Abstract
Manometry, particularly high-resolution manometry is the preferred diagnostic tool used to evaluate esophageal motor function. This investigation is strongly indicated in the setting of dysphagia, but is also useful in gastroesophageal reflux disease (GERD), especially in case of failure of conventional treatment to exclude alternative diagnoses and prior to anti-reflux surgery. Moreover, ineffective esophagogastric junction barrier function and esophageal motor dysfunction are pathophysiological mechanisms in GERD and can be identified by manometry. The recent international guidelines have positioned high-resolution manometry as an important part of functional diagnostic work up in GERD in order to identify the GERD phenotype to guide specific treatment. The proposed manometric identification and measurement is based on the Chicago classification version 4.0 adding with new established metrics for GERD evaluation.
Keywords: Diagnosis, Esophageal motility disorders, Gastroesophageal reflux, Manometry, Phenotype
Introduction
Esophageal manometry is an established standard diagnostic tool for clinical evaluation of esophageal motor dysfunction.1 In the 1990s, high-resolution esophageal manometry (HREM) has been developed to improve diagnostic accuracy, and has now largely replaced conventional manometry.2 HREM systems combine closely spaced pressure sensor recordings (1-cm intervals) and topographic pressure plotting to display esophageal contractions as continuous spatio-temporal maps rather than the previously displayed 2-D line tracing format.3 HREM provides standardized, quantifiable metrics of 10 wet swallows in upright and supine positions, including integrated relaxation pressure (IRP), distal contractile integral (DCI), distal latency (DL), peristaltic breaks, and pressurization patterns. As a result, the manometric diagnosis of disorders of esophagogastric junction (EGJ) outflow and disorders of peristalsis were determined according to the latest international diagnostic criteria, Chicago classification version 4.0 (Table 1).4-6
Table 1.
Chicago Classification of Esophageal Motility Disorders Version 4.0
| Disorders of EGJ outflow | Criteria |
|---|---|
| • Type 1 achalasia | - Elevated median IRP (supine and/or upright) and 100% failed peristalsis |
| • Type 2 achalasia | - Elevated median IRP (supine and/or upright), 100% failed peristalsis, and ≥ 20% swallows with panesophageal pressurization |
| • Type 3 achalasia | - Elevated median IRP (supine and/or upright) and ≥ 20% swallows with premature/spastic contraction and no evidence of peristalsis |
| • EGJ outflow obstruction | - Elevated median IRP (supine and upright), ≥ 20% elevated intrabolus pressure (supine), and not meeting criteria for achalasia |
| - Clinically relevant symptoms with at least one confirmatory non-HRM supportive test (FLIP or TBE) |
| Disorders of peristalsis | Criteria |
|---|---|
| • Absent contractility | - Normal median IRP (supine and upright) and 100% failed peristalsis |
| • Distal esophageal spasm | - Normal median IRP and ≥ 20% swallows with premature/spastic contraction |
| - Clinically relevant symptoms | |
| • Hypercontractile esophagus | - Normal median IRP and ≥ 20% hypercontractile swallows |
| - Clinically relevant symptoms | |
| • Ineffective esophageal motility | - Normal median IRP, with > 70% ineffective swallows or ≥50% failed peristalsis |
EGJ, esophagogastric junction; IRP, integrated relaxation pressure; HRM, high-resolution manometry; FLIP, functional lumen imaging probe; TBE, timed barium esophagogram.
Nowadays, HREM is strongly indicated in the diagnostic evaluation of patients with non-obstructive dysphagia, non-cardiac chest pain, regurgitation7,8 as well as for assessing peristaltic function in patients who undergo surgical esophageal myotomy.9 However, the role of HREM in the context of gastroesophageal reflux disease (GERD) is less established.
In the current narrative review, we aim to summarize the role of HREM in the clinical setting of GERD (Table 2), particularly to emphasize its position as an important part of functional diagnostic work up in GERD in order to identify the GERD phenotype to guide specific treatment. A PubMed literature search was performed that included published articles in English through Dec 31, 2021 with combinations of the terms “high-resolution manometry,” “gastroesophageal reflux disease,” “esophageal motor dysfunction,” and “anti-reflux surgery.” Reference lists of the retrieved articles were also searched for additional articles.
Table 2.
Utility of High-resolution Esophageal Manometry in Gastroesophageal Reflux Disease
| Summarized HREM applications in GERD |
|---|
| • Determine mechanisms of GERD |
| • GERD diagnosis in conjunction with reflux monitoring |
| • Pursue the etiology of PPI-refractory GERD symptoms |
| • Localize the upper border of LES prior to catheter based reflux monitoring |
| • Before anti-reflux surgery |
| • Symptomatic patients after anti-reflux surgery |
HREM, high-resolution esophageal manometry; GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; LES, lower esophageal sphincter.
Mechanisms of Gastroesophageal Reflux Disease
GERD has a complex and multifactorial pathogenesis. Indeed, defective saliva production, esophageal motor dysfunction, presence of a hiatal hernia, the gastric acid pocket, gastric hypersecretory states as well as delayed gastric emptying can all contribute to gastroesophageal reflux occurrence.10-12 A more detailed understanding of GERD pathophysiology could better identify GERD phenotypes and potentially improve treatment outcomes. Among those factors, esophageal motor dysfunction represents a key pathophysiological mechanism and can be identified by HREM. Broadly, esophageal motor dysfunction can be classified into 2 broad categories.13
Impaired Anti-reflux Barrier at the Esophagogastric Junction
Impaired anti-reflux barrier at the EGJ including hypotensive basal lower esophageal sphincter (LES) pressure, low EGJ contractile integral, frequent transient LER relaxations, and the presence of a hiatal hernia. These factors promote repetitive reflux episodes, abnormal pH monitoring, reflux-symptoms association, as well as increasing the likelihood of erosive esophagitis (Table 3).14-20
Table 3.
Esophageal Motor Dysfunction in Gastroesophageal Reflux Disease and its Association With Reflux
| Esophageal motor dysfunction | Reflux relationship |
|---|---|
| Impaired anti-reflux barrier at EGJ | |
| - Frequent TLESR | ↑ Postprandial reflux14 |
| - Hypotensive LES | ↑ Reflux esophagitis15 |
| - Low EGJ-CI | ↑ Acid reflux |
| ↑ Reflux esophagitis17-20 | |
| - Hiatal hernia | ↑ Acid exposure time |
| ↑ Positive reflux-symptom association17,18 | |
| Esophageal body dysmotility | |
| - Ineffective primary peristalsis | ↑ Acid, weakly-acidic reflux |
| ↑ Reflux esophagitis21,25,26 | |
| - Decreased effective secondary peristalsis | ↑ Refractory GERD |
| ↑ Reflux esophagitis27,28 | |
| - Abnormal multiple rapid swallowing | ↑ Acid exposure time29 |
EGJ, esophagogastric junction; TLESR, transient lower esophageal relaxation; LES, lower esophageal sphincter; EGJ-CI, esophagogastric junction-contractile integral; GERD, gastroesophageal reflux disease.
Esophageal Body Dysmotility
Ineffective peristalsis (including ineffective esophageal motility [IEM] and absent contractility), along with impaired contractile reserve after provocative maneuvers (eg, multiple rapid swallows and [semi]solid boluses). The impairment of either primary or secondary esophageal peristalsis contributes to delayed bolus transit and impairs the clearance of the refluxate.21-23 These abnormalities will result in increased esophageal acid exposure time, prolonged esophageal bolus contact time, increased risk of erosive esophagitis and are risk factors for refractory GERD (Table 3).13,24
Gastroesophageal Reflux Disease Diagnosis in Conjunction With Reflux Monitoring
The Role of High-resolution Esophageal Manometry in the Diagnosis of Gastroesophageal Reflux Disease
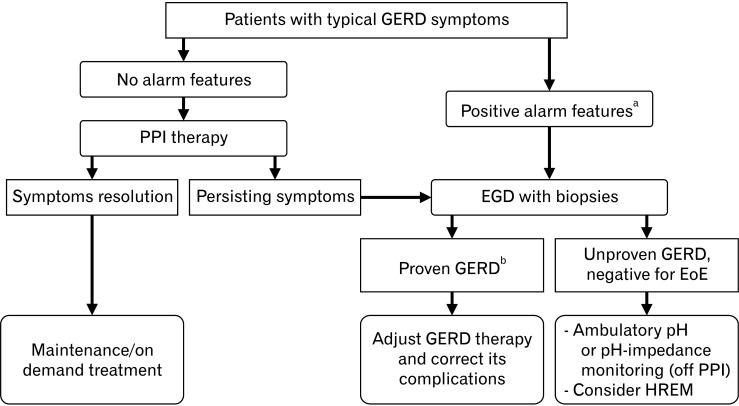
GERD is a condition in which reflux of gastric contents into the esophagus causes troublesome symptoms and/or mucosal damage.24 In general, GERD can be diagnosed on the basis of typical reflux symptoms (heartburn or regurgitation) and a therapeutic trial of proton pump inhibitors (PPIs), which is usually prescribed as the initial management without additional testing. In case of refractory symptoms or in the presence of alarm features (eg, dysphagia, anemia, and weight loss), prompt esophagogastroduodenoscopy (EGD) is warranted and can detect erosive esophagitis, GERD complications (eg, peptic stricture, Barrett’s epithelium, and esophageal carcinoma) as well as identify conditions mimicking GERD such as eosinophilic esophagitis and achalasia. However, EGD has a low diagnostic yield, because erosive reflux disease is present in only 30% of untreated patients with GERD and is very rare in patients on PPI.30,31 Therefore, if EGD shows normal esophageal mucosa or non-specific esophagitis, ambulatory pH monitoring and esophageal manometry should be performed to confirm the diagnosis of GERD (Fig. 1).12,32 At the present time, the interpretation of pH-impedance monitoring is based on the international Lyon consensus of GERD experts in 2018.33 The key metric of pH-monitoring is the esophageal acid exposure time (AET). A total AET level of less than 4% indicates physiological reflux, while a level greater than 6% is considered as pathological acid exposure, ie, GERD. AET values between 4-6% are considered borderline and require adjunctive evidence to establish GERD. This supportive evidence can be retrieved either from esophageal manometry, impedance-based parameters (eg, number of reflux episodes) or mucosal histopathology. With regard to HREM, the Lyon consensus has put forward hypotensive LES, hiatal hernia, IEM, fragmented peristalsis (based on the Chicago 3.0 criteria34 at the time of the consensus) as well as absent contractility as the main supportive findings for GERD.
Figure 1.
Current management algorithm for patients with typical gastroesophageal reflux disease (GERD) symptoms. GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; EGD, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; HREM, high-resolution esophageal manometry. aAlarm features: dysphagia, anemia, weight loss, persistent vomiting, and GI bleeding. bProven GERD: severe reflux esophagitis (Los Angeles classification C or D) or peptic stricture or histology proven Barrett’s mucosa > 1 cm.
High-resolution Gastroesophageal Manometry in the Functional Diagnostic Workup of Gastroesophageal Reflux Disease
The Chicago classification of esophageal motility disorders targets abnormal bolus transit but was not conceptualized to assess motor function in the context of GERD.6 The International GERD Consensus Working Group specifically proposed a new classification for esophageal motor findings in GERD.12,35 These international recommendations highlight the clinical relevance of HREM findings for the diagnosis and treatment of GERD subdivided according to pathophysiological mechanisms.33
Evaluation and reporting of esophageal motor function in GERD can be achieved through 3 hierarchical steps. The first 2 steps can reveal abnormalities of EGJ and esophageal body. The third step only applies when esophageal body dysfunction was illustrated and may have beneficial implications on management outcome. Most metrics utilized in evaluating esophageal motor function in GERD are obtained from esophageal HRM using a standard protocol of ten 5 mL water swallows in the supine position.6
Step 1. Morphology and integrity of esophagogastric junction
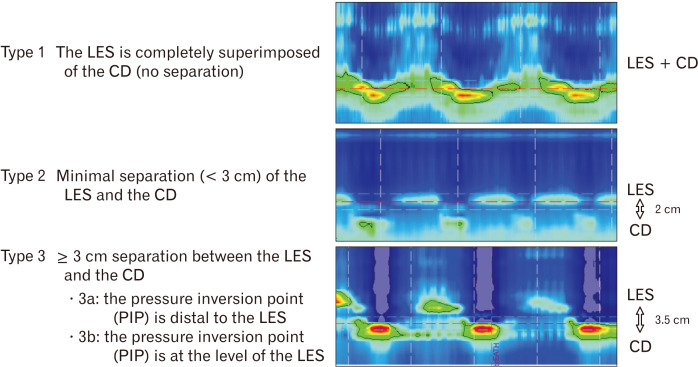
The Lyon consensus proposes 2 metrics in evaluating EGJ competence, one illustrating EGJ morphology and the second quantifying its contractile vigor.33 EGJ morphology, defined by the relationship between the intrinsic LES and the crural diaphragm (CD), has been characterized into 3 subtypes based on HRM35,36 (Fig. 2). Type 1 appearance reveals the physiological setting with superimposed LES and CD, type 2 with separated pressure signals by < 3 cm, and type 3 with ≥ 3 cm separation. Type 3 EGJ morphology is associated with reduced LES pressure and lower inspiratory augmentation, which correlates with reflux severity.17,18,36,37 Type 2 and type 3 EGJ morphology represent hiatal hernia which should be reported, particularly in GERD patients.35,36
Figure 2.
High-resolution manometry demonstrating 3 esophagogastric junction (EGJ) subtypes which represent physiological situation (EGJ type 1), small hiatal hernia (EGJ type 2), and larger hiatal hernia (EGJ type 3a and 3b). In hiatal hernia (EGJ type 2 and 3), there are 2 clearly separated high pressure zone at the level of cardia with the proximal representing the lower esophageal sphincter (LES) and the distal representing the crural diaphragm (CD).
Another HRM metric to evaluate competence of EGJ is the EGJ contractile integral (EGJ-CI) (Fig. 3). The EGJ-CI can be calculated using the DCI tool which is available in current HRM software with the DCI box encompassing the LES and CD over a period of 3 respiratory cycles above a threshold of gastric pressure.20 The measured DCI is then divided by the duration of the 3 respiratory cycles and finally expressed in units of mmHg·cm. This promising metric identifies a subset of patients with severe barrier dysfunction prone to either endoscopic esophagitis or abnormal reflux testing. However, more standardized methodology and further research are needed before widespread application as the variation of normal ranges were observed in previous studies.17,19,20,37,38
Figure 3.
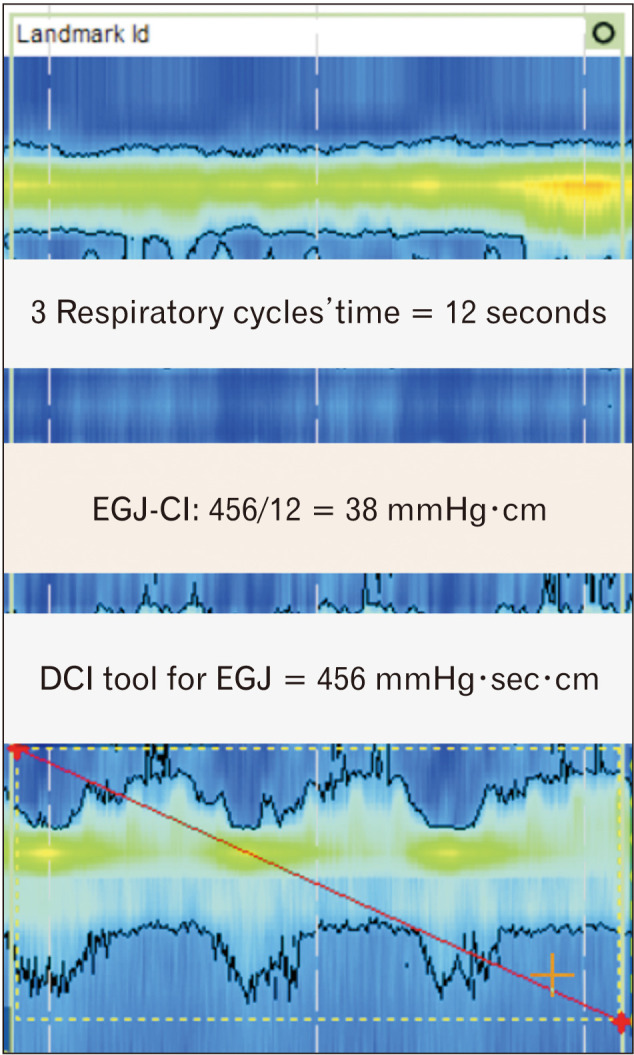
Assessment of esophagogastric junction (EGJ) contractility by EGJ contractile integral (EGJ-CI). For measurement EGJ-CI, upper and lower margins of the EGJ are enclosed in a DCI tool box for 3 consecutive respiratory cycles. The resulting DCI value is then divided by the duration of the 3 respiratory cycles (in seconds).
Step 2. Esophageal body motor function
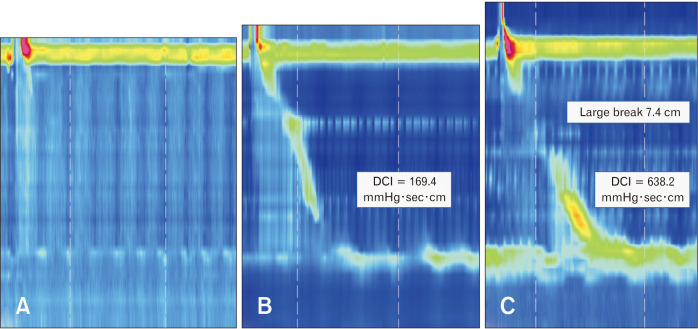
Esophageal dysmotility leads to impaired esophageal clearance which results in a prolonged contact of gastric refluxate to the esophageal mucosa, contributing to GERD pathogenesis (Table 3). Esophageal peristalsis can be characterized by the DCI which summarizes the vigor of the post-transition zone contraction. A DCI threshold of 450 mmHg·sec·cm correlates with an average distal peristaltic amplitude of 30 mmHg defining an ineffective peristalsis.6 Esophageal hypomotility can present with variable severity, ranging from absent contractility over IEM to fragmented peristalsis (Fig. 4). It has been shown that peristaltic dysmotility becomes progressively more common going from nonerosive reflux disease to erosive esophagitis, to Barrett’s esophagus.39,40 In addition, the burden of reflux symptoms and abnormal AET are correlated with the severity of peristaltic disturbances, with the greatest burden in absent contractility.41,42
Figure 4.
High-resolution manometry representing a typical swallow characteristic of esophageal hypomotility disorders. (A) Absent peristalsis (100% of swallows with distal contractile integral [DCI] < 100 mmHg·sec·cm), (B) ineffective esophageal motility (over 70% of swallows with DCI ≤ 450 mmHg·sec·cm), and (C) fragmented peristalsis (at least 50% of swallows with ≥ 5 cm breaks in the peristaltic contour when contraction vigor is preserved) which is not maintained as a diagnostic category in Chicago classification version 4.0.
Step 3. Esophageal body contraction reserve
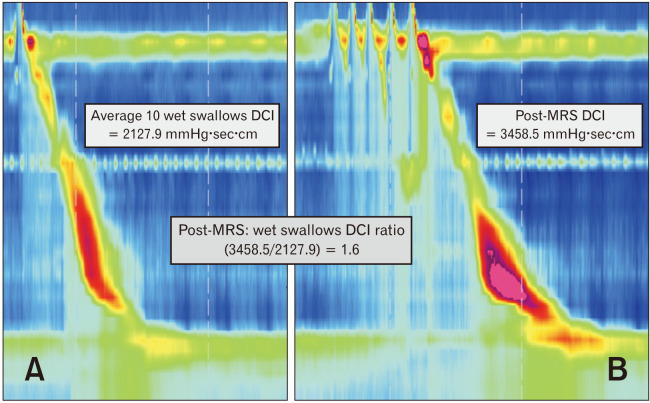
This metric is evaluated when esophageal body motor function is abnormal, especially in case of IEM or absent contractility. For multiple rapid swallow (MRS), five 2 mL swallows are taken < 4 seconds apart, and this is the accurate provocative tests of the integrity of inhibition during HRM in GERD patients.43 Post-MRS contractions are an indicator of esophageal contraction reserve in case of a greater post-MRS DCI compared to pre-MRS DCI (post-MRS: wet swallow DCI ratio > 1)44 (Fig. 5). It is suggested to perform 3 MRS sequences for a reliable assessment for contraction reserve.45 Furthermore, MRS has been adopted into HRM protocols for determining contraction reserve in IEM or absent contractility by the Lyon consensus.33 The absence of contraction reserve in IEM is predictive of the poor efficacy of promotility drugs.46,47 Moreover it has been identified as a risk factor for dysphagia and persistence or development of IEM post anti-reflux surgery.48,49
Figure 5.
Metrics from esophageal high-resolution manometry used in the evaluation of contraction reserve. (A) The vigor of esophageal body contraction is assessed using the distal contractile integral (DCI). (B) The vigor of esophageal body contraction following inhibition of peristalsis during the repetitive swallows by multiple rapid swallows (MRS) provocative test. The DCI of contraction following MRS is higher than the mean DCI from test swallows (post-MRS: wet swallow DCI ratio > 1) indicates contraction reserve.
Pursue the Etiology of Proton Pump Inhibitor-refractory Gastroesophageal Reflux Disease symptoms
PPIs remain the cornerstone of treatment in patients with troublesome reflux symptoms,50,51 but 30-50% of patients have an incomplete response to a standard or double dose PPI course of at least 8-12 weeks,52 which is defined as refractory GERD (rGERD).51,53-55 Previous studies showed that rGERD had a negative impact on both patients’ physical and mental health-related quality of life.56 A variety of conditions have been reported to cause rGERD including non-compliance to PPI, insufficient acid suppression (refractory acid reflux), and persistent weakly acidic reflux or reflux hypersensitivity (normal reflux parameters but positive symptom association). However, in other cases the symptoms cannot be attributed to reflux and are explained by functional heartburn, esophagitis caused by infection or pills, eosinophilic esophagitis, esophageal motor disorders, rumination syndrome and/or excessive (supra-) gastric belching.53-55,57 Among the non-reflux-related causes, major esophageal motility disorders play a crucial role and should be excluded by HREM, especially if anti-reflux surgery is considered. In 2011, Chan et al58 conducted a large cohort study in 1081 patients who were undergoing elective surgery for GERD. Surprisingly, the preoperative HREM revealed major esophageal motility disorders in a number of cases such as absent contractility (3.2%), distal esophageal spasm (3.2%), as well as achalasia and its variants (2.7%).58 In another study in 2017, Jeon et al59 reported the clinical characteristics of 64 achalasia patients and found that 59.4% and 76.6% of them had presented with heartburn and regurgitation symptoms. In addition, results showed that 25.0% of these achalasia patients were misdiagnosed as GERD before undergoing manometry.59 Besides its utility in diagnosing esophageal motility disorders, HREM combined with impedance (high-resolution impedance manometry or HRIM) can also diagnose conditions such as rumination syndrome and excessive (supra-) gastric belching which can mimic GERD.60,61 For this reason, patients with rGERD should undergo a work-up consisting of endoscopy, reflux monitoring, as well as HREM/HRIM to make a correct diagnosis and tailor therapy.32,54
Localize the Upper Border of Lower Esophageal Sphincter Prior to Catheter-based Reflux Monitoring
Ambulatory reflux monitoring can be performed using 2 types of techniques, ie, (1) 48 to 96-hour wireless pH capsule (Bravo) and (2) catheter-based 24-hour pH or pH/impedance monitoring. Although wireless pH monitoring increases the diagnostic yield and is better tolerated by patients, it also comes with several limitations such as the need for endoscopy, significant cost, and lower availability.62 Moreover, the capsule-based method can only pick-up acid events and not weakly or non-acidic reflux, belching or rumination because of the absence of an impedance signal and the presence of only 1 channel. Therefore, catheter-based pH or pH/impedance continues to be the gold standard diagnostic tool for determining pathological reflux. In order to prevent unintentional dislocation of the pH probe into the stomach during swallow-induced esophageal shortening, the pH probe must to be positioned 5 cm above the upper margin of the LES.63 Therefore, manometric localization of the LES is the gold standard method to position the pH probe for accurate and reproducible measurement of acid reflux in the lower esophagus.64,65
High-resolution Esophageal Manometry in the Preoperative Evaluation for Anti-reflux Surgery
Anti-reflux surgery (or surgical fundoplication) is aimed at creating an effective barrier to reflux of gastric contents at the level of the EGJ. To date, several types of fundoplication are available such as Nissen, Toupet, and Dor with variable circumference of the created wrap.66 Surgery is often considered in patients with insufficient symptom relief with medical management, PPI intolerance, unwillingness to continue long-term PPI therapy, or GERD complications such as peptic stricture and Barrett’s esophagus.67,68 HREM or HRIM forms an indispensable part of the pre-operative workup before undergoing surgical fundoplication.69,70 Its role is threefold: (1) distinguish various diseases with symptoms reminiscent to GERD such as major esophageal motility disorders, rumination syndrome, and excessive (supra-)gastric belching; (2) reveal the absolute and relative contraindications for fundoplication ie, achalasia, distal esophageal spasm, and absent contractility; and (3) establish the potential relative contraindications for a 360 degrees Nissen fundoplication (eg, IEM) in case of insufficient peristaltic reserve (post-MRS DCI/pre-MRES DCI ratio < 1) since these conditions may increase the risk of postoperative dysphagia.44
Role of High-resolution Esophageal Manometry in Symptomatic Patients After Anti-reflux Surgery
Most postoperative dysphagia is due to gastroesophageal outlet obstruction, which can be identified using HRM or a barium esophagram.71 The provocative tests during the HREM such as rapid drink challenge and solid test meal can help to demonstrate outlet obstruction which can be missed during evaluation of single wet swallows in patients with post-fundoplication dysphagia.72,73 In addition, a study by Scheffer et al74 demonstrated the benefit of concurrent HREM and fluoroscopy in the post-fundoplication setting. The results showed that the increase in EGJ transit time during liquid and solid bolus significantly correlated with the probability of postoperative dysphagia.74
Conclusion
HRM is now integrated as an important investigation for GERD diagnosis and guided treatment. The 3 hierarchical steps including evaluation of morphology and integrity of EGJ, esophageal body motor function and contraction reserve, are suggested for functional diagnostic work up in GERD. Furthermore, in rGERD, the combination of HREM with other GERD tests, particularly pH-impedance monitoring and endoscopy allow exclusion of alternative diagnosis and to investigate the mechanisms of refractory symptoms to further guide the treatment for these difficult to treat patients.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Sawangpong Jandee: conceptualization, literature search, and drafting of the manuscript; Suriya Keeratichananont: creation of figures and final editing; and Jan Tack: conceptualization and final editing.
References
- 1.Pandolfino JE, Kahrilas PJ American Gastroenterology Association, author. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–224. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Clouse RE, Parks T, Haroian L, Zakko SF. Development and clinical validation of a solid-state high-resolution pressure measurement system for simplified and consistent esophageal manometry. Am J Gastroenterol. 2003;98:S32. doi: 10.1111/j.1572-0241.2003.07830.x. [DOI] [Google Scholar]
- 3.Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 4.Carlson DA, Kahliras PJ. How to effectively use high-resolution esophageal manometry. Gastroenterology. 2016;151:789–792. doi: 10.1053/j.gastro.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohof WOA, Bredenoord AJ. Chicago classification of esophageal motility disorders: lessons learned. Curr Gastroenterol Rep. 2017;19:37. doi: 10.1007/s11894-017-0576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil. 2021;33:e14058. doi: 10.1111/nmo.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Hoeij FB, Bredenoord AJ. Clinical application of esophageal high-resolution manometry in the diagnosis of esophageal motility disorder. J Neurogastroenterol Motil. 2016;22:6–13. doi: 10.5056/jnm15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson DA, Pandolfino JE. High-resolution manometry in clinical practice. Gastroenterol Hepatol. 2015;11:374–384. [PMC free article] [PubMed] [Google Scholar]
- 9.Gyawali CP, de Bortoli N, Clarke J, et al. Indications and interpretation of esophageal function testing. Ann N Y Acad Sci. 2018;1434:239–253. doi: 10.1111/nyas.13709. [DOI] [PubMed] [Google Scholar]
- 10.Boeckxstaens G, El-Serag HB, Smout AJ, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut. 2014;63:1185–1193. doi: 10.1136/gutjnl-2013-306393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. 2015;27:1202–1213. doi: 10.1111/nmo.12611. [DOI] [PubMed] [Google Scholar]
- 12.Savarino E, Bredenoord AJ, Fox M, Pandolfino JE, Sabine R, Gyawali CP International Working Group for Disorders of Gastrointestinal Motility and Function, author. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665–676. doi: 10.1038/nrgastro.2017.130. [DOI] [PubMed] [Google Scholar]
- 13.Lin S, Li H, Fang X. Esophageal motor dysfunctions in gastroesophageal reflux disease and therapeutic perspectives. J Neurogastroenterol Motil. 2019;25:499–507. doi: 10.5056/jnm19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandolfino JE, Zhang QG, Ghosh SK, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology. 2006;131:1725–1733. doi: 10.1053/j.gastro.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Zentilin P, Conio M, Mele MR, et al. Comparison of the main oesophageal pathophysiological characteristics between short- and long-segment barrett's oesophagus. Aliment Pharmacol Ther. 2002;16:893–898. doi: 10.1046/j.1365-2036.2002.01237.x. [DOI] [PubMed] [Google Scholar]
- 16.Jain M, Srinivas M, Bawane P, Venkatarama J. Basal lower esophageal sphincter pressure in gastroesophageal reflux disease: an ignored metric in high-resolution esophageal manometry. Indian J Gastroenterol. 2018;37:446–451. doi: 10.1007/s12664-018-0898-x. [DOI] [PubMed] [Google Scholar]
- 17.Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil. 2015;27:1423–1431. doi: 10.1111/nmo.12638. [DOI] [PubMed] [Google Scholar]
- 18.Ham H, Cho YK, Lee HH, et al. Esophagogastric junction contractile integral and morphology: two high-resolution manometry metrics of the anti-reflux barrier. J Gastroenterol Hepatol. 2017;32:1443–1449. doi: 10.1111/jgh.13720. [DOI] [PubMed] [Google Scholar]
- 19.Xie C, Wang J, Li Y, et al. Esophagogastric junction contractility integral reflected the anti-reflux barrier dysfunction in patients with gastroesophageal reflux disease. J Neurogastroenterol Motil. 2017;23:27–33. doi: 10.5056/jnm16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicodème F, Pipa-Muniz M, Khanna K, Kahrilas PJ, Pandolfino JE. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-contractile integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil. 2014;26:353–360. doi: 10.1111/nmo.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JC, Cheung CM, Wong VW, Sung JJ. Distinct clinical characteristics between patients with nonerosive reflux disease and those with reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5:690–695. doi: 10.1016/j.cgh.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Chen CL, Yi CH, Liu TT. Relevance of ineffective esophageal motility to secondary peristalsis in patients with gastroesophageal reflux disease. J Gastroenterol Hepatol. 2014;29:296–300. doi: 10.1111/jgh.12367. [DOI] [PubMed] [Google Scholar]
- 23.Wang VS, Feldman N, Maurer R, Burakoff R. Esophageal motility in nonacid reflux compared with acid reflux. Dig Dis Sci. 2009;54:1926–1932. doi: 10.1007/s10620-008-0580-8. [DOI] [PubMed] [Google Scholar]
- 24.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group, author. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang L, Ye B, Wang Y, Wang M, Lin L. Esophageal body motility for clinical assessment in patients with refractory gastroesophageal reflux symptoms. J Neurogastroenterol Motil. 2017;23:64–71. doi: 10.5056/jnm16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribolsi M, Balestrieri P, Emerenziani S, Guarino MP, Cicala M. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol. 2014;109:46–51. doi: 10.1038/ajg.2013.373. [DOI] [PubMed] [Google Scholar]
- 27.Martinucci I, de Bortoli N, Giacchino M, et al. Esophageal motility abnormalities in gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5:86–96. doi: 10.4292/wjgpt.v5.i2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daum C, Sweis R, Kaufman E, et al. Failure to respond to physiologic challenge characterizes esophageal motility in erosive gastro-esophageal reflux disease. Neurogastroenterol Motil. 2011;23:517–e200. doi: 10.1111/j.1365-2982.2011.01669.x. [DOI] [PubMed] [Google Scholar]
- 29.Martinucci I, Savarino EV, Pandolfino JE, et al. Vigor of peristalsis during multiple rapid swallows is inversely correlated with acid exposure time in patients with NERD. Neurogastroenterol Motil. 2016;28:243–250. doi: 10.1111/nmo.12719. [DOI] [PubMed] [Google Scholar]
- 30.Hershcovici T, Fass R. Nonerosive reflux disease (NERD)-an update. J Neurogastroenterol Motil. 2010;16:8–21. doi: 10.5056/jnm.2010.16.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayerdörffer E, Bigard MA, Weiss W, et al. Randomized, multicenter study: on-demand versus continuous maintenance treatment with esomeprazole in patients with non-erosive gastroesophageal reflux disease. BMC Gastroenterol. 2016;16:48. doi: 10.1186/s12876-016-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama J, Kuribayashi S, Baeg MK, et al. Current and future perspectives in the management of gastroesophageal reflux disease. Ann N Y Acad Sci. 2018;1434:70–83. doi: 10.1111/nyas.13850. [DOI] [PubMed] [Google Scholar]
- 33.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the lyon consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: conclusions from an international consensus group. Neurogastroenterol Motil. 2017;29:e13104. doi: 10.1111/nmo.13104. [DOI] [PubMed] [Google Scholar]
- 36.Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–1063. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 37.Jasper D, Freitas-Queiroz N, Hollenstein M, et al. Prolonged measurement improves the assessment of the barrier function of the esophago-gastric junction by high-resolution manometry. Neurogastroenterol Motil. 2017;29:e12925. doi: 10.1111/nmo.12925. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Patel A, Mello M, Shriver A, Gyawali CP. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol Motil. 2016;28:639–646. doi: 10.1111/nmo.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savarino E, Gemignani L, Pohl D, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:476–486. doi: 10.1111/j.1365-2036.2011.04742.x. [DOI] [PubMed] [Google Scholar]
- 40.Meneghetti AT, Tedesco P, Damani T, Patti MG. Esophageal mucosal damage may promote dysmotility and worsen esophageal acid exposure. J Gastrointest Surg. 2005;9:1313–1317. doi: 10.1016/j.gassur.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 41.Reddy CA, Patel A, Gyawali CP. Impact of symptom burden and health-related quality of life (HRQOL) on esophageal motor diagnoses. Neurogastroenterol Motil. 2017;29:e12970. doi: 10.1111/nmo.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rengarajan A, Bolkhir A, Gor P, Wang D, Munigala S, Gyawali CP. Esophagogastric junction and esophageal body contraction metrics on high-resolution manometry predict esophageal acid burden. Neurogastroenterol Motil. 2018;30:e13267. doi: 10.1111/nmo.13267. [DOI] [PubMed] [Google Scholar]
- 43.Savarino E, de Bortoli N, Bellini M, et al. Practice guidelines on the use of esophageal manometry - A GISMAD-SIGE-AIGO medical position statement. Dig Liver Dis. 2016;48:1124–1135. doi: 10.1016/j.dld.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol. 2013;108:1706–1712. doi: 10.1038/ajg.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mauro A, Savarino E, De Bortoli N, et al. Optimal number of multiple rapid swallows needed during high-resolution esophageal manometry for accurate prediction of contraction reserve. Neurogastroenterol Motil. 2018;30:e13253. doi: 10.1111/nmo.13253. [DOI] [PubMed] [Google Scholar]
- 46.Min YW, Shin I, Son HJ, Rhee PL. Multiple rapid swallow maneuver enhances the clinical utility of high-resolution manometry in patients showing ineffective esophageal motility. Medicine. 2015;94:e1669. doi: 10.1097/MD.0000000000001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sifrim D, Jafari J, Woodland P, Anggiansah A, Wong T. Effect of azithromycin on esophageal hypomotility (EH) and prediction of response by esophageal stimulations tests during high resolution manometry. Neurogastroenterol Motil. 2015;27:35. doi: 10.1016/s0016-5085(15)30264-x. [DOI] [Google Scholar]
- 48.Stoikes N, Drapekin J, Kushnir V, Shaker A, Brunt LM, Gyawali CP. The value of multiple rapid swallows during preoperative esophageal manometry before laparoscopic antireflux surgery. Surg Endosc. 2012;26:3401–3407. doi: 10.1007/s00464-012-2350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mello MD, Shriver AR, Li Y, Patel A, Gyawali CP. Ineffective esophageal motility phenotypes following fundoplication in gastroesophageal reflux disease. Neurogastroenterol Motil. 2016;28:292–298. doi: 10.1111/nmo.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;2013:CD002095. doi: 10.1002/14651858.CD002095.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarpellini E, Ang D, Pauwels A, De Santis A, Vanuytsel T, Tack J. Management of refractory typical GERD symptoms. Nat Rev Gastroenterol Hepatol. 2016;13:281–294. doi: 10.1038/nrgastro.2016.50. [DOI] [PubMed] [Google Scholar]
- 52.Delshad SD, Almario CV, Chey WD, Spiegel BMR. Prevalence of gastroesophageal reflux disease and proton pump inhibitor-refractory symptoms. Gastroenterology. 2020;158:1250–1261. e2. doi: 10.1053/j.gastro.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fock KM, Talley N, Goh KL, et al. Asia-Pacific consensus on the management of gastro-esophageal reflux disease: an update focusing on refractory reflux disease and barrett's esophagus. Gut. 2016;65:1402–1415. doi: 10.1136/gutjnl-2016-311715. [DOI] [PubMed] [Google Scholar]
- 54.Yadlapati R, DeLay K. Proton pump inhibitor-refractory gastroesophageal reflux disease. Med Clin North Am. 2019;103:15–27. doi: 10.1016/j.mcna.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1240–1254. doi: 10.1136/gutjnl-2011-301897. [DOI] [PubMed] [Google Scholar]
- 56.Tack J, Becher A, Mulligan C, Johnson DA. Systematic review: the burden of disruptive gastro-esophageal reflux disease on health-related quality of life. Aliment Pharmacol Ther. 2012;35:1257–1266. doi: 10.1111/j.1365-2036.2012.05086.x. [DOI] [PubMed] [Google Scholar]
- 57.Jandee S, Jandee K. Diagnostic yield of high-resolution esophageal manometry with Chicago classification version 3.0 in Thai patients. J Neurogastroenterol Motil. 2021;27:533–539. doi: 10.5056/jnm20088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc. 2011;25:2943–2949. doi: 10.1007/s00464-011-1646-9. [DOI] [PubMed] [Google Scholar]
- 59.Jeon HH, Kim JH, Youn YH, Park H, Conklin JL. Clinical characteristics of patients with untreated achalasia. J Neurogastroenterol Motil. 2017;23:378–384. doi: 10.5056/jnm16177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kessing BF, Bredenoord AJ, Smout AJ. Objective manometric criteria for the rumination syndrome. Am J Gastroenterol. 2014;109:52–59. doi: 10.1038/ajg.2013.428. [DOI] [PubMed] [Google Scholar]
- 61.Kessing BF, Bredenoord AJ, Smout AJ. Mechanisms of gastric and supragastric belching: a study using concurrent high-resolution manometry and impedance monitoring. Neurogastroenterol Motil. 2012;24:e573–e579. doi: 10.1111/nmo.12024. [DOI] [PubMed] [Google Scholar]
- 62.Lee JS. Is wireless capsule pH monitoring better than catheter systems? J Neurogastroenterol Motil. 2012;18:117–119. doi: 10.5056/jnm.2012.18.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aksglaede K, Funch-Jensen P, Thommesen P. Intra-esophageal pH probe movement during eating and talking. A videoradiographic study. Acta Radiol. 2003;44:131–135. doi: 10.1034/j.1600-0455.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 64.Monés J, Clavé P, Mearin F. Esophageal pH monitoring: are you sure that the electrode is properly placed? Am J Gastroenterol. 2001;96:975–978. doi: 10.1111/j.1572-0241.2001.03680.x. [DOI] [PubMed] [Google Scholar]
- 65.Trudgill NJ, Sifrim D, Sweis R, et al. British society of gastroenterology guidelines for oesophageal manometry and oesophageal reflux monitoring. Gut. 2019;68:1731–1750. doi: 10.1136/gutjnl-2018-318115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cuenca-Abente F, Oelschlager BK, Pellegrini CA. Principles of successful surgical anti-reflux procedures. In: Ferguson MK, Fennerty MB, eds. Managing failed anti-reflux therapy. London: Springer. 2006:57–65. doi: 10.1007/1-84628-011-7_5. [DOI] [Google Scholar]
- 67.Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD SAGES Guidelines Committee, author. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647–2669. doi: 10.1007/s00464-010-1267-8. [DOI] [PubMed] [Google Scholar]
- 68.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 69.Pauwels A, Boecxstaens V, Andrews CN, et al. How to select patients for antireflux surgery? The ICARUS guidelines (international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery) Gut. 2019;68:1928–1941. doi: 10.1136/gutjnl-2019-318260. [DOI] [PubMed] [Google Scholar]
- 70.Park S, Weg R, Enslin S, Kaul V. Ten things every gastroenterologist should know about antireflux surgery. Clin Gastroenterol Hepatol. 2020;18:1923–1929. doi: 10.1016/j.cgh.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 71.Wilshire CL, Niebisch S, Watson TJ, et al. Dysphagia postfundoplication: more commonly hiatal outflow resistance than poor esophageal body motility. Surgery. 2012;152:584–592. discussion 592–594. doi: 10.1016/j.surg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic esophageal motility disorders: serial diagnostic study. Lancet Gastroenterol Hepatol. 2017;2:654–661. doi: 10.1016/S2468-1253(17)30148-6. [DOI] [PubMed] [Google Scholar]
- 73.Wang YT, Tai LF, Yazaki E, et al. Investigation of dysphagia after antireflux surgery by high-resolution manometry: impact of multiple water swallows and a solid test meal on diagnosis, management, and clinical outcome. Clin Gastroenterol Hepatol. 2015;13:1575–1583. doi: 10.1016/j.cgh.2015.04.181. [DOI] [PubMed] [Google Scholar]
- 74.Scheffer RC, Samsom M, Haverkamp A, Oors J, Hebbard GS, Gooszen HG. Impaired bolus transit across the esophagogastric junction in postfundoplication dysphagia. Am J Gastroenterol. 2005;100:1677–1684. doi: 10.1111/j.1572-0241.2005.42009.x. [DOI] [PubMed] [Google Scholar]