Abstract
Introduction
Takotsubo cardiomyopathy is a transient type of acute heart failure with distinct wall motion abnormalities and unclear pathophysiology. This review focuses on the proposed pathophysiological mechanisms that could be involved in the occurrence takotsubo cardiomyopathy.
Main body
Acute stress and subsequent excessive activation of the sympathetic nervous system are major factors in the pathophysiology of takotsubo cardiomyopathy. The high levels of catecholamine work in a triggering manner, generate reactive oxygen species, release inflammatory cytokines, and induce endothelial injury. The incidence of Takotsubo cardiomyopathy has increased following COVID-19 infection and vaccination, which suggests that neurohormonal and psychological factors (i.e., fear and anxiety of infection or vaccination) may have an additional role in the pathophysiology. In addition, inflammatory state, cytokine storm, augmented sympathetic activity, and endothelial dysfunction during the acute phase of COVID-19 infection may participate in Takotsubo cardiomyopathy. Chronic stress is also linked to this complex mechanism by accelerating cripple of endocrinal hypothalamic-pituitary-adrenal axis activity, which influences the cortisol effect on releasing catecholamine, which is directly related to the pathogenesis of takotsubo cardiomyopathy.
Conclusion
The excessive activation of the sympathetic nervous system and subsequent high levels of catecholamines could initiate the process. The catecholamines, in turn, generate reactive oxygen species and release inflammatory cytokines (i.e., IL-1, IL-2, IL-6, IL-7, IL-8, CXCL1, TNF-α, and IFN-γ), which causes endothelial injury.
Keywords: Takotsubo cardiomyopathy, Takotsubo syndrome, Stress-induced cardiomyopathy, Pathophysiology, Literature review
Highlights
-
•
The excessive activation of the sympathetic nervous system is the major drive for Stress Cardiomyopathy.
-
•
Catecholamines are responsible for the subsequent endothelial injury.
-
•
IL-1, IL-2, IL-6, IL-7, IL-8, CXCL1, TNF-α, and IFN-γ are the major cytokines involved in Stress Cardiomyopathy.
1. Introduction
Takotsubo cardiomyopathy (TTC) is considered a mysterious and attractive entity described in Japan by Sato et al., in 1990 [1]. The term this syndrome is derived from the Japanese word takotsubo (Tako means octopus; tsubo means pot) because the appearance of the left ventricle (LV) at the end of systole which can be visualized in trans-thoracic echocardiogram (TTE) or coronary angiography with left ventriculography is similar to a Japanese octopus fishing pot through the acute phase [1]. TTC is a transient type of acute heart failure with distinct wall motion abnormalities [1]. TTC has a significant economic burden on health care systems. In the US, the hospitalization rate due to TTC increased from 52 cases/million to 178 cases/million adult discharges over six years (i.e., six folds) [2], with an annual nationwide cost burden exceeding 112$ million for the initial admission and readmission within 1-month [3]. It was estimated that the average expenditure of care from hospitalization to six months after discharge was 11,491$ per patient [4]. While the cost for the readmissions was equal to the entire cost of all outpatient and primary care coalesced (€169 739 vs. €170 514) [4].
The classical morphological pattern of TTC is apical akinesia with basal hyperkinesia of the left ventricular myocardium. TTC patients typically presented with symptoms and signs that resemble acute coronary syndrome (ACS) symptoms with elevated cardiac biomarkers but without any coronary lesion on the angiography [5]. It is estimated that 1%–2% of all patients with suspected ACS are ultimately diagnosed with TTC. The presence of localized wall motion abnormalities extending beyond a single epicardial coronary artery perfusion territory is an essential feature to distinguish TTC from ischemic myocardial infarction (MI) [5]. Interestingly, ventricular function improves spontaneously in most patients within one month of presentation. Although several hypotheses have been formulated, the underlying pathophysiological mechanism remains unclear. It predominantly affects postmenopausal women and is often preceded by an emotional or physical trigger, but the absence of the trigger does not preclude the diagnosis [5]. The plasma levels of epinephrine and norepinephrine are significantly elevated in TTC patients. Previous findings have suggested that sympathetic tone activation following an acute stress event plays a significant role in the pathophysiologic process. Many studies have supposed that antidepressive disorders (ADD) and psychiatric diseases may participate in the pathogenesis of TTC [6,7]. This study aims to provide a comprehensive review of Takotsubo syndrome by concentrating on the proposed pathophysiological mechanisms that could be involved in the occurrence of this entity, especially the sympathetic nervous system (SNS) activation hypothesis, which finally leads to catecholamine-mediated myocardial stunning.
2. Epidemiology
Since the first report by the Japanese physicians Sato et al., in 1990, TTC has gained worldwide recognition [1]. The expected incidence of TTC in the USA and Europe is between 50,000 and 100,000 per annum [8,9]. It is estimated to represent 0.02% of all hospitalizations in the United States [8]. TTC forms about 1–2% of all patients presenting with the suspected ACS [10]. The recurrence rate of TTC is 1.8% per-patient year [5]. While the rate of death from any cause is 5.6% per patient-year [5]. The syndrome shows a high predominance toward the female gender, with 89.8% of all cases being women with a mean age of 66.8 years [5]. Females older than 55 years have a 4.8 fold higher possibility of developing TTC than younger females [8]. Contrary, there is no significant correlation between the age and incidence of TTC in the males' population [8]. However, male gender was associated with increased rate of mortality in comparison with female gender [9].
3. Pathophysiology
The pathophysiology of takotsubo syndrome has been addressed in many studies and can be subdivided into four main categories: (1) myocardial ischemia, (2) the role of catecholamine in triggering cardiotoxicity, (3) LV outflow tract obstruction and increased ventricular afterload, and (4) enhanced autonomic nervous system activity [11], and it can be shown in excessive stimulation of SNS, in psychiatric disorders, neurologic disorders and the hormonal changes in women including post-menopause and pre-menopause.
3.1. TTC with catecholamines and SNS stimulation
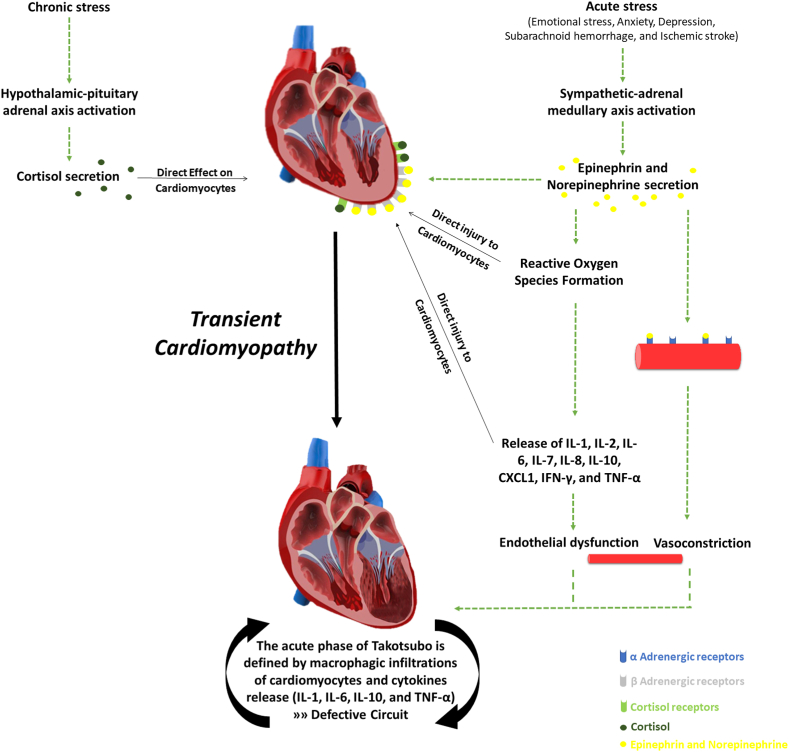
One major factor in the advancement of TTC is the excessive activation of the sympathetic nervous system (SNS) because it was observed that the high level of catecholamine works in a triggering manner [11]. In addition, catecholamines were observed to be responsible for reactive oxygen species (ROS), inflammatory cytokines release (i.e., IL-1, IL-2, IL-6, IL-7, IL-8, CXCL1, TNF-α, and IFN-γ), and subsequent endothelial injury [12,13] (Fig. 1). A series of recent clinical studies have proved a strong correlation between the number of cases triggered by extensive catecholamine and the stressful psychological events which is accelerating the beginning of the clinical symptoms of TTC [14,15]. These findings suggest that the elevated catecholamine responses and more cardiac sympathetic stimulation are induced by psychiatric illness because of the shortness of catecholamine reuptake. In addition to that supraphysiologic adjustments in catecholamines and chronic cardiac sympathetic stimulation are developed because of the aggravation of underlying psychiatric disorders [16]. One of the main characteristic features of TTC is the cardiogenic shock which is a common complication of the change that is happening in peripheral vessels of spasm followed by dilation, which can harm at least the left ventricle systolic. All of these modifications can be surged by the systemic catecholamine to clarify the pathophysiologic mechanism; it can be described as the more density of beta-adrenoreceptors a region has, the more sensitive to circulating catecholamine it will be. Therefore, the apical myocardium of the left ventricle is the most sensitive region in the heart, and it is more susceptible to suffering from TTC than other myocardial regions [14]. Besides, the myocardial characteristics, structure, and cytoskeletal contractile proteins can be altered due to acute catecholamine overload, reversible intracellular modifications, and moderate neutrophilic infiltration; however, all can return to normal after functional recovery [11]. However, the changes that happen after the catecholamine-induced cardiotoxic effects are examined during the acute phase of the TTC syndrome in biopsy samples which approve the direct effects as well as the vascular impact [14]. In the acute phase of TTC, many extracellular clusters were revealed by the immunohistochemical staining for macrophages (CD68) with a little rising in leukocyte counts and C-reactive protein levels in addition to the increasing natriuretic peptide levels related to the elevation in catecholamine concentrations that are linked to the asperity of left ventricular systolic dysfunction [17]. Chronic stress is linked to the accelerating cripple of endocrinal hypothalamic-pituitary adrenal axis (HPAA) activity regarding the psychiatric pressure or the stressful life events patients experience, which influence the promotion of cortisol effect on releasing catecholamine, which is directly related to the pathogenesis of Takotsubo syndrome [18]. Supraphysiologic alterations in catecholamines and chronic cardiac sympathetic activation are suggested to be the intensification of hidden psychiatric disorders which can trigger the onset of the TTC. Chronic psychosocial or traumatic stress can induce constant hyperactivity of HPAA, which can cause chronic hypoactivity [19,20]. However, Takotsubo syndrome patients felt more nervous during a stress experiment than the control group with a pronounced CSR36 [18]. On the other hand, the direct effect of catecholamine on cardiomyocytes results in LV transient dysfunction [21]. Endomyocardial biopsies showed sporadic contractions band necrosis in clinical settings of a high level of catecholamines such as pheochromocytoma or subarachnoid hemorrhage, associated with hypercontracted sarcomeres, dense eosinophilic transverse bands, and interstitial mononuclear inflammation as a reflection of the injury of myocyte [22], which its viability can be decreased because of catecholamines through cyclic adenosine monophosphate (cAMP) mediated Ca2þ overload as it may occur in TTC. It was reported in the medical literature that takotsubo patients have 7 to 34 times as high as published normal values of epinephrine, norepinephrine, and dopamine [23].
Fig. 1.
Illustrate the pathophysiology of Takotsubo cardiomyopathy and the role of cortisol, catecholamines, cytokines, and interleukins.
Noteworthy, there is no accommodation concerning the antiplatelet therapy in TTC patients even though the effects of catecholamines on platelet activation and endothelial dysfunction have been approved [22,24]. To sum it up, recent studies agree that the acute release of catecholamines from the sympathetic nerves or adrenal medulla, or as drug therapy, can lead to chronic, but transient LV dysfunction with secondary myocardial inflammation due to the elevation in susceptibleness of the coronary microcirculation and cardiac myocytes to the stress hormones [21]. It was noticed that the acute phase of TTC defined by macrophagic infiltrations of cardiomyocytes and an increase in systemic proinflammatory cytokines (i.e., IL-1, IL-6, IL-10, and TNF-α), which may further worsen TTC and lead to defective pathologic circuit [12,13] (Fig. 1).
3.2. TTC with neurological disorders and psychiatric disorders
Previous research showed that hidden psychiatric disorders such as depression, anxiety, mania and psychosis, and other neurological disorders including subarachnoid hemorrhage, stroke, transient ischemic attack or seizures, and pheochromocytoma are disorders that can generate stress and physiologic modification which are identical to symptoms expressed in TTC female patients which can lead to a supraphysiological adjustment in catecholamines and longer cardiac sympathetic provocation due to underlying ongoing stress because of the reduced catecholamine reuptake [16,24]. Likewise, cripple CSR and the elevated prevalence of psychiatric overload and nervousness are found to be associated with the long-term pressure and accelerating crippling of endocrinal HPAA activity and the cortisol induced on catecholamine release that can lead to the pathogenesis of Takotsubo syndrome [18]. For example, research has provided evidence for the high prevalence rate of neurological and psychiatric disorders which have been reported to have a higher rate than TTC patients seem to in comparison with ACS patients [5,22,25]. Furthermore, it is remarkable that TTC female patients have experienced more sentimental triggers before the onset of the TTC compared with male TTC patients who have been found to have more physical pre syndrome triggers. Nevertheless, physical events such as acute respiratory failure or central nervous system (CNS) conditions were reported to be independent predictors for in-hospital complications, but they are less frequent than emotional life events such as panic attacks, anxiety, surprise parties, and stress, yet each of those two main categories of life events can trigger TTC in patients [5,25].
Previous recent retrospective studies have emphasized a prevalence rate of depressive symptoms in comparison with a healthy control group. Additionally, a proportion of 43.2% of TTC patients who have enrolled in this study has suffered from a psychiatric disease while half of them had an active disorder [5]. Those diagnosed with chronic anxiety disorders seemed to be prevalent before the onset of the disease, while prior research showed that anxiety preadmission is linked to the occurrence of takotsubo cardiomyopathy syndrome [6]. TTC patients respond to emotional and physical stressors more sympathetically which triggers the stimulation of the autonomic nervous system, this stimulation is expressed by two neurohumoral axes: the sympathetic-adrenal medullary axis which is linked to the catecholamine release in the adrenal medulla which is activated after the immediate stressor and the HPA axis that is activated by chronic stressors during the successive releasing of cortisol from the adrenal cortex [18] (Fig. 1). This has also been explored in an earlier retrospective analysis which recognized that selective norepinephrine reuptake inhibitors, serotonin reuptake inhibitors, or benzodiazepines are medications observed to be taken to treat affective disorders in TTC patients more prevalent than healthy controls [5,22]. Besides, general anxiety levels are significantly higher in healthy controls than in TTC patients contrary to the illness-related anxiety levels which show a high prevalence in TTC patients [18]. Accordingly, there has been a recent study that showed psychiatric illness before the onset of the disease is correlated to the increased risk of TTC, but this does not affect the long-standing mortality [26].
It is worth to mention that the incidence of TTC has increased following COVID-19 infection and vaccination. Patients had no previous physical or emotional triggers, thus, neurohormonal and psychological factors (i.e., fear and anxiety of infection or vaccination) may have an additional role in the pathophysiology [27,28]. In addition, inflammatory state, cytokine storm, augmented sympathetic activity, and endothelial dysfunction that occurs during the acute phase of COVID-19 infection may participate to TTC [13].
4. Clinical features
Takotsubo patients typically presented with symptoms and signs almost similar to ACS symptoms [5,29]. The typical clinical presentation for takotsubo syndrome (TTC) is a post-menopausal woman presented to the emergency department with chest pain, dyspnea, palpitation, nausea, and syncope that is preceded by emotional or physical trigger [30]. However, the absence of this trigger does not exclude the diagnosis [8]. Such patients should prompt urgent clinical evaluation and hasty acquisition of resting 12-lead electrocardiography (ECG). During the acute phase, the findings may include ST-segment elevation (usually in the anterior precordial leads), ST depression, new left bundle branch block (LBBB), T-wave inversion, Q wave abnormalities, and/or QTc prolongation and even complete AV block with ventricular asystole [31,32]. Cardiac biomarkers maybe show elevated levels of serum cardiac troponin and brain natriuretic peptide (BNP) [5,33], while the creatine kinase remains normal or is mildly elevated [5,34]. During the acute phase, the echocardiography shows characteristic features consisting of regional wall motion abnormalities of the LV (or occasionally RV myocardium) that extend beyond a single epicardial coronary artery perfusion territory with a significant reduction in left ventricular ejection fraction (EF) [5,35]. Because of acute left ventricular dysfunction, some patients have pulmonary edema or cardiogenic shock [10,36]. The classical morphological pattern of left ventricular (LV) regional wall motion abnormality is the apical hypokinesia, akinesia, or dyskinesia (apical ballooning) with basal hyperkinesia that occurs in more than two-thirds of TTC patients [5,37]. However, further wall motion abnormalities in TTC have been described, such as basal, mid-ventricular, and lateral akinesia of the left ventricular myocardium and the involvement of the right ventricular myocardium as part of a biventricular involvement or isolated right ventricular wall motion abnormality [5,37]. Various types of Takotsubo cardiomyopathy are summarized in figure (1). Serial echocardiographic assessments of TTC patients reveal a significant improvement in systolic left ventricular function [5]. Most Takotsubo syndrome patients should have urgent coronary angiography to exclude myocardial infarction. However, coronary angiography typically demonstrates normal vessels with no obstructive coronary artery disease (CAD) or plaque rupture corresponding to the wall motion abnormalities seen in this condition [10,30].
5. Differential diagnosis
When approaching a patient with suspected takotsubo cardiomyopathy, it is essential to consider the following differential diagnosis in your evaluation: acute coronary syndrome (ACS), acute coronary syndromes cardiomyopathy associated with pheochromocytoma and in the setting of acute brain injury, ACS related to cocaine abuse and acute myocarditis [36,38].
6. Diagnosis
The diagnosis of TTC is considered a big challenge for physicians because its clinical pattern may closely resemble myocardial infarction (MI) regarding clinical presentations, electrocardiographic (ECG) abnormalities, and biomarkers [5,29]. Therefore, the definitive diagnosis requires diagnostic criteria incorporating clinical and anatomical features, ECG abnormalities, triggers, cardiac biomarkers, and reversibility of myocardial dysfunction. After the first diagnostic criteria for TTC were published by Japanese physicians in 2003, different other criteria have been proposed, including the Gothenburg criteria, Johns Hopkins criteria, Takotsubo Italian Network proposal, and the European Society of Cardiology (ESC) Heart Failure Association (HFA) TTC Taskforce criteria [[39], [40], [41]]. However, the most acceptable and used criteria for TTC diagnosis are the Mayo Clinic Criteria, which were modified in 2008 Since the clinical picture of Takotsubo Syndrome (TTC) mimics the presentation of the acute coronary syndrome (ACS) and due to the lack of recognized non-invasive tool which allows a quick and reliable diagnosis of TTC, the International Takotsubo Registry developed a clinical score to evaluate the probability of TTC and to distinguish TTC from (ACS) in the emergency department [21]. The score consists of the female sex, emotional trigger, physical trigger, absence of ST-segment depression, psychiatric disorders, neurologic disorders, and prolongation of corrected QT (QTc) [21].
7. Prognosis
Most of the patients with takotsubo cardiomyopathy (TTC) recover rapidly after the acute Most patients with takotsubo cardiomyopathy (TTC) recover rapidly after the acute episode without remaining symptoms. Therefore, takotsubo syndrome (TTC) is considered a benign entity, and the prognosis of the patients with (TTC) in the absence of significant underlying comorbidities at admission is good once the acute phase has passed. It is recommended to perform an echocardiographic follow-up approximately 4–6 weeks after discharge from the hospital to document the normalization of the cardiac function. Typically, the systolic dysfunction and the regional wall motion abnormalities are transient and resolve completely within 3–8 weeks [42,43]. Thus, a different diagnosis should be considered for patients with constant contractile cardiac dysfunction. TTC has a 1.8% per patient-year recurrence rate with a span of 25 days up to 9.2 years following the initial event [5], while the death rate is approximately 5.6% per patient-year from any cause [5]. Among all TTC deaths, 38% were directly related to cardiac complications and 62% to underlying comorbid medical conditions [44]. Takotsubo cardiomyopathy complications occur through the acute period of the disease, whereas the late complications are scarce (i.e., Left ventricular aneurysm) as the syndrome is reversible and the injury is temporary [45]. Factors that predict adverse outcomes include TTC related to physical triggers, male gender [5,46], age older than 75 years [47], acute neurologic or psychiatric disorders [5,48]. In an Electrocardiogram (ECG), findings that predict unfavorable outcomes involve corrected QT prolongation since it has been associated with a higher risk of ventricular arrhythmias, especially polymorphic tachycardia [49]. Furthermore, one study reported that the magnitude and extent of ST-segment elevation (ECG) are considered an independent predictors of in-hospital adverse events [50]. The following factors expect adverse in-hospital outcomes 1) initial troponin >10 [5], 2) high values of brain natriuretic peptide (BNP), 3) elevated values of white blood cell counts (WBCs) [51], 4) left ventricular ejection fraction (LVEF) < 45% at admission which may predispose to cardiogenic shock [5], 5) reversible moderate to severe mitral valve regurgitation (MVR) [47], 6) right ventricular (RV) involvement [47], 7) free wall or septal rupture [24], and 8) left ventricular outflow tract obstruction (LVOTO) [47]. The occurrence of life-threatening complications requires rapid intervention with close supervision in critical situations. Until now, studies and data about the long-term follow-up of TTC are limited, and the natural history of the syndrome is still mysterious. Further studies concerning the long-term follow-up are necessary to determine the differences in the outcomes.
8. Management
Since the clinical presentations of takotsubo syndrome (TTC) are similar to those of acute coronary syndrome (ACS), the primary management focuses on the treatment of myocardial ischemia with a continuous electrocardiogram (ECG) monitoring, administration of aspirin, anticoagulants with direct thrombin inhibition and/or glycoprotein IIb/IIa receptor inhibition, intravenous heparin, and β-blockers [34,52,53]. Once the diagnosis of ACS is ruled out, the purpose of treatment of TTC is supportive care to save lives and reduce the incidence of complications; especially in high-risk patients, until complete resolution, which usually occurs in a few weeks [53]. All patients with TTC should be admitted to a coronary care department with close ECG monitoring for the initial 24 h while the investigations and risk stratification are completed [21]. Anti-platelet agents can be ceased unless there is coexisting coronary atherosclerosis or peripheral vascular disease. In addition, statins can be discontinued unless there is any indication. Patients without any complications and who have a left ventricular ejection fraction (LVEF) of more than 45% can be evaluated for early hospital discharge. While patients with LVEF of 35–45%, conventional heart failure treatment should be considered, including beta-blockers unless contraindicated [22,34,52,53]. Furthermore, some patients may develop acute complications such as shock and acute heart failure (HF). It is mandatory to detect whether the cardiogenic shock is caused by significant left ventricular outflow tract obstruction (LVOTO) or primary failure. Management of acute heart failure is generally performed according to the standard guidelines except in the presence of LVOTO. Therefore, If the diagnosis of LVOTO is established in these patients, it is critical to avoid volume depletion and vasodilator therapy [21,34,52,53]. The recommended treatment in patients with LVOTO in the absence of significant pulmonary congestion is cautious fluid resuscitation and parenteral beta-blockers, which may increase cardiac filling and reduces basal hyper-contractility, thus can relieve the obstruction [34,52]. Inotropic therapy such as (dobutamine or dopamine) should be considered as a temporizing measure in patients who develop cardiogenic shock due to failure of the pump [52]. Exogenous Catecholamines should be avoided in more severe cases of cardiogenic shock with progressive end-organs injury since these agents probably aggravate or lengthen the acute phase [52,53]. In this situation, the preferred therapy is mechanical circulatory support such as intra-aortic balloon counter pulsation (IABP) [52]. So far, no specific randomized trials are conducted to identify optimal medical treatment for takotsubo cardiomyopathy. Therefore, further studies considering the effective management in patients with stress cardiomyopathy are needed.
In conclusion, TTC is a transient type of acute heart failure with distinct wall motion abnormalities. The excessive activation of the sympathetic nervous system and subsequent high levels of catecholamines could initiate the process. The catecholamines, in turn, generate reactive oxygen species and release inflammatory cytokines (i.e., IL-1, IL-2, IL-6, IL-7, IL-8, CXCL1, TNF-α, and IFN-γ), which causes endothelial injury. The incidence of TCC increased following the COVID-19 pandemic due to the fear and anxiety of infection or vaccination. In addition, inflammatory state, cytokine storm, augmented sympathetic activity, and endothelial dysfunction that occurs during the acute phase of COVID-19 may participate in TTC.
Funding
The authors have received no funding.
Sources of funding
No funding was required.
Ethical approval
No ethical approval was required.
Consent
N/A.
Author contribution
Hasan Nabil Al Houri, Sami Jomaa, Massa Jabra, and Ahmad Nabil Alhouri wrote the first draft. Hasan Nabil Al Houri and Sami Jomaa designed the illustration figure. Youssef Latifeh reviewed the first draft. All the authors revised and approved the final manuscript.
Guarantor
Hasan Nabil Al Houri.
Declaration of competing interest
The authors have no conflict of interest.
Contributor Information
Hasan Nabil Al Houri, Email: Hasan.Alhouri@Spu.edu.sy.
Sami Jomaa, Email: sami.jomaa1997@gmail.com.
Massa Jabra, Email: massajabra@gmail.com.
Ahmad Nabil Alhouri, Email: alhouri.ahmad@gmail.com.
Youssef Latifeh, Email: yoseflatifa@gmail.com.
References
- 1.Sato H. 1990. Tako-tsubo-like Left Ventricular Dysfunction Due to Multivessel Coronary Spasm, Clinical Aspects of Myocardial Injury : from Ischemia to Heart Failure; pp. 56–64. [Google Scholar]
- 2.Khera R., Light-McGroary K., Zahr F., Horwitz P.A., Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am. Heart J. 2016;172:53–63. doi: 10.1016/j.ahj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah M., Ram P., Lo K.B.U., Sirinvaravong N., Patel B., Tripathi B., Patil S., Figueredo V.M. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin. Cardiol. 2018;41(7):916–923. doi: 10.1002/clc.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallström S., Ekman I., Omerovic E., Ulin K., Gyllensten H. Cohort study of healthcare use, costs and diagnoses from onset to 6 months after discharge for takotsubo syndrome in Sweden. BMJ Open. 2019;9(2) doi: 10.1136/bmjopen-2018-027814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Templin C., Ghadri J.R., Diekmann J., Napp L.C., Bataiosu D.R., Jaguszewski M., Cammann V.L., Sarcon A., Geyer V., Neumann C.A., Seifert B., Hellermann J., Schwyzer M., Eisenhardt K., Jenewein J., Franke J., Katus H.A., Burgdorf C., Schunkert H., Moeller C., Thiele H., Bauersachs J., Tschöpe C., Schultheiss H.P., Laney C.A., Rajan L., Michels G., Pfister R., Ukena C., Böhm M., Erbel R., Cuneo A., Kuck K.H., Jacobshagen C., Hasenfuss G., Karakas M., Koenig W., Rottbauer W., Said S.M., Braun-Dullaeus R.C., Cuculi F., Banning A., Fischer T.A., Vasankari T., Airaksinen K.E., Fijalkowski M., Rynkiewicz A., Pawlak M., Opolski G., Dworakowski R., MacCarthy P., Kaiser C., Osswald S., Galiuto L., Crea F., Dichtl W., Franz W.M., Empen K., Felix S.B., Delmas C., Lairez O., Erne P., Bax J.J., Ford I., Ruschitzka F., Prasad A., Lüscher T.F. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 6.Summers M.R., Lennon R.J., Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J. Am. Coll. Cardiol. 2010;55(7):700–701. doi: 10.1016/j.jacc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Kastaun S., Schwarz N.P., Juenemann M., Yeniguen M., Nef H.M., Moellmann H., Hamm C.W., Sammer G., Hennig J., Bachmann G., Gerriets T. Cortisol awakening and stress response, personality and psychiatric profiles in patients with takotsubo cardiomyopathy. Heart (British Cardiac Society) 2014;100(22):1786–1792. doi: 10.1136/heartjnl-2014-305745. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh A., Kumar G., Pant S., Rihal C., Murugiah K., Mehta J.L. Prevalence of takotsubo cardiomyopathy in the United States. Am. Heart J. 2012;164(1):66–71.e1. doi: 10.1016/j.ahj.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Brinjikji W., El-Sayed A.M., Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am. Heart J. 2012;164(2):215–221. doi: 10.1016/j.ahj.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Gianni M., Dentali F., Grandi A.M., Sumner G., Hiralal R., Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur. Heart J. 2006;27(13):1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 11.Wittstein I.S. The sympathetic nervous system in the pathogenesis of takotsubo syndrome. Heart Fail. Clin. 2016;12(4):485–498. doi: 10.1016/j.hfc.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Fan X., Yang G., Kowitz J., Akin I., Zhou X., El-Battrawy I. Takotsubo syndrome: translational implications and pathomechanisms. Int. J. Mol. Sci. 2022;23(4) doi: 10.3390/ijms23041951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omerovic E., Citro R., Bossone E., Redfors B., Backs J., Bruns B., Ciccarelli M., Couch L.S., Dawson D., Grassi G., Iacoviello M., Parodi G., Schneider B., Templin C., Ghadri J.R., Thum T., Chioncel O., Tocchetti C.G., van der Velden J., Heymans S., Lyon A.R. Pathophysiology of takotsubo syndrome - a joint scientific statement from the heart failure association takotsubo syndrome study group and myocardial function working group of the European society of Cardiology - Part 1: overview and the central role for catecholamines and sympathetic nervous system. Eur. J. Heart Fail. 2022;24(2):257–273. doi: 10.1002/ejhf.2400. [DOI] [PubMed] [Google Scholar]
- 14.Akashi Y.J., Nef H.M., Lyon A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015;12(7):387–397. doi: 10.1038/nrcardio.2015.39. [DOI] [PubMed] [Google Scholar]
- 15.Nef H.M., Möllmann H., Kostin S., Troidl C., Voss S., Weber M., Dill T., Rolf A., Brandt R., Hamm C.W., Elsässer A. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur. Heart J. 2007;28(20):2456–2464. doi: 10.1093/eurheartj/ehl570. [DOI] [PubMed] [Google Scholar]
- 16.Barton D.A., Dawood T., Lambert E.A., Esler M.D., Haikerwal D., Brenchley C., Socratous F., Kaye D.M., Schlaich M.P., Hickie I., Lambert G.W. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J. Hypertens. 2007;25(10):2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 17.Randhawa M.S., Dhillon A.S., Taylor H.C., Sun Z., Desai M.Y. Diagnostic utility of cardiac biomarkers in discriminating Takotsubo cardiomyopathy from acute myocardial infarction. J. Card. Fail. 2014;20(1):2–8. doi: 10.1016/j.cardfail.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Kastaun S., Gerriets T., Tschernatsch M., Yeniguen M., Juenemann M. Psychosocial and psychoneuroendocrinal aspects of Takotsubo syndrome. Nat. Rev. Cardiol. 2016;13(11):688–694. doi: 10.1038/nrcardio.2016.108. [DOI] [PubMed] [Google Scholar]
- 19.Fries E., Hesse J., Hellhammer J., Hellhammer D.H. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Golczynska A., Lenders J.W., Goldstein D.S. Glucocorticoid-induced sympathoinhibition in humans. Clin. Pharmacol. Ther. 1995;58(1):90–98. doi: 10.1016/0009-9236(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 21.Ghadri J.R., Cammann V.L., Jurisic S., Seifert B., Napp L.C., Diekmann J., Bataiosu D.R., D'Ascenzo F., Ding K.J., Sarcon A., Kazemian E., Birri T., Ruschitzka F., Lüscher T.F., Templin C. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur. J. Heart Fail. 2017;19(8):1036–1042. doi: 10.1002/ejhf.683. [DOI] [PubMed] [Google Scholar]
- 22.Ghadri J.R., Wittstein I.S., Prasad A., Sharkey S., Dote K., Akashi Y.J., Cammann V.L., Crea F., Galiuto L., Desmet W., Yoshida T., Manfredini R., Eitel I., Kosuge M., Nef H.M., Deshmukh A., Lerman A., Bossone E., Citro R., Ueyama T., Corrado D., Kurisu S., Ruschitzka F., Winchester D., Lyon A.R., Omerovic E., Bax J.J., Meimoun P., Tarantini G., Rihal C., S Y.H., Migliore F., Horowitz J.D., Shimokawa H., Lüscher T.F., Templin C. International expert consensus document on takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 2018;39(22):2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Känel R., Dimsdale J.E. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur. J. Haematol. 2000;65(6):357–369. doi: 10.1034/j.1600-0609.2000.065006357.x. [DOI] [PubMed] [Google Scholar]
- 24.Di Filippo C., Bacchi B., Di Mario C. Novel aspects of classification, prognosis and therapy in takotsubo syndrome. Eur. Cardiol. 2019;14(3):191–196. doi: 10.15420/ecr.2019.27.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchmann S.J., Lehmann D., Stevens C.E. Takotsubo cardiomyopathy-acute cardiac dysfunction associated with neurological and psychiatric disorders. Front. Neurol. 2019;10:917. doi: 10.3389/fneur.2019.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nayeri A., Rafla-Yuan E., Farber-Eger E., Blair M., Ziaeian B., Cadeiras M., McPherson J.A., Wells Q.S. Pre-existing psychiatric illness is associated with increased risk of recurrent takotsubo cardiomyopathy. Psychosomatics. 2017;58(5):527–532. doi: 10.1016/j.psym.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fearon C., Parwani P., Gow-Lee B., Abramov D. Takotsubo syndrome after receiving the COVID-19 vaccine. J Cardiol Cases. 2021;24(5):223–226. doi: 10.1016/j.jccase.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jani C., Leavitt J., Al Omari O., Dimaso A., Pond K., Gannon S., Chandran A.K., Dennis C., Colgrove R. COVID-19 vaccine-associated takotsubo cardiomyopathy. Am. J. Therapeut. 2021;28(3):361–364. doi: 10.1097/MJT.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 29.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am. Heart J. 2008;155(3):408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Bybee K.A., Kara T., Prasad A., Lerman A., Barsness G.W., Wright R.S., Rihal C.S. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann. Intern. Med. 2004;141(11):858–865. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 31.Akashi Y.J., Nakazawa K., Sakakibara M., Miyake F., Koike H., Sasaka K. The clinical features of takotsubo cardiomyopathy. QJM : monthly journal of the Association of Physicians. 2003;96(8):563–573. doi: 10.1093/qjmed/hcg096. [DOI] [PubMed] [Google Scholar]
- 32.Kown H., Seo J., Kim B.G., Kim G.S., Jin M.N., Lee H.Y., Byun Y.S., Kim B.O. Takotsubo syndrome presenting with high-degree atrioventricular block with ventricular asystole. J Cardiol Cases. 2022;25(3):193–197. doi: 10.1016/j.jccase.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen T.H., Neil C.J., Sverdlov A.L., Mahadavan G., Chirkov Y.Y., Kucia A.M., Stansborough J., Beltrame J.F., Selvanayagam J.B., Zeitz C.J., Struthers A.D., Frenneaux M.P., Horowitz J.D. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am. J. Cardiol. 2011;108(9):1316–1321. doi: 10.1016/j.amjcard.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 34.Ghadri J.R., Wittstein I.S., Prasad A., Sharkey S., Dote K., Akashi Y.J., Cammann V.L., Crea F., Galiuto L., Desmet W., Yoshida T., Manfredini R., Eitel I., Kosuge M., Nef H.M., Deshmukh A., Lerman A., Bossone E., Citro R., Ueyama T., Corrado D., Kurisu S., Ruschitzka F., Winchester D., Lyon A.R., Omerovic E., Bax J.J., Meimoun P., Tarantini G., Rihal C., S Y.H., Migliore F., Horowitz J.D., Shimokawa H., Lüscher T.F., Templin C. International expert consensus document on takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur. Heart J. 2018;39(22):2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citro R., Rigo F., Ciampi Q., D'Andrea A., Provenza G., Mirra M., Giudice R., Silvestri F., Di Benedetto G., Bossone E. Echocardiographic assessment of regional left ventricular wall motion abnormalities in patients with tako-tsubo cardiomyopathy: comparison with anterior myocardial infarction. Eur. J. Echocardiogr. : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12(7):542–549. doi: 10.1093/ejechocard/jer059. [DOI] [PubMed] [Google Scholar]
- 36.Ako J., Sudhir K., Farouque H.M., Honda Y., Fitzgerald P.J. Transient left ventricular dysfunction under severe stress: brain-heart relationship revisited. Am. J. Med. 2006;119(1):10–17. doi: 10.1016/j.amjmed.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Ghadri J.R., Cammann V.L., Napp L.C., Jurisic S., Diekmann J., Bataiosu D.R., Seifert B., Jaguszewski M., Sarcon A., Neumann C.A., Geyer V., Prasad A., Bax J.J., Ruschitzka F., Lüscher T.F., Templin C. Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome: data from the international takotsubo registry. JAMA cardiology. 2016;1(3):335–340. doi: 10.1001/jamacardio.2016.0225. [DOI] [PubMed] [Google Scholar]
- 38.Kassim T.A., Clarke D.D., Mai V.Q., Clyde P.W., Mohamed Shakir K.M. Catecholamine-induced cardiomyopathy. Endocr. Pract. : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2008;14(9):1137–1149. doi: 10.4158/EP.14.9.1137. [DOI] [PubMed] [Google Scholar]
- 39.Abe Y., Kondo M., Matsuoka R., Araki M., Dohyama K., Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J. Am. Coll. Cardiol. 2003;41(5):737–742. doi: 10.1016/s0735-1097(02)02925-x. [DOI] [PubMed] [Google Scholar]
- 40.Schultz T., Shao Y., Redfors B., Bergmann Sverrisdóttir Y., Råmunddal T., Albertsson P., Matejka G., Omerovic E. Stress-induced cardiomyopathy in Sweden: evidence for different ethnic predisposition and altered cardio-circulatory status. Cardiology. 2012;122(3):180–186. doi: 10.1159/000338814. [DOI] [PubMed] [Google Scholar]
- 41.Lyon A.R., Bossone E., Schneider B., Sechtem U., Citro R., Underwood S.R., Sheppard M.N., Figtree G.A., Parodi G., Akashi Y.J., Ruschitzka F., Filippatos G., Mebazaa A., Omerovic E. Current state of knowledge on takotsubo syndrome: a position statement from the Taskforce on takotsubo syndrome of the heart failure association of the European society of Cardiology. Eur. J. Heart Fail. 2016;18(1):8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 42.Wittstein I.S., Thiemann D.R., Lima J.A., Baughman K.L., Schulman S.P., Gerstenblith G., Wu K.C., Rade J.J., Bivalacqua T.J., Champion H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005;352(6):539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 43.Desmet W.J., Adriaenssens B.F., Dens J.A. Apical ballooning of the left ventricle: first series in white patients. Heart (British Cardiac Society) 2003;89(9):1027–1031. doi: 10.1136/heart.89.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh K., Carson K., Shah R., Sawhney G., Singh B., Parsaik A., Gilutz H., Usmani Z., Horowitz J. Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am. J. Cardiol. 2014;113(8):1420–1428. doi: 10.1016/j.amjcard.2014.01.419. [DOI] [PubMed] [Google Scholar]
- 45.Elzieny A.A. Left ventricular aneurysm as long-term complication of Takotsubo cardiomyopathy: is it still a benign disease?-case report. Oxf Med Case Reports. 2022;2022(2):omab148. doi: 10.1093/omcr/omab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobue Y., Watanabe E., Ichikawa T., Koshikawa M., Yamamoto M., Harada M., Ozaki Y. Physically triggered Takotsubo cardiomyopathy has a higher in-hospital mortality rate. Int. J. Cardiol. 2017;235:87–93. doi: 10.1016/j.ijcard.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 47.Citro R., Rigo F., D'Andrea A., Ciampi Q., Parodi G., Provenza G., Piccolo R., Mirra M., Zito C., Giudice R., Patella M.M., Antonini-Canterin F., Bossone E., Piscione F., Salerno-Uriarte J. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC (J. Am. Coll. Cardiol.): Cardiovascular Imaging. 2014;7(2):119–129. doi: 10.1016/j.jcmg.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Ghadri J.R., Kato K., Cammann V.L., Gili S., Jurisic S., Di Vece D., Candreva A., Ding K.J., Micek J., Szawan K.A., Bacchi B., Bianchi R., Levinson R.A., Wischnewsky M., Seifert B., Schlossbauer S.A., Citro R., Bossone E., Münzel T., Knorr M., Heiner S., D'Ascenzo F., Franke J., Sarcon A., Napp L.C., Jaguszewski M., Noutsias M., Katus H.A., Burgdorf C., Schunkert H., Thiele H., Bauersachs J., Tschöpe C., Pieske B.M., Rajan L., Michels G., Pfister R., Cuneo A., Jacobshagen C., Hasenfuß G., Karakas M., Koenig W., Rottbauer W., Said S.M., Braun-Dullaeus R.C., Banning A., Cuculi F., Kobza R., Fischer T.A., Vasankari T., Airaksinen K.E.J., Opolski G., Dworakowski R., MacCarthy P., Kaiser C., Osswald S., Galiuto L., Crea F., Dichtl W., Empen K., Felix S.B., Delmas C., Lairez O., El-Battrawy I., Akin I., Borggrefe M., Horowitz J., Kozel M., Tousek P., Widimský P., Gilyarova E., Shilova A., Gilyarov M., Winchester D.E., Ukena C., Bax J.J., Prasad A., Böhm M., Lüscher T.F., Ruschitzka F., Templin C. Long-term prognosis of patients with takotsubo syndrome. J. Am. Coll. Cardiol. 2018;72(8):874–882. doi: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Madias C., Fitzgibbons T.P., Alsheikh-Ali A.A., Bouchard J.L., Kalsmith B., Garlitski A.C., Tighe D.A., Estes N.A., 3rd, Aurigemma G.P., Link M.S. Acquired long QT syndrome from stress cardiomyopathy is associated with ventricular arrhythmias and torsades de pointes. Heart Rhythm. 2011;8(4):555–561. doi: 10.1016/j.hrthm.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Takashio S., Yamamuro M., Kojima S., Izumiya Y., Kaikita K., Hokimoto S., Sugiyama S., Tsunoda R., Nakao K., Ogawa H. Usefulness of SUM of ST-segment elevation on electrocardiograms (limb leads) for predicting in-hospital complications in patients with stress (takotsubo) cardiomyopathy. Am. J. Cardiol. 2012;109(11):1651–1656. doi: 10.1016/j.amjcard.2012.01.393. [DOI] [PubMed] [Google Scholar]
- 51.Murakami T., Yoshikawa T., Maekawa Y., Ueda T., Isogai T., Konishi Y., Sakata K., Nagao K., Yamamoto T., Takayama M. Characterization of predictors of in-hospital cardiac complications of takotsubo cardiomyopathy: multi-center registry from Tokyo CCU Network. J. Cardiol. 2014;63(4):269–273. doi: 10.1016/j.jjcc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Sattar Y., Siew K.S.W., Connerney M., Ullah W., Alraies M.C. Management of takotsubo syndrome: a comprehensive review. Cureus. 2020;12(1) doi: 10.7759/cureus.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurisu S., Kihara Y. Clinical management of takotsubo cardiomyopathy. Circ. J. 2014;78(7):1559–1566. [PubMed] [Google Scholar]