Introduction
The 2020 Nobel Prize in Medicine or Physiology was awarded to Drs. Harvey Alter, Michael Houghton and Charles Rice for their contributions to the discovery and characterization of the hepatitis C virus (HCV), a small, enveloped, positive-sense RNA virus belonging to the genus Hepacivirus within the Flaviviridae family. Chronic HCV infection is a leading cause of cirrhosis and hepatocellular carcinoma worldwide and an important contributor to global mortality.(1) Dr. Harvey Alter, a hematologist at the Clinical Center of the National Institutes of Health in Bethesda, Maryland, along with his long-term collaborator, Bob Purcell in NIAID, recognized that most cases of posttransfusion hepatitis were unrelated to hepatitis A virus (HAV) or hepatitis B virus (HBV)—hence the term non-A, non-B hepatitis (NANBH)—and showed the NANBH agent was transmissible experimentally. Dr. Michael Houghton, a microbiologist, working at Chiron, a biotechnology company in Emeryville, California, identified HCV as the causative agent of NANBH. Dr. Charles Rice, at Washington University School of Medicine, Saint Louis, Missouri, engineered a replication-competent laboratory strain of the virus that was used to prove that HCV alone was the cause of liver disease and to characterize its viral lifecycle.
The origin of Non-A, Non-B hepatitis
Although the first successful human blood transfusion occurred over two centuries ago, the procedure did not gain widespread acceptance until World War II. Shortly thereafter, cases of posttransfusion hepatitis began to be reported in the medical literature.(2) Following the discoveries of HBV in 1965, the discovery of HAV in 1973, and the switch from paid blood donors to an all-volunteer blood donor system, cases of posttransfusion hepatitis declined dramatically from 33% to 6%. Cases of posttransfusion hepatitis, however, continued to occur that were not caused by HAV or HBV, so-called NANBH.(3) During this period of emerging recognition of and revelations about NANBH, Dr. Alter became involved and conducted seminal natural history studies of posttransfusion hepatitis in patients undergoing open-heart surgery at the NIH Clinical Center. Instrumental to later success was the decision to define cases based on elevated serum alanine aminotransferase (ALT) levels, in recognition of the fact that not all patients with posttransfusion hepatitis presented with jaundice. This fortuitous decision, together with the meticulous collection of samples from both blood donors and recipients, allowed Dr. Alter to establish a carefully pedigreed series of posttransfusion NANBH that, later, would prove invaluable in confirming that HCV was the causative agent. Dr. Alter along with his collaborators used these samples to conduct pioneering chimpanzee inoculation studies to demonstrate that the likely causative agent was a blood borne transmissible agent.(4) Of importance, natural history studies conducted by Dr. Alter and others demonstrated that most cases of NANBH became chronic and that cirrhosis developed in a sizable proportion.(5)
Discovery of HCV
Dr. Michael Houghton joined the search for the causative agent of NANBH in 1982 after he was recruited to Chiron. His initial approach involved creation of a bacterial cDNA library derived from infected human and chimpanzee liver and plasma (based on the assumption that some clones were likely to contain nucleic acid of viral origin).(6) Under the premise of genetic relatedness to the NANBH agent, Dr. Houghton and his team screened the NANBH cDNA library with cDNA probes from flaviviruses, togaviruses, and hepadnaviruses. Despite their screening millions of clones, this approach proved unsuccessful. More conventional approaches to propagate the NANBH agent in culture systems and to visualize the virus by electron microscopy or to separate nucleic acid on electrophoretic gels were unsuccessful as well. A chance discussion with a colleague at Chiron eventually led to the method that did work. Rather than trying to identify the nucleic acid with a complementary nucleic acid probe, Dr. Houghton and his colleagues used a blind cDNA immunoscreening approach in which the protein product of the cloned nucleic acid was identified with an antibody probe. By using a high-infectivity-titer chimpanzee plasma pool obtained from Dr. Dan Bradley at the Centers for Disease Control and Prevention (CDC) and serum from a patient with active hepatitis (the assumption being that more active disease would be associated with higher antibody levels), the Chiron investigators identified a single, small clone of ~150 base pairs termed 5–1-1, which was shown to be derived from the HCV genome.(7) From this initial clone, Dr. Houghton’s team performed additional hybridization studies that extended the cDNA clone, resulting in identification of the remainder of the viral genome. Based on these advances, they developed an antibody test for the virus, which “broke the code” on Dr. Alter’s coded-sample panel and demonstrated a specific serologic relation to HCV infection in a majority of samples from patients with NANBH procured from different geographical regions.(8) The viral agent of NANBH had been found and was christened HCV.
Characterizing HCV
Following the identification of HCV, initial efforts to establish an in vitro culture model system in which clinical HCV isolates were used to infect cultured hepatocytes proved to be unsuccessful. Dr. Rice demonstrated that the explanation for such failed attempts was the absence in the initial cloned viral genome of a terminal 3’ sequence. This discovery enabled the development of an infectious clone that, while not capable of propagating in cell culture, could be introduced into chimpanzees to produce hepatitis, thereby formally proving HCV as a causative agent of hepatitis.
To circumvent the impediments to cell culture propagation of HCV, Dr. Rice’s laboratory, along with Dr. Ralf Bartenschlager’s laboratory, were the first to develop a subgenomic replicon consisting of the HCV non-structural proteins and an internal ribosome entry site (IRES) sequence that was able to replicate in culture, albeit at low level.(9) Replication was enhanced further through the introduction of a series of cell culture adaptive mutations and identification of a human hepatoma cell line (Huh-7.5) that was highly permissive for replication of subgenomic and full-length HCV RNAs.(10) Later, a viral isolate from a Japanese patient with fulminant hepatitis yielded high-level in vitro viral replication without the need for adaptive mutations and, more importantly, infectious virions in the culture media.(11) Together, the replicons and tissue culture infectious models paved the way for development of direct-acting antiviral agents. (12)
A cure at hand
In tandem with this ground-breaking virological work, efforts were ongoing to develop effective treatments for chronic HCV infection. Initially, interferon alpha (IFN) was tried but with limited success. Advancements in therapy were achieved by the addition of ribavirin to standard IFN and then later by the development of a long-acting pegylated form of IFN.(13) Deciphering the crystal structures of the non-structural proteins together with the availability of the replicon system and the infectious tissue culture strain allowed the rapid screening of a large number and variety of inhibitor compounds. These efforts culminated in the development of safe and effective combination all-oral therapy. Currently, virological cure of all HCV strains can be achieved in over 95% of patients with 8 to 12 weeks of treatment and with few side effects.(14)
It took only four decades from the first description of NANBH to the identification of HCV and the development of curative therapy. This achievement represents a remarkable triumph of biomedical science, and, fittingly, the three pioneers of this nascent field were awarded the 2020 Nobel Prize. Much unfinished work, however, remains. Only a fraction of the estimated 72 million persons with chronic HCV infection have been diagnosed and received curative therapy. The goal now is to identify and treat the remaining sizable cohort of infected persons. To this end, the World Health Organization has challenged the stewards of the world’s health systems to eliminate chronic HCV infection by 2030. Such an achievement will require creative models of health care delivery suitable to different geographical regions, likely in conjunction with the development of an HCV vaccine. The accomplishments of the three laureates provide a solid foundation and inspiration to spur efforts to finish the job of HCV elimination.
A Conversation with the Nobel Laureates
The journey from discovery to cure involved many researchers and was chronicled by countless research presentations and workshops over the years, many of them held at the annual meetings of the American Association for the Study of Liver Diseases (AASLD). Building on this history, the Association hosted a unique forum at the 2020 Liver Meeting Digital Experience, during which the three laureates offered their perspectives in a moderated discussion, excerpts of which are presented here.
The Call from Stockholm
[Alter]: First, it is a great honor to share this award with Michael and Charlie, both longtime friends and collaborators. They’ve taken my early findings and brought them to new clinical relevance, to global impact. There are many collaborators that I am so dependent on, in particular Bob Purcell, Patrizia Farci, and Steve Feinstone. And a patient that we’ve all shared serum from, the initial non-A, non-B case, Mr. Hutchinson, now deceased. And the NIDDK Liver Service, starting with Jay Hoofnagle and Adrian Di Bisceglie, to Jake Liang and Marc Ghany and a host of liver fellows, each of whom have stuck needles into the livers of my patients. Lastly, to thank AASLD, who has morphed me from a hematologist into a hepatologist by osmosis, and I owe very much to this great organization.
Back to that morning, I got called at 4:15 AM. As usual, I ignored the call, but was very perturbed by it. Five minutes later I got called again, and was getting increasingly angry. Finally, the third call I got up, and was about to scream when he said this is Stockholm calling. I was dumbfounded and told them that was the best alarm clock I’d ever had.
[Rice]: My phone rang at about 4:30 AM. I stumbled to grab the phone and thought who could be calling at this time of the morning. I hadn’t gotten too far, and the phone rang again. I was irked and thought it might have been one of our ultra-cold freezers warming up. Someone with a Swedish accent said something about the Nobel Prize for the discovery of HCV. And I thought, this has got to be a prank. He persisted and mentioned Mike Houghton and Harvey Alter. Even then I was skeptical. And he said, well, if you don’t believe me now you can listen to the formal announcement. It came as a total surprise. I was stunned.
[Houghton]: My story was a little unusual. I was woken up at 3:00 AM, Pacific time, by my colleague, Dr. Lorne Tyrrell. He said congratulations and I said, why? He said, because you’re a co-recipient of the Nobel Prize. I said, well, I haven’t heard anything from Sweden. And he said, go online and it was there. I got the call from Sweden around 6:00 AM, it was obviously very nice.
Journey to Discovery: What inspired you?
[Alter]: It didn’t start with the idea that we would discover a new virus. It started with just the realization that a lot of people were getting hepatitis after blood transfusion. We had established at the NIH a prospective study of open-heart surgery patients who were multiply transfused. We were astounded to find that 30% were developing hepatitis, most of that asymptomatic, and the only thing we had was ALT elevations. We kept studying these patients to find out why so many people were getting it. And the first thing that became apparent was the source of the blood transfusion, and paid donors were not good donors. By 1970, we were able to go to an all-volunteer donor system and introduced the first test for hepatitis B. The striking finding was that only about 25% of the cases were hepatitis B-related. And when the hepatitis A test was discovered by Feinstone, Kapikian and Purcell, we looked at all our non-A, non-B cases and not one of them was hepatitis A.
In this brilliant sense of deductive reasoning, we said if these are not A and not B, we will call this non-A, non-B. We really thought we would find the virus within a short time. As you know, that did not happen. But we were able to learn a lot about it, because we had that great patient, Mr. Hutchinson, from whom I had gotten an apheresis unit when he was on the upswing of his ALT curve. We had this infectious inoculum and the chimp model. With Steve Feinstone, we showed that the agent was lipid encapsulated, and filtration studies showed it was small, probably an RNA virus, or a totally new virus. It was Dan Bradley at CDC who first said it’s most like a flavivirus. We muddled along until Michael came up with the cloning. This is one of the big things the NIH Liver service did. We showed this was not just ALT elevations, this was serious liver disease, 20% of our patients got cirrhosis, and some died. We knew we had a potentially fatal disease and asymptomatic blood donors were transmitting a small infectious agent. Steve Feinstone was doing subtractive cloning but Chiron snuck in with their discovery. At the time this was groundbreaking molecular biology.
[Houghton]: I was in England, all ready to apply to University, but I wasn’t sure if I wanted to be a physicist or something else. Then I read a book about Louis Pasteur. And that made me realize this is what I want to do. I want to do microbiology. At that time, they were showing on Sunday morning programs the elucidation of the structure of DNA, by Watson and Crick. I used to get up early to watch that, so I think I was committed to doing molecular medicine. I had been working on human interferon genes in England. Dino Dina, who was the head of virology in Chiron at that time, said to me, “do you think you could use your molecular biology approaches to try to identify the etiology of NANBH”. I was very attracted to that idea and got involved.
I would like to add that my own contributions to hepatitis C discovery were dependent on two colleagues at Chiron, Qui-Lim Choo and George Kuo, and a collaboration with Dan Bradley at the CDC who supplied us with an incessant supply of chimpanzee materials. Without that, I would not be talking to you today.
[Rice]: As an undergraduate, I had my focus on developmental biology. When I went to graduate school, I got placed into a virology lab, James Strauss’s, that happened to be studying togaviruses that included alphaviruses and flaviviruses. Towards the end of my stint at Caltech, we got interested in the flaviviruses, and we started working with the vaccine strain of yellow fever virus. It was the 17D vaccine strain that Max Tyler developed in the mid 30s, for which he won a Nobel Prize in 1951.
The next step was having my own lab at Wash U. The Science paper came out in 1989 from Michael, and as Dan Bradley said, “it looks like a flavivirus, very similar in terms of genome organization to yellow fever virus.” We initially waffled whether or not to get into this, because we figured that biotech companies, like Chiron, and pharma companies would be rushing to do more with the virus, and develop vaccines and therapies. And what could we possibly contribute?
It really started off as a chance phone call from Steve Feinstone. He had seen our paper on making an infectious clone for yellow fever. He thought we could just pack the envelope proteins of HCV on the yellow fever vaccine and we’ll have a vaccine for hepatitis C. We never got around to doing that, but it piqued my interest in figuring out how HCV proteins are produced. One of the things needed to study RNA viruses is a genetic system, so we attempted to make infectious clones for hepatitis C. We were limited to testing them in the chimpanzee model, because we didn’t have a validated cell culture system. We could not get it to work until the discovery of this missing piece at the 3’ end, and we used Harvey’s famous Hutchinson H77 inoculum to build a consensus sequence.
Advice for Young Investigators
[Alter]: For young people just starting out, I emphasize the message on a congratulatory card I got that said there is no elevator to success. You have to take the stairs, and that I think epitomizes research. It’s a step by step, slow process. Not too many eureka moments, a lot of plodding. You have to be willing to put in the time and effort. Young people need to find an institution that fosters research and a mentor. It’s very hard to start out on your own with a great project. Do not jump around, get into something where you become an expert, a particular knowledge that other people want, or a particular patient population, or data, or technology that makes you a good collaborator. And then find collaborators, because you just can’t do things on your own anymore. Collaborations are great, because they not only help get the work done, collaborators can become mentors as well. The last thing is to be persistent. I learned that from Dr. Blumberg. The Australia antigen story was a very long process, but he just kept at it until eventually he showed that this precipitin line (on the Ouchterlony diffusion assay) was the surface of the HBV. He taught me if you are working on something, follow it out until it’s clear it’s not going anywhere. Chance findings can often have the biggest payoffs.
[Houghton]: I see hepatology as a great field to be in as a young medical doctor and as a young scientist, because there are many challenges that are very important in the future. We need to make progress on non-alcoholic fatty liver disease and autoimmune liver diseases. We also have the challenge of curing hepatitis B and of preventing the development of hepatocellular carcinoma. The advice I would give is progress depends so much on the technology, so make sure to keep up to date with all the new technologies. Spending a morning every week looking at what’s happening in the field of molecular biology, immunology, and so forth is absolutely key. One of the most outstanding examples is the RNA vaccine technology that has just come out of nowhere in the last couple of years, and is now one of the leading technologies for vaccines against COVID-19.
[Rice]: I think one of the driving forces of success is to identify a problem. Be curious. Observations are important. That’s obviously key for the contributions that Harvey has made. And I agree with Mike on technology. But for me, curiosity and persistence, that was certainly important with hepatitis C. Mike and his team toiled for six or seven years before they found this sequence. When you believe in what you’re doing you will be willing to put up with some setbacks, and try again.
What’s Left to Accomplish in Hepatitis C?
[Alter]: The quasispecies nature of the virus makes it easy to escape the immune response, so making a vaccine is difficult. The question is do we need a vaccine? My short answer is yes - it would be the best way to get global eradication. With hepatitis C, we have curative therapies. The impediment is getting everybody tested, and getting those testing positive to get the treatment. It would take a massive global effort but if there was the political will, the philanthropic will, and the moral courage, we could conceivably over decades get virtually everybody treated. If a vaccine comes along, still better. It could happen, but it would probably take several decades.
[Houghton]: It would be nice if these very potent antivirals for hepatitis C were cheaper, so all countries around the world could use them. But even for diseases like syphilis, we have very good drugs and they’re cheap, but we still have outbreaks. So I think we’re going to need a vaccine. I think it’s absolutely feasible. There are several highly conserved epitopes and you can design a vaccine to elicit antibodies to those highly conserved epitopes to get broad neutralization of most HCV strains around the world. I’m sure HCV vaccine will appear over the next 10 years. I see it being combined with HBV vaccine against blood-borne hepatitis.
[Rice]: I was a bit naive being a molecular virologist not thinking about the challenges that we face in public health. I thought with the approval of these miraculous antivirals, we can cure most people of the virus. I’ve been dismayed at how slow this process is. We need a global plan to overcome the barriers. I am enthusiastic about the possibilities of developing a vaccine for hepatitis C. I am hopeful that this unprecedented effort to develop a COVID-19 vaccine with so many different platforms, will inform us what platforms might be worth trying out for hepatitis C.
[Alter]: I agree that the vaccine is the Holy Grail, but if the price of DAAs is affordable for each country I could conceive of having people come in and have a rapid test. While still at a testing facility, be given DAAs to take home, no monitoring is needed except for a blood test later to confirm cure. This is potentially achievable.
AASLD and the Nobel Laureates
None of the three Nobel Laureates is a hepatologist, yet all three have been long term members as well as being distinguished awardees.
[Alter]: I was trained as a hematologist, then morphed into liver disease because of what we found by chance. But being in AASLD has been just remarkable, because it’s given me so many new colleagues, and so many great meetings. I have slowly stopped going to hematology meetings, and focused only on hepatology. I’ve been part of the HEPATOLOGY journal. I’ve gotten some very nice awards. What I appreciated most was to be a hematologist getting an award from a hepatology group. AASLD is a great organization. The teaching is astounding. I see what goes on in the meeting, even as a virtual meeting. It is I think the best teaching group ever. It fosters young people. Just to say I’m very proud to be associated with it, and in my next life I’ll come back as a hepatologist.
[Houghton]: AASLD has been an outstanding organization not only to keep abreast of what’s new, but also as a discussion forum with peers. I’m a molecular biologist, a molecular virologist, but I’ve learned so much from talking to hepatologists. Interface between scientists and clinicians is absolutely vital, and AASLD has been truly outstanding at doing that.
[Rice]: Coming into this, I really didn’t know much about the liver. This interface between basic scientists and clinicians to me was really very important. My clinical partner over the last 20 years, Ira Jacobson’s perspective of the challenges and problems was very helpful. Having exposure to the hepatology community through AASLD and other venues was really an important education, and for me, a very important motivational push. There were periods when things were not working, having an increased awareness of the severity of this disease and the problem that it can cause, was very important.
Personal Legacy
[Alter]: I hope my legacy will be I played a role in improving the safety of the blood supply to virtually zero risk for hepatitis viruses. That I laid the groundwork with my many collaborators, particularly Bob Purcell, for the work that Michael and Charlie have carried on.
[Houghton]: I would hope my legacy would be to encourage young medical doctors and young scientists to spend time and effort on unmet medical need. We have so much information today, but I’d still like to see more of that information apply to major diseases. That sounds simple but it’s not, because the typical researcher has to work on things in the lab that students and postdocs can produce data and papers, to enable the careers of those young people. I’d like to see more time focused on unmet medical needs.
[Rice]: One of the things that this field has really epitomized has been a lot of people working towards a common goal, and part of this has to do with the belief that what you’re doing is important and it can have impact. I guess another part is what importance unexpected things can have. There is a lot of chance involved in my career, a lot of serendipity. You’ve got to keep your eyes open. We have to encourage people to have a passion for biological discovery, because you just never know what kind of impact things like clustered regularly interspaced short palindromic repeats (CRISPR) can have. We need a multi-pronged approach, targeted work and exploratory, curiosity-driven research.
Poet Laureate – Ode to HCV
Many in the hepatitis field know Harvey is also a talented poet and his favorite topic, not surprisingly, is hepatitis C. He shared part of a poem written after Dr. Houghton and his group cloned HCV (and incorrectly predicted the future):
“There’s No Sense Chiron over Spilt Milk.”
It was in Dr. Houghton’s antiviral prescription to make clones by reverse transcription.
Well, Chiron’s finance department had another conniption.
Michael said this was not a rehearsal.
The RNA had to be changed by transcription reversal.
Like Jack the Ripper at his most vindictive, he chopped up the codon with an enzyme restriction.
Could these tiny little pieces be of an antigen predictive?
Corporate executives prayed that it would on their knees been addictive.
Showing in science and religion are not contradicted.
As for Chiron, I hold no resentment.
In the test support of my claims, I’ll find my contentment.
For coming in second, I make no apologies.
I can only turn to the bench and do blood bank serologies.
For me there will be no Nobel Prize, but there’s always another virus on the horizon.
Perspectives from others in the field
Others in the hepatology community, major contributors in their own right, have worked closely with one or more of the laureates. We share some of their reflections and anecdotes.
Stephen Feinstone, Center for Biologics Evaluation and Research/Food and Drug Administration (CBER/FDA):
I feel privileged to know the three 2020 Nobel recipients and to have worked with two of them. Before I joined Bob Purcell’s group (we were only 3) in 1971, Bob had developed an extremely sensitive radioimmunoprecipitation test for anti-HBs that along with Jay Hoofnagle’s anti-HBc assay, revolutionized our understanding of the epidemiology of hepatitis B. Using that assay, he and Harvey Alter tested Harvey’s samples from his long-term, prospective study of posttransfusion hepatitis patients and found that after exclusion of HBsAg positive donors, there was residual hepatitis not caused by HBV. Later, Bob, Al Kapikian and I identified hepatitis A virus by immune electron microscopy. We tested Harvey’s non-B post-transfusion hepatitis patients - none of them had hepatitis A and Harvey coined the term NANBH. This initiated a 14-year quest for the causative agent that finally ended with Michael Houghton’s discovery of HCV. The proof of specificity was accomplished with Harvey’s collection of well characterized samples.
Houghton’s discovery was exciting for all of us in the field. At last we could begin to study the virology, immunology, treatment and prevention of hepatitis C. About the time of the HCV discovery, I opened my own lab in the CBER/FDA. Early on we began a collaboration with Charlie’s group. My entire lab benefited from this relationship especially when we wrote a joint NIH grant that was a major support my lab for over six years. Just writing that grant with Charlie was an educational experience. Charlie is a brilliant and creative researcher as well as a tough competitor while also being one of the most generous and ethical scientists I have known. The 45 years between the demonstration of the existence of a third form of viral hepatitis and this Nobel Prize has been a fascinating time enabled and enriched by these three Nobel laureates.
Jay Hoofnagle, National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK):
I have known and collaborated with Harvey Alter for almost 50 years, and it has been a pleasure. Harvey has many distinctive qualities. A major one is his talent as a speaker and ability to present data clearly and with humor. Harvey has become the most liked and most sought-after speaker in viral hepatitis. His presentations are not only entertaining, they are also always informative, clear and solidly data based.
When I had to organize symposia on viral hepatitis, I always tried to have Harvey give a major presentation, but importantly to give the last one at the meeting. First, if the audience knows that Harvey is the last speaker, they will stay to the end to hear what he has to say and what new jokes or poems he has written. Second, no one should be placed at the disadvantage to follow Harvey Alter on the podium. He is a hard act to follow.
A famous example occurred in 1993 when Harold Varmus (another Nobel laureate) was appointed NIH Director and, shortly after, asked to give grand rounds. NIH Grand Rounds, however, always has two speakers. For Dr. Varmus, Harvey Alter was chosen, which I knew was a mistake. Every seat in the auditorium was taken. Harvey gave an exceptionally engaging presentation, tracing the history of posttransfusion hepatitis at the Clinical Center, from the high rates in the 1960s, which were incrementally reduced, first by exclusion of commercial donors, then donor screening for HBsAg, and later using ALT and anti-HBc, surrogate markers for the elusive “NANBH.” The coup-de-grace came with the donor screening for anti-HCV, after which there were no cases. Harvey depicted this with a tombstone engraved with: “Post-Transfusion Hepatitis, R.I.P.” As usual, he had peppered his talk with humor. One slide asked in bold letters: “Is life worth living?” The next answered: “It depends upon the liver”. The audience roared. A final slide asked, “Why was I chosen to precede Dr. Varmus in Grand Rounds?” The answer: “To Varm-up the audience” and the warmed-up audience exploded in applause.
Jules Dienstag, Massachusetts General Hospital:
Like everyone else who has had a long career devoted to working on hepatitis viruses, I was thrilled to learn that the 2020 Nobel Prize was awarded to Harvey, Michael, and Charlie. Many of my colleagues and I have had the unusual and unique privilege of having had our careers bookended by the recognition of what started out as an intangible abstraction, NANBH, in the mid-1970s and the achievement of nearly-100% cures with DAAs by the mid-2010s. That the Nobel Committee recognized the incalculably huge impact this advance in science and medicine represents for humanity is so gratifying; that these three outstanding, inspirational investigators were chosen for the prize allows the dedicated scientists and physicians who worked on hepatitis C to bask in their glow.
Many of us have had personal ties to the Nobel troika. When I began my research training in Bob Purcell’s Laboratory in 1974, the first thing he did was ship me off to Harvey Alter’s lab to learn how to perform immunoassays for hepatitis B. Harvey’s colleagueship and friendship over these four-plus decades have been a precious gift. When Michael Houghton canvassed the NANBH community for pedigreed serum samples, I was so gratified to play a small role in confirming the sensitivity and specificity of the Chiron assay.(8) What a learning experience it was for me to serve on the Scientific Advisory Committee of the Rockefeller University Center for the Study of Hepatitis C, led by Charlie. Like many other investigators, I have been enriched by connections with “our” Nobelists, whose creativity and generosity have touched us all.
I could not help but chuckle to recall Harvey’s humility in 1989 about his foundational role in the discovery of HCV. In the second-to-the-last line (“For me there will be no Nobel Prize”) of his poem (above) about Chiron’s cloning of HCV, Harvey underestimated utterly his role in the quest to identify, understand, and conquer hepatitis C. He had already been skipped over for inclusion in the 1976 Nobel Prize awarded to Baruch Blumberg for his work on the discovery of HBV. Few recall that Harvey was the second author on Blumberg’s ground-breaking report on the discovery of Australia antigen.(15) Good things come to those who wait!
Leonard Seeff, NIDDK:
At the Washington DC VA Medical Center in 1967, my mentor, Hyman J Zimmerman, assigned me to oversee a multicenter VA study of gamma globulin prophylaxis, first for posttransfusion and later, needlestick hepatitis, long before identification of any hepatitis virus. Finding gamma globulin ineffective, the study goal changed to the conduct of a long-term natural history study of what proved to be NANBH.
Concurrently, at the NIH, Harvey Alter was also conducting a study of posttransfusion hepatitis. I arranged to meet with him for help and advice. I immediately discovered him to be a deeply serious, committed and knowledgeable person who was also extraordinarily witty with an appealing sense of humor. Happily, he consented to offer advice and to collaborate on the VA studies. He, of course, later went on to conduct brilliant research seeking surrogate markers of potentially infectious donors for NANBH, with extreme success, but short of identifying the actual HCV. Indeed, I recall hearing him say at an AASLD meeting many years after starting his studies that the virus would not be identified in his lifetime. Enter Michael Houghton and collaborators who identified the HCV, and, eureka, the Nobel ensued! For me, reading his many publications and listening to his presentations shows why he, together with Michael Houghton and Charlie Rice, deserved the Nobel Prize. But I contend that he deserved another Nobel award, for Mirth. At AASLD meetings, attendees would rush to hear a presentation from Harvey, partly for the research findings, but equally to hear his latest poem. I count myself lucky to have received a personal poem from him.
T. Jake Liang, NIDDK:
Among the few experiences that truly made a difference to my life, I would unequivocally count my friendship with Harvey Alter, Michael Houghton and Charlie Rice as one. I had known of Harvey for many years but first met him in person when I was being recruited to NIH more than 20 years ago. His humility and soft-spoken manner also impressed me on how one could be so accomplished as he was, but in the meantime had his feet firmly on the ground as a normal person. During my time at NIH, we worked closely together and published many important works with his guidance and tutelage. I truly consider him as a mentor that has had the most influence on me as a person, a scientist and a physician.
My first interaction with Michael Houghton came at a basic science meeting on viral hepatitis when I was a fellow. In this meeting, he disclosed the discovery of HCV that he and his colleagues at Chiron had worked on for many years. That unique experience etched an indelible impression on my nascent scientific mind to follow my dreams. Michael has been openly generous with all of us in the field and provided reagents, support and advice that made much of our research possible. Not being content with the discovery of HCV, Michael has been singularly focused on developing an HCV vaccine, and in my mind, probably has the best chance of success.
My encounter with Charlie began at one of the earlier international HCV meetings. Charlie quickly established himself as the leading researcher in the HCV field and published countless seminal papers over the ensuing 20 years. Many of us cringed at competing with his lab, which published papers an order-of-magnitude better than what we could do. Fortunately, Charlie is equally generous and has been open in sharing his work and reagents with the rest of the community.
I realize how lucky the HCV community has been with Harvey, Michael and Charlie as the vanguards. They set the tone and forged the model we all tried to emulate. It has been the most collegial and spirited field I have worked in, and I would say with some bravado that it has been the most scientifically successful and medically impactful field of research over the last 30 years.
Conclusion:
Fueled by their curiosity, creativity, persistence and passion, Drs. Alter, Houghton, and Rice have had a huge impact on hepatology and medicine. By discovering and explicating the mysteries of HCV, they have shown definitively that science truly delivers cures. The AASLD is proud to salute these pioneers.
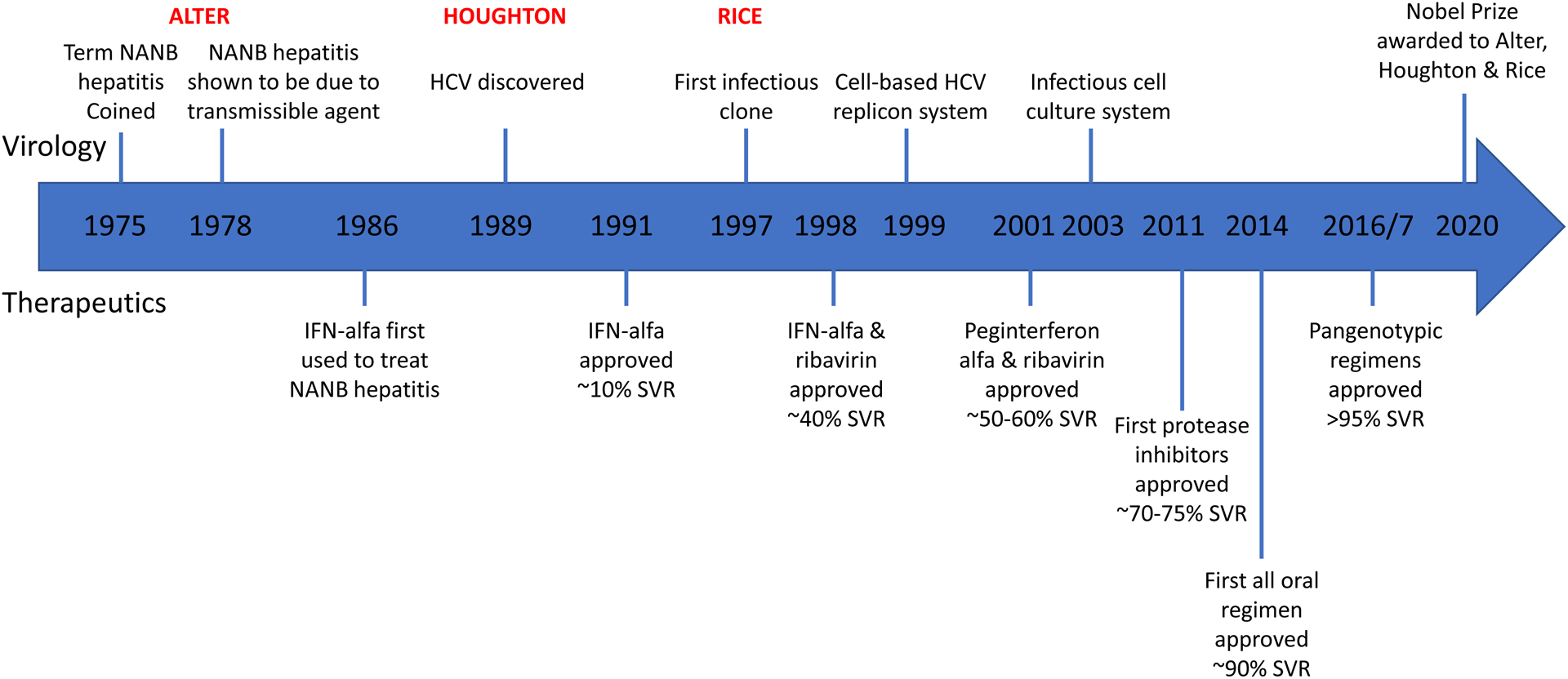
Figure 1:
The Nobel Laureates. Left Panel, Dr. Harvey Alter, middle panel, Dr. Michael Houghton, right panel, Dr. Charles Rice.
Figure 2:

Key Milestones in HCV Virology and Therapeutics. NANB=Non-A, non-B hepatitis, SVR=sustained virological response
Funding:
This work was supported by the Intramural Research Program of NIDDK, NIH,
Abbreviations:
- HCV
Hepatitis C virus
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- NANBH
non-A, non-B hepatitis
- ALT
alanine aminotransferase
- CDC
Centers for Disease Control and Prevention
- IFN
interferon alpha
- AASLD
American Association for the Study of Liver Diseases
- CRISPR
clustered regularly interspaced short palindromic repeats
- CBER
Center for Biologics Evaluation and Research
- FDA
Food and Drug Administration
- NIDDK
National Institutes of Diabetes and Digestive and Kidney Diseases
Footnotes
Conflict of interest statement:
Marc G. Ghany, has nothing to disclose.
Anna S.F. Lok, has nothing to disclose related to this manuscript.
Jules L. Dienstag has nothing to disclose related to this manuscript.
Stephen M. Feinstone, has nothing to disclose related to this manuscript.
Jay H. Hoofnagle, has nothing to disclose related to this manuscript.
T. Jake Liang, has nothing to disclose related to this manuscript.
Leonard B. Seeff, has nothing to disclose related to this manuscript.
David E. Cohen, has nothing to disclose related to this manuscript.
Jorge A. Bezerra, has nothing to disclose related to this manuscript.
Raymond T. Chung has no relevant disclosures.
(For access to the AASLD virtual session with the Nobel Laureates at TLMdX clinic here)
References:
- 1.Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaundice PB occurring one to four weeks after transfusion of blood or plasma. Report of seven cases. JAMA. 1943;121:1332–4. [Google Scholar]
- 3.Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med. 1975;292(15):767–70. [DOI] [PubMed] [Google Scholar]
- 4.Alter HJ, Purcell RH, Holland PV, Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1(8062):459–63. [DOI] [PubMed] [Google Scholar]
- 5.Berman M, Alter HJ, Ishak KG, Purcell RH, Jones EA. The chronic sequelae of non-A, non-B hepatitis. Ann Intern Med. 1979;91(1):1–6. [DOI] [PubMed] [Google Scholar]
- 6.Houghton M The long and winding road leading to the identification of the hepatitis C virus. J Hepatol. 2009;51(5):939–48. [DOI] [PubMed] [Google Scholar]
- 7.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244(4902):359–62. [DOI] [PubMed] [Google Scholar]
- 8.Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244(4902):362–4. [DOI] [PubMed] [Google Scholar]
- 9.Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290(5498):1972–4. [DOI] [PubMed] [Google Scholar]
- 10.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76(24):13001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato T, Date T, Miyamoto M, Furusaka A, Tokushige K, Mizokami M, et al. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology. 2003;125(6):1808–17. [DOI] [PubMed] [Google Scholar]
- 12.Chung RT, Baumert TF. Curing chronic hepatitis C--the arc of a medical triumph. N Engl J Med. 2014. Apr 24;370(17):1576–8. [DOI] [PubMed] [Google Scholar]
- 13.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444–51. [DOI] [PubMed] [Google Scholar]
- 14.Liang TJ, Ghany MG. Therapy of hepatitis C--back to the future. N Engl J Med. 2014;370(21):2043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumberg BS, Alter HJ, Visnich S. A “new” antigen in leukemia sera. JAMA 1965;191:541–6 [DOI] [PubMed] [Google Scholar]


