Abstract
Objective
Gliomas are one of the most common brain tumors in adults with a poor prognosis in most patients. Magnetic Resonance Imaging (MRI) plays a critical role in the diagnosis, management, and follow-up of gliomas. The aim of this study is to assess the sensitivity and specificity of MRI in the preoperative grading of supratentorial gliomas in comparison to histopathology.
Methods
A cross-sectional study included 39 patients, aged between 40 and 75 years with histologically diagnosed supratentorial gliomas who underwent conventional MR imaging, which included T1, T2, and FLAIR sequences from November 2018–December 2019 in the Department of Neurosurgery, Tishreen University Hospital, Lattakia. The histopathological typing and grading of the tumor were done by using 2016 WHO classification. The sensitivity, specificity, predictive value, and accuracy of MRI in determining tumor grade were calculated. The comparison was done between MRI findings and WHO histopathological grading.
Results
The overall sensitivity and specificity of MRI findings in the assessment of high-grade gliomas were 100% and 91% respectively. The positive predictive value (PPV) was 66.6%, and the negative predictive value (NPV) was 100%. The overall accuracy was 94.9%. The agreement between histopathological and MRI findings was 72%.
Conclusions
MRI plays an essential role in the initial diagnosis and grading of supratentorial gliomas with high sensitivity and specificity. It is considered a non-invasive method and is useful in cases where the biopsy procedure is a contraindication or rejected by the patient.
Keywords: Gliomas, Glioblastoma, Magnetic resonance imaging, Histopathology
Highlights
-
•
Magnetic Resonance Imaging (MRI) plays a critical role in the diagnosis, management, and follow-up of gliomas.
-
•
We report a study of 39 patients with histologically diagnosed supratentorial gliomas who underwent conventional MR imaging.
-
•
MRI plays an essential role in the diagnosis and grading of supratentorial gliomas with high sensitivity and specificity.
1. Introduction
Gliomas are the most common primary malignant brain tumors that are derived from glial cells [1]. Based on the histopathological analysis, gliomas are divided into three main types: astrocytomas, oligodendrogliomas, and ependymomas [1,2]. The World Health Organization (WHO) classifies gliomas based on histologic criteria, which are cytological atypia, mitotic activity, microvascular proliferation, and necrosis, into four grades: grade I (pilocytic astrocytoma), grade II (diffuse astrocytoma), grade III (anaplastic astrocytoma), and grade IV (glioblastoma) [3]. Glioblastoma multiforme accounts for almost half of the cases and it is the most aggressive form of these tumors with a poor prognosis. The annual incidence of malignant gliomas is approximately 5 cases per 100,000 people [2]. Glioblastoma, in particular, has a significant cost in the health care system because of both the severity of neurologic dysfunction and the wide range of treatment options [4]. Studies have found that patients with lower socioeconomic status often have more advanced diseases at the time of diagnosis [4]. The accurate histological diagnosis of gliomas is of great importance for determining a prognosis and guiding therapy. Histologic grading is based on findings of nuclear atypia, proliferative activity, microvascular proliferation, and necrosis [1,2]. Although Histopathological diagnosis remains the gold standard for gliomas diagnosis; however, diagnostic difficulty may arise from tumor heterogeneity, overlapping morphologic features, and tumor sampling [5]. Magnetic resonance imaging (MRI) in particular has emerged as the imaging modality most frequently used to evaluate gliomas, and it continues to have an ever-expanding multifaceted role in the diagnosis, characterization, and management of gliomas. Improved MRI techniques have shown many potentials in evaluating the key pathological features of gliomas, including cellularity, invasiveness, mitotic activity, angiogenesis, and necrosis, hence, further shedding light on glioma grading before treatment [6]. MRI is the most sensitive medical imaging procedure for all brain tumors. Low-grade gliomas usually present as small lesions without contrast enhancement, while high-grade gliomas have a different degree of enhancement and they appear as irregular, unmarked masses with angioedema, and necrotic core [7]. Although pathological contrast enhancement is generally associated with more aggressive lesions, up to one-third of non-enhancing gliomas are malignant, so contrast enhancement alone is, therefore, a limited differentiator between high-grade and low-grade gliomas in an individual patient [8]. There were very few studies that attempted to correlate the histopathological grade of the tumor with the findings of MRI. The aim of the current study is to assess the sensitivity and specificity of MRI and to find if it can be used for a better assessment of glioma grade.
2. Methods
A cross-sectional study was conducted at the department of neurosurgery, Tishreen University Hospital, Lattakia, Syria from November 2018–December 2019. This study was approved by the ethics review committee of Tishreen University Hospital. All patients aged 40–75 years of either gender with histologically diagnosed supratentorial gliomas. Participants had previous brain surgery, children, recurrent cases, diagnosed with glioma by MRI but histopathology showed meningioma, and cases, where MRI and histopathological results were not available at the same time, were excluded. The final sample size came to be 39 patients. All the patients were evaluated by a standard conventional contrast-enhanced study on Siemens 1.5 T MRI. Brain MRI was performed using the following protocols: pre-injection: Sagittal T1 (Tse), Axial T1 (Tse), Coronal FLAIR, After injection: Axial T1 (Tse), and Sagittal T1 (Tse). The MRI device of the Siemens model, MAGNETOM ESSENZA ATIM + DOT SYSTEM, was made by Siemens, Germany. The strength of the magnetic field is 1.5 T, the type of coil used is the Head Matrix ATIM coil, and the number of channels is 16. After the MRI and additional workup, a tumor biopsy was done to achieve a histopathological diagnosis. The histopathological grading of the tumor was done according to the 2016 WHO classification [9]. The histopathological examination was done by a pathologist who was blinded to the MRI findings of the tumors. The sensitivity, specificity, predictive value, and accuracy of MRI in determining glioma grade were calculated. A comparison between MRI findings and histopathological results was done. The statistical analysis was performed using SPSS version 22. Range and median were computed for quantitative variables. Frequency and percentages were calculated for qualitative variables. Sensitivity, Specificity, PPV (positive predictive value), negative predictive value (NPV), and accuracy were calculated by taking histopathology findings as a gold standard. The coefficient of concordance (kappa) to study the compatibility between magnetic resonance and histopathology was also calculated. To estimate the significant correlations, we calculated the p-values using Fisher's exact test. P ≤ 0.05 was considered to indicate a statistically significant difference.
This work has been reported in line with the STROCSS criteria [10].
3. Results
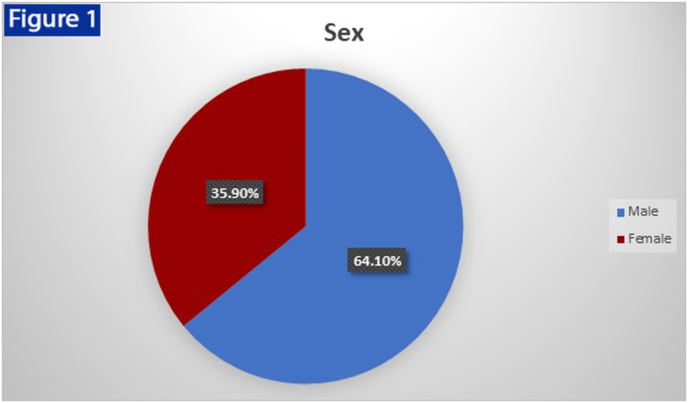
39 patients with ages ranging from 40 to 60 years (mean 55 years). Majority of the patients were males (n = 25, 64.10%) and 14 (35.90%) were females. (Fig. 1). Twenty-four (61.5%) patients had glioblastoma multiform and they had grade Ⅳ of the tumor. Fifteen patients had astrocytoma (38.5%). Three cases (7.7%) had a grade Ⅱ and twelve cases (30.8%) had a grade Ⅲ. Table 1.
Fig. 1.
Distribution of the study sample by gender: the number of males 25 and females 14 with Sex Ratio (M: F) 1.8:1.
Table 1.
Distribution of a sample of 39 patients according to the type and grade of the tumor.
| Variables | Number | Percentage |
|---|---|---|
| Type of tumor | ||
| Glioblastoma multiform | 24 | 61.5% |
| Astrocytoma | 15 | 38.5% |
| Grade | ||
| Grade Ⅰ | 0 | 0.0% |
| Grade Ⅱ | 3 | 7.7% |
| Grade Ⅲ | 12 | 30.8% |
| Grade Ⅳ | 24 | 61.5% |
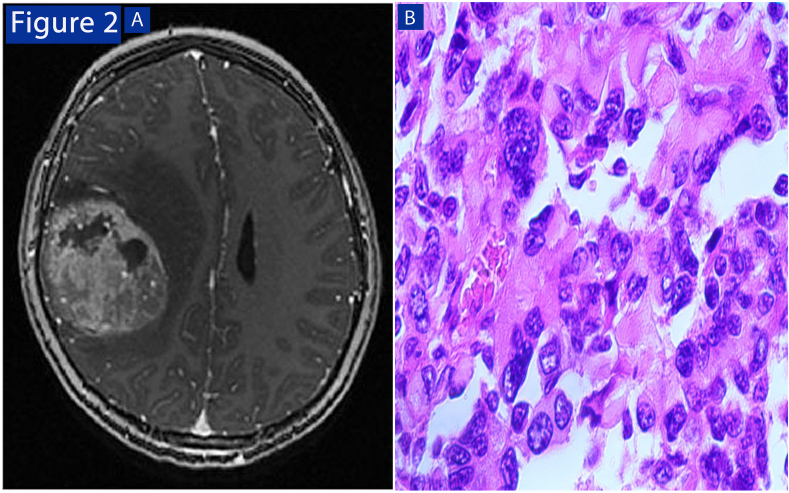
From the previous table, we noticed that there were no cases of grade Ⅰ tumors, which may be due to too-small samples or because of the delay in health care which leads to late detection. High-grade tumors were associated with the following results: T2 with a hyperintense, hyperintense FLAIR, and T1 hypointense, with contrast enhancement occurring in cases of grade Ⅳ complete, and all are heterogeneous except for one case (homogeneous enhancement). As well as accompanying findings (necrosis, hemorrhage, edema) were of high-grade tumors. Table 2 (Fig. 2).
Table 2.
Correlation between the histopathological grade of the gliomas and MRI findings.
| MRI findings | Histopathological grade |
||
|---|---|---|---|
| Grade Ⅱ | Grade Ⅲ | Grade Ⅳ | |
| T1: | |||
| Hypointense | 3 | 12 | 22 |
| Isointense | 0 | 0 | 2 |
| T2 (hyperintense) | 3 | 12 | 24 |
| FlAIR (hyperintense) | 3 | 12 | 24 |
| Enhancement: | |||
| homogeneous | 2 | 0 | 0 |
| heterogeneous | 1 | 12 | 24 |
| Hemorrhage | 0 | 0 | 5 |
| Necrosis | 0 | 0 | 12 |
| Meningitis | 0 | 1 | 3 |
| Edema | 2 | 10 | 24 |
| Hiccups | 0 | 0 | 2 |
Fig. 2.
High-grade tumor: (A (MRI brain T1 imaging after injection showing the presence of heterogeneous enhancement with edema and hemorrhage. (B) Histopathological features include necrosis and vascular infiltration (x 400).
The overall sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 100%, 94.4%, 60%, and 100% respectively. The overall accuracy was 94.4%. The compatibility between the findings of magnetic resonance and pathological results was also studied through the compatibility factor Kappa. The value of the compatibility factor reached 72%, and it is considered a high-degree compatibility factor. Table 3.
Table 3.
Distribution of a sample of 39 patients according to the histopathological and MRI grade of the tumors.
| No. of patients | Histopathological grade |
MRI | |
|---|---|---|---|
| High grade | Low grade | ||
| 5 | 2 | 3 | Low grade |
| 34 | 34 | 0 | High grade |
| 39 | 3 | 36 | Total no. of patients |
The relationship between the tumor type according to the histopathological result and sex was studied by Fisher's exact test. Table 4.
Table 4.
The relationship between the tumor type according to the histopathological result and sex.
| Sex |
Tumor type | |
|---|---|---|
| Females | Males | |
| 9 | 15 | Glioblastoma multiform |
| 5 | 10 | Astrocytoma |
| 14 | 25 | Total no. of patients |
From the previous table, it was found that there was no statistical relationship between them with a p-value = 0.8.
4. Discussion
Gliomas are the most common primary malignant brain tumors in adults and include a variety of histologic typing and grading as follows: Grade I (pilocytic astrocytoma), grade II (diffuse astrocytoma), grade III (anaplastic astrocytoma), and grade IV (glioblastoma) [1,2]. Conventional magnetic resonance imaging with T1, T2, FLAIR, and contrast enhancement are basic tests for its diagnosis and determine its grade [6]. All gliomas were hyperintense in T2 and in FLAIR, while 92% of them were hypointense in T1. High-grade tumors were heterogeneous contrast enhancement with other medical imaging aspects of MRI such as bleeding, necrosis, and edema [7,11]. Glioblastoma multiforme represented 61.5% of the supratentorial gliomas in our study and is similar to their proportion in the Zena Study., Et al (2014) 56,25% [12], but it was less than 39% in the study of Al-Najjar., Et al (2003) [13], and this can be explained by the difference in the target age group where patients younger than 40 years was used in the study of Al-Najjar where the pilocytic astrocytoma is more common than GBM. In our study the compatibility of the MRI findings and histopathological results was large and in contrast to the study by K Abul-Kasim (2013); the compatibility was moderate using conventional imaging and the rate of it was increased to complete using multimodal MRI. While compatibility was average in the Arian Lasocki (2018) study, this may be attributed to the use of conventional MRI and the clinical experience of the examiner radiologist. Table 5.
Table 5.
Comparison between MRI findings and pathological results between studies.
| Study | MRI and pathological results |
|---|---|
| K Abul-Kasim., Et al (2013), Sweden | K = 92% (MRI New Sequences) K = 38% (traditional MRI) |
| Tishreen Hospital - Lattakia (2019) | 72% (conventional MRI) |
| Arian Lasocki (2018) – Melbourne [14]. | 58% (traditional MRI) |
In our study, sensitivity and specificity were 100% and 91% respectively. The PPV and NPV were 66.6%% and 100% respectively. The overall accuracy was 94.9% which was consistent with the study by Zena., Et al (2014) – Erbil. Comparing our study results with the previous study suggests that radiological biology predicts the glioma histopathological grade better. But in the Dehghani F MD (2011) -Iran study the quality was very low 25% and this indicates the presence of many false-positive cases with MRI. Table 6.
Table 6.
Comparing the results of sensitivity, specificity, PPV, and NPV for MRI in the diagnosis and classification of gliomas among studies.
| Study | predicts | Specificity | PPV | NPV |
|---|---|---|---|---|
| Dehghani F MD (2011) -Iran | 92% | 25% | 93% | 2% |
| Study Zena., Et al (2014) - Erbil | 100% | 96% | 97% | 100% |
| Tishreen Hospital study Lfalse-positive | 100% | 94.4% | 60% | 100% |
Abbreviations: PPV, Positive Predictive Value; NPV, Negative Predictive Value.
4.1. Limitations of the study
The study duration was short and MRI and histopathological results were not available at the same time of some cases.
5. Conclusions
All supratentorial gliomas were hyperintense in T2, while the T1 signal was hypointense in 92% of them. In FLAIR it was observed that tumors were hyperintense in 100% of cases. Contrast enhancement, necrosis, hemorrhage, and edema were seen with high-grade gliomas. Peritumoral edema was the most frequently associated feature of high-grade gliomas. There was a strong agreement between the pathological results and MRI findings in determining the grade of gliomas (72%). Traditional MRI has a high sensitivity with up to 96% accuracy in diagnosing gliomas and determining their grade in comparison to histopathology. Histopathological diagnosis remains the gold standard for diagnosing gliomas and determining their grade, but MRI imaging is considered a non-invasive method and is useful in cases where the biopsy procedure is a contraindication or rejected by the patient.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Sources of funding
None declared.
Ethical approval
All participants gave their consent to anonymously publish their entered data at Tishreen university hospital, Latakia, Syria which was compatible with the Declaration of Helsinki.
Consent
All participants gave their consent to anonymously publish their entered data at Tishreen university hospital, Latakia, Syria which was compatible with the Declaration of Helsinki.
Author statement
Nisreen Haydar: study design, data analysis, and writing.
Khatoun Alyousef: data analysis, and writing.
Moatasem Hussein Al-janabi: study design, data collections, data analysis, writing, and reviewing the manuscript.
Rana Issa, Fawaz Baddour, and Zuheir Al-shehabi: in reviewing the manuscript.
Registration of research studies
We registered our study in Research Registry in accordance with the Declaration of Helsinki. Our number registry is researchregistry8086 (https://www.researchregistry.com/browse-the-registry#home/registrationdetails/62cd643dd6d298001ec43f72/).
Guarantor
Zuheir Al-shehabi.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104679.
Contributor Information
Nisreen Haydar, Email: nisrinehay79@gmail.com.
Khatoun Alyousef, Email: khatounalyousef@gmail.com.
Usama Alanan, Email: usamaniphro@gmail.com.
Rana Issa, Email: ranissa@gmail.com.
Fawaz Baddour, Email: baddourf@gmail.com.
Zuheir Al-shehabi, Email: alshehabizuheir08@gmail.com.
Moatasem Hussein Al-janabi, Email: dr.3esami2022@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shoaib Y., Nayil K., Makhdoomi R., Asma A., Ramzan A., Shaheen F., Wani A. Role of diffusion and perfusion magnetic resonance imaging in predicting the histopathological grade of gliomas - a prospective study. Asian J. Neurosurg. 2019;14(1):47–51. doi: 10.4103/ajns.AJNS_191_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen P Y., Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008 July;359:5. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Vigneswaran K., Neill S., Hadjipanayis C G. Beyond the World Health Organization grading of infiltrating gliomas: advances in the molecular genetics of glioma classification. Ann. Transl. Med. 2015 May;3(7):95. doi: 10.3978/j.issn.2305-5839.2015.03.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su YouRong Sophie. 2016. Glioblastoma || National and Global Economic Impact of Glioblastoma; pp. 271–278. [DOI] [Google Scholar]
- 5.Nikiforova M N., Hamilton R L. Molecular diagnostics of gliomas. Arch. Pathol. Lab Med. 2011;135(5):558–568. doi: 10.5858/2010-0649-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 6.Wen Kao H., Chiang S., Chung H., Tsai F., Chen C. Advanced MR imaging of gliomas: an update. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/970586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horská A., Barker P.B. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin. 2010 Aug;20(3):293–310. doi: 10.1016/j.nic.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upadhyay N., Waldman A.D. Conventional MRI evaluation of gliomas. Br. J. Radiol. 2011 Dec;84(Spec Iss 2):S107–S111. doi: 10.1259/bjr/65711810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis D.N., Ohgaki H., Wiestler O.D. fourth ed. IARC; Lyon: 2016. WHO Classification of Tumors of the Central Nervous System. [DOI] [PubMed] [Google Scholar]
- 10.Mathew G., Agha R., for the STROCSS Group STROCSS 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 11.Munir S., Khan S.A., Hanif H., Khan M. Diagnostic accuracy of magnetic resonance imaging in detection of intra-axial gliomas. Pakistan J. Med. Sci. 2021;37(1):125–130. doi: 10.12669/pjms.37.1.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zena Akram Jarjees Ahmad M.D., Saeed Nadhim Younis M.D., Mohammed Sami Saeed M.D. MRI findings of supratentorial gliomas: a radiopathological comparative study. J. Arab Board Health Specializations. 2017;18(2–15) No.1. [Google Scholar]
- 13.Al-Najjar F.M. Bagdad; 2003. MRI Findings in Supra-tentorial Gliomas. A Thesis Submitted to the Scientific Council of Diagnostic Radiology in Partial Fulfillment for the Degree of Fellowship of the Iraqi Commission for Medical Specialization in Diagnostic Radiology. [Google Scholar]
- 14.Lasocki Arian. The University of Melbourne; 2018. The Preoperative MRI Assessment of Adult Intracranial Diffuse Gliomas. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.