Abstract
Background/Aims
We aim to assess the influence of obesity on gastroparesis (GP) hospitalizations in the United States (US).
Methods
The National Inpatient Sample was analyzed from 2007-2017 to identify all adult hospitalizations with a primary discharge diagnosis of GP. They were subdivided based on the presence or absence of obesity (body mass index > 30). Hospitalization characteristics, procedural differences, all-cause inpatient mortality, mean length of stay (LOS), and mean total hospital charge (THC) were identified and compared.
Results
From 2007-2017, there were 140 293 obese GP hospitalizations accounting for 13.75% of all GP hospitalizations in the US. Obese GP hospitalizations were predominantly female (76.11% vs 64.36%, P < 0.001) and slightly older (51.9 years vs 50.8 years, P < 0.001) compared to the non-obese cohort. Racial disparities were noted as Blacks (25.49% vs 22%, P < 0.001) had higher proportions of GP hospitalizations with obesity compared to the non-obese cohort. Furthermore, we noted higher rates of inpatient upper endoscopy utilization (6.05% vs 5.42%, P < 0.001), longer mean LOS (5.71 days vs 5.32 days, P < 0.001), and higher mean THC ($53 373 vs $45 040, P < 0.001) for obese GP hospitalizations compared to the non-obese group. However, obese GP hospitalizations had lower rates of inpatient mortality (0.92% vs 1.33%, P < 0.001), and need for nutritional support with endoscopic jejunostomy (0.25 vs 0.56%, P < 0.001) and total parenteral nutrition (1.46% vs 2.33%, P < 0.001) compared to the non-obese cohort.
Conclusions
In the US, compared to non-obese, a higher proportion of obese GP hospitalizations were female and Blacks. Obese GP hospitalizations also had higher THC, LOS, and rates of upper endoscopy.
Keywords: Cost, Gastroparesis, Mortality, Obesity, Outcomes
Introduction
Gastroparesis (GP) is a chronic motility disorder of the stomach characterized by objectively delayed gastric emptying in the absence of mechanical obstruction.1,2 First described by Kassander et al3 in 1958, the exact pathogenic mechanisms implicated in the development of GP are currently unknown. It can be classified into several distinct categories based on the underlying etiology, with idiopathic GP (35%) being the most common.4 Clinical features commonly seen in patients with GP include nausea, vomiting, early satiety, and post-prandial fullness often leading to significant weight loss.2,5 However, in recent literature, growing evidence suggests that patients with GP may in fact be overweight (body mass index [BMI] ≥ 25 kg/m2 but < 30 kg/m2) or obese (BMI > 30 kg/m2) secondary to the acquisition of maladaptive dietary habits to cope with the symptoms of GP.6 Additionally, the presence of obesity in these patients may further lead to the development of refractory GP.6 Although numerous studies focus on the epidemiology, pathogenesis, and management aspects of GP, there are significant gaps in knowledge on obese GP hospitalizations. Therefore, in this study, we identified hospitalization characteristics of obese GP hospitalizations. Furthermore, we also compared demographic and patient characteristics, adverse outcomes, and healthcare utilization between obese GP patients and those without obesity.
Materials and Methods
Design and Data Source
This retrospective national study utilized the National Inpatient Sample (NIS) database which consists of hospitalizations derived from billing data submitted by hospitals to state-wide data organizations covering more than 97% of the United States (US) population.7 It approximates a 20% stratified sample of discharges from US community hospitals (excluding rehabilitation and long-term acute care hospitals). The dataset is weighted to obtain national estimates.8 The database was coded using the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification/Procedure Coding System for the study period.
Study Population
We included all adult (≥ 18 years) hospitalizations with a primary discharge diagnosis of GP from the NIS database between January 2007-December 2017. Individuals < 18 years of age were excluded from the analysis. These hospitalizations were further subdivided into 2 distinct groups based on the presence or absence of obesity, which was defined as a BMI > 30 kg/m2.
Statistical Analysis and Outcome Measures
Statistical analysis was conducted using SAS 9.4 (SAS Institute Inc, Cary, NC, USA) to account for weights in the stratified survey design. The weights were considered in the statistical estimating process when incorporating the variables for strata which is the stratum used to post-stratify hospital, for cluster which is the Healthcare Cost and Utilization Project hospital identification number, and for weight which is the weight to discharges in the NIS universe. Descriptive statistics were provided and included the mean for age, length of stay (LOS) and total hospital charge (THC), and count (percentage) for other categorical variables. The Rao-Scott design-adjusted chi-square test, which takes the stratified survey design into account, examined the association between obesity status and a categorical variable. The difference of the means for age, LOS, and THC between obesity and non-obesity groups were tested by F-statistics from a weighted regression model. All analytical results were considered significant when P-values ≤ 0.05. We do not report any missing data for the variables and outcomes analyzed.
Ethical Considerations
The NIS database lacks patient and hospital-specific identifiers. Hence, this study did not require Institutional Review Board approval for analysis as per guidelines put forth by our Institutional Review Board for research on database studies.
Data Availability Statement
The NIS is one of the largest, publicly available, multi-ethnic inpatient database in the US. The database can be accessed at: https://www.hcup-us.ahrq.gov.
Results
Hospitalizations Characteristics for Obese and Non-obese Gastroparesis Hospitalizations
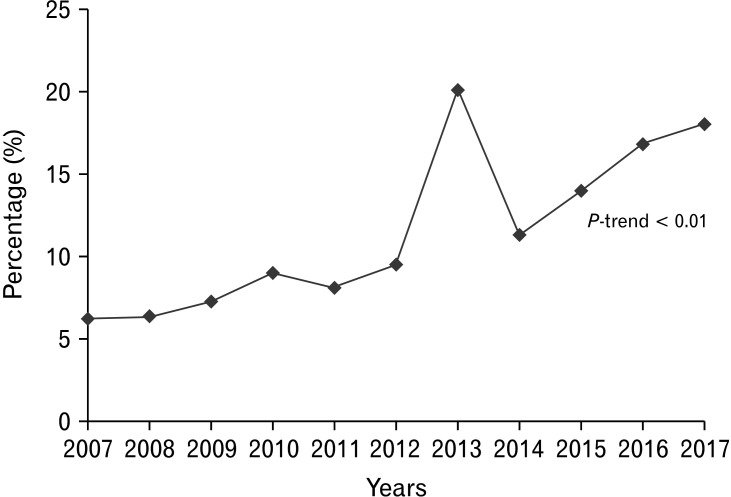
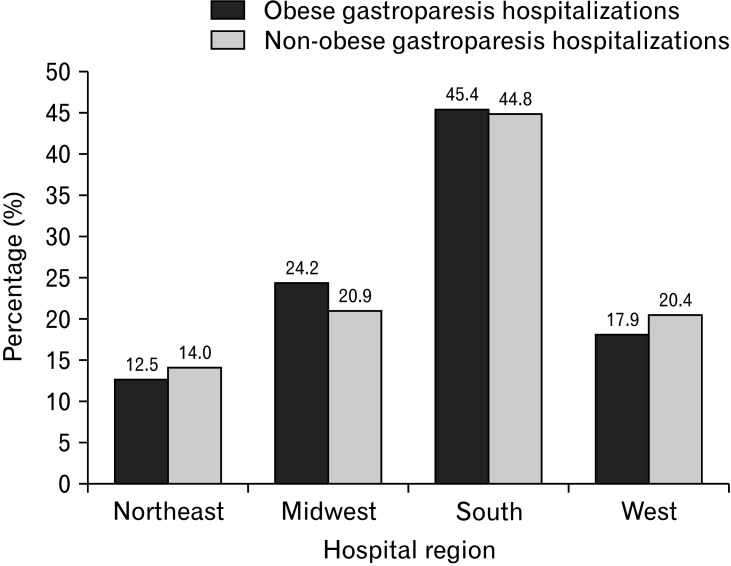
There were 140 293 adult obese GP hospitalizations, accounting for 13.75% of all GP hospitalizations in the US from 2007-2017, while 879 950 GP hospitalizations without obesity served as controls. Obese GP hospitalizations were on a rise from 2007-2017 in the US (Fig. 1). Obese GP hospitalizations were also slightly older (51.9 years vs 50.8 years, P < 0.001) compared to those without obesity. The 50-64 age group had the highest obese and non-obese GP hospitalizations, followed by the 35-49, 65-79, 18-34, and the ≥ 80 age group (Table 1). A female predominance was observed for all GP hospitalizations, but obese GP hospitalizations had a higher proportion of females (76.11% vs 64.36%, P < 0.001) compared to the non-obese cohort. Racial differences were also prominent in the study. White patients made up a majority of all GP hospitalizations, and we noted a higher proportion of hospitalizations for Whites in the non-obese cohort (63.18% vs 60.88%, P < 0.001) compared to the obese cohort. On the other hand, Blacks (25.49% vs 22.00%, P < 0.001) and Native Americans (0.85% vs 0.73%, P < 0.001) had a higher proportion of GP hospitalizations with obesity compared to the non-obese subgroup (Table 1). From a hospital perspective, the Southern hospital region (45.39%), urban teaching (63.32%), and large bed-size (53.68%) hospitals were observed to have the highest obese GP hospitalizations (Table 1). Medicare was the largest payer for all GP hospitalizations. Additionally, we noted a higher proportion of low-income (first quartile) patients in the obese cohort (36.91% vs 34.74%, P < 0.001) compared to the non-obese subgroup (Table 1).
Figure 1.
Increasing trend (P-trend < 0.01) of obese gastroparesis hospitalizations in the United States from 2007-2017.
Table 1.
Comparative Analysis of Hospitalization Characteristics for Gastroparesis With and Without Obesity in the United States From 2007-2017
| Variable | Obese gastroparesis hospitalizations | Non-obese gastroparesis hospitalizations | P-value |
|---|---|---|---|
| Total number of hospitalizations (n) | 140 293 | 879 950 | |
| Proportions of hospitalizations | 13.75% | 86.25% | |
| Mean age (yr) | 51.93 | 50.84 | < 0.001 |
| Age groups (yr) | < 0.001 | ||
| 18-34 | 18 263 (13.02%) | 196 276 (22.31%) | |
| 35-49 | 43 281 (30.85%) | 227 723 (25.88%) | |
| 50-64 | 49 467 (35.26%) | 242 506 (27.56%) | |
| 65-79 | 25 458 (18.15%) | 151 903 (17.26%) | |
| ≥ 80 | 3824 (2.73%) | 61 541 (6.99%) | |
| Gender | < 0.001 | ||
| Male | 33 504 (23.89%) | 313 503 (35.64%) | |
| Female | 106 759 (76.11%) | 566 176 (64.36%) | |
| Race | < 0.001 | ||
| White | 81 938 (60.88%) | 523 049 (63.18%) | |
| Black | 34 304 (25.49%) | 182 146 (22.00%) | |
| Hispanic | 13 510 (10.04%) | 84 499 (10.21%) | |
| Asian | 1061 (0.79%) | 12 982 (1.57%) | |
| Native American | 1139 (0.85%) | 6048 (0.73%) | |
| Other | 2638 (1.96%) | 19 209 (2.32%) | |
| Charlson comorbidity index | < 0.001 | ||
| CCI = 0 | 17 813 (12.70%) | 208 272 (23.67%) | |
| CCI = 1 | 17 986 (12.82%) | 128 304 (14.58%) | |
| CCI = 2 | 18 036 (12.86%) | 111 724 (12.70%) | |
| CCI ≥ 3 | 86 458 (61.63%) | 431 649 (49.05%) | |
| Hospital Region | < 0.001 | ||
| Northeast | 17 505 (12.48%) | 122 823 (13.96%) | |
| Midwest | 33 989 (24.23%) | 183 481 (20.85%) | |
| South | 63 674 (45.39%) | 394 522 (44.83%) | |
| West | 25 126 (17.91%) | 179 124 (20.36%) | |
| Hospital bed-size | 0.016 | ||
| Small | 22 829 (16.29%) | 147 623 (16.83%) | |
| Medium | 42 071 (30.03%) | 252 596 (28.80%) | |
| Large | 75 206 (53.68%) | 476 896 (54.37%) | |
| Hospital location and teaching status | < 0.001 | ||
| Rural | 11 912 (8.50%) | 96 455 (11.00%) | |
| Urban non-teaching | 39 483 (28.18%) | 269 592 (30.74%) | |
| Urban teaching | 88 711 (63.32%) | 511 067 (58.27%) | |
| Expected primary payer | < 0.001 | ||
| Medicare | 66 823 (47.70%) | 384 925 (43.81%) | |
| Medicaid | 28 598 (20.41%) | 185 516 (21.12%) | |
| Private | 35 753 (25.52%) | 227 472 (25.89%) | |
| Self-pay | 5568 (3.97%) | 53 711 (6.11%) | |
| Other | 3359 (2.40%) | 26 905 (3.06%) | |
| Median household income (quartile) | < 0.001 | ||
| 1st (0-25th) | 51 003 (36.91%) | 300 524 (34.74%) | |
| 2nd (26-50th) | 37 345 (27.02%) | 229 468 (26.53%) | |
| 3rd (51-75th) | 31 140 (22.53%) | 195 688 (22.62%) | |
| 4th (76-100th) | 18 709 (13.54%) | 139 360 (16.11%) | |
| Co-morbidities | |||
| Diabetes Mellitus type 1 | 9121 (6.50%) | 136 360 (15.50%) | < 0.001 |
| Diabetes Mellitus type 2 | 89 535 (63.82%) | 300 118 (34.11%) | < 0.001 |
| Hypertension | 59 937 (42.72%) | 298 555 (33.93%) | < 0.001 |
| Myocardial infarction | 3074 (2.19%) | 14 456 (1.64%) | < 0.001 |
| Cardiomyopathy | 4882 (3.48%) | 21 831 (2.48%) | < 0.001 |
| Congestive heart failure | 30 929 (22.05%) | 106 041 (12.05%) | < 0.001 |
| Atrial fibrillation | 9474 (6.75%) | 44 405 (5.05%) | < 0.001 |
| Dyslipidemia | 53 588 (38.20%) | 202 383 (23.00%) | < 0.001 |
| Anemia | 48 157 (34.33%) | 282 604 (32.12%) | < 0.001 |
| Peripheral vascular disease | 5793 (4.13%) | 32 990 (3.75%) | 0.003 |
| Chronic kidney disease | 43 035 (30.67%) | 209 150 (23.77%) | < 0.001 |
| Acute kidney injury | 29 653 (21.14%) | 159 218 (18.09%) | < 0.001 |
| Chronic obstructive pulmonary disease | 24 245 (17.28%) | 98 537 (11.20%) | < 0.001 |
| Malnutrition | 7202 (5.13%) | 92 472 (10.51%) | < 0.001 |
CCI, Charlson comorbidity index.
Associated Comorbidities for Obese and Non-obese Gastroparesis Hospitalizations
Higher proportion of obese GP hospitalizations were noted to have a Charlson comorbidity index score ≥ 3 (61.63% vs 49.05%, P < 0.001) compared to the non-obese cohort. Furthermore, obese GP hospitalizations also had a higher proportion of patients with type-2 diabetes mellitus, hypertension, dyslipidemia, anemia, peripheral vascular disease, myocardial infarction, cardiomyopathy, congestive heart failure, atrial fibrillation, chronic obstructive pulmonary disease, acute kidney injury, and chronic kidney disease; however, a higher proportion of patients with type-1 diabetes mellitus and malnutrition were noted in the non-obese cohort compared to the obese cohort (Table 1).
Outcomes for Obese and Non-obese Gastroparesis Hospitalizations
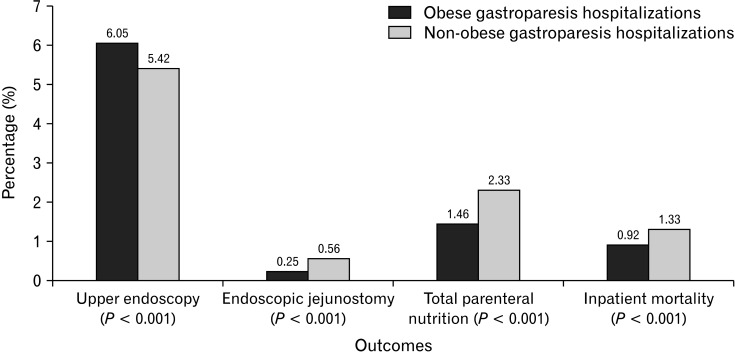
Higher proportion of patients in the obese cohort had inpatient upper endoscopy (6.05% vs 5.42%, P < 0.001); however, the need for nutritional support with endoscopic jejunostomy (EJ) (0.25% vs 0.56%, P < 0.001) and total parenteral nutrition (TPN) (1.46% vs 2.33%, P < 0.001) was more common in the non-obese cohort (Table 2). We noted a longer LOS (5.71 days vs 5.32 days, P < 0.001) and higher mean THC ($53 373 vs $45 040, P < 0.001) for GP hospitalizations with obesity compared to those without. Although most GP hospitalizations were discharged home, we noted a higher proportion of obese GP hospitalizations discharged with home health care or transfer to another facility (including skilled nursing facility or an intermediate care facility) compared to the non-obese cohort (Table 2). From a mortality perspective, non-obese GP hospitalizations had higher all-cause inpatient mortality (1.33% vs 0.92%, P < 0.001) compared to the obese cohort.
Table 2.
Comparative Analysis of Outcomes for Gastroparesis With and Without Obesity in the United States From 2007-2017
| Outcomes | Obese gastroparesis hospitalizations | Non-obese gastroparesis hospitalizations | P-value |
|---|---|---|---|
| Upper endoscopy | 8486 (6.05%) | 47 700 (5.42%) | < 0.001 |
| Endoscopic jejunostomy | 347 (0.25%) | 4953 (0.56%) | < 0.001 |
| Total Parenteral Nutrition | 2052 (1.46%) | 20 498 (2.33%) | < 0.001 |
| Length of stay (day) | 5.71 | 5.32 | < 0.001 |
| Total hospital charge (USD) | 53 373 | 45 040 | < 0.001 |
| Disposition | < 0.001 | ||
| Discharge home | 94 774 (67.59%) | 616 626 (70.12%) | |
| Transfer to short-term hospital | 2409 (1.72%) | 16 972 (1.93%) | |
| Transfer to another facility (includes SNF and ICF) | 18 044 (12.87%) | 94 327 (10.73%) | |
| Home health care | 21 405 (15.26%) | 114 145 (12.98%) | |
| Discharge against medical advice | 2274 (1.62%) | 25 283 (2.88%) | |
| Inpatient mortality | 1297 (0.92%) | 11 735 (1.33%) | < 0.001 |
USD, United States dollars; SNF, skilled nursing facility; ICF, intermediate care facility.
Discussion
Obese GP hospitalizations made up 13.75% of all GP hospitalizations in the US. Compared to patients without obesity, obese GP hospitalizations had a higher proportion of females (76.11% vs 64.35%, P < 0.001), less Whites (60.88% vs 63.18%, P < 0.001), and more Blacks (25.49% vs 22.00%, P < 0.001) and Native Americans (0.85% vs 0.73%, P < 0.001). GP hospitalizations with obesity also had higher inpatient upper endoscopy rates (6.05% vs 5.42%, P < 0.001), longer LOS (5.71 days vs 5.32 days, P < 0.001), and mean THC ($53 373 vs $45 040, P < 0.001); however, they had a lower need for nutritional support via EJ (0.25% vs 0.56%, P < 0.001) and TPN (1.46% vs 2.33%, P < 0.001), and lower all-cause inpatient mortality rates (0.92% vs 1.33%, P < 0.001) compared to the non-obese cohort.
The exact prevalence of GP in the general population is currently unknown due to significant clinical overlap with conditions such as functional dyspepsia.2,9 From 1996-2006, a community-based study on GP from Olmsted County, MN estimated the age-adjusted prevalence of 9.6 and 37.8 per 100 000 persons for males and females, respectively.10 Another study that evaluated medical records for GP from 340 hospitals across the US reported a prevalence rate of 0.16% in the general population.11 Furthermore, it was estimated that the hospitalizations for GP as a primary diagnosis increased by 158.00% and as a secondary diagnosis increased by 136.00% from 1995 to 2004 in the US.12 Initially thought to be associated with weight loss, current literature describes a rising association between obesity and gastroparesis.6 This association is particularly important in light of a well-documented national obesity pandemic in the US. Literature reports a continuous increase in the prevalence of age-adjusted obesity from 30.50% for the 1999-2000 period to 42.40% through 2017-2018 for individuals ≥ 20 years.13 It has also been estimated that by 2030, approximately 1 in 2 adults will be obese in the US.14 Numerous studies have attempted to estimate the prevalence of obesity in individuals with GP. A multi-center study that enrolled patients from 7 large tertiary centers in the US reported that 29% of the patients with GP were obese.15 Additionally, over a 48-week follow-up period, about 30% of these patients had ≥ 5% increase in body weight.15 In 2012, a NIS-based study reported an inpatient mortality rate of 1.50% for obese patients with GP and these patients had lower odds of inpatient mortality compared to that of non-obese gastroparesis patients.16 The exact mechanism implicated in the development of obesity in individuals with GP is currently unknown and an area of active research. However, recent studies have demonstrated that obesity and GP may cause overlapping but antagonistic changes in the gastric muscularis transcriptome.17 Obesity increases transcription of mRNA encoding smooth muscle contractile proteins thereby increasing gastric motility, whereas GP has the opposite effect.17 Clinically, this translates to a less severe loss of appetite and improvement in the inability to finish meals.15 Furthermore, the acquisition of maladaptive dietary habits to cope with the symptoms of GP, imbalance between calorie intake and energy expenditure, lack of physical activity and decreased severity of symptoms may also promote obesity in patients with GP.6,15 It is paramount to identify the impact of obesity in patients with GP as it may lead to an increased risk of adverse outcomes and place a significant burden on the US healthcare system. Efforts must be directed towards patient education on dietary changes, lifestyle modification, and self-directed/structured weight loss programs along with the management of GP to reduce BMI which may lead to a reduction of adverse outcomes and healthcare burden.
In this study, we noted a significant female predominance for all GP hospitalizations, but there were a higher proportion of females in the obese GP cohort compared to the non-obese subgroup. These findings were in line with current literature which reports a higher prevalence of both GP and obesity in females.10,11,12 We observed that the 50-64 age group had a higher proportion of obese GP hospitalizations compared to the non-obese cohort (Table 1). We also noted significant racial differences in this study. Although Whites made up a majority of the study population for both the obese and non-obese cohorts, we noted a higher proportion of Blacks in the obese GP cohort compared to the non-obese cohort. The exact reason for this finding is unknown as the effects of race on GP have not yet been fully studied, but studies have reported a higher likelihood of hospitalization for Blacks with GP.18,19 The possible reasons for higher hospitalization rates for Blacks could be related to an overall lack of access to outpatient medical care and greater severity of disease compared to other races. Furthermore, we noted lower all-cause inpatient mortality for GP hospitalizations with obesity compared to those without (Table 2). This was in line with current literature and may partially be attributed to the “obesity paradox” which hypothesizes that the presence of obesity is protective against adverse inpatient outcomes such as mortality.16,20,21
Moreover, obese GP hospitalizations had a higher comorbidity burden (Charlson comorbidity index ≥ 3) compared to the non-obese cohort. This may be explained by the presence of obesity in these patients as obesity has known associations with a wide spectrum of comorbid conditions.22 This was further reflected when we analyzed individual associations and found that obese GP hospitalizations had a higher proportion of patients with associated comorbidities (Table 1). Furthermore, we noted that obese GP hospitalizations had a longer mean LOS at 5.71 days and higher mean THC compared to the non-obese cohort. This is likely due to the presence of multiple co-morbidities in the obese group which requires additional management, intervention, and a multidisciplinary healthcare team.16
From a procedural standpoint, higher proportions of patients in the obese cohort required inpatient upper endoscopy (Fig. 2). This may, in part, be due to the fact that obese patients may develop refractory gastroparesis which in turn requires additional endoscopic intervention or because these patients may have additional symptoms and associations of obesity (such as gastroesophageal reflux disease) necessitating upper endoscopy.6,23 Furthermore, non-obese GP hospitalizations had higher rates of EJ and TPN requirements compared to the obese cohort (Table 2). This may be attributed to a higher association of non-obese GP hospitalizations with malnutrition (Table 1) which requires additional nutritional support in terms of enteral feeding via jejunostomy and in severe cases TPN.
Figure 2.
Outcomes for obese and non-obese gastroparesis hospitalizations in the United States from 2007-2017.
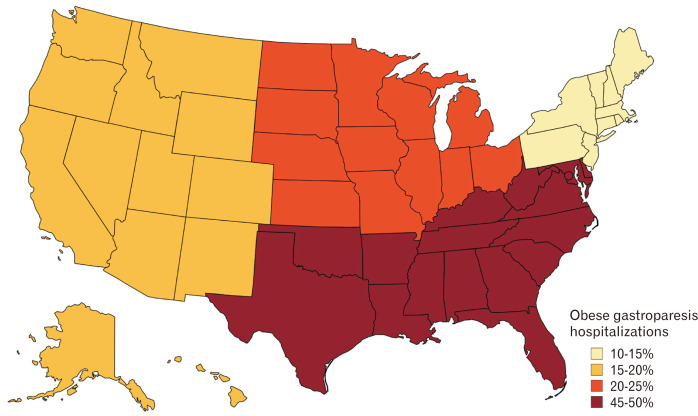
Southern hospital regions had the most obese GP hospitalizations, followed by the Midwest, West, and Northeast regions (Fig. 3 and 4). This distribution may be secondary to increased consumption of the “southern diet” consisting of added fats, fried food, eggs, organ and processed meats, and sugar-sweetened beverages.24 This diet promotes obesity which in turn may lead to the development of GP. Additionally, large bed-size hospitals had a higher number of GP hospitalizations for both cohorts. This may be because larger hospitals have a higher capacity for in-patient admissions compared to smaller or medium-bed-sized hospitals. Furthermore, urban teaching hospitals had a higher proportion of obese GP hospitalizations compared to the non-obese cohort. This may be due to the fact that these hospitals are usually tertiary care referral centers accepting complex patients from large geographical areas. Hence, they are equipped with the necessary resources and specialists to adequately manage these hospitalizations and their complications. Moreover, their location in an urban area with a high population density favors higher hospitalization rates as compared to hospitals located in non-urban/rural areas.
Figure 3.
Hospital region distribution (P < 0.001) of obese and non-obese gastroparesis hospitalizations in the United States from 2007-2017.
Figure 4.
Map demonstrating the distribution of obese gastroparesis hospitalizations in the United States from 2007-2017.
Prior studies have reported that low household income and high unemployment rates are associated with GP and Obesity.5,25-27 Our study echoed similar findings as we noted a higher proportion of low-income patients in the obese cohort (36.9% vs 34.74%, P < 0.001) compared to the non-obese group (Table 1). However, a reversal of proportions was seen for the highest income patients (fourth quartile).
Our study possesses several strengths. A key strength of our study is the study population, which is derived from one of the largest, multi-ethnic, publicly available databases in the US. The unique methodology and an 11-year study duration also help us critically assess key differences between the 2 subgroups of patients. Additionally, through the study design, we focused on hospitalization characteristics and numerous outcome-oriented facets allowing for a comprehensive comparative analysis thereby adding substantial meaningful information to current literature. Furthermore, as NIS collects data from hospitals across the US, the results of our study are applicable to most hospitals in the US. However, we do acknowledge all the limitations. The NIS database does not contain data on the severity of the disease, methods/tests used to establish diagnosis, the hospital course, and treatment aspects of GP in obese patients. Furthermore, due to the retrospective study design, all biases associated with retrospective studies are applicable to this study. Additionally, NIS stores information based on the diagnosis rather than for individual patients. Therefore, patients admitted on numerous occasions for the same diagnosis may have been included several times in the data set. Finally, NIS is an administrative database using codes to store information; hence, the possibility of coding errors cannot be excluded. Despite these limitations, we believe that the large sample size, unique methodology, and comprehensive analysis technique help us better understand the topic in question. This study aims to stimulate further research on GP in obese individuals.
In conclusion, the presence of obesity significantly influences GP hospitalizations. In our study, GP hospitalizations with obesity accounted for 13.75% of all GP hospitalizations. These patients were older and had a higher proportion of females and Black patients when compared to the non-obese cohort. Obese GP hospitalizations had lower all-cause inpatient mortality rates, but higher mean LOS and mean THC compared to non-obese GP hospitalizations without obesity. It is interesting to note that there were higher rates of less invasive measures in the non-obese cohort including TPN and EJ. The factors contributing to this discrepancy may include early access to healthcare, disparities in techniques, and incorporation of innovation and evidence-based care into current practice. However, these findings need to be studied further as they may result in improvements and standardization of care, and lead to more universal outcomes.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Conception and design: Dushyant S Dahiya, Sumant Inamdar, and Abhilash Perisetti; administrative support: Dushyant S Dahiya, Chin-I Cheng, and Asim Kichloo; revision of key components of manuscript: Dushyant S Dahiya, Abhilash Perisetti, Hemant Goyal, Sumant Inamdar, Mohammad Al-Haddad, and Neil Sharma; and provision, collection, assembly, and interpretation of data, review of literature, drafting the manuscript, final approval of manuscript, and agreement to be accountable for all aspects of the work: Dushyant S Dahiya, Sumant Inamdar, Abhilash Perisetti, Hemant Goyal, Amandeep Singh, Rajat Garg, Chin-I Cheng, Asim Kichloo, Mohammad Al-Haddad, and Neil Sharma.
References
- 1.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L American College of Gastroenterology, author. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–38. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahiya DS, Kichloo A, Shaka H, et al. Gastroparesis with cannabis use: a retrospective study from the nationwide inpatient sample. Postgrad Med. 2021;133:791–797. doi: 10.1080/00325481.2021.1940219. [DOI] [PubMed] [Google Scholar]
- 3.Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum) Ann Intern Med. 1958;48:797–812. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 4.Liu N, Abell T. Gastroparesis updates on pathogenesis and management. Gut Liver. 2017;11:579–589. doi: 10.5009/gnl16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:1056–1064. doi: 10.1016/j.cgh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumhagen J, Volckmann E. Managing gastroparesis in the setting of obesity. In: Ibele A, Gould J, editors. Gastroparesis. Springer; Cham: 2020. [DOI] [Google Scholar]
- 7.Healthcare Cost and Utilization Project, author. Introduction to the HCUP national inpatient sample (NIS) [accessed 30 August, 2022]. Available from URL: https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2018.jsp .
- 8.Houchens R, Ross D, Elixhauser A, et al. Nationwide inpatient sample (NIS) redesign final report. Report # 2014-04. [accessed 30 August, 2022]. Available from URL: https://www.hcup-us.ahrq.gov/reports/methods/2014-04.pdf .
- 9.Baumgardner DJ. Suffering in silence: is gastroparesis underdiagnosed? J Patient Cent Res Rev. 2019;6:133–134. doi: 10.17294/2330-0698.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syed AR, Wolfe MM, Calles-Escandon J. Epidemiology and diagnosis of Gastroparesis in the United States: a population-based study. J Clin Gastroenterol. 2020;54:50–54. doi: 10.1097/MCG.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States; trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313–322. doi: 10.1111/j.1572-0241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- 13.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020:1–8. [PubMed] [Google Scholar]
- 14.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 15.Parkman HP, Van Natta M, Yamada G, et al. Body weight in patients with idiopathic gastroparesis. Neurogastroenterol Motil. 2021;33:e13974. doi: 10.1111/nmo.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okeke F. Gastroparesis impacts mortality and resource utilization in patients admitted with obesity: a nationwide analysis: 560. Am J Gastroenterol. 2016;111:S255. doi: 10.14309/00000434-201610001-00560. [DOI] [Google Scholar]
- 17.Herring BP, Chen M, Mihaylov P, et al. Transcriptome profiling reveals significant changes in the gastric muscularis externa with obesity that partially overlap those that occur with idiopathic gastroparesis. BMC Med Genomics. 2019;12:89. doi: 10.1186/s12920-019-0550-3.e43e310fe144496aba46ddc71acabcff [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garawi F, Devries K, Thorogood N, Uauy R. Global differences between women and men in the prevalence of obesity: is there an association with gender inequality? Eur J Clin Nutr. 2014;68:1101–1106. doi: 10.1038/ejcn.2014.86. [DOI] [PubMed] [Google Scholar]
- 19.Parkman HP, Yamada G, Van Natta ML, et al. Ethnic, racial, and sex differences in etiology, symptoms, treatment, and symptom outcomes of patients with gastroparesis. Clin Gastroenterol Hepatol. 2019;17:1489–1499. e8. doi: 10.1016/j.cgh.2018.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedenberg FK, Kowalczyk M, Parkman HP. The influence of race on symptom severity and quality of life in gastroparesis. J Clin Gastroenterol. 2013;47:757–761. doi: 10.1097/MCG.0b013e3182819aae. [DOI] [PubMed] [Google Scholar]
- 21.Cereda E, Klersy C, Hiesmayr M, et al. Body mass index, age and in-hospital mortality: the nutrition day multinational survey. Clin Nutr. 2017;36:839–847. doi: 10.1016/j.clnu.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(suppl 2):S276–S281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guh DP, Zhang W, Bansback N, Amarsi Z, Brimingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shikany JM, Safford MM, Newby PK, Durant RW Brown TM, Judd SE. Southern dietary pattern is associated with hazard of acute coronary heart disease in the reasons for geographic and racial differences in stroke (REGARDS) study. Circulation. 2015;132:804–814. doi: 10.1161/CIRCULATIONAHA.114.014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9–19. doi: 10.1016/j.gtc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielefeldt K, Raza N, Zickmund SL. Different faces of gastroparesis. World J Gastroenterol. 2009;15:6052–6060. doi: 10.3748/wjg.15.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TJ, von dem Knesebeck O. Income and obesity: what is the direction of the relationship? A systematic review and meta-analysis. BMJ Open. 2018;8:e019862. doi: 10.1136/bmjopen-2017-019862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The NIS is one of the largest, publicly available, multi-ethnic inpatient database in the US. The database can be accessed at: https://www.hcup-us.ahrq.gov.