Abstract
Introduction
Large cystic masses are rare in pregnancy. Corpus luteum cysts and theca lutein cysts are common are most common among all. Ovarian masses are usually discovered as an incidental finding during routine obstetric ultrasonography. Management depends upon the size of the mass, and the symptoms produced by the mass.
Case presentation
Our case describes an incidental finding of a large ovarian cyst during the second trimester, in a 24-year-old female patient. Per abdominal examination revealed a huge mass in the epigastrium, left hypogastrium, and left lumbar region, along with 20 weeks sized uterus. Ultrasonography revealed a cystic mass of 11.9 cm × 11.7 cm X 15.9 cm, with multiple septations and cystic areas. After other baseline investigations, she was planned for surgical removal of the mass. Upon histopathologic examination after surgical excision, the mass was found to be mucinous cystadenoma.
Discussion
Large ovarian masses are uncommon during pregnancy. The most common complications of ovarian masses in pregnancy are torsion, rupture, infection, or malpresentation of the fetus. Surgical management can be done if the mass presents with acute symptoms like torsion, or if the size of the mass is greater than 5 cm. Non-obstetric surgery for cyst removal can be done electively during the second trimester, or irrespective of the period of gestation if there are acute symptoms.
Conclusion
Large ovarian masses are usually rare during pregnancy. It is necessary to properly evaluate the case of ovarian masses during pregnancy, to decide the appropriate line of management.
Keywords: Ovarian cyst, Mucinous cystadenoma, Second trimester, Case report
Highlights
-
•
Large cystic ovarian masses are rare in pregnancy.
-
•
Usually discovered as an incidental findings during routine obstetric ultrasonography.
-
•
The most common complication of ovarian cyst during pregnancy is torsion.
-
•
Non-obstetric surgery for cyst removal can be performed electively on 2nd trimester, or at any time if acute symptoms are present.
1. Introduction
Though adnexal masses during pregnancy are common, large cystic tumors are not frequently seen during pregnancy [1,2]. The incidence of adnexal masses during pregnancy is about 0.2–2%, depending upon the stage of pregnancy. the majority of them are benign [3,4]. The most commonly seen ovarian masses during pregnancy are corpus luteum cysts and theca lutein cysts [2]. The adnexal masses are usually discovered as an incidental finding during routine obstetric ultrasonography [2]. The main complications of adnexal masses during pregnancy are torsion. Surgical management is done usually in the second trimester unless the masses present with features of acute abdomen (like torsion), in which urgent interventions are required irrespective of the period of gestation [5].
We, herein, present a case of a large mucinous cystadenoma in pregnancy, which was operated on in the second trimester. The case has been reported in line with SCARE 2020 criteria [6].
2. Case presentation
A 24-years-old married Hindu female was referred to our tertiary care center from a peripheral center with an incidental finding of a cyst in the abdomen for 2 weeks. She was pregnant for 4.5 months. She had a spontaneous planned pregnancy which was diagnosed at 2 months by a urinary pregnancy test kit at home. She did not take folic acid. There was no history of radiation exposure and teratogenic drug intake in the first trimester. The first trimester was uneventful. She followed up in the local health post for an ANC checkup and started to take iron and calcium in the second trimester. USG was done in a private hospital, which revealed a huge cystic mass, and then, she was referred to our center.
Her menstrual cycles were regular. She was married for 1.5 years, and this was her first pregnancy. There is no history of abortions. There was no history of use of any contraception methods. She did not have any significant past medical/surgical histories.
On presentation to our center, her period of gestation was 26 weeks +6 days, as calculated from her LMP. On examination, her general condition was fair and there was no pallor, edema, or dehydration. Her vitals were stable. On chest examination, there was decreased air entry on the left infra-scapular and midaxillary region. There were no added sounds heard. CVS examination was normal. Per abdominal examination revealed a distended abdomen with bilateral flanks full and the presence of linea nigra and striae gravidarum on inspection. On palpation, the abdomen was soft and non-tender, with no local rise in temperature. Deep palpation revealed 20 weeks sized uterus, with a symphysio-fundal height of 19 cm. A huge mass of 18 cm × 15 cm was felt occupying the left hypochondrium, epigastrium, and the left lumbar region, with a smooth surface, ill-defined margins, cystic consistency, and slightly restricted mobility felt. The lower pole of the mass could be reached, and the mass was non-tender. On percussion, there was a fluid thrill and a dull note was noted all over the abdomen. Per speculum examination revealed posterior, and nulliparous type cervix with pinkish and minimally discharging external os. On bimanual examination, cervix was posterior, uterus was 20 weeks size, slightly deviated to the right, with bilateral vaginal fornices non-tender.
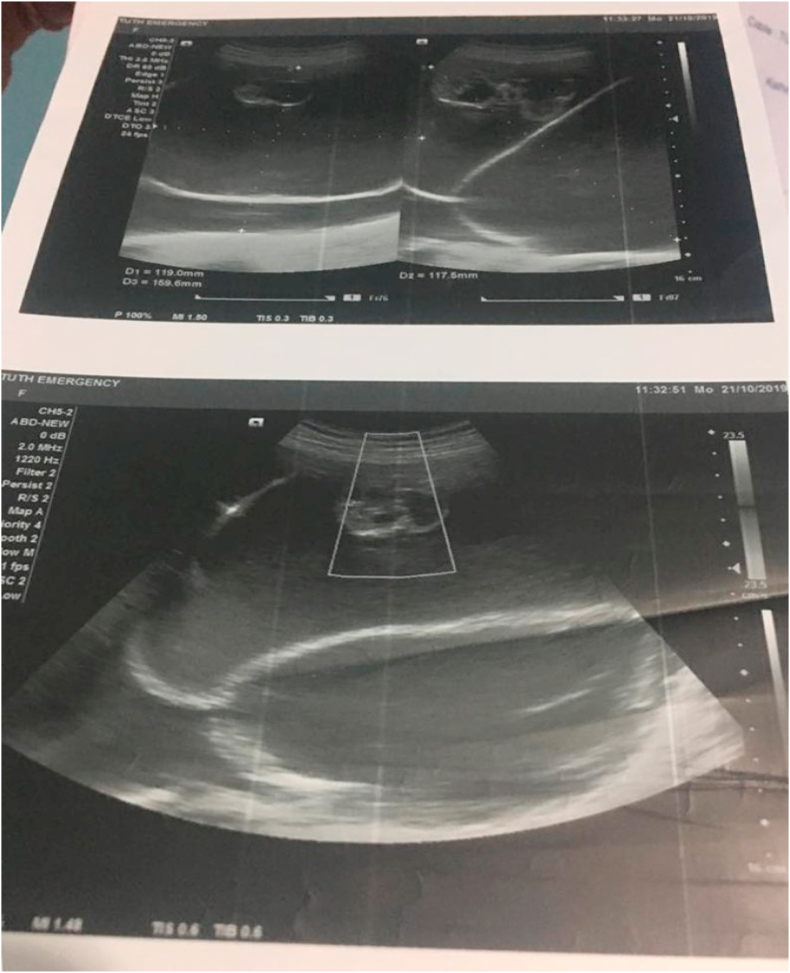
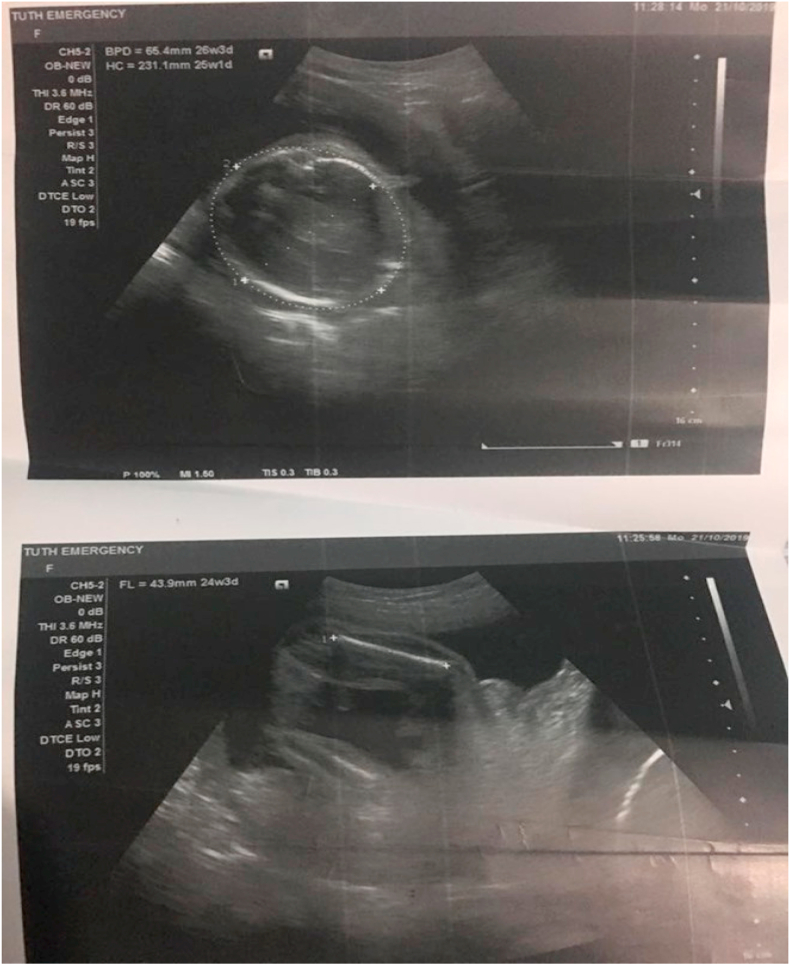
The patient was admitted to the in-patient department for surgery, and all the pre-operative investigations were done. Ultrasonography revealed a single live intrauterine fetus with 25 weeks +2 days of gestation (from imaging), with cephalic presentation. A large cystic lesion of size approximately 11.9 cm × 11.7 cm X 15.9 cm with multiple thick septations and cystic areas within, with no vascularity noted in the abdomen and pelvis. The USG picture is shown in Fig. 1, Fig. 2. With these findings, a provisional diagnosis was made as an ovarian cystadenoma.
Fig. 1.
Figure showing the USG picture depicting a large ovarian mass during pregnancy.
Fig. 2.
Figure showing the USG picture depicting a large ovarian mass during pregnancy.
Her investigations findings are shown in the following Table 1.
Table 1.
Table showing investigation reports of the patient.
| Investigations | Reports |
|---|---|
| Hb (gm %) | 10.9 |
| Platelet count (per cubic mm) | 1,61000 |
| WBC count (per cubic mm) | 9200 |
| PT (sec)/INR | 12/1.09 |
| Random sugar (mmol/L) | 5.6 mmol/L |
| Na/K (mEq/L) | 136/3.4 |
| Urea/Creatinine | 3.5/60.0 |
| HIV Ab/HBsAg/HCV Ab | Non-Reactive |
| Urine R/M/E | Normal |
| Echinococcus Ab ELISA | Negative |
| Liver Function Test | Normal |
She was advised for an MRI abdomen and pelvis. MRI revealed approximately 28 × 25 × 14 cm sized, well-defined, large, thin-walled, abdominopelvic multi-loculated cystic lesion with multiple thin septa, with no evidence of solid component. The lesion was seen abutting the uterus antero-inferiorly, compressing and displacing the bowel loop right laterally, and compressing the left kidney postero-superiorly. Bilateral ovaries were not visualized separately.
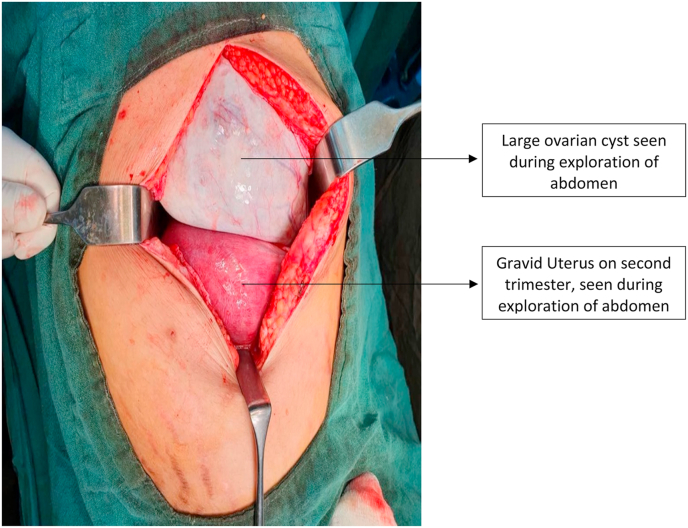
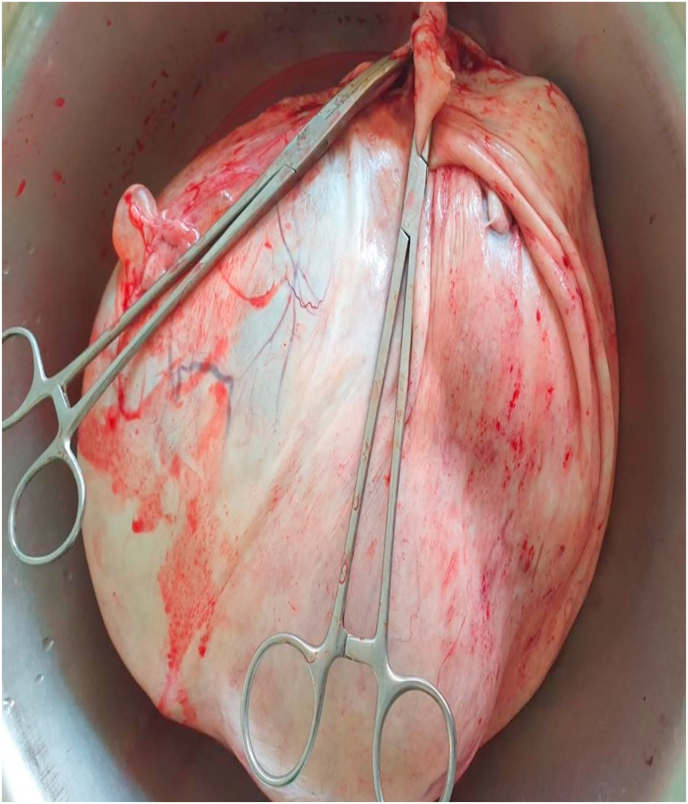
She was planned for surgery. Inj. Proluton depot (hydroxyprogesterone) 500 mg IM stat was given half-hour before surgery. Exploratory laparotomy with left salpingo-ophorectomy with omental biopsy and peritoneal cytology was done. Around 200 ml of ascitic fluid was noted in the peritoneal cavity and was sent for cytology. A huge cyst of 28 cm × 25 cm occupying the top of the uterus, left & right hypochondrium, left lumbar, and left iliac fossa, and arising from the left ovary was found per-operatively, as shown in Fig. 3. The uterus was 26 weeks in size, and the right ovary and right fallopian tube were normal looking. Under surface of the diaphragm, and the liver surface were normal looking. The ovarian mass is shown in Fig. 4. Cross-section of the specimen released 7 L of mucinous fluid. Multiple daughter cysts were noted without solid components or papillary projections.
Fig. 3.
Picture showing a large ovarian cyst upon exploration of abdomen.
Fig. 4.
Large ovarian cyst after being removed from abdomen.
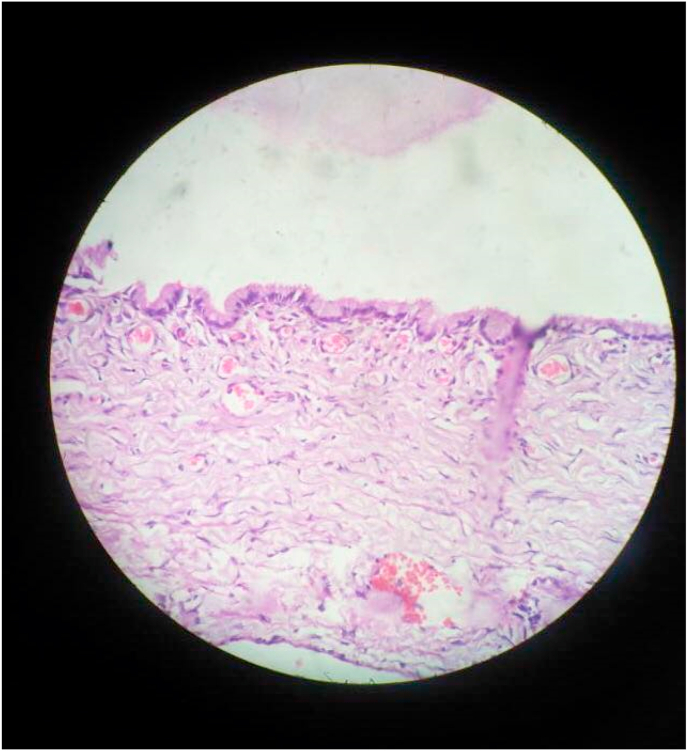
The specimen was sent for histopathologic examination, which showed a cyst wall lined by a single layer of mucinous epithelium with a basally located nucleus and apical mucin. Pseudo-stratification and nuclear atypia were not found. The histopathologic image is shown in Fig. 5. Sections from the omentum showed multiple lobules of mature adipose tissue and focal mesothelial linings, and no deposits were found.
Fig. 5.
Picture showing histopathologic image of the large ovarian cyst.
The final diagnosis after histopathology was made as left ovary mucinous cystadenoma with unremarkable omentum.
The post-operative period was uneventful. The patient was discharged on the 4th postoperative day, on medications. She is on regular follow-up.
3. Discussion
Adnexal masses in premenopausal women are usually benign and incidentally discovered [7]. They can be ovarian or non-ovarian masses. Functional cysts like follicular cysts or corpus luteum cysts are the most common adnexal masses in premenopausal women. The most common non-functional ovarian masses in premenopausal women are dermoid. Cystadenomas are also common and account for about 40–50% of all benign adnexal lesions, the most common being serous cystadenomas [2]. Ovarian mucinous cystadenoma is common in the third to sixth decades of life, unlike its malignant form ovarian mucinous carcinoma which occurs in a young female under 40 years of age [8]. Our case is a case of huge ovarian mucinous cystadenoma in pregnancy.
During pregnancy, the same ovarian masses can be found as in non-pregnant women. In addition to above mentioned ovarian masses, a few other pregnancy-associated masses like cysts of the corpus luteum of pregnancy may also occur [2]. Mucinous cystadenomas are benign epithelial neoplasm that can rapidly grow during pregnancy, as seen in our case.
Complications of the ovarian cysts in pregnancy include torsion of the cyst, rupture, infection, malignancy, impaction of cyst in the pelvis causing retention of urine, malpresentation of fetus, and obstructed labor [5]. Most common ovarian tumors associated with torsion are dermoid. The torsion rate of adnexal masses during pregnancy is about 10–15%. The majority of the cases of ovarian torsion during pregnancy are seen during 8–16 weeks of gestation, the period during which the uterus grows faster [9]. A study showed that the rate of torsion in pregnancy was about 75% in the first trimester and 30% of those masses were mature teratomas [10]. Complete torsion causes total blockade of venous and lymphatic supply leading to venous congestion, hemorrhage, and necrosis, subsequently cyst becomes tense and may rupture [11]. Patient usually presents with features of the acute abdomen like acute pain in the lower abdomen and pelvic examination may reveal a tender cystic mass separate from the uterus.
Asymptomatic adnexal masses which present as a simple cyst with a dimension of 5 cm or smaller are very likely to resolve themselves and hence no further follow-up and treatment during pregnancy is mandatory. Patients having asymptomatic cysts of size 5–10 cm ought to be carefully reviewed after 16 weeks of pregnancy by ultrasonography, along with color doppler and possibly MRI. On review, if the cysts are persistent, further follow-ups are necessary to plan for surgical management. For asymptomatic cysts of size greater than 10 cm, because of the substantial risk of torsion, malignancy, and labor obstruction, surgical removal is reasonable [12,13]. It is necessary for the clinician to make a careful decision about when to operate, as too early or too late interventions can lead to adverse maternal and fetal outcomes. Too early interventions can increase the risk of miscarriage and loss of luteal function whereas, too late interventions can increase the risk of complications like torsion, rupture or bleeding, progression into malignancy, or premature labor [2]. The recommendation to perform surgery during the second trimester rather than the third trimester is primarily mechanical. The early second-trimester uterus is still small enough to not obliterate the abdominal operative field, and the risk of preterm labor may be lower when surgery is performed during the second trimester than compared in the third trimester [14]. Symptomatic masses producing features of acute abdomen due to torsion, or rupture require immediate surgical management, irrespective of the period of gestation [2].
The incidence of non-obstetric surgery in pregnancy is about 0.75%. Though such surgeries do not increase the risk of congenital anomalies, the adverse fetal outcome includes increased incidence of prematurity and IUGR [15]. The non-obstetric surgical intervention has been reported to cause an incidence of miscarriage in about 10.5% of the patients in the first trimester. Some studies have reported higher rates of miscarriage in women who undergo first-trimester abdominal surgery. However, it is not clear whether these higher rates were due to surgery itself, or the underlying maternal conditions like infections, high-grade fever, prompting the surgery or due to maternal characteristics like cigarette smoking, older age etc., or other factors like excision of the corpus luteum early in gestation [15]. During the surgery, there are no proven benefits for the routine administration of prophylactic tocolytics. No anesthetic drugs are proven to cause teratogenic effects in humans when used in standard dosing [16]. For postoperative pain management, opioids can be used as per need. Post-operative progesterone supplementation is recommended when the corpus luteum is removed before 7–9 weeks [15].
4. Conclusion
Ovarian cysts or masses during pregnancy should be appropriately evaluated in order to decide the most appropriate treatment option. Ultrasound & MRI are safe and allow to distinguish between benign and malignant masses. The treatment options including surgical procedures should be discussed for each patient individually.
Ethical approval
Case reports are exempt from ethical approval in our institution, Tribhuvan University Institute of Medicine, Maharajgunj.
Sources of funding
There are no any sources of funding.
Author contributions
Diptee Poudel(DP), Sandip Kuikel (SK): Concept of study, study design, and data acquisition.
Kshitiz Acharya (KA), Sampada Dahal (SD): Writing the manuscript.
Diptee Poudel (DP), Ashmita Adhikari (AA): Surgical therapy and patient management.
All the authors individually did the final proof-reading of the manuscript before submission.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Registration of research studies
1. Name of the registry:
2. Unique identifying number or registration ID:
3. Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Kshitiz Acharya, Maharajgunj Medical Campus, Tribhuvan University Institute of Medicine, Maharajgunj, Kathmandu, Nepal, Email: kshitiz21@iom.edu.np Phone number: +977-9860297999 P.O. Box: 1524.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
There are no any conflicts of interest.
Contributor Information
Kshitiz Acharya, Email: kshitiz21@iom.edu.np.
Diptee Poudel, Email: poudeldiptee@gmail.com.
Sampada Dahal, Email: sampadahal45@gmail.com.
Sandip kuikel, Email: kuikelsandip@iom.edu.np.
Ashmita Adhikari, Email: ashmitaadhikary0@gmail.com.
References
- 1.Fujimoto Y., Takahashi H., Horie K., Nakaya T., Niki T., Fujiwara H., et al. Rapid growth of pelvic cyst during pregnancy: a case report. Case Rep Obstet Gynecol. 2019 May 13;2019:1–5. doi: 10.1155/2019/3120921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haan J de, Verheecke M., Amant F. Management of ovarian cysts and cancer in pregnancy. Facts, Views Vis ObGyn. 2015;7(1):25. [Internet] Available from:/pmc/articles/PMC4402440/ [PMC free article] [PubMed] [Google Scholar]
- 3.Nn A., Rg O., Doppa G., Bh U. Antenatal laparoscopic management of ovarian cyst: a case report. Int J Reprod Contraception, Obstet Gynecol. 2021 Mar 24;10(4) https://www.ijrcog.org/index.php/ijrcog/article/view/9715 [Internet] 1734–8. Available from: [Google Scholar]
- 4.Hoover K., Jenkins T.R. Evaluation and management of adnexal mass in pregnancy. Am. J. Obstet. Gynecol. 2011 Aug;205(2):97–102. doi: 10.1016/j.ajog.2011.01.050. https://pubmed.ncbi.nlm.nih.gov/21571247/ [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 5.Kolluru V., Gurumurthy R., Vellanki V., Gururaj D. Torsion of ovarian cyst during pregnancy: a case report. Cases J. 2009 Dec;2(12):9405. doi: 10.1186/1757-1626-2-9405. [Internet] Available from:/pmc/articles/PMC2809077/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., et al. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec 1;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Chiang G., Levine D. Imaging of adnexal masses in pregnancy. J. Ultrasound Med. 2004 Jun 1;23(6) doi: 10.7863/jum.2004.23.6.805. https://onlinelibrary.wiley.com/doi/full/10.7863/jum.2004.23.6.805 [Internet] 805–19. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Poudel D., Acharya K., Poudel N., Adhikari A., Khaniya B., Maskey S. Bilateral ovarian mucinous carcinoma (stage III) with omental involvement and incidental hydronephrosis: a rare case report. Int J Surg Case Rep. 2022 Aug 1;97 doi: 10.1016/j.ijscr.2022.107415. https://linkinghub.elsevier.com/retrieve/pii/S2210261222006617 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usui R., Minakami H., Kosuge S., Iwasaki R., Ohwada M., Sato I. A retrospective survey of clinical, pathologic, and prognostic features of adnexal masses operated on during pregnancy. J. Obstet. Gynaecol. Res. 2000;26(2):89–93. doi: 10.1111/j.1447-0756.2000.tb01289.x. https://pubmed.ncbi.nlm.nih.gov/10870299/ [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 10.Chang S.D., Yen C.F., Lo L.M., Lee C.L., Liang C.C. Surgical intervention for maternal ovarian torsion in pregnancy. Taiwan. J. Obstet. Gynecol. 2011 Dec 1;50(4):458–462. doi: 10.1016/j.tjog.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 11.B A., P S. Adnexal torsion in pregnancy. http://www.jpgo.org/2017/12/adnexal-torsion-in-pregnancy.html Available from:
- 12.Glanc P., Salem S., Farine D. Adnexal masses in the pregnant patient: a diagnostic and management challenge. Ultrasound Q. 2008 Dec;24(4) doi: 10.1097/RUQ.0b013e31819032f. https://pubmed.ncbi.nlm.nih.gov/19060689/ [Internet] 225–40. Available from: [DOI] [PubMed] [Google Scholar]
- 13.Wang P.H., Chang W.H., Cheng M.H., Horng H.C. Management of adnexal masses during pregnancy. J. Obstet. Gynaecol. Res. 2009 Jun 1;35(3):597–598. doi: 10.1111/j.1447-0756.2009.01048.x. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1447-0756.2009.01048.x [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Visser B.C., Glasgow R.E., Mulvihill K.K., Mulvihill S.J. Safety and timing of nonobstetric abdominal surgery in pregnancy. Dig. Surg. 2001;18(5) doi: 10.1159/000050183. https://www.karger.com/Article/FullText/50183 [Internet] 409–17. Available from: [DOI] [PubMed] [Google Scholar]
- 15.Mazze R.I., Källén B. Reproductive outcome after anesthesia and operation during pregnancy: a Registry study of 5405 cases. Am. J. Obstet. Gynecol. 1989 Nov 1;161(5) doi: 10.1016/0002-9378(89)90659-5. http://www.ajog.org/article/0002937889906595/fulltext [Internet] 1178–85. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Committee Opinion No. 696 Nonobstetric surgery during pregnancy. Obstet. Gynecol. 2017 Apr 1;129(4) doi: 10.1097/AOG.0000000000002014. https://pubmed.ncbi.nlm.nih.gov/28333816/ [Internet] 777–8. Available from: [DOI] [PubMed] [Google Scholar]