Abstract
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that is characterized by abdominal pain and disordered bowel habits. The etiology of IBS is multifactorial, including abnormal gut-brain interactions, visceral hypersensitivity, altered colon motility, and psychological factors. Recent studies have shown that the intestinal microbiota and its metabolites short chain fatty acids (SCFAs) may be involved in the pathogenesis of IBS. SCFAs play an important role in the pathophysiology of IBS. We discuss the underlying mechanisms of action of SCFAs in intestinal inflammation and immunity, intestinal barrier integrity, motility, and the microbiota-gut-brain axis. Limited to previous studies, further studies are required to investigate the mechanisms of action of SCFAs in IBS and provide more precise therapeutic strategies for IBS.
Keywords: Fatty acids, volatile; Gastrointestinal microbiome; Irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder (FGID) characterized by abdominal pain and changes in stool form or frequency. According to the Rome IV criteria, IBS can be classified into 4 subtypes based on the predominant clinical symptoms: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), IBS with a mixed stool pattern (IBS-M), and IBS unclassified.1 It was estimated that the prevalence of IBS among different countries ranged from 10% to 20%.2 IBS affects the patients’ quality of life and places a heavy burden on both the healthcare systems and society.
The pathophysiology of IBS remains poorly understood, complex, and multifactorial. Abnormal gut-brain interactions, visceral hypersensitivity, altered colon motility, and psychological factors are considered as the triggers of IBS.3 In addition, the gut microenvironment has been implicated in the pathophysiology of IBS. Patients with IBS have a different gastrointestinal microbiome to that of healthy controls.4-9 Jeffery et al6 analyzed fecal microbiota and found that subjects with IBS had lower microbiota diversity than healthy controls. They showed that the microbiota composition and IBS subtypes were associations. They found an increase in Firmicutes and a depletion in Bacteroidetes in patients with IBS-C and IBS-M. This resulted from an increase in Dorea, Ruminococcus, and Clostridium spp., and a decrease in the number of Bacteroidetes, Bifidobacterium, and Faecalibacterium spp.9 A meta-analysis8 indicated that bacterial colonization, including Lactobacillus, Bifidobacterium, and Faecalibacterium prausnitzii, was significantly downregulated in patients with IBS-D. Moreover, Botschuijver et al5 found a loss of mycobiome diversity in patients with IBS compared to healthy volunteers. The results showed that the ratio of the predominant species, Saccharomyces cerevisiae and Candida albicans, increased, but the richness and evenness of the total species decreased.
Recently, gut microbiota-derived metabolites, such as short chain fatty acids (SCFAs), amino acid-derived metabolites, and bile acids, have been proposed as the possible etiologies of IBS, and may play an important role in the development of IBS.10,11 SCFAs are the end products of non-absorbed carbohydrates fermented by obligate anerobic bacteria in the intestine.12 The most abundant SCFAs in the colon were acetate (C2), propionate (C3), and butyrate (C4), which occurred in a molar ratio of 3:1:1. SCFAs concentrations are high in the proximal colon and cecum (70-140 mM for total SCFAs and 8-40 mM for individual SCFAs) and low in the distal colon (10-70 mM for total SCFAs and 1-20 mM for individual SCFAs).13,14 Previous reports have indicated that SCFAs show promising effects against various diseases, including obesity, diabetes, cancer, inflammation, immunodeficiency, pain, and depression.10,15 Increasing evidence has shown altered levels of SCFAs and abundance of SCFAs-producing bacteria in patients with IBS compared to healthy controls, revealing that SCFAs may affect the pathogenesis of IBS.16
Although intestinal bacteria and their metabolites, SCFAs, may be involved in the pathogenesis of IBS, their potential mechanism is still unclear. This review aim to summarize the alterations in intestinal bacteria and SCFAs in patients with different IBS subtypes and explore their underlying mechanisms in the development of the disease.
Altered Short Chain Fatty Acids and Short Chain Fatty Acids-producing Bacteria in Irritable Bowel Syndrome
It was reported that fecal samples from patients with IBS expressed significantly higher levels of acetate, propionate, and total SCFAs than controls, which positively related to the severity of symptoms.17 Altered fecal levels of SCFAs appeared to be associated with different IBS subtypes. Compared to controls, acetate, propionate, and butyrate levels were reduced in patients with IBS-C and increased in patients with IBS-D.18,19 SCFAs in feces can become non-invasive and reliable biomarkers for the primary diagnosis of IBS, especially propionate and butyrate.20
Altered levels of SCFAs in feces is related to the distribution of intestinal bacteria in patients with IBS. It was reported that patients with IBS showed significantly higher counts of Veillonella and Lactobacillus than controls, which are producers of acetate and propionate.17 The number of butyrate-producing Roseburia-Eubacterium rectale group was lower in patients with IBS-C than in controls.21 Ruminococcaceae, Clostridiales, and Erysipelotrichaceae, which are butyrate-producing bacteria, decreased in patients with IBS-D and IBS-M.22
Short Chain Fatty Acid Receptors
G protein coupled receptors (GPRs), GPR41(known as FFA3), GPR43(known as FFA2) and GPR109A, are known SCFAs receptors (Table 1). GPR41 and GPR43 can be activated by all the 3 SCFAs, whereas GPR109A is activated only by butyrate.23 All the receptors are coupled to Gi-type proteins, and GPR43 to Gq proteins.24,25 Both GPR41 and GPR43 receptors are expressed in enterocytes and enteroendocrine L-cells, which release glucagon-like peptide 1 (GLP-1) and peptide YY (PYY).26,27 GPR43 is expressed in 5-hydroxytryptamine (5-HT)-containing mucosal mast cells, enterochromaffin cells,27,28 and immune cells such as neutrophils and eosinophils. GPR41 is specifically expressed in neuronal cells of the submucosal and myenteric ganglia29 and autonomic ganglia such as the vagal, spinal dorsal root, and trigeminal ganglia.30 GPR109A is reported to be present in the intestinal epithelial and immune cells such as neutrophils and macrophages.25,31
Table 1.
Short Chain Fatty Acids Receptors Involved in Irritable Bowel Syndrome
| Receptor | G protein | Ligand | Cell | Refs |
|---|---|---|---|---|
| GPR41 (FFA3) | Gi/o | Acetate, propionate, and butyrate | Enteroendocrine cells (L cells) and neuronal cells | 23-24, 26, 29, 30 |
| GPR43 (FFA2) | Gi/o, Gq | Acetate, propionate, and butyrate | Enteroendocrine cells, (enterochromaffin cells and L cells), mast cells, and immune cells (neutrophils and eosinophils) | 23-24, 27, 28, 29 |
| GPR109A | Gi | Butyrate | Intestinal epithelial cells and immune cells (neutrophils and macrophages) | 23-25, 31 |
GPR, G protein coupled receptors; FFA, free fatty acid receptors.
Potential Mechanism of Short Chain Fatty Acids
Immunity and Inflammation
SCFAs binding to GPR43 and GPR109A in colonic epithelial cells induced an increase in intracellular Ca2+ and stimulated K+ efflux and hyperpolarisation, thus leading to nucleotide-binding domain and leucine-rich repeat protein-3 (NLRP3) inflammasome activation.32 The NLRP3 inflammasome triggers caspase-1-dependent processing of inflammatory mediators such as IL-18and IL-1β, which play key roles in the maintenance of intestinal homeostasis and protection from colitis development.33,34 Through GPR41 and GPR43, SCFAs activate the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signalling pathways in colonic epithelial cells. This recruits leukocytes and activates effector T cells in the gut, inducing the production of chemokines (C-X-C motif chemokine ligand 1 [CXCL1], CXCL2, and CXCL10) and cytokines (IL-6).35 Chemokines and cytokines promoted by SCFAs are critical for immune response, the early clearance of pathogen or late excessive inflammatory response.36 SCFAs can promote the differentiation of effector T cells and regulatory T cells, such as T helper type 1 (Th1) cells, T helper type 17 (Th17) cells, and IL-10-producing T cells, which produce IFN-γ, IL-17, and IL-10. This regulation could be independent of GPR41 and GPR43 through the inhibition of histone deacetylase (HDAC) and regulation of the mechanistic target of rapamycin (mTOR)-S6K pathway in T cells.37 Alternatively, GPR43-dependent activation of the mTOR-STAT3 pathway promotes the expression of B lymphocyte-induced maturation of protein 1 in T-cells.38
Butyrate can act on GPR109A in colonic macrophages and dendritic cells, enabling them to induce the differentiation of regulatory T cells and IL-10-producing T cells, and promote the expression of IL-10 and IL-18, thereby suppressing intestinal inflammation.39 Binding to GPR41, butyrate inhibits HDAC, activates the mTOR-STAT3 pathway, and increases the expression of aryl hydrocarbon receptor and hypoxia-inducible factor 1α, thus upregulating IL-22 production by CD4+ T cells and innate lymphocytes.40 IL-22 is central to host mucosal antimicrobial defense. The direct inhibition of IL-22 in intestinal innate lymphoid cells increases the risk of pathogen-mediated diarrhoea.41,42 In addition, butyrate downregulates lipopolysaccharide-induced pro-inflammatory cytokine production by neutrophils and macrophages, including IL-6, IL-12, and nitric oxide.43
These studies have shown that SCFAs can regulate immune and inflammatory responses of the intestinal epithelium, protect the intestinal mucosa, and maintain intestinal homeostasis.
Intestinal Barrier Integrity
SCFAs, mainly butyrate, increase the secretion of the goblet cell-specific mucin 2 (MUC2) and promote reassembling of tight junctions, improving the protective effect of the intestinal epithelium and enhancing the integrity of the intestinal barrier.44-46 However, this protective effect was dose-dependent, with small doses of SCFAs increasing MUC2 secretion and vice versa at high doses. Propionate and butyrate at concentrations of 1-15 mM have been reported to increase MUC2 expression. The effect of butyrate on MUC2 mRNA level is mediated through active activating protein-1 cis-element, acetylation of histone H3 and H4, and methylation of histone H3 at the promoter.44 One study showed that butyrate stimulated MUC2 production in individual cells by HDAC inhibition,45 while another study indicated that a decrease in MUC2 was associated with the ability of butyrate to repress HDAC.47 This was presumably related to the different butyrate concentrations used in these studies. SCFAs stimulate the expression of MUC2 in intestinal epithelial cells by regulating prostaglandin (PG) production in subepithelial myofibroblasts and increasing the PG1/PG2 ratio.48 These myofibroblasts are an important source of PGs and are therefore crucial for mucoprotection. Sodium butyrate has been reported to promote the reassembling of tight junctions by inhibiting the myosin light chain kinase/myosin II regulatory light chain pathway and phosphorylation of PKCβ2 in Caco-2 cells.49 It acts on the Akt signaling pathway to increase the expression of tight junction proteins claudin-3, occludin, and zonula occludens-1 in the colon in a GPR109A-dependent manner.50
Motility
SCFAs play an important role in the regulation of gut motility. This modulation varies depending on the type and dose of SCFAs, animal species, and experimental models. In IBS-D mice, the fecal SCFA levels were higher and colonic contractions were stronger than those in controls. SCFAs dose-dependently (0.5-30 mM) reduced the tonic tone, frequency, and amplitude of proximal colonic contractions. Exogenous administration of butyrate (5 mM) increased the colonic transit rate.51 In a rat model of IBS, total SCFAs potentiated proximal colonic contractions at low concentrations (5-50 mM) and inhibited contractions at high concentrations (50-150 mM).52 Studies have investigated the effects of SCFAs on proximal and distal colonic contractions in guinea pig models. In the proximal colon, butyrate increased the frequency of contractions, whereas propionate and acetate decreased the frequency of contractions. In the distal colon, butyrate increased and propionate decreased the rate of colonic propulsion.53
SCFAs (100 mM) promotes the secretion of 5-HT from enterochromaffin cells, acted on 5-HT3 receptors on sensory fibres of the vagus nerve, and stimulates the colonic submucosal plexus and myenteric plexus. Increased Ca2+ signalling triggers action potential generation in neurones, resulting in the release of acetylcholine and muscle contraction, which contribute to proximal colonic contractions.54-56 However, another study came to the opposite conclusion that SCFAs (> 5 mM) inhibited the distal colon contraction frequency through the above mechanism, which may be related to the different concentrations of SCFAs and the different segments of the colon.57 SCFAs promote the release of 5-HT from intestinal mucosal cells and activated 5-HT4/5-HT1p receptors in intrinsic calcitonin gene-related peptide-containing sensory neurones. It causes proximal colon contraction and distal colon relaxation, which enhances the peristaltic reflex induced by mechanical stimulation of the colonic mucosa and accelerates colonic transit.13,58,59 SCFAs increase intestinal contractility by upregulating L-type calcium channels in intestinal smooth muscle cells and/or increasing the number of interstitial cells of Cajal through 5-HT2B receptors.60-62 The long-term increase in butyrate can significantly increase the number of nitrogenic and cholinergic neurones that promote submucosal and myenteric neuromuscular signal transmission in the colon and enhance intestinal contraction and peristalsis.63-65
The short chain fatty acid receptors GPR41 and GPR43 are considered to be involved in intestinal motility.29 Activation of GPR41 in nitrergic and cholinergic neurones in the submucosal and myenteric plexus suppresses nicotinic acetylcholine receptor-mediated neural activity and reduces intestinal motility.66,67 Moreover, the activation of GPR43/GPR41 located in enteroendocrine cells releases anorectic PYY and GLP-1, which functionally inhibit gut transit.68-71 GPR43 selective agonist stimulated GLP-1 secretion in vivo and PYY secretion in the colonic mucosa.71 Colonic infusion of SCFAs, such as propionate, stimulated PYY release via GPR3.68,70 Contrary to many previous reports, one study showed that SCFAs increased colonic GLP-1/PYY secretion, but this seemed to be independent of GPR41 and GPR43.72
These studies suggest that the effects of SCFAs on colonic motility are not absolute. Promotion or inhibition depends on the homeostasis of SCFA concentrations in different colonic segments.
Microbiota-Gut-Brain Axis
The microbiota-gut-brain axis is crucial in maintaining homeostasis and may impact psychiatric disorders and IBS. Psychological disorders appear to be risk factors for IBS.73 The gut microbiota communicates with the brain through the neural (autonomic and enteric nervous system), endocrine (hypothalamic-pituitary-adrenal axis and enteroendocrine cells), and immune signaling channels.74-76 SCFAs have been implicated in microbiota-gut-brain axis interactions. However, the results of different studies vary. Studies have shown negative correlations between the levels of SCFAs (eg, acetate and propionate) and the degree of depression in a patient’s stool.77,78 Exogenous SCFA supplementation reduces stress-induced psychological and behavioral deficits.79 Another study suggested that emotional problems were significantly related to higher fecal butyrate levels.80
Activation of the hypothalamic-pituitary-adrenal-axis is critical for psychoneurological-related diseases, such as depression and anxiety.81 SCFAs attenuate stress-induced behavioral and physiological alterations by downregulating stress signaling and reducing the responsiveness of the hypothalamic-pituitary-adrenal axis.79 SCFAs modulate the activity of the sympathetic nervous system at the sympathetic ganglion level via GPR41. Propionate was reported to promote sympathetic activation and adrenaline secretion by activating GPR41.82 In PC12 cells, the administration of propionate and butyrate increased the expression of tyrosine hydroxylase and the ability of cells to produce catecholamines.83 In mouse models, gut microbiota dysbiosis and its reduction in SCFAs adversely affect epinephrine release, and oral SCFA supplementation improves the stress-induced epinephrine response.84 Vagus nerve stimulation was a form of neuromodulation which provided a treatment for chronic pain and depression.85,86 SCFAs such as butyrate can directly activate vagal afferent nerve terminals in the gut.87 They stimulated vagal afferents by activating GPR41 in vagal neurones.30,88,89
Taken together, SCFAs coordinate pain transmission, depression, anxiety, and stress through neuroendocrine mechanisms.
Future Perspectives
The concentration of SCFAs is susceptible to external conditions such as dietary patterns, antibiotics, and probiotics. A low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet was able to improve bloating, flatulence, diarrhea, and systemic symptoms of IBS by reducing microbial fermentation products, including SCFAs.90 Dietary fiber intake is beneficial for regulating gut bacteria and increasing the production of SCFAs.91 The use of antibiotics could lead to sustained changes in the intestinal microbiota composition and lower concentrations of SCFAs, which is accompanied by a decrease in the immunoreactivity of GPR41 and GPR43 in the intestinal mucosa.92,93 Although probiotics increase the number of bifidobacteria in the gut, they have no effect on SCFA levels.94,95 Fecal microbiota transplantation increased the concentration of SCFAs in the stool of patients with IBS and improved their symptoms. Further studies have shown that increased butyrate levels are inversely associated with IBS symptoms after fecal microbiota transplantation.96-98
In addition, a series of agonists of SCFA receptors can affect gut function through different mechanisms (Table 2).66,71,99-102 Selective GPR41 agonists are expected to become promising targets for the treatment of neurogenic diarrheal disorders because of their anti-dynamic and anti-secretory functions.66 Selective GPR43 agonists inhibit gut transit via the PYY pathways.71 The selective GPR43 agonist phenylacetamide-1 stimulates enterochromaffin cells and releases 5-HT, which enhances intestinal mucosal defences. However, excessive phenylacetamide-1 leads to injury of the intestinal mucosa by decreasing the blood flow.102
Table 2.
Selective Short Chain Fatty Acid Receptor Agonists Involved in Irritable Bowel Syndrome
| Agonist | Receptor | Function | Model | Refs |
|---|---|---|---|---|
| AR420626 | GPR41 | Stimulate anion secretion, suppress neural activity, and inhibit muscle contractions | Rat | 66, 99 |
| 4-CMTB | GPR43 | Stimulate anion secretion | Rat | 99 |
| AZ1729 | GPR43 | Activated and desensitize of neutrophils | Human neutrophil | 101 |
| phenylacetamide-1 | GPR43 | Modulate the intestinal mucosa protection | Mice | 102 |
| Compound 1 | GPR43 | Slow intestinal transit | Mice | 71 |
| Compound 58 | GPR43 | Activate and desensitize of neutrophils | Human neutrophil | 101 |
| Compound 110 | GPR43 | Attenuate intestinal inflammation | Mice | 100 |
| Compound 187 | GPR43 | Attenuate intestinal inflammation | Mice | 100 |
GPR, G protein coupled receptors.
Conclusions
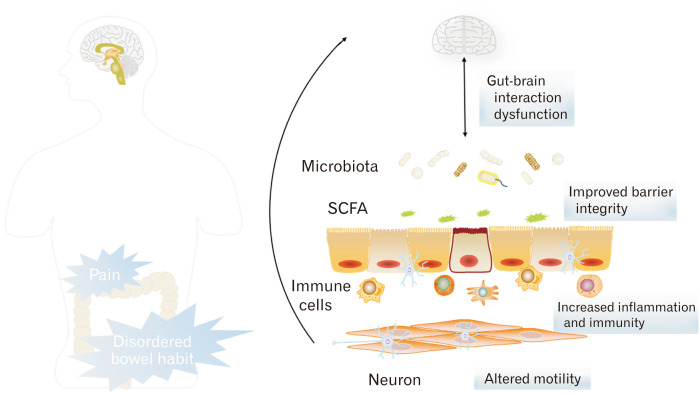
In the past decade, many studies have explored the relationship between SCFAs and IBS. Increasing evidence has revealed an important role of altered SCFAs in the pathophysiology of IBS. This review demonstrates the possible mechanism of SCFAs in IBS in terms of inflammation and immunity, intestinal barrier integrity, motility, and the microbiota-gut-brain axis (Figure). As discussed in this review, SCFAs were considered to exert a vital impact to the development of IBS, and modulating the concentration of SCFAs or the activity of SCFA receptors may be a new strategy for treating IBS. However, limited to different models and conditions of previous studies, further studies are required to investigate the mechanism of SCFAs in IBS and to provide more precise therapeutic strategies for IBS.
Figure.
The mechanism of short chain fatty acids (SCFAs) in irritable bowel syndrome (IBS). The metabolites of gut microbiota SCFAs can modulate intestinal epithelial immunity and inflammation, maintain gut barrier integrity, alter gut motility, and act as part of the microbiota-gut-brain axis, which may underlie the potential mechanisms of SCFAs in the pathogenesis of IBS.
Footnotes
Financial support: This study was supported by Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Conflicts of interest: None.
Author contributions: Zhe Shen: guarantor of the article; Shefeng Zhu and Linying Xin completed the literature search; Jiali Wu and Wenxi Jiang wrote the paper; and Chaohui Yu and Zhe Shen reviewed and edited the article. All authors approved the final version of the manuscript.
References
- 1.Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675–1688. doi: 10.1016/S0140-6736(20)31548-8. [DOI] [PubMed] [Google Scholar]
- 2.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075–1082. doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 3.Simrén M, Törnblom H, Palsson OS, Van Oudenhove L, Whitehead WE, Tack J. Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient-reported outcomes in irritable bowel syndrome. Gastroenterology. 2019;157:391–402. e2. doi: 10.1053/j.gastro.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Kassinen A, Krogius-Kurikka, Mäkivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Botschuijver S, Roeselers G, Levin E, et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology. 2017;153:1026–1039. doi: 10.1053/j.gastro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery IB, O'Toole PW, Öhman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 7.Durbán A, Abellán JJ, Jiménez-Hernández N, et al. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ Microbiol Rep. 2012;4:242–247. doi: 10.1111/j.1758-2229.2012.00327.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig Liver Dis. 2017;49:331–337. doi: 10.1016/j.dld.2017.01.142. [DOI] [PubMed] [Google Scholar]
- 9.Rajilić-Stojanović M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Hua D, Wang Q, et al. The role of bacteria and its derived metabolites in chronic pain and depression: recent findings and research progress. Int J Neuropsychopharmacol. 2020;23:26–41. doi: 10.1093/ijnp/pyz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao L, Liu Q, Luo M, Xiong L. Gut microbiota-derived metabolites in irritable bowel syndrome. Front Cell Infect Microbiol. 2021;11:729346. doi: 10.3389/fcimb.2021.729346.b1d18af4988f4d0f89e16248f66132f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29:700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:G429–G437. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- 14.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu T, Wu X, Liu J, et al. The regulatory roles of dietary fibers on host health via gut microbiota-derived short chain fatty acids. Curr Opin Pharmacol. 2021;62:36–42. doi: 10.1016/j.coph.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Carco C, Young W, Gearry RB, Tally NJ, McNabb WC, Roy NC. Increasing evidence that irritable bowel syndrome and functional gastrointestinal disorders have a microbial pathogenesis. Front Cell Infect Microbiol. 2020;10:468. doi: 10.3389/fcimb.2020.00468.ecb0df1563e644f38b00623cc929c196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519. e114–e115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun Q, Jia Q, Song L, Duan L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14513. doi: 10.1097/MD.0000000000014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gargari G, Taverniti V, Gardana C, et al. Fecal Clostridiales distribution and short-chain fatty acids reflect bowel habits in irritable bowel syndrome. Environ Microbiol. 2018;20:3201–3213. doi: 10.1111/1462-2920.14271. [DOI] [PubMed] [Google Scholar]
- 20.Farup PG, Rudi K, Hestad K. Faecal short-chain fatty acids - a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016;16:51. doi: 10.1186/s12876-016-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 22.Pozuelo M, Panda S, Santiago A, et al. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015;5:12693. doi: 10.1038/srep12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 24.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 25.Kostylina G, Simon D, Fey MF, Yousefi S, Simon HU. Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A) Cell Death Differ. 2008;15:134–142. doi: 10.1038/sj.cdd.4402238. [DOI] [PubMed] [Google Scholar]
- 26.Tazoe H, Otomo Y, Karaki SI, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- 27.Karaki SI, Mitsui R, Hayashi H, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 28.Akiba Y, Inoue T, Kaji I, et al. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2015;593:585–599. doi: 10.1113/jphysiol.2014.280792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nøhr MK, Pedersen MH, Gille A, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 30.Nøhr MK, Egerod KL, Christiansen SH, et al. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol. 2013;13:869–874. doi: 10.1016/j.coph.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 33.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirota SA, Ng J, Lueng A, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Di, 2011;17:1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. e1–e10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 36.Dunsmore G, Koleva P, Ghobakhloo N, et al. Lower abundance and impaired function of CD71+ erythroid cells in inflammatory bowel disease patients during pregnancy. J Crohns Colitis. 2019;13:230–244. doi: 10.1093/ecco-jcc/jjy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, Wu W, Chen L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2.0ba5e8cba7f0472bb973f7f9800301ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang W, Yu T, Huang X, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11:4457. doi: 10.1038/s41467-020-18262-6.c31a59e08ef446ffa06978b9286f2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu R, O'Quinn DB, Silberger DJ, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo X, Qiu J, Tu T, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40:25–39. doi: 10.1016/j.immuni.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger-van Paassen N, Vincent A, Puiman PJ, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 45.Hatayama H, Iwahita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun. 2007;356:599–603. doi: 10.1016/j.bbrc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 46.Finnie IA, Dwarakanath AD, Taylor BA, Rhodes JM. Colonic mucin synthesis is increased by sodium butyrate. Gut. 1995;36:93–99. doi: 10.1136/gut.36.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augenlicht L, Shi L, Mariadason J, Laboisse C, Velcich A. Repression of MUC2 gene expression by butyrate, a physiological regulator of intestinal cell maturation. Oncogene. 2003;22:4983–4992. doi: 10.1038/sj.onc.1206521. [DOI] [PubMed] [Google Scholar]
- 48.Willemsen LEM, Koetsier MA, van Deventer SJH, van Tol EAF. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miao W, Wu X, Wang K, et al. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCβ2. Int J Mol Sci. 2016;17:1696. doi: 10.3390/ijms17101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng W, Wu Y, Ghen G, et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell Physiol Biochem. 2018;47:1617–1629. doi: 10.1159/000490981. [DOI] [PubMed] [Google Scholar]
- 51.Shaidullov IF, Sorokina DM, Sitdikov FG, Hermann A, Abdulkhakov SR, Sitdikova GF. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol. 2021;21:37. doi: 10.1186/s12876-021-01613-y.4f4591c468384eefb3c3b7ea6b2af93a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan F, Tan W, Ren H, Yan L, Wang Y, Luo H. The effects of short-chain fatty acids on rat colonic hypermotility induced by water avoidance stress. Drug Des Devel Ther. 2020;14:4671–4684. doi: 10.2147/DDDT.S246619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26:1586–1596. doi: 10.1111/nmo.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 55.Fung C, Cools B, Malagola S, et al. Luminal short-chain fatty acids and 5-HT acutely activate myenteric neurons in the mouse proximal colon. Neurogastroenterol Motil. 2021;33:e14186. doi: 10.1111/nmo.14186. [DOI] [PubMed] [Google Scholar]
- 56.Bertrand PP, Kunzee WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/S0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 57.Ono S, Karaki S, Kuwahara A. Short-chain fatty acids decrease the frequency of spontaneous contractions of longitudinal muscle via enteric nerves in rat distal colon. Jpn J Physiol. 2004;54:483–493. doi: 10.2170/jjphysiol.54.483. [DOI] [PubMed] [Google Scholar]
- 58.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol. 1996;270(5 Pt 1):G778–G782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 59.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- 60.Jin B, Ha SE, Wei L, et al. Colonic motility is improved by the activation of 5-HT2B receptors on interstitial cells of Cajal in diabetic mice. Gastroenterology. 2021;161:608–622. e7. doi: 10.1053/j.gastro.2021.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tharayil VS, Wouters MM, Stanich JE, et al. Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil. 2010;22:462–469. e109–e110. doi: 10.1111/j.1365-2982.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wouters MM, Gibbons SJ, Roeder JL, et al. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Suply E, de Vries P, Soret R, Cossais F, Neunlist M. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1373–G1380. doi: 10.1152/ajpgi.00338.2011. [DOI] [PubMed] [Google Scholar]
- 64.Soret R, Chevalier J, De Coppet P, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 65.Vicentini FA, Keenan CM, Wallace LE, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9:210. doi: 10.1186/s40168-021-01165-z.c218aabe31c34611bbed7773547b1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaji I, Akiba Y, Furuyama T, et al. Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon. Neurogastroenterol Motil. 2018;30:e13157. doi: 10.1111/nmo.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaji I, Akiba Y, Konno K, et al. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J Physiol. 2016;594:3339–3352. doi: 10.1113/JP271441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tough IR, Forbes S, Cox HM. Cox, Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate. Neurogastroenterol Motil. 2018;30:e13454. doi: 10.1111/nmo.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wichmann A, Allahyar A, Greiner TU, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Cherbut C, Ferrier L, Rozé C, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275:G1415–G1422. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- 71.Forbes S, Stafford S, Coope G, et al. Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake but does not improve glucose tolerance in mouse models. Diabetes. 2015;64:3763–3771. doi: 10.2337/db15-0481. [DOI] [PubMed] [Google Scholar]
- 72.Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315:G53–G65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 73.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284–1290. doi: 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- 74.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skonieczna-Żydecka K, Grochans E, Maciejewska D, et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients. 2018;10:1939. doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelly JR, Borre Y, O' Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 79.van de Wouw M, Boehme M, Lyte JM, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michels N, Van de Wiele T, De Henauw S. Chronic psychosocial stress and gut health in children: associations with calprotectin and fecal short-chain fatty acids. Psychosom Med. 2017;79:927–935. doi: 10.1097/PSY.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 81.Wiley NC, Dinan TG, Ross RP, Stanton C, Glarke G, Cryan JF. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: implications for human and animal health. J Anim Sci. 2017;95:3225–3246. doi: 10.2527/jas2016.1256. [DOI] [PubMed] [Google Scholar]
- 82.Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nankova BB, Agarwal R, MacFabe DF, La Gamma EF. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells--possible relevance to autism spectrum disorders. PLoS One. 2014:9e103740. doi: 10.1371/journal.pone.0103740.7bd7d25af02d48b28b788cadf9dba55e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LaGamma EF, Hu F, Cruz FP, Bouchev P, Nankova BB. Bacteria - derived short chain fatty acids restore sympathoadrenal responsiveness to hypoglycemia after antibiotic-induced gut microbiota depletion. Neurobiol Stress. 2021;15:100376. doi: 10.1016/j.ynstr.2021.100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chakravarthy K, Chaudhry H, Williams K, Christo PJ. Review of the uses of vagal nerve stimulation in chronic pain management. Curr Pain Headache Rep. 2015;19:54. doi: 10.1007/s11916-015-0528-6. [DOI] [PubMed] [Google Scholar]
- 86.Thompson SL, O'Leary GH, Austelle CW, et al. A review of parameter settings for invasive and non-invasive vagus nerve stimulation (VNS) applied in neurological and psychiatric disorders. Front Neurosci. 2021;15:709436. doi: 10.3389/fnins.2021.709436.4c6814b059564443be14e84149f63f5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 88.Goswami G, Iwasaki Y, Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem. 2018;57:130–135. doi: 10.1016/j.jnutbio.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 89.Cook TM, Gavini CK, Jesse J, et al. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol Metab. 2021;54:101350. doi: 10.1016/j.molmet.2021.101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66:1517–1527. doi: 10.1136/gutjnl-2017-313750. [DOI] [PubMed] [Google Scholar]
- 91.Salamone D, Rivellese AA, Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetol. 2021;58:1131–1138. doi: 10.1007/s00592-021-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holota Y, Dovbynchuk T, Kaji I, et al. The long-term consequences of antibiotic therapy: role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS One. 2019;14:e0220642. doi: 10.1371/journal.pone.0220642.7f2c24d7fd6e4d5c8b880cae2fea4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao X, Jiang Z, Yang F, et al. Sensitive and simplified detection of antibiotic influence on the dynamic and versatile changes of fecal short-chain fatty acids. PLoS One. 2016;11:e0167032. doi: 10.1371/journal.pone.0167032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merenstein D, Fraser CM, Roberts RF, et al. Bifidobacterium animalis subsp. lactis BB-12 protects against antibiotic-induced functional and compositional changes in human fecal microbiome. Nutrients. 2021;13:2814. doi: 10.3390/nu13082814.36a4993c43494c279dfb8a6960e44d22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee Y, Ba Z, Roberts R, et al. Effects of Bifidobacterium animalis subsp. lactis BB-12® on the lipid/lipoprotein profile and short chain fatty acids in healthy young adults: a randomized controlled trial. Nutr J. 2017;16:39. doi: 10.1186/s12937-017-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.El-Salhy M, Valeur J, Hausken T, Hatlebakk JG. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e13983. doi: 10.1111/nmo.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El-Salhy M, Kristoffersen AB, Valeur J, et al. Long-term effects of fecal microbiota transplantation (FMT) in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2021;34:e14200. doi: 10.1111/nmo.14200. [DOI] [PubMed] [Google Scholar]
- 98.Mazzawi T, Hausken T, Hov JR, et al. Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scand J Gastroenterol. 2019;54:690–699. doi: 10.1080/00365521.2019.1624815. [DOI] [PubMed] [Google Scholar]
- 99.Ballout J, Akiba Y, Kaunitz JD, Diener M. Short-chain fatty acid receptors involved in epithelial acetylcholine release in rat caecum. Eur J Pharmacol. 2021;906:174292. doi: 10.1016/j.ejphar.2021.174292. [DOI] [PubMed] [Google Scholar]
- 100.Park BO, Kang JS, Paudel S, et al. Novel GPR43 agonists exert an anti-inflammatory effect in a colitis model. Biomol Ther (Seoul) 2022;30:48–54. doi: 10.4062/biomolther.2021.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lind S, Holdfeldt A, Mårtensson J, et al. Interdependent allosteric free fatty acid receptor 2 modulators synergistically induce functional selective activation and desensitization in neutrophils. Biochim Biophys Acta Mol Cell Res. 2020;1867:118689. doi: 10.1016/j.bbamcr.2020.118689. [DOI] [PubMed] [Google Scholar]
- 102.Akiba Y, Maruta K, Narimatsu K, et al. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am J Physiol Gastrointest Liver Physiol. 2017;313:G117–G128. doi: 10.1152/ajpgi.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]