Abstract
A gastrointestinal explant culture system was developed and compared to the mononuclear cell extraction and enzyme-linked immunospot assay method for measurement of immunoglobulin A (IgA) and IgG antibody-secreting cells (ASCs) in gastric antral and duodenal biopsies of non-Helicobacter pylori-infected volunteers. IgA and IgG were detected in explant supernatants during 6 to 7 days of culture in all subjects. IgA containing secretory component was also detected throughout the culture period, although peak production occurred only in the first 3 days. During 7 days of culture, the cumulative geometric mean IgA levels produced were 2.2 and 8.02 μg/ml/10 mg of antral and duodenal biopsy tissues, respectively, while the cumulative geometric mean IgG levels were 1.54 and 2.92 μg/ml/10 mg of antral and duodenal biopsy tissues, respectively. Cycloheximide treatment resulted in a >90% reduction in both immunoglobulin classes after 6 days of treatment compared to levels in untreated controls. The detection of IgA and IgG ASCs extracted from biopsies on days 1 and 6 of culture confirmed that the antibody detected was derived from mucosal lamina propria. The IgA and IgG ASC responses were positively correlated with antibody concentrations detected in culture supernatants (r = 0.87 and 0.85, respectively). These results validate the potential usefulness of our gastrointestinal explant system for the evaluation of mucosal effector B-cell function.
Studies of mucosal immunity in general and of gastrointestinal immunity in particular have been hampered by difficulties in obtaining accessible and representative samples of mucosal effector function, specifically at the cellular level. Detection of antibody-secreting cells (ASCs) from peripheral blood and detection of immunoglobulin A (IgA) from intestinal secretions such as jejunal fluids or stool give only limited information about gastrointestinal-tract immunity following vaccination or disease. While measurement of specific ASC responses (cells that represent antigen-stimulated immunoblasts migrating from mucosal lymphoid follicles into the systemic circulation) can accurately reflect mucosal priming, these responses may be absent or suppressed during secondary immunologic exposures (5, 8). Specific IgA antibody analysis from secretions may be affected by local antibody degradation from intestinal proteases and sialidases (2, 6). In addition, neither method gives information about where in the gastrointestinal tract the specific antibody response is occurring. This deficiency has specific relevance for the development of vaccines targeting specific mucosal sites in the gastrointestinal tract.
Recently, assays that measure mucosal effector B-cell function at the cellular level in humans, using lymphocyte extraction of intestinal biopsy samples adapted to B-cell-based enzyme-linked immunospot assays (ELISPOTs), have been described (10, 13, 14). While these methods allow for the analysis of mucosal B cell function at the single-cell level, they are somewhat limited to specialized laboratories since they require a large number of biopsies and complicated tissue disruption and cell extraction techniques to produce the cell yields needed to measure specific immune responses. In addition, cell extraction inevitably leads to cell loss, which may significantly affect the accuracy of the immune responses detected. These considerations have prompted us to examine the use of an explant culture system of gastrointestinal biopsies as an alternative method for studying mucosal B-cell function. An explant system has the potential to be a more efficient and easy way to measure in situ gastrointestinal antibody production than lymphocyte extraction and ELISPOT methods. Since the whole biopsy sample is used as is and requires no special processing, the likelihood of contamination or poor cell yields is decreased. In addition, because the mucosal microenvironment is left intact, the cytokines and the accessory cells needed to produce antibody responses are present (15). Whole-explant culture systems have had limited development for the evaluation of human mucosal-tissue immune responses (3, 16, 17). We present the validation of a gastrointestinal explant system for the measurement of mucosal antibody in humans and compare it to the mucosal-tissue cell extraction B-cell ELISPOT technique.
MATERIALS AND METHODS
Subjects.
This study was approved by the Institutional Review Board of the University of Maryland. Ten healthy volunteers (8 males and 2 females, aged 18 to 43 years, with a mean age of 28 years) who had no history of ulcers or current gastrointestinal illnesses and who were found to be seronegative for Helicobacter pylori by enzyme-linked immunoassay (Wampole Laboratories, Cranbury, N.J.) participated in the study. Written informed consent was obtained after each volunteer passed a written test of understanding of the research procedures. H. pylori-seronegative status was confirmed by the rapid urease test of a gastric antral biopsy at the time of endoscopy (Clo-test; Delta West Ltd., Perth, Australia). In addition, histologic evaluation of a gastric and duodenal biopsy sample showed no evidence of inflammation or disease.
Collection of specimens.
Antral and duodenal biopsy samples were collected at the Endoscopy Suite in the Division of Gastroenterology at the University of Maryland Medical System from volunteers who fasted overnight. Volunteers were sedated with midazolam and meperidine during the procedure, and they received a local anesthetic to facilitate the passing of the video endoscope. After visual examination of the esophagus, stomach, and duodenum, 6 to 12 pinch biopsy samples were obtained from both the antrum and the duodenum of each volunteer. Each sample, which consisted of epithelium and lamina propria, was 1 to 2 mm in diameter and weighed 6.3 to 19.5 mg (mean, 13.5 mg).
Explant culture system.
Our culture system for the measurement of gastrointestinal antibody was adapted from published methods for the culture of mouse lymph nodes and for the evaluation of lymphocyte markers in human tonsillar tissue (3, 7). Biopsy fragments were washed twice in culture medium, which consisted of cold RPMI 1640 containing 10 mM HEPES supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), streptomycin, (100 μg/ml), and gentamicin (100 μg/ml). Each biopsy fragment was floated on a 7- to 10-mm gel foam slab (The Upjohn Co., Kalamazoo, Mich.), covered with a 0.45-μm-pore-size sterile membrane (Gelman Sciences, Ann Arbor, Mich.), and placed in an individual tissue culture well of a sterile six-well plate (Nunc; VWR Scientific) containing 5 ml of culture medium. Fragments were cultured for 1, 2, 3, 5 or 6, and 7 days under constant agitation (65 rpm) at 37°C in an atmosphere of 95% O2–5% CO2. At each time point, 3 ml of supernatant was removed for immunologic analyses and stored at −70°C, and the culture medium was replaced. Specimens were run in duplicate to compare variability of responses for each set of experiments.
Immunologic analysis of explant supernatants.
Culture supernatants from each time point for each specimen were assayed for total IgA by using previously described methods with affinity-purified human colostrum IgA (Sigma Chemical, St. Louis, Mo.) as the standard (4). In addition, secretory IgA (SIgA) measurements were performed by using a modification of the IgA detection assay in which a mouse monoclonal antibody against secretory component (Nordic Immunological Laboratories, San Clemente, Calif.) was used as a detector antibody. A purified SIgA human immunoglobulin preparation of known concentration (Nordic) was used as a positive control in this assay to quantitate SIgA. A low-level IgG antibody assay was adapted from our IgA assay, by using a chicken yolk anti-IgG (Kirkegaard and Perry, Gaithersburg, Md.) antibody as a capture antibody and a goat anti-human IgG alkaline phosphatase-labeled antibody (Kirkegaard and Perry) as a detector antibody. A standard curve was established with affinity-purified serum IgG (Accurate Chemical, Westbury, N.Y.) as the standard, with a sensitivity of detection of 5 ng/well.
Inhibition of protein synthesis in the explant culture system.
Biopsy fragments from each anatomic site were used to confirm that antibody was being actively produced in our explant system. At 24 h of culture, 3 ml of supernatant was removed from each well and cycloheximide was added to each well to a final concentration of 25 μg/ml in 5 ml of culture medium. Removal of supernatant then proceeded for the control tissues, except that a cycloheximide concentration of 25 μg/ml was maintained in the cycloheximide-treated wells. IgA and IgG were assayed in these supernatants.
Lymphocyte extraction of biopsy samples for ASC assay.
Cellular extractions were performed on explant specimens cultured for 1 and 6 days in order to evaluate cell viability and on day 1 cultures to compare cell activity with IgA and IgG recovered in culture supernatants. All specimens were run in duplicate. Gastric and intestinal biopsy fragments were dispersed in 5 ml of cold RPMI 1640 with 1% l-glutamine, 10% heat-inactivated fetal bovine serum, 40 μg of gentamicin per ml, and 0.01% soybean trypsin inhibitor by vigorous pipetting with siliconized sterile pipettes and gentle teasing of the tissue with 1-ml sterile tuberculin syringes. Supernatants were put in siliconized glass tubes on ice. Tissues were further digested by using an extraction buffer consisting of RPMI 1640, 10% heat-inactivated fetal bovine serum, 40 μg of gentamicin per ml, 0.01% trypsin soybean inhibitor, and 40 IU of collagenase (#C2139; Sigma) per ml. This buffer was constantly shaken at 37°C for 1 h at 189 cycles/min. After incubation, supernatants from the first two procedures were pooled, fresh extraction buffer was added, and the mixture was shaken at 37°C for an additional hour. Afterwards, all supernatants were combined and debris was removed by centrifugation on a Ficoll-Hypaque gradient. Cells were then washed with 10 ml Hanks balanced salt solution at 1,000 rpm for 10 min. Viable and total mononuclear cells (MNCs) were then counted in a cell chamber by trypan blue exclusion. Total IgA and IgG cells were enumerated by an ELISPOT assay as previously described, except that 10-fold dilutions of cell suspensions were counted, starting at 104 MNCs/well (8). Each well was run in duplicate.
The effect of this extraction procedure on the ability to accurately detect specific ASCs was initially verified by using peripheral blood MNC and ASC responses to lipopolysaccharide in volunteers participating in a typhoid vaccine study. During treatment with collagenase, there were insignificant declines (0 to 10%) in IgA ASC responses to lipopolysaccharide in peripheral blood MNCs treated with this extraction procedure compared to those not so treated.
Data analysis.
Antibody levels for duplicate specimens from each site and subject were calculated as geometric mean concentrations (in micrograms per milliliter) of total IgA or IgG; IgA- and IgG-bearing B cells were calculated as geometric mean number of specific cells per 106 MNCs. All values were standardized to 10 mg of tissue weight. For antibody quantitation and kinetics analysis, data were represented as cumulative mean total antibody produced in culture. This was calculated by determining the geometric mean amount (in micrograms) of total IgA or IgG antibody present in 5-ml culture supernatants of duplicate biopsy specimens (taken from 10 subjects at each time point), subtracting the total amount of antibody remaining from the previous time point (antibody that was present in culture and measured but was not newly made), and adding the result to the previous total value. To evaluate the variability of responses in duplicate specimens from each anatomic site at each time point for each subject, the geometric mean difference in levels of antibody detected between samples and the standard deviation were determined. To evaluate the variability in extracted IgA and IgG cell numbers in duplicate specimens, the geometric mean fold differences between specimens ([maximum value − minimum value]/geometric mean) and the standard deviations were calculated. Comparisons of site-specific antibody levels or cell numbers were analyzed by one- or two-tailed t tests as appropriate. Correlation coefficients were determined for geometric mean IgA and IgG levels detected in duplicate specimen culture supernatants and their corresponding geometric mean viable IgA and IgG B-cell counts from extracted biopsy samples.
RESULTS
Quantitation and kinetics of IgA and IgG in explant supernatants.
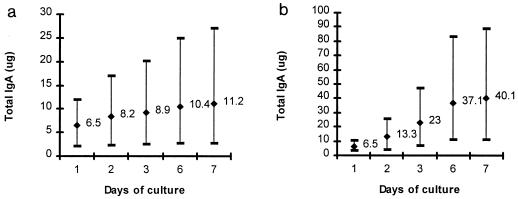
The kinetics and amounts of IgA antibody produced in gastric antrum and duodenum explant supernatants are presented in Fig. 1. IgA was detected in gastric and duodenal supernatants throughout the 7-day culture period in all subjects. Although IgA levels in gastric and duodenal cultures varied from subject to subject, maximal amounts of IgA were produced in the first 2 days of culture for antral tissues and in days 3 and 6 for duodenal cultures in all subjects. After 7 days of culture, four times as much IgA was produced in duodenal tissue cultures as in antral cultures. The geometric mean amount of IgA produced in antral cultures each day was 0.7 μg/ml per 10 mg of tissue (range, 0.16 to 1.6 μg/ml/10 mg of tissue) and for duodenal cultures was 2.4 μg/ml per 10 mg of tissue (range, 0.7 to 5.2 μg/ml/10 mg of tissue) (P = 0.001, two-tailed t test).
FIG. 1.
Cumulative amount of IgA detected in antral (a) and duodenal (b) explant supernatants from 10 healthy North American volunteers over 7 days of culture. Data are represented as cumulative geometric mean (GM) total antibody levels (in micrograms per 10 mg of tissue) and range produced in culture at each time point tested.
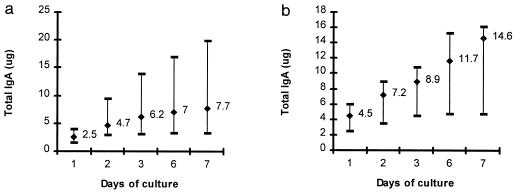
The kinetics and amounts of IgG antibody detected in gastric antrum and duodenum explant supernatants are presented in Fig. 2. IgG was detected in all subjects in antral and duodenal cultures for each day of culture. Although levels of IgG increased in all subjects for the first 6 days of culture, maximal amounts of IgG were excreted in the first 2 days of culture. The geometric mean amount of IgG produced in antral cultures each day was 0.4 μg/ml/10 mg of tissue (range, 0.2 to 1 μg/ml/10 mg of tissue) and in duodenal cultures was 0.9 μg/ml/10 mg of tissue (range, 0.3 to 1.4 μg/ml/10 mg of tissue) (P = 0.09, two-tailed t test).
FIG. 2.
Cumulative amount of IgG detected in antral (a) and duodenal (b) explant supernatants from 10 healthy North American volunteers over 7 days of culture. Data are cumulative geometric mean (GM) total antibody levels (in micrograms per 10 mg of tissue) and range produced in culture at each time point tested.
The levels of IgA and IgG detected in duplicate biopsy samples taken from the same anatomic site for the first 3 days of culture were analyzed for specimen variability (Table 1). The levels of IgA and IgG in samples taken from the antrum and duodenum were relatively consistent over the first 3 days of culture, with geometric mean differences ranging from 0.27 to 0.4 μg/ml on day 1 of culture to 0.08 to 0.27 μg/ml on day 3. Although the differences in IgG antibody levels between samples were somewhat higher than in those of IgA, they were not significant (P > 0.05, two-tailed t test). These variations in antibody levels represent 0.26 (±0.07)- and 0.33 (±0.21)-fold differences in the levels of IgA and IgG antibody between samples, respectively, over all 3 days of culture.
TABLE 1.
Variability in IgA and IgG concentrations in 10 healthy subjects
| Site and antibody | Difference in antibody level
(μg/ml/10 mg of tissue) on culture daya
|
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Antrum | |||
| IgA | 0.27 (0.08) | 0.12 (0.1) | 0.08 (0.14) |
| IgG | 0.38 (0.3) | 0.13 (0.2) | 0.1 (0.15) |
| Duodenum | |||
| IgA | 0.33 (0.17) | 0.23 (0.15) | 0.23 (0.24) |
| IgG | 0.4 (0.36) | 0.27 (0.1) | 0.27 (0.09) |
Duplicate specimens from the antrum and duodenum were cultured, and supernatants were tested on days 1, 2, and 3. Values are geometric means (standard deviations are in parentheses).
SIgA determinations.
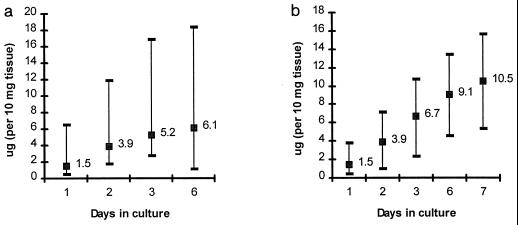
SIgA determinations were performed on antral and duodenal specimens from five subjects; the results are presented in Fig. 3. Although SIgA production occurred throughout the entire culture period in all subjects, maximal SIgA levels were detected in the first 2 to 3 days in both antral and duodenal explant cultures. Although levels of SIgA detected in duodenal explant cultures were somewhat higher than that seen in antral cultures, differences were not significant (P = 0.2, two-tailed t test). At peak excretion, SIgA represented 70 and 62% of the total IgA detected in the supernatants of antral and duodenal cultures, respectively.
FIG. 3.
Cumulative amount of SIgA produced in antral (a) and duodenal (b) explant culture supernatants over 7 days of culture. Data are cumulative geometric mean (GM) total antibody levels (in micrograms per 10 mg of tissue) and range produced in culture at each time point tested.
Effect of protein synthesis inhibition.
Results of cycloheximide treatment to inhibit IgA and IgG production in antral and duodenal explant cultures are seen in Table 2. There was a decrease in the amounts of IgA and IgG detected in antral and duodenal explant cultures within 2 days of cycloheximide treatment compared to levels in untreated control cultures. At 6 days of culture, there was a 78 to 98% reduction of IgG and IgA detected in all treated explant cultures compared to levels in untreated cultures (P < 0.05, one-tailed t test).
TABLE 2.
Effect of cycloheximide treatment of explant cultures on recovery of IgA and IgG in explant supernatants
| Tissue site | Antibody level with
cycloheximide/level without cycloheximide [%
inhibition]a
|
|||
|---|---|---|---|---|
| IgA
|
IgG
|
|||
| Day 3 | Day 6 | Day 3 | Day 6 | |
| Antrum | 0.4 (0.12)/0.9 (0.4) [60] | 0.05 (0.05)/0.65 (0.1) [92]b | 0.12 (0.3)/0.34 (0.2) [66] | 0.09 (0.03)/0.4 (0.04) [78]b |
| Duodenum | 0.16 (0.1)/1.2 (0.2) [87]b | 0.02 (0.05)/0.94 (0.08) [98]b | 0.2 (0.2)/0.52 (0.2) [62] | 0.03 (0.02)/0.6 (0.09) [95]b |
Values are geometric means (standard deviations are in parentheses).
Significant difference between levels of antibody recovered from supernatants following cycloheximide treatment and those obtained from nontreated control explants (P < 0.05, one-tailed t test).
Extraction of ASCs from gastrointestinal biopsies.
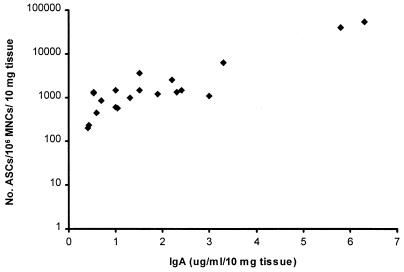
The cell viability, the numbers of IgA- and IgG-bearing lymphocytes recovered from antral and duodenal explants after 1 and 6 days of culture, and the geometric mean differences in viable-cell numbers between duplicate samples from day 1 extractions are shown in Table 3. Although the range of IgA and IgG cells extracted from subjects was broad, the variation in viable-cell numbers between all duplicate samples was less than a onefold difference (based on the geometric mean plus 2 standard deviations). All samples had over 80% cell viability after 24 h of culture. Cell death increased by day 6 of culture in both antral and duodenal explants. Similar numbers of IgA and IgG MNCs were recovered after days 1 and 6 of culture of duodenal explants. However, there were fewer IgA and IgG ASCs recovered on day 6 than on day 1 of culture of antral biopsies (P = 0.02 and 0.002, respectively, two-tailed t test). There was an association between IgA ASC numbers and the amount of antibody detected in explant supernatants; higher concentrations of antibody were detected in explants that had higher yields of viable ASCs (r = 0.87) (Fig. 4). A similar correlation was found for IgG ASC numbers and antibody concentrations (r = 0.85) (data not shown).
TABLE 3.
Characteristics of ASCs recovered from explant tissuea
| Site and culture day | % MNC viability | No. of IgA ASCsb | Variation in IgA cell no.c | No. of IgG ASCsb | Variation in IgG cell no.c |
|---|---|---|---|---|---|
| Antrum | |||||
| 1 | 82 | 572 (70–2,612) | 0.25 (0.25) | 162 (26–1,323) | 0.3 (0.21) |
| 6 | 68 | 142 (35–999) | ND | 82 (10–683) | ND |
| Duodenum | |||||
| 1 | 84 | 2,188 (514–21,700) | 0.38 (0.3) | 242 (18–1,179) | 0.2 (0.2) |
| 6 | 76 | 2,787 (304–14,853) | ND | 199 (90–566) | ND |
Values are geometric means.
Number of cells per 106 viable MNCs per 10 mg of tissue. Values in parentheses are ranges.
Fold difference in cell number between duplicates. Values in parentheses are standard deviations.
FIG. 4.
Association of IgA ASCs and IgA recovered in antral and duodenal explant supernatants after 1 day of culture (r = 0.87).
DISCUSSION
These studies were initiated to evaluate the feasibility of maintaining gastric and intestinal biopsy samples in tissue culture and to compare this technique with lymphocyte extraction methods for the measurement of mucosal B-cell antibody. We have shown that both IgA and IgG antibodies can be detected in our gastrointestinal explant system during the first week of culture, verifying in humans the findings from the early 1970s that described IgA levels in rabbit small intestinal organ cultures (4). IgA and IgG levels increased maximally over the first 72 h of culture in all samples, with lower rates of production at later culture time points, suggesting that active production was occurring in culture. The almost complete inhibition of de novo IgA and IgG production and secretion by cycloheximide compared to levels in untreated cultures also provides proof that the antibody measured in supernatants during culture was produced actively rather than as the result of antibody released by injured or dying cells.
Additional verification of active antibody production during culture was confirmed by the recovery of secretory component attached to IgA in culture supernatants with levels increasing over culture duration. Although 60 to 70% of the IgA recovered in culture supernatants was attached to secretory component, this was below our initial expectations. It has been shown that 90% of gastrointestinal IgA antibody is in dimeric form and that such IgA is associated with secretory component during epithelial transport to the luminal surface of the gastrointestinal tract (11, 12). Our data suggest that there is surface epithelial cell loss or derangement in function during culture. Several biopsy samples that we examined histologically showed a progressive loss and a flattening of epithelium over several days of culture. Epithelial cell loss has been described for human gastric explant systems developed for metabolic rather than for antibody investigations in gastric carcinoma (17). Surface epithelial cell loss is not an impediment to the detection of lamina B-cell antibody activity, since we were able to detect both IgG and IgA that were actively synthesized during culture. However, a system such as ours could not be used for measurement of epithelial cell activity in gastrointestinal biopsy samples.
Our detection of IgA- and IgG-secreting B lymphocytes in biopsy tissue offers confirmatory evidence that the antibody detected in culture supernatants was due to mucosally derived B cells rather than to serum transudation. Although the levels of IgA and IgG detected in culture supernatants were highly correlated with the number of specific-immunoglobulin-bearing lymphocytes detected by ELISPOT, there was quite a broad range of secreting-cell numbers (from 2 to 3 logs) associated with relatively low levels of total antibody (<1 μg/ml/10 mg of tissue) detected in culture supernatants. It is unclear why this is, but it may reflect differences in the antibody-producing activity of B cells present in mucosal lamina propria. It has been shown in cholera protection experiments in mice, for example, that specific-IgA-producing cells may produce quite different amounts of specific IgA antibody when multiple intestinal sites are examined (9). In these mouse studies, differences in the relative amounts of specific antibody detected were influenced by where antigen stimulation occurred. These differences in local antibody production directly influenced protection in the mouse cholera challenge model. These data plus ours suggest that quantifying the amount of specific antibody produced, instead of or in conjunction with counting the number of specific ASCs extracted during mucosal biopsies, may be critical in the evaluation of the immunogenicity of mucosally administered vaccines.
The majority of our samples (80%) had >1 μg of total IgA per ml per day produced in culture supernatants. At this level of antibody production coupled with the known limits of detection of antibody by our enzyme-linked immunosorbent assay (3 to 5 ng/ml), the sensitivity of detection of specific antibody by using pooled culture supernatants from 6 to 7 days of culture should achieve a level of 0.01% of the total antibody content. The lowest level of IgA antibody produced daily in our cultures was 0.4 μg/ml (seen in antral cultures), representing only 10% of the total samples tested. Even at this level of production, similarly high levels of sensitivity of detection of specific antibody could be attained by a simple concentration of pooled tissue culture supernatants. Current work is aimed at application of this system to specific antibody measurements.
The immunocyte profile of IgA and IgG in gastric antral and duodenal explant cultures revealed, not surprisingly, that IgA cells predominate and, likewise, that IgA is preferentially present in culture supernatants. Interestingly, although the numbers of IgA ASCs in gastric and duodenal biopsies were 4 to 10 times higher and 10 to 20 times higher, respectively, than the number of IgG ASCs, IgA levels in culture supernatants were, overall, only 1 to 2 times higher and 4 times higher than IgG levels detected in gastric and duodenal cultures, respectively. The ASC data are in agreement with other published reports (1, 13). In contrast, the levels of IgG detected in antral culture supernatants were higher than one would expect from the numbers of ASCs extracted. The consistency of this finding will have to be verified. If true, it would suggest that IgG antibody production may be upregulated in gastric mucosa. Whether IgG, which is more easily subjected to enzymatic degradation in the gastrointestinal lumen, has any mucosal function at the gastrointestinal submucosal or mucous layer remains to be determined.
In summary, we report the validation of the use of a gastrointestinal explant culture system for the measurement of mucosally derived IgA and IgG antibody. Future studies are ongoing to evaluate its use in measuring specific immune responses in vaccine studies and clinical infections.
ACKNOWLEDGMENTS
This work was supported by Research Contract NO1-AI-65299 from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We acknowledge the clinical support provided by Karen Kotloff and Kathy Palmer in recruiting some of the volunteers participating in this study.
REFERENCES
- 1.Brandzaeg P, Kett K, Rognum T O, Soderstrom J, Bjorkander J, Soderstrom T, Petrusson B, Hanson L A. Distribution of mucosal IgA and IgG subclass-producing immunocytes and alterations in various disorders. Monogr Allergy. 1986;20:179–201. [PubMed] [Google Scholar]
- 2.Cauci S, Monte R, Driussi S, Lanzafame P, Quadrifoglio F. Impairment of the mucosal immune system: IgA and IgM cleavage detected in vaginal washings of a subgroup of patients with bacterial vaginosis. J Infect Dis. 1998;178:1698–1706. doi: 10.1086/314505. [DOI] [PubMed] [Google Scholar]
- 3.Ferro L M, Weedon H M, Flego L R, Beroukas D, Zola H. An organ fragment culture model to study lymphocyte activation in human lymphoid tissue. Immunobiology. 1993;188:51–61. doi: 10.1016/S0171-2985(11)80486-9. [DOI] [PubMed] [Google Scholar]
- 4.Kagnoff M F, Donaldson R M, Trier J S. Organ culture of rabbit small intestine: prolonged in vitro steady state protein synthesis and secretion and secretory IgA secretion. Gastroenterology. 1972;63:541–551. [PubMed] [Google Scholar]
- 5.Kantele A, Makela P H. Different profiles of the human immune response to primary and secondary immunization with an oral Salmonella typhi Ty21a vaccine. Vaccine. 1991;9:423–425. doi: 10.1016/0264-410x(91)90129-t. [DOI] [PubMed] [Google Scholar]
- 6.Kilian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan A C, Chow K-P N, George A, Weinstein P D, Cebra J J. Use of Peyer’s patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect Immun. 1991;59:1024–1031. doi: 10.1128/iai.59.3.1024-1031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losonsky G A, Tacket C O, Wasserman S S, Kaper J B, Levine M M. Secondary Vibrio cholerae-specific cellular antibody responses following wild-type homologous challenge in people vaccinated with CVD 103-HgR live oral cholera vaccine: changes with time and lack of correlation with protection. Infect Immun. 1993;61:729–733. doi: 10.1128/iai.61.2.729-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lycke N, Bromander A K, Holmgren J. Role of local IgA antitoxin-producing cells for intestinal protection against cholera toxin challenge. Int Arch Allergy Appl Immunol. 1989;88:273–279. doi: 10.1159/000234806. [DOI] [PubMed] [Google Scholar]
- 10.Lycke N, Kilander A, Nilsson L A, Tarkowski A, Werner N. Production of antibodies to gliadin in intestinal mucosa of patients with coeliac disease: a study at the single cell level. Gut. 1989;30:72–77. doi: 10.1136/gut.30.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mestecky J, Lue C, Russell M W. Selective transport of IgA: cellular and molecular aspects. Gastroenterol Clin N Am. 1991;20:441–471. [PubMed] [Google Scholar]
- 12.Moldoveanu Z, Egan M L, Mestecky J. Cellular origins of human polymeric and monomeric IgA: intracellular and secreted forms of IgA. J Immunol. 1984;133:3156–3160. [PubMed] [Google Scholar]
- 13.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson L A, Holmgren J, Czerkinsky C. Oral cholera vaccination induces strong intestinal antibody responses and interferon-γ production and evokes local immunoglobulin memory. J Clin Investig. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quiding-Järbrink M, Granström G, Nordström I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrader C E, Kerlin R L, George A, Cebra J J. Dendritic cells support the production of IgA and other non-IgM isotypes in clonal culture. Int Immunol. 1990;2:563–570. doi: 10.1093/intimm/2.6.563. [DOI] [PubMed] [Google Scholar]
- 16.Trier J S. Organ culture methods in the study of gastrointestinal mucosal function and development. N Engl J Med. 1976;295:150–155. doi: 10.1056/NEJM197607152950308. [DOI] [PubMed] [Google Scholar]
- 17.Wilson N W, McCartney J C. Organ culture of human gastric mucosa and gastric cancers. J Pathol. 1986;150:127–134. doi: 10.1002/path.1711500207. [DOI] [PubMed] [Google Scholar]