Abstract
The live attenuated Brucella abortus strain RB51 is a rifampin-resistant, lipopolysaccharide (LPS) O-chain-deficient mutant of virulent B. abortus 2308. The reduced O-chain content in RB51 prevents this bacterium from inducing antibodies detectable by the conventional serologic tests for bovine brucellosis diagnosis that mainly identify antibodies to LPS. The absence of available serologic tests for RB51 also complicates the diagnosis of possible RB51 infections in humans exposed to this strain. The purpose of this study was to evaluate the suitability of a complement fixation (CF) test performed with the rough strain B. abortus RB51, previously deprived of anticomplementary activity, in detecting anti-B. abortus RB51 antibodies in cattle and sheep experimentally vaccinated with this strain. The results of this study showed that a CF test with RB51 as the antigen is able to specifically detect antibodies following RB51 vaccination in cattle and sheep. In addition, this method could be a useful tool for detecting B. abortus RB51 infection in humans.
Strain RB51 of Brucella abortus is a live rough rifampin-resistant mutant of the standard virulent strain B. abortus 2308 (12). This strain lacks most of the lipopolysaccharide O side chain present in both S19 and naturally occurring field strains of virulent B. abortus; thus, it does not result in measurable titers of antibody to B. abortus in standard serologic tests. Results of previous studies indicated that strain RB51 can protect cattle against infection with virulent B. abortus strains to at least the same extent as does strain 19, but it does not interfere with the serologic diagnosis of field infections (4, 10, 13). In addition, experiments performed with a mouse model showed that vaccination with strain RB51 provided protection against challenge with heterologous Brucella species, including Brucella melitensis (6). Cattle vaccinated with B. abortus RB51 had negative results for all routine serologic tests, including a rapid plate agglutination test, standard tube agglutination test, card test, ethacridine lactate (Rivanol) test, and complement fixation (CF) test, using whole-cell antigens of B. abortus 1119-3 prepared according to the method of Alton et al. (2). In addition, sera from RB51-vaccinated female cattle were evaluated by an agar gel immunodiffusion test with O polysaccharide isolated from smooth strains of B. abortus, and a ring test was carried out on samples of milk obtained after first calving. The results of these studies confirmed that the vaccination of cattle with B. abortus RB51, administered once or twice, does not induce seroconversion detectable by serologic surveillance tests, including the agar gel immunodiffusion test (8).
The purpose of this study was to perform a CF test with a B. abortus rough strain, RB51, previously deprived of its anticomplementary activity and to evaluate the ability of this test to specifically detect antibody responses of cattle and sheep vaccinated with the B. abortus RB51 strain.
MATERIALS AND METHODS
B. abortus RB51 vaccine suspension.
For sheep and cattle vaccinations, a suspension of B. abortus RB51, containing 5 × 109 CFU per ml, was used. This suspension was prepared with bacteria from an unlicensed veterinary product, labelled “B. abortus vaccine strain RB51, live culture” (Investigation Services, Mantova, Italy), biochemically and serologically tested at Istituto Superiore di Sanità.
Animals and vaccination.
For this study, six 7-month-old Frisona heifers and six 5-month-old sheep, all obtained from brucellosis-free herds, were used, and each group was kept in a concrete isolation room. After an acclimation period, four cows were vaccinated subcutaneously in the neck region with 2 ml each of B. abortus RB51 vaccine while four sheep received 1 ml each of the same suspension. Strain RB51 vaccine was administered twice; the second dose was given, as described above, 110 days after the first vaccination. The remaining unvaccinated animals were used as controls.
Collection of sera.
Blood from all of the vaccinated cattle and sheep was collected by venipuncture before vaccination (time zero), at 7, 15, 30, 48, 76, and 110 days postvaccination, and at 7, 15, 30, and 60 days after the booster. All unvaccinated animals were sampled at the same times. Blood was collected into sterile 10-ml tubes, allowed to clot for 20 h at 4°C, centrifuged, and stored at −20°C until use.
Preparation of B. abortus RB51 antigen for the CF test.
Unlike smooth brucellae, the RB51 rough strain of B. abortus shows a considerable anticomplementary activity as the antigen in a CF test. To eliminate this effect, the following RB51 suspension was prepared. Single colonies of B. abortus RB51 cloned from a vaccine suspension were cultured for 48 h at 37°C on tryptose agar supplemented with 5% bovine serum. After incubation, bacteria were harvested with physiologic saline (0.15 M NaCl, pH 7.2), washed twice by centrifugation at 1,475 × g for 20 min (Megafuge 3.0R; Heraeus Instruments, Hanau, Germany), and adjusted to a concentration of about 1.3 × 108 CFU per ml with calcium-magnesium-Veronal buffer (pH 7.2) (bioMérieux, Marcy l’Etoile, France). Before heat inactivation at 65°C for 1 h, the RB51 suspension was tested for the desired morphological and biochemical characteristics (rifampin resistance and rough colonial morphology).
(i) Incubation with negative serum.
Twofold dilutions of concentrated B. abortus RB51 suspension were prepared, and in a block titration plate, 25 μl of each dilution was added to 25 μl of an equal dilution of negative bovine serum previously inactivated at 58°C for 30 min. After incubation overnight at room temperature, each well was tested for anticomplementary activity by adding 25 μl containing 2 CH100 of guinea pig complement (1 CH100 is equal to 1 U of complement lysing 100% of the erythrocytes) produced in our laboratory. After incubation at 37°C for 30 min, 25 μl of sensitized erythrocytes was added as previously described (2). The optimal concentration of RB51 antigen to be used in the CF test was determined to be the dilution of RB51 suspension with the lowest concentration that caused the complete absence of anticomplementary activity when incubated with negative serum.
(ii) Titration of RB51 antigen.
The most sensitive dilution of the RB51 antigen was determined in a block titration CF test against anti-B. abortus RB51 bovine sera of high to low titer obtained from experimentally vaccinated cattle. In the same test, the second international standard anti-B. abortus serum (ISaBS) of the Veterinary Laboratories Agency of Weybridge, containing 1,000 international CF test units per ml, and bovine sera from brucellosis-free herds were tested to evaluate the specificity of the reaction. Each antigen dilution was also tested for anticomplementary activity against 2, 1, and 0.5 U of complement as described above.
Serologic analysis.
Serum samples from the vaccinated cattle and sheep and from the controls were tested for the presence of antibodies to B. abortus RB51 by conventional CF and rose bengal plate tests with the official S-type B. abortus strain 99 from the Veterinary Laboratories Agency of Weybridge, according to the Italian Decree (5, 5a), and by the CF test with RB51 as the antigen. In all CF assays performed with both antigens, the following sera were tested: previously titrated anti-B. abortus RB51 bovine serum, the ISaBS, and highly positive anti-B. abortus serum from naturally infected cattle. Finally, 1,000 IU of the international standard anti-Brucella ovis serum of the Veterinary Laboratories Agency of Weybridge and a pool of ovine sera from naturally B. ovis-infected sheep were tested against RB51 in the CF test to verify the efficiency of this antigen to also detect B. ovis infection in sheep.
RESULTS
Elimination of anticomplementary activity in B. abortus rough strain RB51.
Both heat and chloroform treatments failed to reduce the anticomplementary activity of strain RB51, while complete elimination was obtained by incubating a suspension of B. abortus RB51 with an equal volume of previously heat-inactivated negative bovine serum.
Titration of RB51 antigen for the CF test.
The results of the RB51 antigen titration indicated that the most sensitive dilution used in the CF test corresponded to a suspension of B. abortus RB51 containing about 1.6 × 107 CFU per ml, which was incubated with an equal volume of negative bovine serum diluted 1:8 in Veronal buffer.
Serologic responses.
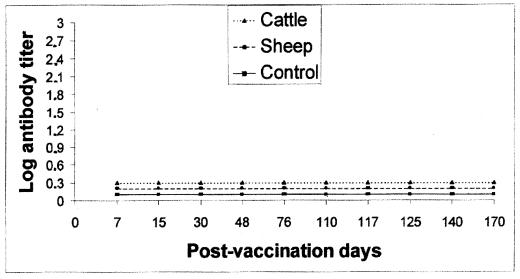
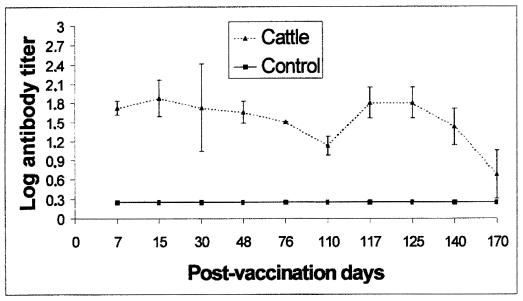
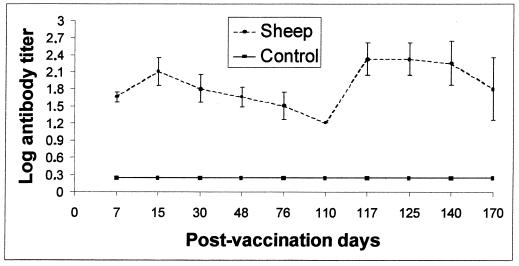
None of the cows or sheep vaccinated with strain RB51 developed antibodies detected by B. abortus 99-based CF and rose bengal plate surveillance tests (Fig. 1), but all of the animals developed antibodies to B. abortus RB51 that reacted in the CF test with RB51 as the antigen. Serologic responses of the vaccinated cattle and sheep to B. abortus RB51 in the CF assay are presented in Fig. 2 and 3, respectively. As shown in Fig. 2, anti-RB51 antibodies were produced in the cattle at 7 days postvaccination, and the highest titers occurred at 15 to 30 days and decreased at 110 days, when two cows were still weakly seropositive. After the booster, administered 110 days after the first dose of the vaccine, the highest titers occurred at 7 days; titers decreased 60 days later, when only one cow showed detectable anti-RB51 antibodies. Sheep vaccinated with RB51 (Fig. 3) developed peak antibody titers at 15 days postvaccination; the anti-RB51 antibodies remained for a more extended period than did the antibodies induced in cattle, and high titers were still present in all of the vaccinated sheep at 110 days, when the booster was administered. The antibody responses following the booster peaked at 7 days, the high titers remaining up to 60 days after the booster.
FIG. 1.
Serum antibody responses of cattle and sheep vaccinated with B. abortus RB51 and given a booster at 110 days, measured by the CF test with conventional B. abortus 99 antigen. The results are means.
FIG. 2.
Serum antibody responses of cattle after vaccination with B. abortus RB51 and after a booster given at 110 days, determined by the CF test with RB51 antigen. The results are means ± standard deviations.
FIG. 3.
Serum antibody responses of sheep after vaccination with B. abortus RB51 and after a booster given at 110 days, measured by the CF test with RB51 antigen. The results are means ± standard deviations.
The titers of the ISaBS and of serum from infected cattle were 1:4 and 1:2, respectively, when tested in the CF test with RB51 as the antigen.
The international standard anti-B. ovis serum yielded a titer of 1:128 against RB51 antigen while positive sera from B. ovis-infected sheep had titers similar to those detectable by the hot saline extract conventional antigen for the serodiagnosis of B. ovis infection in sheep (data not shown).
None of the samples from the unvaccinated cattle or sheep reacted in the CF test.
DISCUSSION
The results of this study confirm that currently available serologic surveillance tests for B. abortus 99 do not detect seroconversion following B. abortus RB51 vaccination in cattle or sheep. In many European countries, including Italy, vaccination against brucellosis is not allowed. The eradication programs for bovine and ovine-caprine brucellosis (5, 5a) have centered on the serologic screening of herds to detect infected animals and on a surveillance system on the vaccine’s use. In this situation, the limitations of standard serologic tests in identifying vaccinated animals are emphasized. In addition, the detection of possible human infections with the RB51 vaccine strain is also complicated by the absence of available serologic tests for RB51, and the recommendations for antibiotic treatment for prophylaxis after exposure to RB51 are difficult to make because the virulence of the strain in humans is unknown and the strain is resistant to rifampin in vitro (3, 7). An experimental dot blot assay to detect anti-RB51 antibodies has been evaluated under experimental and field conditions for cattle, but it has not yet been validated for humans (9). More recently, studies have demonstrated that the gamma interferon test, using inactivated B. abortus RB51 as the specific stimulus, may be a useful method to assess cell-mediated responses in RB51-vaccinated cattle (1). Our results show that the CF test with B. abortus RB51, previously deprived of its anticomplementary activity by incubating it with negative serum, as the antigen is able to specifically detect antibodies to RB51 in cattle and sheep experimentally vaccinated with this strain.
Based on our results, the serologic responses of sheep to RB51, despite the lower doses of the vaccine that were administered, were similar to those of cattle except that the CF peak titers in sheep were higher and their seropositivity persisted longer after vaccination. The weak responses of standard anti-B. abortus serum and serum from naturally B. abortus-infected cattle against RB51 were not unexpected, because the outer membrane proteins of Brucella species have been documented to be similar (11). However, in contrast with results reported by other authors that compared serologic responses of B19- and RB51-vaccinated cattle assessed by an RB51 dot enzyme-linked immunosorbent assay (14), the anti-RB51 antibody titers in S-type Brucella antisera were significantly lower than those in the sera of RB51-vaccinated animals, enabling a distinction between vaccinated and S-type Brucella-infected animals. In addition, the high titers induced by both the international standard anti-B. ovis serum and serum from B. ovis-infected sheep demonstrate that the CF test with RB51 antigen is also able to detect infection due to a B. ovis rough strain in sheep.
In conclusion, despite the low number of animals used in this study, our results indicate that the CF test with RB51 as the antigen may identify cattle and sheep vaccinated with strain RB51 and is able to identify B. ovis-infected sheep. These data suggest the possible use of strain RB51 as the official antigen, to be used alone or combined with the B. abortus 99 strain, to improve the efficiency of the serologic identification of cattle or sheep vaccinated or infected with rough strains of Brucella in the brucellosis eradication program. Finally, this test could be a useful tool to diagnose RB51 infection in humans.
REFERENCES
- 1.Adone R, Ciuchini F, Piccininno G, Pistoia C. Uso del gamma-interferon test per il rilievo della risposta cellulo-mediata indotta nei bovini da ceppi di Brucella spp. Salsomaggiore Terme: Convegno Società Italiana di Diagnostica di Laboratorio Veterinaria; 1998. [Google Scholar]
- 2.Alton G C, Jones L M, Angus R D, Verger J M. Techniques for the brucellosis laboratory. Paris, France: Institut National de la Recherche Agronomique; 1988. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Human exposure to Brucella abortus strain RB51. Kansas, 1997. Morbid Mortal Weekly Rep. 1998;47:172–175. [PubMed] [Google Scholar]
- 4.Cheville N F, Olsen S C, Jensen A E, Stevens M G, Palmer M V, Florance A M. Effects of age at vaccination on efficacy of Brucella abortus strain RB51 to protect cattle against brucellosis. Am J Vet Res. 1996;57:1153–1156. [PubMed] [Google Scholar]
- 5.Decreto Ministeriale. 1994. 27 August, no. 651. GU no. 277, 6 November 1994.
- 5a.Decreto Ministeriale. 1995. 31 May, no. 292. GU no. 169, 21 July 1995.
- 6.Jiménez de Bagüés M P, Elzer P H, Jones S M, Blasco J M, Enright F M, Schurig G G, Winter A J. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994;62:4990–4996. doi: 10.1128/iai.62.11.4990-4996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karhel S C. Brucella abortus strain RB51 vaccine: its advantages and risks. J Am Vet Med Assoc. 1998;213:12. , 22. [PubMed] [Google Scholar]
- 8.Lord V R, Schurig G S, Cherwonogrodzky J W, Marcano M J, Melendez G E. Field study of vaccination of cattle with Brucella abortus strain RB51 and 19 under high and low disease prevalence. Am J Vet Res. 1998;59:1016–1020. [PubMed] [Google Scholar]
- 9.Olsen S C, Stevens M G, Cheville N F, Schurig G G. Experimental use of a dot-blot assay to measure serologic responses of cattle vaccinated with Brucella abortus strain RB51. J Vet Diagn Investig. 1997;9:363–367. doi: 10.1177/104063879700900404. [DOI] [PubMed] [Google Scholar]
- 10.Palmer M V, Olsen S C, Cheville N F. Safety and immunogenicity of Brucella abortus strain RB51 vaccine in pregnant cattle. Am J Vet Res. 1997;58:472–477. [PubMed] [Google Scholar]
- 11.Santos J M, Verstreate D R, Perera V Y, Winter A J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984;46:188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schurig G G, Roop II R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 13.Stevens M G, Olsen S C. Antibody responses to Brucella abortus 2308 in cattle vaccinated with B. abortus RB51. Infect Immun. 1996;64:1030–1034. doi: 10.1128/iai.64.3.1030-1034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens M G, Olsen S C, Cheville N. Comparative analysis of immune responses in cattle vaccinated with Brucella abortus strain 19 or strain RB51. Vet Immunol Immunopathol. 1995;44:223–235. doi: 10.1016/0165-2427(94)05311-f. [DOI] [PubMed] [Google Scholar]