This cohort study uses data from the 26th annual nationwide survey on Kawasaki disease in Japan to identify differences between incidence rates of the disease before and after the start of the COVID-19 pandemic.
Key Points
Question
Did the COVID-19 pandemic and its accompanying mitigation measures affect the incidence of Kawasaki disease (KD) in Japan?
Findings
In this cohort study of 28 520 patients, the number of patients diagnosed with KD decreased by approximately one-third across Japan in 2020 (after the COVID-19 pandemic) compared with 2019 (before the pandemic), with no indication suggesting parental behavior to avoid a hospital visit. Differences were found in the percentage reduction among patients diagnosed with KD who were younger than 12 months vs 24 months or older.
Meaning
Findings of this study appear to support the hypothesis of a pathogenesis involving transmission of KD among children, especially among older children.
Abstract
Importance
Global studies have reported that the incidence of Kawasaki disease (KD) declined during the COVID-19 pandemic. These studies suggest that the global pandemic and its accompanying mitigation measures may provide an important opportunity to explore the hypothesis of a KD pathogenesis.
Objective
To compare changes in KD incidence in Japan before and after the start of the COVID-19 pandemic.
Design, Setting, and Participants
This cohort study was conducted using the data set from Japan’s 26th nationwide KD survey that obtained information on patients who were diagnosed with KD in Japan from January 1, 2019, through December 31, 2020.
Main Outcomes and Measures
Kawasaki disease incidence rates were calculated by referring to the national population data in the vital statistics data for Japan.
Results
A total of 28 520 patients were identified (16 236 male individuals [56.9%]; median [IQR] age, 26 [14-44] months). A total of 17 347 patients were diagnosed with KD in 2019 and 11 173 were diagnosed in 2020, representing a 35.6% reduction in the number of patients diagnosed in 2020 compared with the previous year. Patient distributions for days of illness at the first hospital visit were almost identical in 2019 and 2020, suggesting that the decrease in KD incidence likely was not associated with pandemic-related delays in seeking treatment. The proportion of patients diagnosed with KD who were younger than 12 months was significantly larger in 2020 than in 2019 (21.6% vs 19.4%; P < .001). Compared with KD incidence among younger patients, the incidence among those 24 months and older declined rapidly after initiation of COVID-19 special mitigation measures, with a greater percentage reduction (58.3% reduction in July), but rebounded faster after the end of the special mitigation period. By contrast, the incidence among patients younger than 12 months declined moderately after the initiation of the special mitigation period, with a lower percentage reduction (40.3% reduction in October), and rebounded at a later phase.
Conclusions and Relevance
In this cohort study, the number of patients diagnosed with KD decreased by approximately one-third across Japan in 2020, with no indication that parents avoided a hospital visit. Differences in KD incidence reduction patterns before and after the initiation of COVID-19 pandemic mitigation measures were found in patients with KD aged younger than 12 months compared with those 24 months or older, suggesting a potential KD pathogenesis involving transmission among children.
Introduction
Half a century has passed since the first reports were published on patients with Kawasaki disease (KD) from Japan.1,2 Recently, KD has been reported in more than 60 countries as well as in children of all races and ethnicities.3,4,5,6,7,8,9 This acute febrile disease, which is characterized by systemic inflammation in medium-sized arteries as well as multiple organs and tissues, affects young children. Some patients with KD develop coronary artery abnormalities, followed by lifelong cardiac sequelae after acute illness; this is a substantial issue in high-income countries as it is the current leading cause of acquired heart disease in children.10,11,12,13
Although the cause of KD remains unidentified, the hypothesis that unidentified infectious agents trigger an abnormal acute immune response of KD is supported by epidemiological characteristics, such as age, with the highest incidence among children aged 12 to 23 months but the lowest incidence in those younger than 6 months. This finding is compatible with the hypothesis of infection by a ubiquitous agent associated with increasing immunity with age and with transplacental immunity14,15,16; seasonal differences in incidence17,18,19,20,21,22; temporal- and regional-level clustering22,23,24; and potential sibling-to-sibling transmission within households.25,26,27 A recent study suggested the possibility that KD may be triggered by unidentified respiratory pathogens that may be acquired from both inside and outside the household.28
In Japan, a nationwide KD survey has been conducted biennially since 1970, aiming to provide epidemiological evidence that may suggest clues that could help identify KD pathogenesis. The most recent survey was completed in 2021, updating previous epidemiological findings of KD in Japan. The most recent survey results indicated a large epidemiological change before and after the start of the COVID-19 pandemic, suggesting potential implications of COVID-19 mitigation measures for the incidence of KD. Similar findings have been reported from other countries—the incidence of KD was largely reduced during the COVID-19 pandemic period compared with the corresponding period in previous years.29,30,31,32 These studies suggest that the global pandemic and its accompanying mitigation measures may provide an important opportunity to explore the hypothesis of a potential KD pathogenesis involving transmission via human-to-human contact. The present study aims to compare the changes in KD incidence before and after the start of the COVID-19 pandemic in Japan, using the largest global data set from the nationwide KD surveys in Japan.
Methods
Nationwide KD Survey in Japan
This cohort study used data from a survey that has been conducted biennially since 1970 to collect information on patients who were diagnosed with KD during the preceding 2 years across Japan.3,33,34,35,36 Survey respondents were nationwide hospitals specializing in pediatrics and those with 100 or more beds and a pediatric department. These hospitals include nearly all of those in Japan where patients are appropriately diagnosed and eventually treated by pediatricians with expertise in KD. The survey outline has not been revised since the first survey was conducted in 1970, and response rates are high, at more than 70% for the past 2 decades.3,33,34,35,36 The most recent survey, the 26th in the series, was completed in September 2021 and obtained information on patients diagnosed with KD across Japan from January 1, 2019, through December 31, 2020. The Jichi Medical University Clinical Research Ethics Committee approved the study and waived the requirement for informed consent because only deidentified data were used. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
To accurately select the target hospitals, we used the 2019 to 2020 listing of hospitals compiled by the Committee on Studies of Health Policies at the Ministry of Health, Labour and Welfare in Japan, from which we identified 1745 eligible hospitals nationwide. Questionnaires were sent by mail or email to representative pediatricians at the hospitals, and each was asked to document all patients diagnosed with KD at their hospitals during 2019 and 2020. The diagnosis of KD was based on the current Japanese diagnostic guideline, revised in 2019.37 The 6 principal signs of KD are fever, bilateral conjunctival injection, oral mucosal changes, polymorphous skin rash, peripheral extremity changes, and nonsuppurative cervical lymphadenopathy.37 We sent reminders twice to prompt responses. Incomplete or unclear questionnaires were reviewed and sent back to respondents for correction to avoid reporting bias.
Measurements
The demographic characteristics collected in the survey included patient age, sex, recurrent status (for those with a history of KD), sibling and parental history of KD, principal KD signs, and day of illness at the first hospital visit. The first day of illness was defined as the first day with signs of KD (ie, the day of KD onset). Patients with 5 or 6 principal signs were defined as having complete KD, whereas those with 4 or fewer were defined as having incomplete KD.11,37 The survey also obtained information on initial intravenous immunoglobulin (IVIG) treatment and cardiac complications associated with KD. Initial IVIG treatment failure was defined as recurrent or persistent fever (≥37.5 °C) at least 24 hours after the end of the initial standard IVIG administration. Cardiac complications, including coronary artery abnormalities (ie, coronary artery dilatations and aneurysms) and valvular lesions, were identified by 2-dimensional echocardiography. Coronary artery abnormalities were evaluated based on either the z score38,39,40,41 or the Japanese Circulation Society criteria.38 Any cardiac complications were recorded during the acute illness period (<30 days from KD onset) and during the postacute illness period (≥30 days from KD onset, typically noted at the earliest echocardiographic assessment after 30 days); the latter were defined as cardiac sequelae. In addition, the survey obtained the polymerase chain reaction test result for SARS-CoV-2 to identify patients with KD who also had COVID-19.
Statistical Analysis
First, we described annual patterns in the incidence rates of KD corresponding with the total number of patients during the past 2 decades (2001-2020) using data from the 17th through 26th nationwide surveys. The incidence rates were calculated by referring to the national population data in the vital statistics data for Japan.42 Second, we compared characteristics between patients diagnosed with KD during 2019 and 2020 using χ2 tests for age groups (<12, 12-35, 36-59, and ≥60 months), sex, recurrent status, sibling and parental history of KD, the numbers of principal signs (≤4 and 5 or 6), days of illness at the first hospital visit (1-4, 5-7, 8-10, and ≥11 days), initial IVIG treatment and days of illness at administration (1-4, 5-7, 8-10, and ≥11 days), initial IVIG treatment failure, and cardiac complications. Median age in months was compared using Mann-Whitney U tests. Third, we described differences in days of illness at the first hospital visit to test the hypothesis that parents of patients with KD may have avoided visiting a hospital during the initial COVID-19 pandemic period in 2020 because of the potential risk of hospital-related COVID-19 infection.43
Fourth, we assessed differences in monthly patterns in the number of patients diagnosed with KD in 2020 and the preceding 3 control years (2017, 2018, and 2019). The Japanese government implemented a nationwide school closure, including daycare centers for younger children, beginning March 2, 2020, and subsequently announced a state of emergency beginning April 7, 2020. The nationwide school closure continued until the state of emergency was officially lifted on May 25, 2020. We defined this period from the beginning of March through the end of May as the COVID-19 special mitigation period. Furthermore, we described any reduction in the number of patients diagnosed with KD during 2020 compared with the patient numbers during control years according to age groups (age <12, 12-23, ≥24 months). The monthly percentage reduction in the number of patients with KD was evaluated for each month. In this analysis, percentage reductions in the number of patients who developed KD in 2020 were compared with the mean for the corresponding month in the years 2017 to 2019.
All categorical variables are presented as percentage of patients, and ages are presented as medians with IQRs (25th-75th percentile) in addition to the percentage of patients in categorized groups. The significance threshold for statistical tests was set at 2-sided P < .01. All analyses were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp).
Results
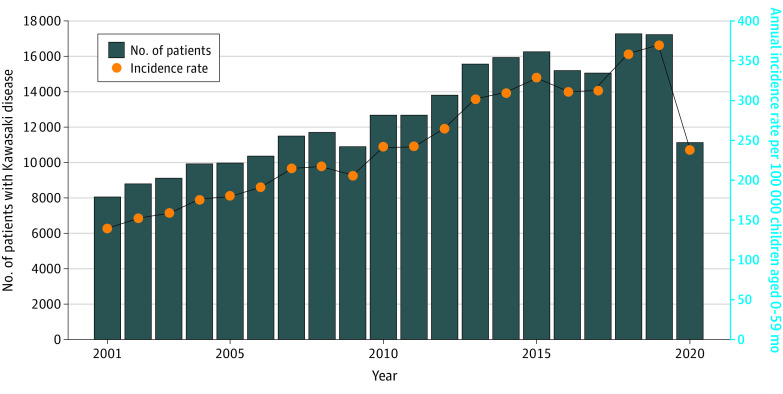
The annual numbers of patients with KD (28 520 patients; 16 236 male individuals [56.9%] and 12 284 female individuals [43.1%]; median [IQR] age, 26 [14-44] months) revealed increasing incidence of KD from 2001 through 2019, but clear and substantial reductions occurred in both the number of cases and incidence rates in 2020 (Figure 1). For example, a total of 17 347 patients were diagnosed with KD in 2019 and 11 173 were diagnosed in 2020, representing a 35.6% reduction. The highest annual KD incidence rate for all survey years was recorded in 2019 at 371 cases per 100 000 children aged 0 to 59 months per year, but the rate also greatly declined in 2020 almost to the level observed in 2001.
Figure 1. Annual Number of Patients With Kawasaki Disease in Japan and Incidence Rates From 2001 Through 2020.
Differences in patient characteristics between 2019 and 2020 are shown in the Table. The mean (IQR) age in patients diagnosed with KD was significantly younger in 2020 than in 2019 (25 [13-44] vs 26 [14-44] months; P = .003), and the proportion of patients younger than 12 months was significantly larger in 2020 than in 2019 (21.6% vs 19.4%; P < .001). Significant differences were found in other characteristics, including incomplete KD (20.3% vs 18.9%; P = .003) and initial IVIG treatment failure (21.8% vs 19.5%; P < .001); these characteristics tended to be more common in patients with KD younger than 12 months than in older patients. Although patients aged 12 to 23 months were a major population in both 2019 and 2020 with no proportional differences between years, when patients were distributed by age between 2019 and 2020 (eFigure 1 in the Supplement), the proportion of those younger than 12 months in 2020 was higher than in 2019, but the proportion of those aged 24 months or older was lower. In 2020, a total of 2586 patients (23.1%) with KD underwent SARS-CoV-2 polymerase chain reaction testing (Table), but only 4 patients (0.2%) had a positive test result; all 4 patients were diagnosed as having complete KD.
Table. Patient Characteristics.
| Characteristic | Patients, No. (%) | P valuea | |
|---|---|---|---|
| 2019 (n = 17 347) | 2020 (n = 11 173) | ||
| Age, mo | |||
| Median (IQR) | 26 (14-44) | 25 (13-44) | .003 |
| <12 | 3369 (19.4) | 2417 (21.6) | <.001 |
| 12-35 | 7927 (45.7) | 4893 (43.8) | |
| 36-59 | 3884 (22.4) | 2488 (22.3) | |
| ≥60 | 2167 (12.5) | 1375 (12.3) | |
| Sex | |||
| Male | 9830 (56.7) | 6406 (57.3) | .27 |
| Female | 7517 (43.3) | 4767 (42.7) | |
| Had recurrent KD | 759 (4.4) | 529 (4.7) | .16 |
| Had sibling history of KD | 421 (2.4) | 248 (2.2) | .26 |
| Had parental history of KD | 273 (1.6) | 182 (1.6) | .72 |
| Principal 6 signs of KD | |||
| Fever | 17 240 (99.4) | 11 135 (99.7) | <.001 |
| Bilateral conjunctival injection | 15 217 (87.7) | 9566 (85.6) | <.001 |
| Oral mucosal changes | 14 960 (86.2) | 9599 (85.9) | .44 |
| Polymorphous skin rash | 14 750 (85.0) | 9573 (85.7) | .13 |
| Peripheral extremity changes | 14 028 (80.9) | 8858 (79.3) | <.001 |
| Cervical lymphadenopathy | 12 529 (72.2) | 7904 (70.7) | .007 |
| No. of principal signs of KD | |||
| 5 or 6 (Complete KD) | 14 072 (81.1) | 8906 (79.7) | .003 |
| 1-4 (Incomplete KD) | 3275 (18.9) | 2267 (20.3) | |
| Days of illness at first hospital visit | |||
| 1-4 | 10 977/17 343 (63.3) | 7157 (64.1) | .63 |
| 5-7 | 5708/17 343 (32.9) | 3600 (32.2) | |
| 8-10 | 510/17 343 (2.9) | 324 (2.9) | |
| ≥11 | 148/17 343 (0.9) | 92 (0.8) | |
| Had initial IVIG administration | 16 541 (95.4) | 10 674 (95.5) | .48 |
| Days of illness at initial IVIG administration | |||
| 1-4 | 6316/16 536 (38.2) | 4305/10 672 (40.3) | .005 |
| 5-7 | 9264/16 536 (56) | 5756/10 672 (53.9) | |
| 8-10 | 813/16 536 (4.9) | 522/10 672 (4.9) | |
| ≥11 | 143/16 536 (0.9) | 89/10 672 (0.8) | |
| Had initial IVIG treatment failureb | 3222/16 536 (19.5) | 2323/10 672 (21.8) | <.001 |
| Cardiac complicationsc | |||
| Acute illness (<30 d of illness) | |||
| Coronary artery abnormalities | 1291 (7.4) | 884 (7.9) | .15 |
| Valvular lesions | 298 (1.7) | 205 (1.8) | .46 |
| Sequelae (after 30 d of illness) | |||
| Coronary artery abnormalities | 391 (2.3) | 263 (2.4) | .58 |
| Valvular lesions | 77 (0.4) | 50 (0.4) | .96 |
| Underwent SARS-CoV-2 PCR testing | NA | 2586 (23.1)d | NA |
Abbreviations: IVIG, intravenous immunoglobulin; KD, Kawasaki disease; NA, not applicable; PCR, polymerase chain reaction.
Mann-Whitney U tests for median age; χ2 tests for other categorical variables, including age groups (age <12, 12-35, 36-59, and ≥60 months), sex, recurrent status, sibling and parental history of KD, the numbers of principal signs (1-4 and 5 or 6), days of illness at the first hospital visit (1-4, 5-7, 8-10, and ≥11 days), initial IVIG treatment and days of illness at IVIG administration (1-4, 5-7, 8-10, and ≥11 days), initial IVIG treatment failure, and cardiac complications.
Excluding patients who did not receive initial IVIG administration.
Coronary artery abnormalities include coronary artery dilatations and aneurysms; some patients had both coronary artery abnormalities and valvular lesions.
Of the 2586 patients who underwent the testing, only 4 (0.2%) had a positive result.
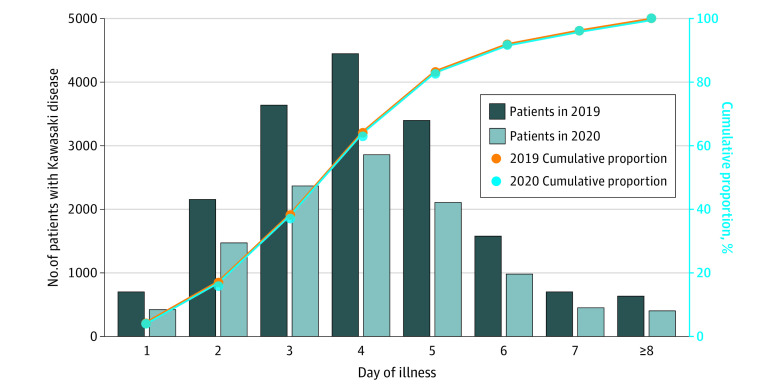
Figure 2 shows patient distributions according to days from KD onset until the first hospital visit in 2019 and 2020. Although the numbers of patients were consistently lower in 2020 than in 2019 across all days of illness, patient proportions for each day of illness as well as the cumulative proportions were almost identical between 2019 and 2020, indicating that potential presentation delays in 2020 were not evident. In each of these years, 83% of patients first visited a hospital by day 5 and 96% by day 7 of illness.
Figure 2. Days of Illness at First Hospital Visit in 2019 vs 2020.
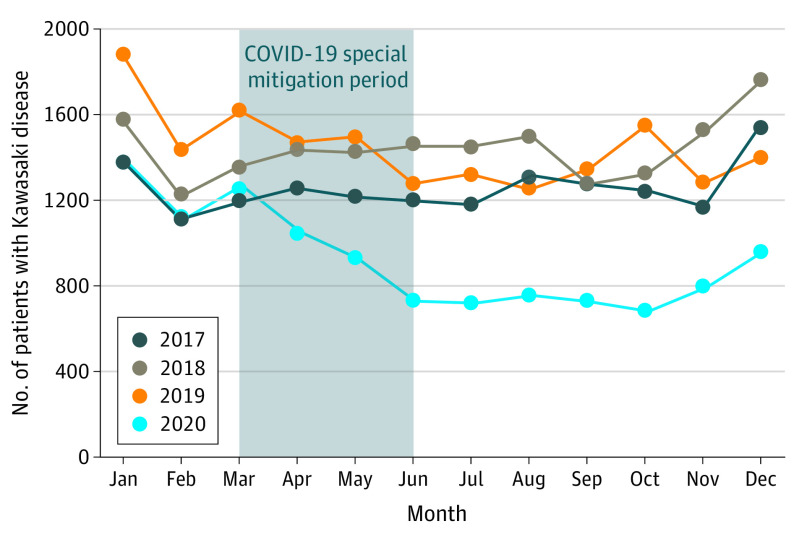
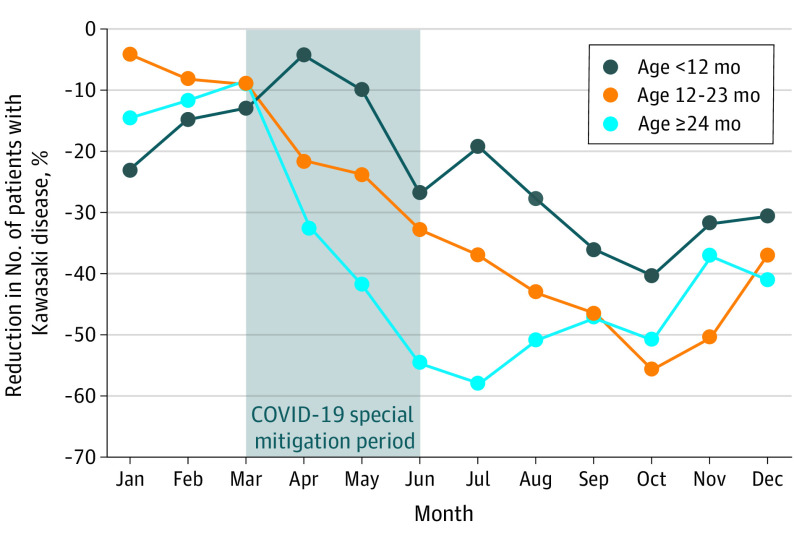
A distinct pattern was observed in the monthly number of patients who developed KD in 2020 compared with those observed in 2017 through 2019 (Figure 3). The monthly number of patients in 2020 showed a large reduction after the beginning of April and remained much lower than the monthly numbers in previous years. The monthly number decreased markedly during the COVID-19 special mitigation period, remained at a minimal level from June (n = 723) through October (n = 689) 2020, and increased moderately after the beginning of November 2020. Figure 4 shows the percentage reductions in the monthly number of patients during 2020 compared with the mean number of patients recorded from 2017 through 2019, with stratification for 3 age groups. Of these patients, the group 24 months or older had the largest reduction after the beginning of April through September, suggesting a prompt response to the initiation of the COVID-19 special mitigation measures. The percentage reduction for this group rapidly reached the lowest point (58.3%) in July 2020 and rebounded beginning in August 2020. By contrast, patients younger than 12 months had a moderate percentage reduction beginning in May 2020, 1 month later than patterns observed in older patients. Patients younger than 12 months had a lower percentage reduction (40.3%) in October 2020 and a rebound beginning in November 2020, 3 months later than those in the group aged 24 months and older. Notably, the reduction pattern observed in patients aged 12 to 23 months showed features intermediate between those of patients aged 24 months or older and younger than 12 months. Additional analyses were separately performed with stratification by age groups, with similar results suggesting confirmation of the primary result (eFigures 2 and 3 in the Supplement).
Figure 3. Monthly Pattern in Number of Patients Diagnosed With Kawasaki Disease, 2017 Through 2019 vs 2020.
A nationwide school closure began March 2, 2020, and a state of emergency was announced April 7, 2020, to restrict human-to-human contact in Japan. These measures continued until the state of emergency was officially lifted on May 25, 2020.
Figure 4. Monthly Percentage Reductions in Number of Patients Diagnosed With Kawasaki Disease in 2020.
The Figure shows monthly percentage reductions in 2020 compared with the mean number of patients diagnosed with Kawasaki disease from 2017 through 2019. During the COVID-19 special mitigation period, a nationwide school closure began March 2, 2020, and a state of emergency was announced April 7, 2020, to restrict human-to-human contact in Japan. These measures continued until the state of emergency was officially lifted on May 25, 2020.
Discussion
Compared with 2019, the number of patients diagnosed with KD in 2020 decreased by approximately one-third across Japan. Results of the present study indicate that parents of patients did not hesitate to visit a hospital during the COVID-19 pandemic, suggesting a true reduction in the number of patients who developed KD in 2020. These results were consistent with results from our preliminary study28 conducted using web-based KD surveillance data, which included much smaller data samples than the nationwide KD survey. Moreover, the present study found that the patterns of reduction in 2020 (ie, degree of reduction as well as months in which reduction and rebound phases began) largely differed by age. Compared with younger patients, KD incidence in patients aged 24 months and older more rapidly declined just after initiation of the COVID-19 special mitigation measures, with a greater percentage reduction, but rebounded earlier after the end of the special mitigation period. By contrast, KD incidence in patients aged younger than 12 months declined moderately beginning 1 month after the initiation of the special mitigation period, with a smaller percentage reduction, and rebounded later after the end of the special mitigation period in May 2020.
Common pediatric infectious diseases were reduced worldwide during the COVID-19 pandemic in 2020.44,45 However, throughout 2020, some children with fever and other infectious symptoms may not have gone to a hospital to avoid hospital-related transmission of COVID-19,43 which may have explained the decreased numbers of patients presenting with pediatric infectious diseases. In contrast, the present study confirmed that, for patients with KD, potential delayed hospital visits were not found in 2020 compared with the previous year, indicating that parents of patients did not hesitate to visit a hospital when they perceived something was wrong with their children who were febrile, even during the COVID-19 pandemic. Shulman et al30 suggested that because of the severity of clinical presentation in KD, parental concern for hospital-related transmission during the COVID-19 pandemic was unlikely to have contributed to the reduction in patients diagnosed with KD. Results of the present study are in line with that reasoning and therefore appear to reflect a true reduction in the number of patients who developed KD after the start of the COVID-19 pandemic in 2020.
Findings of this study suggest that COVID-19 mitigation measures may have been associated with a reduction in the number of patients diagnosed with KD; however, the reduction patterns differed between patients younger than 12 months and those 24 months or older. One potential reason for such differences is mask wearing. Given the existing hypothesis that KD may be triggered by inhalation of an unidentified ubiquitous respiratory agent,14,30,46,47 mask wearing might help prevent triggering KD mostly in children 24 months or older, whereas younger children are discouraged from wearing masks because of suffocation risk.28 Another possible reason is that younger children are much less likely to use daycare centers in Japan. According to Japanese government statistics in 2020,48 daycare use rates were 16.9% for children younger than 1 year, 50.4% for children aged 1 to 2 years, and 55.4% for children 3 years or older. Among all children using daycare centers in Japan (2.74 million children aged 0-5 years), children younger than 1 year accounted for only 5.5% (151 000 children).48 Both use rates and the number of users of daycare centers greatly differed between children younger than 12 months and older children. Therefore, nationwide school closures may have suddenly reduced human contact (and potential risk of acquiring unidentified KD pathogens) more dominantly among children 12 months or older, meaning a more prompt response and greater percentage reduction compared with patients younger than 12 months. Given the hypothesis that potential KD pathogenesis can transmit asymptomatically among children (via human-to-human contact),14,28 thorough infection control measures, such as mask wearing, hand washing, and social distancing, could help prevent development of KD among older children.
Multisystem inflammatory syndrome in children has been increasingly reported as a serious health condition associated with SARS-CoV-2 infection.49,50,51,52 Such patients might have been misdiagnosed as having KD in our study; however, it is unlikely that this misdiagnosis markedly impacted our study population for the following reasons. First, our survey identified only 4 patients with a positive SARS-CoV-2 polymerase chain reaction test result. Second, the Japanese KD Surveillance Team reported that no association was observed between KD and COVID-19 in Japan during 2020 because of the small number of children aged 10 years or older who were diagnosed with COVID-19.53 Third, another study reported that multisystem inflammatory syndrome in children was a rare complication associated with SARS-CoV-2 infection, with an incidence of only 316 per 1 000 000 SARS-CoV-2 infections.52 Based on these findings, those with multisystem inflammatory syndrome in children did not affect our results because the incidence of this complication in Japan during 2020 was estimated to be low.
Limitations
This study has limitations. First, our study could not include information on the status of human-to-human contact. Assuming that KD may be triggered by agents that can be transmitted from human to human, such information would have contributed to the identification of KD pathogenesis. Second, all patients in the present study were diagnosed based on the Japanese diagnostic guidelines for KD, which were revised in 2019.37 Some of these minor revisions for principal signs and coronary artery assessments could have affected the results. Third, although standard mitigation measures, such as mask wearing and social distancing, were mandated nationwide in Japan throughout 2020, there might have been some regional variation in adherence to these mitigation practices during the study period, which might have affected the results. Fourth, because this study was based on patients in Japan only, the conclusions may not apply to populations outside Japan.
Conclusions
Compared with 2019, the number of patients diagnosed with KD in 2020 decreased by approximately 35% across Japan, with no indication of parents delaying or avoiding visiting a hospital in the setting of the global COVID-19 pandemic. We found differences in KD incidence reduction patterns between patients younger than 12 months and those 12 months or older. These findings suggest a potential KD pathogenesis involving transmission among children via human-to-human contact, especially among older children.
eFigure 1. Age Distribution in 2019 vs 2020
eFigure 2. Monthly Percentage Reductions in No. of Patients in 2020 According to 3 Age Groups
eFigure 3. Monthly Percentage Reductions in No. of Patients in 2020 According to 7 Age Groups
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222. [PubMed] [Google Scholar]
- 2.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54(3):271-276. doi: 10.1542/peds.54.3.271 [DOI] [PubMed] [Google Scholar]
- 3.Ae R, Makino N, Kosami K, Kuwabara M, Matsubara Y, Nakamura Y. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the nationwide survey in Japan, 2017-2018. J Pediatr. 2020;225:23-29.e2. doi: 10.1016/j.jpeds.2020.05.034 [DOI] [PubMed] [Google Scholar]
- 4.Tulloh RMR, Mayon-White R, Harnden A, et al. Kawasaki disease: a prospective population survey in the UK and Ireland from 2013 to 2015. Arch Dis Child. 2019;104(7):640-646. doi: 10.1136/archdischild-2018-315087 [DOI] [PubMed] [Google Scholar]
- 5.Lin MT, Wu MH. The global epidemiology of Kawasaki disease: review and future perspectives. Glob Cardiol Sci Pract. 2017;2017(3):e201720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100(11):1084-1088. doi: 10.1136/archdischild-2014-307536 [DOI] [PubMed] [Google Scholar]
- 7.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79-85. doi: 10.2188/jea.JE20110131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holman RC, Christensen KY, Belay ED, et al. Racial/ethnic differences in the incidence of Kawasaki syndrome among children in Hawaii. Hawaii Med J. 2010;69(8):194-197. [PMC free article] [PubMed] [Google Scholar]
- 9.Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112(3 Pt 1):495-501. doi: 10.1542/peds.112.3.495 [DOI] [PubMed] [Google Scholar]
- 10.McCrindle BW, Rowley AH. Improving coronary artery outcomes for children with Kawasaki disease. Lancet. 2019;393(10176):1077-1078. doi: 10.1016/S0140-6736(18)33133-7 [DOI] [PubMed] [Google Scholar]
- 11.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927-e999. doi: 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 12.Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67(14):1738-1749. doi: 10.1016/j.jacc.2015.12.073 [DOI] [PubMed] [Google Scholar]
- 13.Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544. doi: 10.1016/S0140-6736(04)16814-1 [DOI] [PubMed] [Google Scholar]
- 14.Rowley AH, Shulman ST. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374. doi: 10.3389/fped.2018.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley AH. Is Kawasaki disease an infectious disorder? Int J Rheum Dis. 2018;21(1):20-25. doi: 10.1111/1756-185X.13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010;8(2):197-203. doi: 10.1586/eri.09.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kido S, Ae R, Kosami K, et al. Seasonality of i.v. immunoglobulin responsiveness in Kawasaki disease. Pediatr Int. 2019;61(6):539-543. doi: 10.1111/ped.13863 [DOI] [PubMed] [Google Scholar]
- 18.Ozeki Y, Yamada F, Saito A, et al. Epidemiologic features of Kawasaki disease distinguished by seasonal variation: an age-specific analysis. Ann Epidemiol. 2018;28(11):796-800. doi: 10.1016/j.annepidem.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 19.Ozeki Y, Yamada F, Kishimoto T, Yashiro M, Nakamura Y. Epidemiologic features of Kawasaki disease: winter versus summer. Pediatr Int. 2017;59(7):821-825. doi: 10.1111/ped.13293 [DOI] [PubMed] [Google Scholar]
- 20.Kim GB, Park S, Eun LY, et al. Epidemiology and clinical features of Kawasaki disease in South Korea, 2012-2014. Pediatr Infect Dis J. 2017;36(5):482-485. doi: 10.1097/INF.0000000000001474 [DOI] [PubMed] [Google Scholar]
- 21.Du ZD, Zhao D, Du J, et al. ; Beijing Kawasaki Research Group . Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr Infect Dis J. 2007;26(5):449-451. doi: 10.1097/01.inf.0000261196.79223.18 [DOI] [PubMed] [Google Scholar]
- 22.Burns JC, Cayan DR, Tong G, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16(2):220-225. doi: 10.1097/01.ede.0000152901.06689.d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano T, Makino N, Aoyama Y, et al. Temporal and geographical clustering of Kawasaki disease in Japan: 2007-2012. Pediatr Int. 2016;58(11):1140-1145. doi: 10.1111/ped.12970 [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi S, Yanagawa H, Kawasaki T, Yanase Y. An outbreak of Kawasaki disease in Miyako Island in Okinawa prefecture. Pediatr Int (Roma). 1983;25(4):436-437. doi: 10.1111/j.1442-200X.1983.tb01741.x [DOI] [Google Scholar]
- 25.Banday AZ, Bhattacharya D, Pandiarajan V, Singh S. Kawasaki disease in siblings in close temporal proximity to each other—what are the implications? Clin Rheumatol. 2021;40(3):849-855. doi: 10.1007/s10067-020-05328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita Y, Nakamura Y, Sakata K, et al. Kawasaki disease in families. Pediatrics. 1989;84(4):666-669. doi: 10.1542/peds.84.4.666 [DOI] [PubMed] [Google Scholar]
- 27.Hayashida K, Ae R, Masuda H, et al. Clinical characteristics of patients with Kawasaki disease whose siblings had the same disease. Pediatr Infect Dis J. 2021;40(6):531-536. doi: 10.1097/INF.0000000000003074 [DOI] [PubMed] [Google Scholar]
- 28.Ae R, Shibata Y, Kosami K, Nakamura Y, Hamada H. Kawasaki disease and pediatric infectious diseases during the coronavirus disease 2019 pandemic. J Pediatr. 2021;239:50-58.e2. doi: 10.1016/j.jpeds.2021.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shulman ST, Rowley AH. An unintended consequence of pandemic control measures: fewer cases of Kawasaki disease. J Pediatr. 2021;239:11-14. doi: 10.1016/j.jpeds.2021.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shulman S, Geevarghese B, Kim KY, Rowley A. The impact of social distancing for COVID-19 upon diagnosis of Kawasaki disease. J Pediatric Infect Dis Soc. 2021;10(6):742-744. doi: 10.1093/jpids/piab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang JM, Kim YE, Huh K, et al. Reduction in Kawasaki disease after nonpharmaceutical interventions in the COVID-19 era: a nationwide observational study in Korea. Circulation. 2021;143(25):2508-2510. doi: 10.1161/CIRCULATIONAHA.121.054785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175(2):176-184. doi: 10.1001/jamapediatrics.2020.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makino N, Nakamura Y, Yashiro M, et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015-2016. Pediatr Int. 2019;61(4):397-403. doi: 10.1111/ped.13809 [DOI] [PubMed] [Google Scholar]
- 34.Makino N, Nakamura Y, Yashiro M, et al. Epidemiological observations of Kawasaki disease in Japan, 2013-2014. Pediatr Int. 2018;60(6):581-587. doi: 10.1111/ped.13544 [DOI] [PubMed] [Google Scholar]
- 35.Makino N, Nakamura Y, Yashiro M, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239-245. doi: 10.2188/jea.JE20140089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura Y, Yashiro M, Uehara R, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol. 2012;22(3):216-221. doi: 10.2188/jea.JE20110126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi T, Ayusawa M, Suzuki H, et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr Int. 2020;62(10):1135-1138. doi: 10.1111/ped.14326 [DOI] [PubMed] [Google Scholar]
- 38.Fukazawa R, Kobayashi J, Ayusawa M, et al. ; Japanese Circulation Society Joint Working Group . JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J. 2020;84(8):1348-1407. doi: 10.1253/circj.CJ-19-1094 [DOI] [PubMed] [Google Scholar]
- 39.McCrindle BW, Li JS, Minich LL, et al. ; Pediatric Heart Network Investigators . Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174-179. doi: 10.1161/CIRCULATIONAHA.107.690875 [DOI] [PubMed] [Google Scholar]
- 40.Tan TH, Wong KY, Cheng TK, Heng JT. Coronary normograms and the coronary-aorta index: objective determinants of coronary artery dilatation. Pediatr Cardiol. 2003;24(4):328-335. doi: 10.1007/s00246-002-0300-7 [DOI] [PubMed] [Google Scholar]
- 41.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133(2):254-258. doi: 10.1016/S0022-3476(98)70229-X [DOI] [PubMed] [Google Scholar]
- 42.e-Stat Portal Site of the Government Statistics of Japan . Statistics of Japan. Accessed March 1, 2022. https://www.e-stat.go.jp/en
- 43.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ae R, Shibata Y, Furuno T, Sasahara T, Nakamura Y, Hamada H. Human mobility and droplet-transmissible pediatric infectious diseases during the COVID-19 pandemic. Int J Environ Res Public Health. 2022;19(11):6941. doi: 10.3390/ijerph19116941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. doi: 10.1542/peds.2020-006460 [DOI] [PubMed] [Google Scholar]
- 46.Rowley AH. Multisystem inflammatory syndrome in children and Kawasaki disease: two different illnesses with overlapping clinical features. J Pediatr. 2020;224:129-132. doi: 10.1016/j.jpeds.2020.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowley AH, Baker SC, Arrollo D, et al. A protein epitope targeted by the antibody response to Kawasaki disease. J Infect Dis. 2020;222(1):158-168. doi: 10.1093/infdis/jiaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabinet Office, Government of Japan . Annual report on the declining birthrate. White paper. 2021. Accessed March 1, 2022. https://www8.cao.go.jp/shoushi/shoushika/whitepaper/
- 49.Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45-54.e1. doi: 10.1016/j.jpeds.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abrams JY, Oster ME, Godfred-Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323-331. doi: 10.1016/S2352-4642(21)00050-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US Children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175(8):837-845. doi: 10.1001/jamapediatrics.2021.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Payne AB, Gilani Z, Godfred-Cato S, et al. ; MIS-C Incidence Authorship Group . Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4(6):e2116420. doi: 10.1001/jamanetworkopen.2021.16420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura Y; Kawasaki Disease Surveillance Team in Japan . No relationship was observed between Kawasaki disease and COVID-19 in Japan. Pediatr Int. 2021;63(8):977. doi: 10.1111/ped.14515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Age Distribution in 2019 vs 2020
eFigure 2. Monthly Percentage Reductions in No. of Patients in 2020 According to 3 Age Groups
eFigure 3. Monthly Percentage Reductions in No. of Patients in 2020 According to 7 Age Groups