Abstract
Previously, our laboratory showed that bovine and caprine mammary secretions are chemotactic and that chemoattractants found in these secretions are qualitatively different according to infection status and/or lactation stage. However, the cellular source of the chemoattractants has not been defined. In this study we used a modified Boyden chamber assay to examine the ability of previously established caprine mammary epithelial cell (CMEC) and myoepithelial cell (CMMyoEC) lines to produce chemoattractants for neutrophils. We found that CMEC culture supernatants, but not those of CMMyoEC cultures, induced in vitro neutrophil chemotaxis. Further characterization showed that chemotactic activity was produced when the cells underwent contact-induced differentiation. Neutrophil migration was chemotactic, not chemokinetic, and was augmented when the epithelial and myoepithelial cells were cocultured. Additionally, chemotactic activity was inducible by Staphylococcus aureus plus alpha-toxin, Escherichia coli, and interleukin-1β (IL-1β) in CMEC cultures. However, CMMyoEC cultures could not be induced to produce chemotactic activity. Anti-IL-8 antibody was able to block some constitutively produced chemotactic activity and chemotactic activity induced by IL-1β and S. aureus plus alpha-toxin. These results indicate that epithelial cells may play a major role in producing chemoattractants, specifically IL-8, in the mammary gland.
Mastitic, nonmastitic, and mammary secretions from different lactation stages induce migration of neutrophils (1, 16). The migration of neutrophils from the peripheral blood, through the mammary tissue, and into the mammary secretions is called chemotaxis (24). Briefly, chemotaxis is a highly regulated process in which selectins, integrins, and chemoattractants interact to generate cell migration (32). Selectins are adhesion molecules on leukocyte membranes that have an N-terminal domain homologous to that of Ca2+-dependent lectins, and they are responsible for attachment of leukocytes to vessel walls (4). Integrins are responsible for leukocyte-endothelial cell interactions preceding migration into tissue (13, 14). Lastly, chemoattractants are soluble mediators released at or near the site of chemotaxis. They function to regulate integrins, bind leukocytes, and modulate migration (24, 32). The cytokine interleukin-8 (IL-8) is one such chemotactic factor.
IL-8 is a chemokine that is produced by numerous cell types, including lymphocytes (10), neutrophils (35), monocytes/macrophages (29, 34), and epithelial cells (8, 9), including human mammary gland epithelial cells (2, 17, 20). IL-8 has several biological roles, including the following: recruiting and activating neutrophils (11), inducing neutrophil degranulation (29), stimulating phagocytosis of opsonized particles (7), and recruiting T lymphocytes (17, 36). In addition, IL-8 has been detected in human mammary secretions, and human maternal cells in breast milk express mRNA for IL-8 (33). IL-8 has also been detected in mammary secretions from glands challenged with Escherichia coli (30, 31) and in mastitic mammary secretions (1).
In this study we examined whether caprine mammary epithelial cells (CMEC) and caprine mammary myoepithelial cells (CMMyoEC) were able to produce chemoattractants for caprine neutrophils, whether the chemokine IL-8 was present, and whether chemoattractant production by these cells was inducible by a variety of agents. The cell lines used have been previously described (21–23). Briefly, the CMEC show functional differentiation when grown on a plastic substratum by expressing lactation-specific proteins preferentially in cells which form dome-like structures. Morphologic differentiation is observed with the formation of duct-like and acinus-like structures when cells are grown within a collagen matrix. CMEC proliferate in response to insulin, insulin-like growth factor 1, transforming growth factor alpha, hydrocortisone, and the ovarian steroid estradiol, when estradiol is combined with triiodothyronine. The complementing syngeneic CMMyoEC line (21) was derived from the same primary mixed mammary cell culture as CMEC. CMMyoEC have been shown to be alpha-smooth muscle actin positive and to have a contractile response to exogenous oxytocin. Coculture and culture supernatant bioassay experiments with epithelial and myoepithelial cells suggest the presence of paracrine-cell-mediated epithelial modulation of mammary myoepithelial cells. CMEC culture supernatants are able to augment myoepithelial-cell proliferation and are chemotactic for myoepithelial cells. However, myoepithelial-cell culture supernatants are not chemotactic for epithelial or myoepithelial cells. Our previous studies have shown that epithelial and myoepithelial cell lines are a relevant in vitro model in which to study mammary gland function.
In this study, we found that CMEC but not CMMyoEC culture supernatants were chemotactic for neutrophils. In confluent cultures, the chemotactic activity was inhibited by anti-IL-8 antibodies. Also, chemotactic activity of CMEC cultures was induced by the proinflammatory cytokine IL-1β, by Staphylococcus aureus plus alpha-toxin, and to a lesser degree by E. coli. In contrast, CMMyoEC cultures could not be induced to produce chemotactic factors. Additionally, the induced chemotactic activities of CMEC cultures stimulated with IL-1β and S. aureus plus alpha-toxin was inhibited by anti-IL-8 antibodies. These studies indicate that epithelial cells, but not myoepithelial cells, produce IL-8 in the mammary gland.
MATERIALS AND METHODS
Reagents.
All reagents were obtained from Sigma Chemical Co., St. Louis, Mo., unless otherwise noted. Anti-human IL-8 antiserum that was produced in chickens and that was found to cross-react with (ruminant) bovine IL-8 (25) was kindly provided by Donald L. Kreutzer (Departments of Pathology and Surgery, School of Medicine, University of Connecticut, Farmington).
Cells and culture conditions.
The CMEC and CMMyoEC established by our laboratory were used in all experiments. The cell lines were originally derived from a biopsy specimen of a mammary gland from a lactating (114 days postparturition) Anglo-Nubian (Capra hircus) goat. The established cultures showed no evidence of a transformed phenotype and maintained anchorage dependence and contact inhibition. Cells were routinely cultured in phenol red-free Dulbecco modified Eagle medium–Ham’s or F-12 medium (1:1), both supplemented with 2.2 mg of sodium bicarbonate per ml, 5 mM sodium acetate, 5 μg of holotransferrin per ml, 0.5 mM ethanolamine (Fisher Scientific Co., Pittsburgh, Pa.), 10 μg of bovine insulin (Intergen Co., Purchase, N.Y.) per ml, 5 μg of hydrocortisone (Fisher) per ml, 5% fetal bovine serum (FBS) (Rehatuin; Intergen Co.), 100 U of penicillin G per ml, and 100 μg of streptomycin sulfate per ml. Myoepithelial cells were routinely grown in culture media as described above for CMEC, except that the media were supplemented (1:1) with sterile-filtered conditioned media from log-phase CMEC cultures. Cultures were incubated at 37°C with saturated humidity and 5% CO2. Prior to confluence, media were aseptically siphoned and cells were detached with 0.05% trypsin–0.04% EDTA, washed three times with modified Hanks balanced salt solution, and passaged at a threefold dilution.
Culture supernatant for constitutive chemokine expression.
Experiments to determine proliferation- and differentiation-dependent neutrophil chemokine production by epithelial and myoepithelial cells used culture supernatants obtained as follows. Epithelial and myoepithelial cells were cultured for one passage, and during the experiment, were cultured in media with a reduced serum concentration (2.5% FBS) (sample media). Cells were seeded at 5 × 104 cells/well/2 ml−1 in 6-, 12-, or 24-well flat-bottom tissue culture plates (Falcon; Becton Dickinson & Co., Lincoln Park, N.J.). Supernatants were collected each day, sterile filtered, and stored at −20°C until assayed. Cell number and viability were determined for quadruplicate wells each day for 7 to 10 days by trypan blue exclusion. For negative controls, media were added to tissue culture plates without cells and collected as described above.
Coculture of epithelial and myoepithelial cells.
To evaluate modulation of chemokine production through cell-to-cell interaction, epithelial and myoepithelial cells were cocultured and the supernatants were assayed for chemotactic activity. Epithelial, myoepithelial, or epithelial and myoepithelial (1:1) cells were seeded at a total of 5 × 104 cells/well/2 ml−1 in 12-well flat-bottom tissue culture plates (Falcon). Culture supernatants from confluent cultures and negative control media were collected as described above.
Inducible chemokine expression.
CMEC and CMMyoEC were assayed for chemotaxis activity induction by recombinant human IL-1β, alpha-hemolysin (alpha-toxin), heat-killed S. aureus, Staphylococcus epidermidis, and E. coli. The bacteria were from isolates of mastitic caprine mammary gland secretions submitted to the Diagnostic Testing Service, Department of Pathobiology, University of Connecticut. The organisms were heat killed at 56°C for 30 min, resulting in no growth on blood agar plates. Cells were plated at 105 cells/well/ml−1 in 12-well flat-bottom tissue culture plates (Falcon) and allowed to attach overnight. The media were removed and replaced with 10 ng of IL-1β (Pepro Tech, Inc., Rocky Hill, N.J.) per ml or 1 U of alpha-toxin (Sigma) with or without 107 S. aureus, 107 S. epidermidis, or 107 E. coli bacteria per ml of new culture media. Bacteria were enumerated by using the BBL Prompt Inoculation System (Becton Dickinson, Cockeysville, Md.). The cells were incubated for 18 h and the supernatants were collected.
Isolation of responder cells.
Caprine blood was collected via venipuncture into an EDTA Vacutainer (Fisher). Whole blood was centrifuged at 400 × g for 20 min. The plasma and buffy coat layers were aspirated and the erythrocyte pellet, which contained neutrophils, was subjected to hypotonic lysis to remove the erythrocytes. Neutrophils were recovered (500 × g for 5 min), washed three times with RPMI 1640, and resuspended at a final concentration of 2 × 106 cells/ml in RPMI 1640. Typically, the viability was greater than 98%, as shown by the trypan blue exclusion test.
Chemotactic assays.
Neutrophil migration-inducing activity in culture supernatants was assayed by using a microchemotaxis technique and a 48-well modified Boyden chamber (Neuroprobe, Cabin John, Md.) (5). A 5.0-μm-pore-size polycarbonate membrane (Poretics Corp., Livermore, Calif.) was used. A total of 105 neutrophils in a volume of 50 μl were added in the top wells of the chamber. The bottom wells contained 30 μl of the sample to be measured for chemotactic activity. Cell migration proceeded in an incubator with humidified air with 5% CO2 for 30 min. The filters were then removed; the side facing the top well was rinsed with phosphate-buffered saline and wiped clean to rid it of any nonmigrating cells. Filters were fixed with methanol (30 s), allowed to air dry, stained with Diff-Quick (Fisher), mounted with the side facing the bottom well orientated up onto a glass slide (75 by 50 mm) (Corning Glass Works, Corning, N.Y.), rubbed with immersion oil, and counted under a light microscope with a 40× objective and a 10× ocular. Neutrophil migration was expressed as chemotactic index (CI), defined as the number of neutrophils which migrated towards a sample divided by the number of neutrophils which migrated towards a medium control. Dulbecco modified Eagle medium–F-12 medium, both plus supplements, was used as a negative control and to ensure the assay was working properly. Lipopolysaccharide (GIBCO, Grand Island, N.Y.)-stimulated caprine macrophage culture supernatant was used as a positive control with RPMI 1640 as its appropriate negative control in chemotaxis assays. Briefly, 2 × 106 blood monocytes were isolated by a Ficoll-diatrizoate gradient (specific density, 1.090) and allowed to adhere onto plastic. Adherent macrophages were stimulated with lipopolysaccharide (25 μg/ml) in RPMI 1640 with 10% FBS at 37°C for 24 h. The supernatant was collected and stored at −20°C. Positive controls yielded a CI (mean + standard error of the mean [SEM]) of 3.7 + 0.81.
Treatment of supernatants with anti-IL-8 antibodies.
To determine the effect of anti-IL-8 antibodies, supernatants were treated with chicken anti-bovine IL-8 antibodies as described previously (1). Briefly, supernatants were treated with an appropriate dilution of antibodies just prior to being placed in the Boyden chamber. Chemotaxis was allowed to occur as described above. Preimmune chicken immunoglobulin and media with IL-8 did not effect chemotaxis.
Checkerboard analysis.
Checkerboard analysis (37) to differentiate between chemotactic activity (gradient-specific migration) and chemokinesis (random movement) was performed by placing 30 μl of various dilutions of samples in the lower wells of the Boyden chamber and by also placing 50 μl of various dilutions in the upper wells with 105 responder cells. Migration assay was carried out as described above.
Data analysis.
Data were expressed as means + SEMs. Significance was determined by using a two-tailed Student t test, analysis of variance (when appropriate), or a Kruskal-Wallis analysis of variance on ranks (when appropriate).
RESULTS
Chemotactic activities of CMEC and CMMyoEC culture supernatants.
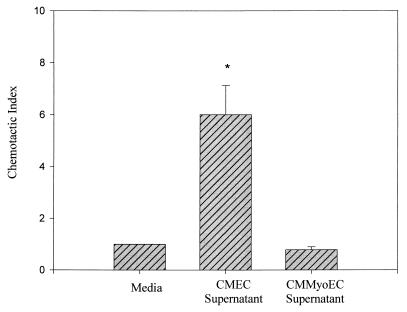
Figure 1 shows the chemotactic activities of CMEC and CMMyoEC culture supernatants. CMEC supernatants had a CI of 4.0 + 1.1, whereas CMMyoEC supernatants had a CI of 0.8 + 0.0. The data indicate that CMEC, but not CMMyoEC, produce neutrophil chemoattractants.
FIG. 1.
Chemotactic activities of CMEC and CMMyoEC culture supernatants. Cells were plated at 5 × 104, and supernatants were collected on day 5. The data are from three separate experiments and are expressed as means + SEMs. ∗, P < 0.05 compared to medium control.
Time course changes of chemotactic activity in culture.
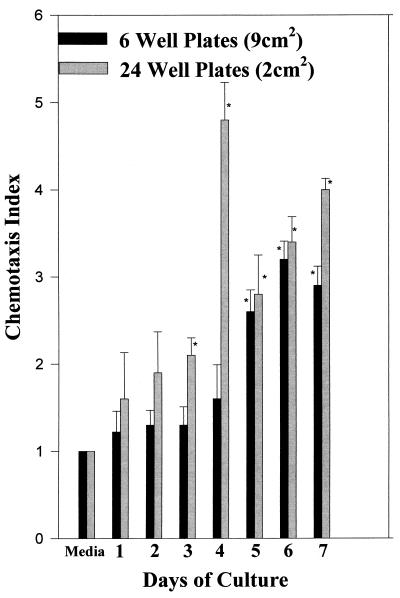
Earlier experiments showed that supernatants of preconfluent CMEC cultures were not able to induce in vitro neutrophil migration (data not shown). In order to determine if a building-up of factors was needed for detection or if factors were produced at a certain time during culture, cells with the same density were plated onto different-size (area) plates. Figure 2 shows that the supernatants collected from plates with the smaller area were able to induce in vitro migration earlier and to a higher degree. This indicates that contact-induced differentiation mediates the release of chemoattractants.
FIG. 2.
Chemokine activity is preferentially expressed by nonproliferating, confluent mammary epithelial cells. A total of 5 × 105 CMEC were plated onto 6- or 24-well tissue culture plates. The culture supernatant was harvested daily and assayed for neutrophil migration. The data are from three separate experiments and are expressed as means + SEMs. ∗, P < 0.05 compared to the medium control.
Checkerboard analysis of CMEC culture supernatants.
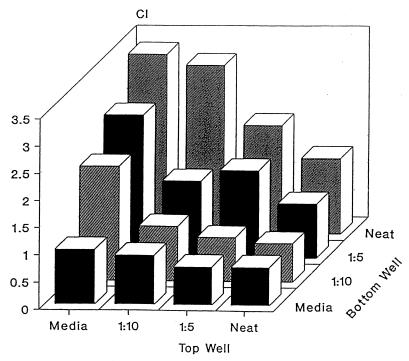
Checkerboard analysis was performed to determine whether neutrophil migration was due to either chemotaxis or chemokinesis. Dilutions of CMEC culture supernatants were added to the upper and lower wells of the Boyden chamber and neutrophil migration was quantitated. As shown in Fig. 3, neutrophil migration generally increased as the concentration gradient between the upper and lower chambers increased. This indicates that the mammary secretions were chemotactic rather than chemokinetic.
FIG. 3.
Checkerboard analysis of CMEC culture supernatants. Various concentration gradients were established by placing different dilutions (neat = undiluted) of sample (along with responder cells) in the bottom and top wells of the Boyden chamber. The data indicate that migration is dependent on an increasing concentration gradient. Data are expressed as means of triplicates.
Chemotactic activity of CMEC and CMMyoEC cocultures.
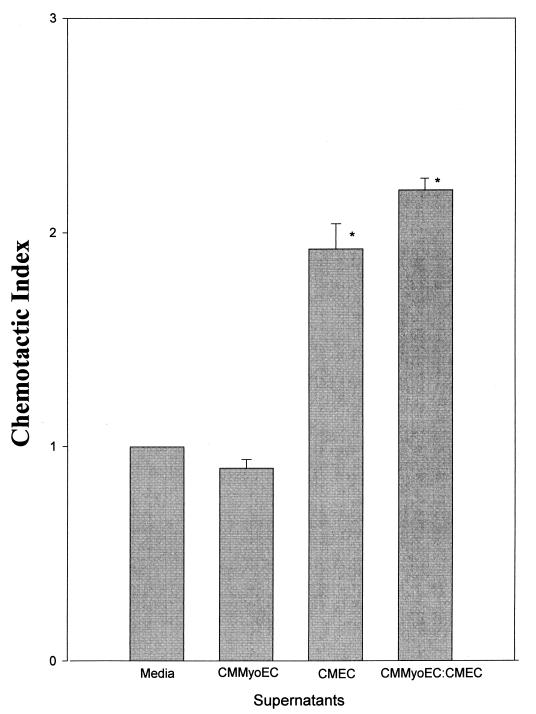
The chemotactic activity of CMEC and CMMyoEC coculture supernatants was examined. Figure 4 shows that the supernatants of CMEC-CMMyoEC cocultures had a significantly increased level of chemotactic activity. Expected chemotactic activity was calculated as follows: (CI of CMEC + CI of CMMyoEC)/CI of control. This indicates that, when the cells are cultured together, one cell population is induced to produce chemoattractants above what it constitutively produces.
FIG. 4.
Chemotactic activity of the supernatants of confluent CMEC, CMMyoEC, and CMEC-CMMyoEC cocultures. Data are from three separate experiments and are expressed as means + SEMs. ∗, P < 0.001 compared to the medium control. Additionally, the CI of CMEC supernatants is statistically different from that of CMMyoEC-CMEC coculture supernatants (P < 0.05).
Effect of anti-IL-8 antibodies on neutrophil chemotaxis towards CMEC culture supernatants.
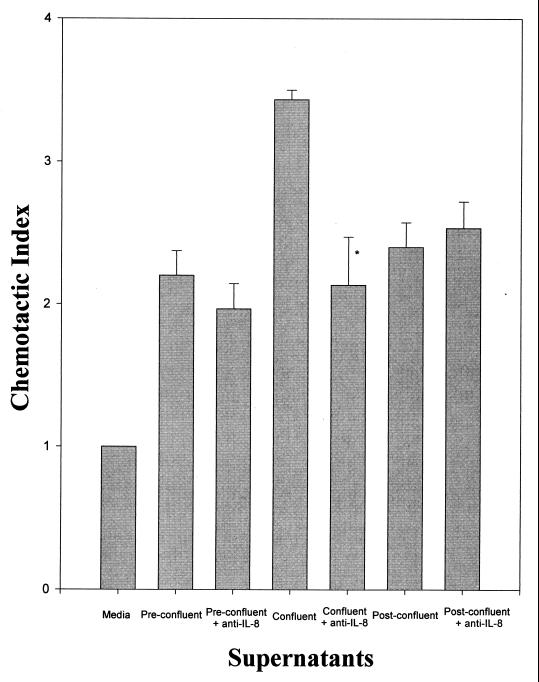
Since IL-8 is a potent inducer of neutrophil chemotaxis and has been shown to be produced by numerous cell types, we examined whether IL-8 played a role in our system. Figure 5 illustrates that anti-IL-8 antibodies did not reduce any of the chemotactic activity in supernatants collected from preconfluent and postconfluent CMEC cultures. However, it did reduce the chemotactic activity of confluent cultures.
FIG. 5.
Effect of anti-IL-8 antibodies on neutrophil chemotaxis towards CMEC culture supernatants. Cells were plated at 5 × 104 and the supernatants were harvested when the cultures reached confluence, usually on day 5. Data are means of triplicates from a representative experiment and are expressed as means + SEMs. ∗, P < 0.05 compared to the same treatment without the addition of antibodies.
Induction of chemotactic activity.
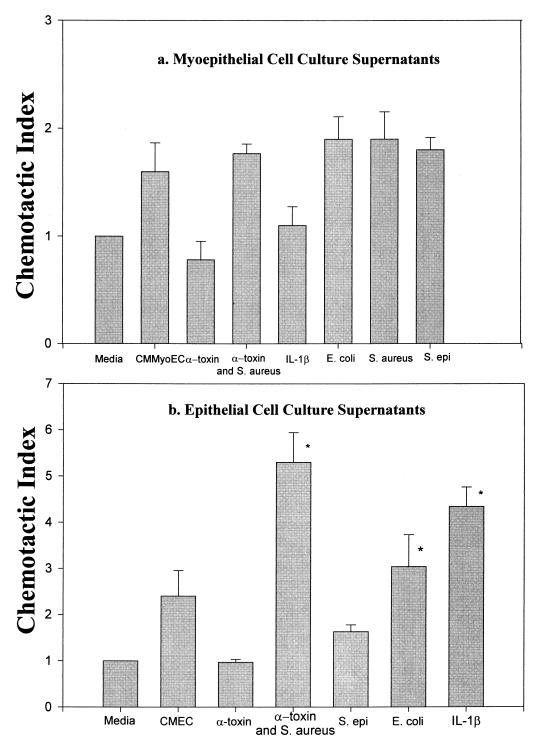
We examined whether a proinflammatory cytokine (IL-1β) or treatments that mimic mastitic conditions could either induce the secretion of chemoattractants in CMMyoEC or augment the production of chemoattractants in CMEC. Figure 6a shows that none of the treatments induced chemoattractant production from CMMyoEC. However, Fig. 6b shows that IL-1β, S. aureus plus alpha-toxin, and E. coli significantly augmented the chemotactic activity of CMEC cultures. With the exception of E. coli, which slightly increased chemotactic activity, none of the treatments alone with media caused neutrophil migration above the medium control (data not shown).
FIG. 6.
Abilities of IL-1β and heat-killed S. aureus, S. epidermidis (S. epi), E. coli, and S. aureus plus alpha-toxin to induce CMMyoEC and CMEC to secrete chemotactic factors. Data are from three separate experiments and are expressed as means + SEMs. ∗, P < 0.05 compared to CMEC cultures.
Effect of anti-IL-8 antibodies on the CMEC supernatants treated with cytokine or heat-killed bacteria.
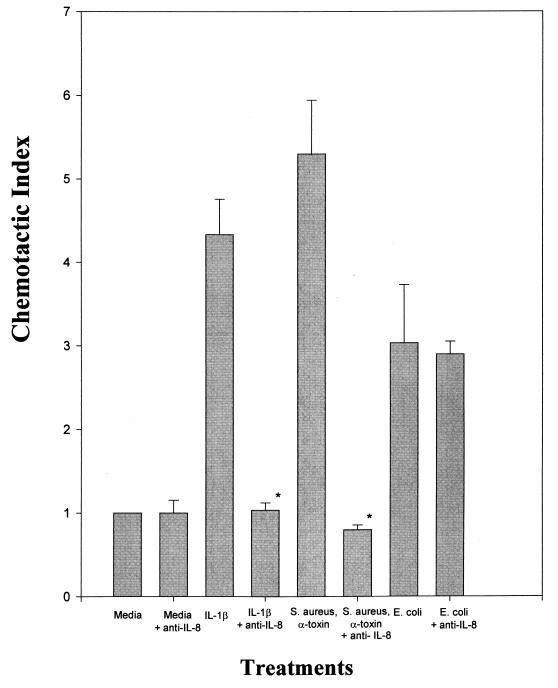
Figure 7 shows that anti-IL-8 significantly blocked chemotactic activity in supernatants from IL-1β-treated cultures and from cultures treated with S. aureus plus alpha-toxin. It did not, however, block chemotactic activity induced by E. coli. Anti-IL-8 alone did not have any effect on neutrophil migration, nor did preimmune immunoglobulin (data not shown).
FIG. 7.
Effect of anti-IL-8 antibodies on the induced chemotactic activity of treated CMEC culture supernatants. Cells were treated as for Fig. 6. Prior to the assay for chemotactic activity, anti-IL-8 antibodies were added to the supernatants. Data are from three separate experiments and are expressed as means + SEMs. ∗, P < 0.01 compared to media plus IL-8.
DISCUSSION
Migration of immune cells into the mammary gland serves to prevent ascending infection of the gland and to provide passive immunity to the suckling offspring. Additionally, these cells may aid in the restructuring of the mammary gland that occurs during involution (i.e., apoptosis). Migration of immune cells from the vasculature system is not a random event, as the leukocyte population in mammary secretions does not mimic that in blood (3, 15). The highly regulated process of immune cell migration is called chemotaxis and is controlled by the secretion of soluble mediators called chemoattractants. Numerous studies have shown the presence of chemotactic regulators in mammary secretions (1, 17, 26–28). Secretion of these soluble mediators can come from numerous cell types, including, but not limited to, epithelial cells and leukocytes (6). In this study, we examined the ability of mammary epithelial and myoepithelial cells to produce soluble chemotaxis mediators. Others have found that epithelial cells produce IL-8 (6, 17, 20, 34) and that IL-8 production can be induced by bacteria (2, 8, 12). Additionally, Michie et al. found that epithelial cells from lactating mammary glands express IL-8 (17).
Previously, we have shown that bovine mastitic mammary secretions contained IL-8 (1). In this study, we wanted to further characterize the immune response to mastitic conditions by elucidating whether mammary epithelial cells or mammary myoepithelial cells produce chemoattractants, whether IL-8 is involved, and whether we could induce chemoattractant production using the proinflammatory cytokine IL-1β, bacteria, or bacterial products. We found that CMEC but not CMMyoEC culture supernatants had chemotactic activity. The highest level of activity occurred when the cultures reached confluence. Also, most of the activity was inhibited by anti-IL-8 antibodies. This data correlated with what other researchers have seen using immunohistochemistry in normal human lactating breast tissue (17).
We also examined whether coculturing CMEC and CMMyoEC would have any effect on the production of chemoattractants. CMEC culture supernatants support the growth of CMMyoEC, and we have found that CMEC culture supernatants are chemotactic for CMMyoEC (21). In this experiment, there was an increase in chemotactic activity when the cells were cultured together, indicating that an additive effect may occur in vivo. Also, in vivo, the effect of other cells and connective tissue may augment the ability of cells to secrete chemoattractants. Lastly, we examined the ability of IL-1β and bacteria or bacterial products to induce chemotactic activity in both CMEC and CMMyoEC cultures. None of the treatments tried could induce CMMyoEC to produce chemoattractants. However, IL-1β, S. aureus plus alpha-toxin, and E. coli all induced an augmented chemotactic response by neutrophils towards the stimulated supernatants. Neither S. epidermidis, S. aureus, nor alpha-toxin alone augmented the activity. The increased activity of the cultures stimulated with IL-1β and S. aureus plus alpha-toxin, but not that of E. coli-stimulated cultures, was inhibited by IL-8, indicating that the increased activity was due to the presence of IL-8. Increased IL-8 levels are seen in most inflammatory events and are preceded and stimulated by IL-1 production. The activity caused by E. coli needs to be studied further, as others have found that E. coli can induce IL-8 production in mammary secretions from challenged glands (30, 31). That this was not observed in cultures may have been due to the lack of other cell types and complex tissue organizations that may be responsible for releasing factors responsible for subsequent IL-8 production by the epithelial cells.
It appears that in the mastitic mammary gland, epithelial cells but not myoepithelial cells are major sources for IL-8. The increased IL-8 levels may be responsible for the large influx of neutrophils into the gland during infection. This does not rule out the possibility that IL-8 may also be produced by leukocytes found in the mammary secretions. However, IL-8 produced by immune cells may have more of an effect on the suckling newborn than on the recruitment of immune cells into the gland. One theory suggests that ingested chemokines play a role in the trafficking of ingested leukocytes (17–19). More work needs to be done to definitively prove this.
Identifying which cells produce chemotactic mediators and when they produce them will help in designing therapies to modulate (either down regulate or up regulate) the immune response to pathogens, allergic responses, and injuries that occur as a result of the influx of cells (reperfusion injury). In summary, we describe an in vitro model combining mammary epithelial and myoepithelial cells that allowed us to study the expression and modulation of chemokines. Epithelial-cell-specific secretion of chemotactic factors may play a role in homeostasis by contributing to the constant influx of immune cells that function to protect the mammary gland from infection. Although it has not been described, chemokine expression, to be effective, would be expected at the basal surfaces of epithelial cells that line the intralobular ducts and acini of the lactating gland. This would facilitate site-specific recruitment of neutrophils and lymphocytes. During mammary gland involution, with the breakdown of interepithelial-cell tight junctions, lumen-derived chemokine would be important for the recruitment of immune cells into the regressing acini, facilitating glandular remodeling.
ACKNOWLEDGMENTS
This work was supported by grants from the South Vermont Dairy Goat Association, USDA AMD 9404477, and the Storrs Agricultural Experiment Station NE-112 project.
M. R. Barber and A. G. Pantschenko contributed equally to the research and writing of the manuscript.
We thank Donald Kreutzer for the gift of anti-IL-8 antibodies.
REFERENCES
- 1.Barber M R, Yang T J. Chemotactic activities in nonmastitic and mastitic mammary secretions: presence of interleukin-8 in mastitic but not nonmastitic secretions. Clin Diagn Lab Immunol. 1998;5:82–86. doi: 10.1128/cdli.5.1.82-86.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basolo F, Conaldi P G, Fiore L, Calvo S, Toniolo A. Normal breast epithelial cells produce interleukins 6 and 8 together with tumor-necrosis factor: defective IL6 expression in mammary carcinoma. Int J Cancer. 1993;55:926–930. doi: 10.1002/ijc.2910550609. [DOI] [PubMed] [Google Scholar]
- 3.Bertotto A, Gerdi R, Fabietti G, Crupi S, Arcangeli C, Scalisi F, et al. Human breast milk T lymphocytes display the phenotype and functional characteristics of memory T cells. Eur J Immunol. 1990;20:1877–1880. doi: 10.1002/eji.1830200838. [DOI] [PubMed] [Google Scholar]
- 4.Bevilacque M P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 5.Boyden S. Chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med. 1962;115:453–459. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curfs J H A J, Meis J F G M, Hoogkamp-Korstanje J A A. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detmers P A, Powell D E, Walz A, Clark-Lewis I, Baggionlini M, Cohn Z A. Differential effects of neutrophil-activating peptide/IL-8 and its homologues on leukocyte adhesion and phagocytosis. J Immunol. 1991;147:4211–4217. [PubMed] [Google Scholar]
- 8.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elner V M, Streiter R M, Elner S G, Baggionlini M, Lindley I, Kunkel S L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–750. [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory H, Young J, Schroder J M, Mrowietz U, Christophers E. Structure determination of a human lymphocyte derived neutrophil activating peptide (LYNAP) Biochem Biophys Res Commun. 1988;151:883–890. doi: 10.1016/s0006-291x(88)80364-4. [DOI] [PubMed] [Google Scholar]
- 11.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 12.Huang J, O’Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson R S, Springer T A. Structure and function of leukocyte integrins. Immunol Rev. 1990;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 14.Leonard E J, Skeel A, Yoshimura T, Noer K, Kutvirt S, Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990;144:1323–1330. [PubMed] [Google Scholar]
- 15.Lindstrand A, Troye-Bloomberg M. Selective compartmentalization of γδ-T lymphocytes in human breast milk. Acta Paediatr. 1997;86:890–891. doi: 10.1111/j.1651-2227.1997.tb08617.x. [DOI] [PubMed] [Google Scholar]
- 16.Manlongat N, Yang T J, Hinckley L S, Bendel R B, Krider H M. Physiologic-chemoattractant-induced migration of polymorphonuclear leukocytes in milk. Clin Diagn Lab Immunol. 1998;5:375–381. doi: 10.1128/cdli.5.3.375-381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michie C A, Tantscher E, Schall T, Rot A. Physiological secretion of chemokines in human breast milk. Eur Cytokine Netw. 1998;9:123–129. [PubMed] [Google Scholar]
- 18.Michie C A, Harvey D. Maternal milk lymphocytes engraft the fetal gut. Pediatr Res. 1995;37:129A. [Google Scholar]
- 19.Ogra S S, Weintraub D, Ogra P L. Immunological aspects of human colostrum and milk. III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J Immunol. 1977;119:245–248. [PubMed] [Google Scholar]
- 20.Palkowitz K H, Royner C L, Garofalo R, Rudolff H E, Schmalstieg F C, Jr, Goldman A S. Production of interleukin-6 and interleukin-8 by human mammary gland epithelial cells. J Reprod Immunol. 1994;26:57–64. doi: 10.1016/0165-0378(93)00867-s. [DOI] [PubMed] [Google Scholar]
- 21.Pantschenko, A. G., M. R. Barber, J. Woodcock-Mitchell, and T. J. Yang. Establishment and characterization of a caprine mammary myoepithelial cell line (CMMyoEC) and its interaction with epithelial cells. Submitted for publication. [DOI] [PubMed]
- 22.Pantschenko, A. G., J. Woodcock-Mitchell, S. L. Bushmich, and T. J. Yang. Establishment and characterization of a caprine mammary epithelial cell line (CMEC). In Vitro Cell. Dev. Biol., in press. [DOI] [PubMed]
- 23.Pantschenko A G, Yang T J. Mitogenic responsiveness of caprine mammary epithelial cells to endocrine and cytokine factors. Endocrine. 1999;10:123–130. doi: 10.1385/ENDO:10:2:123. [DOI] [PubMed] [Google Scholar]
- 24.Picker L J. Control of lymphocyte homing. Curr Opin Immunol. 1994;6:394–396. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 25.Qi J, Kreutzer D L. Fibrin activation of vascular endothelial cells: induction of IL-8 expression. J Immunol. 1995;155:867–876. [PubMed] [Google Scholar]
- 26.Rewinski M J, Yang T J. Lactation stage-dependent changes in levels of tumor necrosis factor/cachectin in milk. Am J Reprod Immunol. 1994;31:170–176. doi: 10.1111/j.1600-0897.1994.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 27.Rot A, Jones A P, Webb L M C. Some aspects of NAP-1/IL-8 pathophysiology. II. chemokine secretion by exocrine glands. In: Lindley I J D, editor. The chemokines. New York, N.Y: Plenum Press; 1993. pp. 77–85. [DOI] [PubMed] [Google Scholar]
- 28.Rudloff H E, Schmalstieg F C, Palowitz K H, Goldman A S. Interleukin-8 in human milk. J Reprod Immunol. 1993;23:13–18. doi: 10.1016/0165-0378(93)90023-b. [DOI] [PubMed] [Google Scholar]
- 29.Schroder J M, Mrwietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- 30.Shuster D E, Lee E K, Kehrli M E. Bacterial growth, inflammatory cytokine production, and neutrophil recruitment during coliform mastitis in cows within ten days after calving, compared with cows at midlactation. Am J Vet Res. 1996;57:1569–1575. [PubMed] [Google Scholar]
- 31.Shuster D E, Kehrli M E, Jr, Rainard P, Paape M. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect Immun. 1997;65:3286–3292. doi: 10.1128/iai.65.8.3286-3292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–304. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava M D, Srivastava A, Brouhard B, Saneto R, Groh-Wargo S, Kubit J. Cytokines in human milk. Res Commun Mol Pathol Pharmacol. 1996;93:263–287. [PubMed] [Google Scholar]
- 34.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch III J P, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Investig. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strieter R M, Kasahara K, Allen R, Showell H J, Standiford T J, Kunkel S L. Human neutrophils exhibit disparate chemotactic factor gene expression. Biochem Biophys Res Commun. 1990;173:725–730. doi: 10.1016/s0006-291x(05)80095-6. [DOI] [PubMed] [Google Scholar]
- 36.Tan J, Deleuran B, Gesser B, Maare H, Deleuran M, Larsen C G, Thestrup-Pedersen K. Regulation of human T lymphocyte chemotaxis in vitro by T cell-derived cytokines IL-2, IFN-γ, IL-4, IL-10, and IL-13. J Immunol. 1995;154:3742–3752. [PubMed] [Google Scholar]
- 37.Zigmond S H, Hirsch J G. Leukocyte locomotion and chemotaxis: new methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973;137:387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]