammam E, Lundblad LC, Macey PM, McKenzie DK, Henderson LA and Macefield VG. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin. 2014;6:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shantha GP and Pancholy SB. Effect of renal sympathetic denervation on apnea-hypopnea index in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2015;19:29–34. [DOI] [PubMed] [Google Scholar]
- 61.Kario K, Hettrick DA, Prejbisz A and Januszewicz A. Obstructive Sleep Apnea-Induced Neurogenic Nocturnal Hypertension: A Potential Role of Renal Denervation? Hypertension. 2021;77:1047–1060. [DOI] [PubMed] [Google Scholar]
- 62.Kario K, Ikemoto T, Kuwabara M, Ishiyama H, Saito K and Hoshide S. Catheter-Based Renal Denervation Reduces Hypoxia-Triggered Nocturnal Blood Pressure Peak in Obstructive Sleep Apnea Syndrome. J Clin Hypertens (Greenwich). 2016;18:707–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, Ojima T and Shimada K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–8. [DOI] [PubMed] [Google Scholar]
- 64.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE and Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–6. [DOI] [PubMed] [Google Scholar]
- 65.Hoshide S, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, Eguchi K, Ishikawa J, Kario K and Group JHS. Morning and Evening Home Blood Pressure and Risks of Incident Stroke and Coronary Artery Disease in the Japanese General Practice Population: The Japan Morning Surge-Home Blood Pressure Study. Hypertension. 2016;68:54–61. [DOI] [PubMed] [Google Scholar]
- 66.Kario K, Kanegae H, Tomitani N, Okawara Y, Fujiwara T, Yano Y and Hoshide S. Nighttime Blood Pressure Measured by Home Blood Pressure Monitoring as an Independent Predictor of Cardiovascular Events in General Practice. Hypertension. 2019;73:1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kario K, Hoshide S, Mizuno H, Kabutoya T, Nishizawa M, Yoshida T, Abe H, Katsuya T, Fujita Y, Okazaki O, Yano Y, Tomitani N, Kanegae H and Group JS. Nighttime Blood Pressure Phenotype and Cardiovascular Prognosis: Practitioner-Based Nationwide JAMP Study. Circulation. 2020;142:1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]


