Abstract
Background
The addition of cetuximab significantly increased the antitumor effect of programmed cell death protein 1 (PD-1) inhibitors in recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). However, preliminary analyses suggested that human papillomavirus (HPV)-positive disease benefited less than HPV-negative disease. Therefore, we conducted a meta-analysis to assess whether the efficacy of the combination therapy varied according to HPV status in HNSCC.
Methods
We identified clinical trials of patients with recurrent or metastatic HNSCC who received PD-1 inhibitor monotherapy or the combination therapy of cetuximab plus a PD-1 inhibitor. The participants were divided into four groups based on the type of therapy (combination vs monotherapy) and HPV status (positive vs negative). We focused on three comparisons (monotherapy vs combination therapy by HPV status and HPV-positive vs HPV-negative disease in combination therapy). The primary and secondary endpoints were objective response rate (ORR) and 1-year overall survival (OS) rate, respectively. The ORR and 1-year OS rate were pooled using random-effects models for each group and were compared for the different comparisons.
Results
Overall, 802 patients from seven trials were eligible for the ORR assessment; of which, 684 patients received PD-1 inhibitor monotherapy and 118 patients underwent the combination therapy. Compared with PD-1 inhibitor monotherapy, the addition of cetuximab improved the ORR in HPV-negative disease (pooled ORR in monotherapy vs combination therapy: 15% vs 46%, p<0.001) but not in HPV-positive disease (17% vs 18%, p=0.686). The efficacy of adding cetuximab was consistent for the 1-year OS rate in HPV-negative disease (pooled 1-year OS rate in monotherapy vs combination therapy: 36% vs 59%, p<0.001) and in HPV-positive disease (40% vs 55%, p=0.252). After the combination therapy, HPV-positive disease had a significantly lower ORR than HPV-negative disease (odds ratio: 0.29, p=0.004), but no differences were shown in the 1-year OS rate.
Conclusions
Our meta-analysis suggests that the addition of cetuximab to a PD-1 inhibitor is more effective compared with PD-1 inhibitor monotherapy only in patients with HPV-negative HNSCC. Despite the retrospective nature of this meta-analysis, these findings should help in designing relevant clinical trials rationally.
Keywords: Biomarkers, Tumor; Head and Neck Neoplasms; Immunotherapy
Background
Head and neck squamous cell carcinomas (HNSCCs) are two etiologically distinct types of human papillomavirus (HPV)-negative and HPV-positive disease, respectively. The HPV-positive tumor occurs primarily in the oropharynx. The HPV status is tested using HPV gene detection (eg, in situ hybridization or PCR assay) or, more frequently, using p16 (a surrogate biomarker) immunohistochemistry. Programmed cell death protein 1 (PD-1) inhibitors (eg, pembrolizumab and nivolumab) and epidermal growth factor receptor (EGFR) inhibitors (eg, cetuximab) are US Food and Drug Administration (US FDA) approved agents for recurrent or metastatic HNSCC, irrespective of HPV status. Monotherapy of these agents is associated with modest response rates (pembrolizumab: 16%–18%,1–4 nivolumab: 13%,5 6 and cetuximab: 6%–13%7).
Cetuximab prevents EGFR signal transduction leading to the promotion of antigen presentation and the increase of immunotherapy efficacy in preclinical models.8 Therefore, prior exposure to or combination with cetuximab may impact the efficacy of PD-1 inhibitors. In a post hoc analysis of the phase 3 CheckMate 141 trial of nivolumab versus single agent chemotherapy in recurrent or metastatic HNSCC post-platinum therapy, the overall survival (OS) benefit of nivolumab appeared lower in patients with prior cetuximab exposure than in those without prior cetuximab exposure.9 Afterwards, the combination therapy of cetuximab with a PD-1 inhibitor was tested in two clinical trials in patients with recurrent or metastatic HNSCC, with both demonstrating a markedly improved antitumor effect.10 11 Post hoc analyses of the two trials showed that HPV-negative disease was associated with a more favorable response rate relative to HPV-positive disease.10 11 However, neither had been adequately powered nor designed to reveal the difference in the response rates by HPV status. We identified all related clinical trials and assessed whether the efficacy of the combination therapy was increased compared with PD-1 inhibitor monotherapy by HPV status and whether the efficacy of the combination therapy varied by HPV status for HNSCC.
Methods
Study selection and data extraction
We searched PubMed, Web of Science, Embase, ClinicalTrials.gov, and Chinese databases (Database for Chinese Technical Periodicals, Wan Fang, and China National Knowledge Infrastructure) on April 1, 2022, for clinical trials of PD-1 inhibitor monotherapy or of the combination therapy of cetuximab plus a PD-1 inhibitor. The search criteria included key terms “MK-3475” OR “pembrolizumab” OR “Keytruda” OR “BMS-936558” OR “nivolumab” OR “Opdivo” AND “recurrent” OR “metastatic” AND “head and neck squamous cell carcinoma” OR “HNSCC” OR “SCCHN”. To identify trials for the combination therapy, the following keywords “cetuximab” OR “Erbitux” were added to the above search terms. No limits were applied to the search for language or date. In addition, we manually searched the references of the retrieved review and primary articles for complete coverage. If a trial was reported by several publications, we included the most recent results.
Clinical trials eligible for inclusion met the following criteria: (1) squamous cell carcinoma originating from the oral cavity, oropharynx, larynx, or hypopharynx; (2) the treatment regimen was PD-1 inhibitor monotherapy or the combination of cetuximab plus a PD-1 inhibitor, and the PD-1 inhibitor must be US FDA approved for HNSCC (pembrolizumab or nivolumab); and (3) the study provided objective response rate (ORR) according to HPV or p16 status or reported information to calculate these measures.
Two authors independently conducted the literature search, study selection, and data extraction. Discrepancies were resolved by reaching a consensus among the authors, with an additional reference to a third reviewer whenever necessary. The trial name; phase; the PD-1 inhibitor used; the number of patients; clinical endpoint; inclusion criterion; prior therapy; HPV status; the number of patients with complete response, partial response, stable disease, and progressive disease; time for assessment of response; criteria for the assessment of reported response; duration of follow-up; and 1-year OS rate were obtained from each study that was included.
Statistical analysis
The primary endpoint was ORR, including the complete and partial response. The secondary endpoint was the 1-year OS rate. Patients were categorized into four groups by both therapy (combination vs monotherapy) and HPV status (positive vs negative). We conducted three different comparisons including monotherapy vs combination therapy stratified by HPV status and HPV-positive versus HPV-negative disease in the combination therapy. Exact binomial distribution was used to compute the 95% CIs of the ORR for the groups in every trial included in the study. The ORR and 1-year OS rate for each group were combined using a random-effects model (the DerSimonian and Laird method), separately. A forest plot was constructed, including the overall effect, Cochran’s Q test, and I2 statistics. The Cochran’s Q test and I2 statistics were used to determine heterogeneity across the included trials, and I2 values of 25%, 50%, and 75% were considered to indicate low, moderate, and high inconsistencies, respectively. The Cochran-Mantel-Haenszel test, stratified by the PD-1 inhibitor, was used to compare ORRs between the different comparisons. The results were presented as odds ratios (ORs) with the corresponding 95% CIs. The Breslow-Day test was conducted to assess the homogeneity among the trials. The test of interaction proposed by Altman and Bland12 was used to compare the 1-year OS rate between the different comparisons. The results were presented as the ratio of the 1-year OS rate with its corresponding 95% CIs. Statistical analyses were performed using the STATA V.14.0 program (Stata Corporation). Statistical significance was defined as a two-sided p value <0.05.
Results
Seven trials were identified, including 978 patients after PD-1 inhibitor monotherapy or PD-1 inhibitor plus cetuximab combined therapy (trial selection process shown in figure 1).1–3 5 6 10 11 13 The study characteristics are described in table 1. The primary endpoint was the ORR in two phase 1b and two phase 2 trials,2 3 10 13 1-year OS rate in one phase 2 trial (two cohorts),11 and OS in two phase 3 trials.1 6 HPV status was mostly assessed by p16 immunohistochemistry in all the trials.
Figure 1.
Flow chart showing trial selection procedure. Note: no eligible trial was identified in Chinese biomedical databases. EGFR, epidermal growth factor receptor; HPV, human papillomavirus; ORR, objective response rate; PD-1, programmed cell death protein 1.
Table 1.
Characteristics of included trials
| Trial identifier/name | Trial type | Therapy | No. of patients | Trial period | ORR | Inclusion criterion | HPV test | Median follow-up (months) |
| NCT03082534/not applicable10 | Non-randomized, multicenter, phase 2 trial | Cetuximab+pembrolizumab | 33 | 2017–2019 | Primary endpoint, per RECIST 1.1 by investigators. | No previous immunotherapy or EGFR inhibition; platinum-resistant or platinum-ineligible. | Oropharyngeal tumors: (methods not specified) by local institution. Non-oropharyngeal tumors: HPV negative. | 7.3 (IQR: 3.9–10.9) |
|
NCT03370276/not applicable Cohort A11 |
Non-randomized, multicenter, phase 1/2 trial | Cetuximab+nivolumab | 47* | 2017–2019 | Secondary endpoint, per RECIST 1.1 (not specified by investigators or central review). | Prior exposure to any systemic therapy including cetuximab or PD-1 inhibitors; platinum resistant. |
p16 immunohistochemistry (no other information). | 32.1 |
|
NCT03370276/not applicable Cohort B11 |
Non-randomized, multicenter, phase 2 trial | Cetuximab+nivolumab | 48† | Not report | Secondary endpoint, per RECIST 1.1 (not specified by investigators or central review). | No systemic therapy. | p16 immunohistochemistry (no other information). | 15.9 (95% CI 12.2 to 18.8)‡ |
| NCT02105636/CheckMate 1415 6 | Randomized, multicenter, phase 3 trial | Nivolumab versus single-agent systemic therapy | 240§ | 2014–2015 | Secondary endpoint, per RECIST 1.1 by investigators. | No previous immunotherapy; platinum resistant. | Oropharyngeal tumors: p16 immunohistochemistry by local institution. Non-oropharyngeal tumors: unknown HPV status. |
≥ 24.2¶ |
| NCT02252042/KEYNOTE-0401** | Randomized, multicenter, phase 3 trial | Pembrolizumab versus single-agent systemic therapy | 247†† | 2014–2016 | Secondary endpoint, per RECIST 1.1 by central review. | No previous immunotherapy; platinum resistant. | Oropharyngeal tumors: p16 immunohistochemistry by local or central testing. Non-oropharyngeal tumors: HPV negative. |
8.4 (range: 3.3–14.5) in the pembrolizumab group |
| NCT01848834/KEYNOTE-0122 | Non-randomized, multicenter, phase 1b trial | Pembrolizumab | 60 hours‡‡ | 2013.6–2013.10 | Primary endpoint, per RECIST 1.1 by central review. | No previous immunotherapy. | Oropharyngeal tumors: mostly p16 immunohistochemistry by local institution. Non-oropharyngeal tumors: HPV negative. |
13 months (range: 1–26) |
| NCT01848834/KEYNOTE-012 expansion13 | Non-randomized, multicenter, phase 1b trial | Pembrolizumab | 132§§ | 2014–2015 | Primary endpoint, per RECIST 1.1 by central review. | No previous immunotherapy. | Oropharyngeal tumors: mostly p16 immunohistochemistry by local institution. Non-oropharyngeal tumors: HPV negative. |
9 (IQR: 3–11) |
| NCT02255097/KEYNOTE-0553 | Non-randomized, multicenter, phase 2 trial | Pembrolizumab | 171¶¶ | 2014–2015 | Primary endpoint, per RECIST 1.1 by central review. | No previous immunotherapy; platinum-resistant; cetuximab-resistant. | Oropharyngeal tumors: mostly p16 immunohistochemistry by local institution. Non-oropharyngeal tumors: HPV negative. |
7 (range: 0–17) |
*Forty-three and 45 patients were evaluable for the ORR and the 1-year OS rate analysis, respectively.
†Forty-two and 43 patients were evaluable for the ORR and the 1-year OS rate analysis, respectively.
‡For patients including both cohort A and cohort B, and the duration of follow-up was not reported in the cohort B group.
§Among the 240 patients assigned to receive nivolumab monotherapy (121 to receive standard therapy), 120 patients were included for the ORR and the 1-year OS rate analysis, respectively.
¶For patients in both nivolumab and single-agent systemic therapy group, and the duration of follow-up was not reported in the nivolumab group.
**The relevant data was reported in the documents submitted to the European Medicines Evaluation Agency for approval.24
††The 247 patients were assigned to receive pembrolizumab monotherapy.
‡‡Forty-five and 56 patients were evaluable for the ORR and the 1-year OS rate analysis, respectively.
§§104 patients with tumor location in the oral cavity, oropharynx, larynx, and hypopharynx were included for the ORR analysis, and no data were reported for the 1-year OS rate analysis, respectively.
¶¶168 patients were evaluable for the ORR and the 1-year OS rate analysis, respectively.
CI, confidence interval; EGFR, epidermal growth factor receptor; HPV, human papillomavirus; IQR, interquartile range; ORR, objective response rate; PD-1, programmed cell death protein 1; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
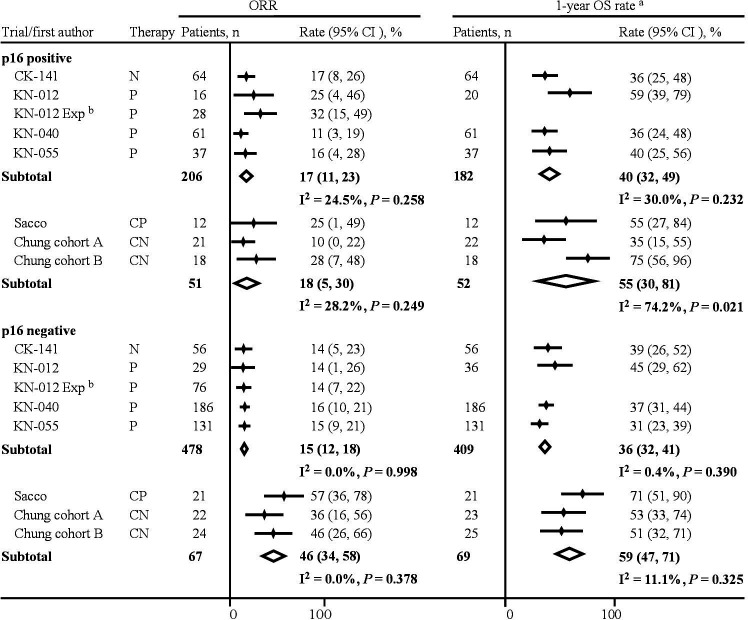
Of the 978 patients included in this meta-analysis, 802 (82%) patients were eligible for tumor response assessment. Five trials with 684 patients (206 p16-positive and 478 p16-negative) investigated PD-1 inhibitor monotherapy. Two trials (three cohorts) with 118 patients (51 p16-positive and 67 p16-negative) investigated the combination therapy, including 29 (24.6%) patients with prior exposure to either checkpoint inhibitor or cetuximab. The pooled ORR after PD-1 inhibitor monotherapy was 17% (95% CI 11% to 23%) and 15% (95% CI 12% to 18%) in patients with p16-positive and p16-negative disease, respectively. The pooled ORR after the combination therapy was 18% (95% CI 5% to 30%) and 46% (95% CI 34% to 58%) in patients with p16-positive and p16-negative disease, respectively (figure 2). Low and moderate heterogeneity was observed in the meta-analysis of the monotherapy and the combination therapy in p16-positive disease, respectively. There was a significant difference in the pooled ORR between PD-1 inhibitor monotherapy and the combination therapy in patients with p16-negative disease (OR: 5.45, 95% CI 2.79 to 10.64, p<0.001, table 2), whereas no differences were detected in patients with p16-positive disease (1.19, 95% CI 0.51 to 2.74, p=0.686). After the combination therapy, patients with p16-positive disease had a significantly lower ORR compared with that of patients with p16-negative disease (0.29, 95% CI 0.13 to 0.68, p=0.004). The Breslow-Day test detected no heterogeneity.
Figure 2.
Pooled estimates of clinical benefits according to p16 status and therapy. aThe 1-year OS rates and their 95% CIs were reported in the KEYNOTE-040 trial and were calculated by the life-table analysis proposed by Anderson et al25 in the other trials. bKEYNOTE-012 expansion reported neither 1-year OS rate nor provided information to calculate it. CK-141, CheckMate-141; CN, cetuximab plus nivolumab; CP, cetuximab plus pembrolizumab; KN, KEYNOTE; N, nivolumab; ORR, objective response rate; OS, overall survival; P, pembrolizumab.
Table 2.
Clinical benefits from adding cetuximab to a PD-1 inhibitor therapy according to p16 status
| Comparison | ORR | 1-year OS rate * | |||||
| Rate (%) | OR (95% CI) | P effect | P homogeneity | Rate (%) | Ratio of rate (95% CI) | P effect | |
| Cetuximab plus anti-PD-1 versus anti-PD-1 in p16-positive patients | 18 versus 17 | 1.19 (0.51 to 2.74) | 0.686 | 0.695 | 55 versus 40 | 1.37 (0.80 to 2.36) | 0.252 |
| Cetuximab plus anti-PD-1 versus anti-PD-1 in p16-negative patients | 46 versus 15 | 5.45 (2.79 to 10.64) | <0.001 | 0.389 | 59 versus 36 | 1.62 (1.26 to 2.07) | <0.001 |
| p16-positive versus p16-negative patients after cetuximab plus anti-PD-1 therapy | 18 versus 46 | 0.29 (0.13 to 0.68) | 0.004 | 0.818 | 55 versus 59 | 0.95 (0.55 to 1.62) | 0.850 |
*Due to the lack of data, tests for heterogeneity could not be conducted.
OR, odds ratio; ORR, Objective response rate; OS, overall survival; PD-1, programmed cell death protein 1.
Six trials with 712 patients were eligible for the survival analysis. Four trials with 591 patients (182 p16-positive and 409 p16-negative) investigated PD-1 inhibitor monotherapy. Two trials with 121 patients (52 p16-positive and 69 p16-negative) investigated the combination therapy. The pooled 1-year OS rate after PD-1 inhibitor monotherapy was 40% (95% CI 32% to 49%) and 36% (95% CI 32% to 41%) in the p16-positive and p16-negative cohorts, respectively. The pooled 1-year OS rate after the combination therapy was 55% (95% CI 30% to 81%) and 59% (95% CI 47% to 71%) in the p16-positive and p16-negative cohorts, respectively (figure 2). Moderate and high heterogeneity was observed in the meta-analysis of the monotherapy and the combination therapy in p16-positive disease, respectively. There was a significant difference in the pooled 1-year OS rate between PD-1 inhibitor monotherapy and the combination therapy in the p16-negative cohort (ratio of 1-year OS rate: 1.62, 95% CI 1.26 to 2.07, p<0.001, table 2), whereas no differences were detected in the p16-positive cohort (1.37, 95% CI 0.80 to 2.36, p=0.252). After the combination therapy, the 1-year OS rate was not different between the p16-positive and p16-negative cohorts (0.95, 95% CI 0.55 to 1.62, p=0.850).
Discussion
To the best of our knowledge, this is the first meta-analysis to evaluate the effectiveness of combining cetuximab, an EGFR inhibitor, with a PD-1 inhibitor based on tumor HPV status in patients with recurrent or metastatic HNSCC. The combination therapy was shown to be significantly better in terms of ORR and 1-year OS rate than the PD-1 inhibitor monotherapy in HPV-negative disease. However, the addition of cetuximab improved neither ORR nor 1-year OS rate compared with PD-1 inhibitor monotherapy in patients with HPV-positive recurrent or metastatic HNSCC.
Our results raise an important question of whether tumor HPV status is a potential predictor of cetuximab plus a PD-1 inhibitor in recurrent or metastatic HNSCC. To the best of our knowledge, EGFR inhibitor monotherapy, either cetuximab or afatinib, appears to be less active in HPV-positive than in HPV-negative disease in all clinical trials of subsequent-line recurrent or metastatic HNSCC.7 14–16 On the contrary, the combination of cetuximab with radiotherapy in the curative setting (the IMCL-9815 trial) or with chemotherapy in the palliative setting (the EXTREME trial (a randomized phase 3 trial comparing platinum/5-FU alone versus combined with cetuximab as first-line treatment in recurrent or metastatic HNSCC)) shows a significant OS benefit regardless of the HPV status.17 18 Nevertheless, in the phase 3 SPECTRUMtrial that compared the same chemotherapy as the EXTREME trial versus combined with panitumumab as first-line treatment in recurrent or metastatic HNSCC, the significant OS improvement was shown only in the p16-negative cohort but not in the p16-positive cohort or the overall population.19 Due to this controversy, tumor HPV status should be an important consideration in the design of future trials of cetuximab combined therapy in recurrent or metastatic HNSCC.
Two methodological issues of the present study are worth noting. (1) Trials of afatinib plus PD-1 inhibition for HNSCC was ineligible for this meta-analysis, because afatinib has not been approved by US FDA. Although afatinib is approved by the National Comprehensive Cancer Network guidelines in the management of recurrent or metastatic disease, the level of evidence (category 2B recommendation) for this approval is poorer than that for cetuximab (category 2A recommendation).20 In addition, there is only one trial (n=29) investigating afatinib plus PD-1 inhibition in HNSCC, which did not report ORR according to HPV status.21 (2) Among five out of the seven trials included in this meta-analysis, non-oropharyngeal tumors were considered HPV-negative unless reported.1–3 10 13 There is evidence that non-oropharyngeal tumors were considered HPV-negative. The presence of HPV has not been clearly established to be associated with carcinogenesis or outcomes in patients with non-oropharyngeal tumors.22 We could not conduct stratified analyses based on both tumor subsite and HPV status due to the lack of information. However, the incidence of HPV positivity was much lower in non-oropharyngeal tumors than oropharynx tumors,22 and thus an analysis of HPV status in non-oropharyngeal tumors would be unlikely to materially change the results of our meta-analysis.
The present analysis had several limitations. First, treatment lines were not uniform between the combination therapy groups and the PD-1 inhibitor monotherapy groups. Ninety (70.3%) out of the 128 patients received the combination therapy as the first-line treatment, whereas only 31 (3.7%) out of the 850 patients received the PD-1 inhibitor monotherapy as the first-line treatment. However, ORR did not differ between the various treatment lines in recurrent or metastatic HNSCC across the KEYNOTE trials: the ORR of pembrolizumab monotherapy was 17% in first-line treatment in KEYNOTE-048,4 15% and 16% in second-line or beyond line treatment in KEYNOTE-0401 and KEYNOTE-0553 respectively, and 18% in mixed-line treatment in both KEYNOTE-0122 and KEYNOTE-012 expansion.13 Second, caution should be taken when interpreting the results of the 1-year OS rate analyses. A larger sample size is needed for the OS analysis than for the ORR analysis in a clinical trial. The sample size (n=121) of the two trials (three cohorts) of the combination therapy was unlikely to have sufficient power for a definite conclusion to be drawn from the analyses of the 1-year OS rate. In addition, there was moderate to high heterogeneity in the meta-analyses of the 1-year OS rate in HPV-positive disease. The small sample size prohibited any investigations on sources of heterogeneity. Nonetheless, the 1-year OS rate was 49% in the total population with any HPV status after the first-line treatment of pembrolizumab monotherapy in KEYNOTE-048,4 compared with the 1-year OS rate of 59% in the HPV-negative group after the combination therapy in our meta-analysis. Because 238 (79%) out of 301 patients had HPV-negative tumors in the pembrolizumab monotherapy group in KEYNOTE-048,4 the difference between the 1-year OS rates, at least in part, supported our findings that the addition of cetuximab was associated with a better 1-year OS rate than PD-1 inhibitor monotherapy in HPV-negative disease. Third, no clinical trials have been conducted to compare the efficacy of the combination therapy of cetuximab plus a PD-1 inhibitor with that of PD-1 inhibitor monotherapy; therefore, we conducted across-trial comparisons. Although across-trial comparisons are prone to bias, the tests of heterogeneity showed no significant heterogeneity in the analyses of ORR, thereby suggesting the reliability of these results. Finally, the present meta-analysis was based on summarized data instead of individual patient data. However, results from summarized data are generally in agreement with those from individual patient data.23
Although clinical trials suggested a synergistic effect from cetuximab plus a PD-1 inhibitor compared with PD-1 inhibitor monotherapy in recurrent or metastatic HNSCC, preliminary analyses showed a more favorable response rate in HPV-negative disease than HPV-positive disease. Our meta-analysis confirms these results and furthermore showed that the combination therapy was likely to be effective in terms of response rate and 1-year OS rate only in patients with HPV-negative disease. These findings support that tumor HPV status should be an important consideration, for example, as a standard stratification factor, in future trials of cetuximab plus a PD-1 inhibitor in patients with HNSCC.
Acknowledgments
We would like to thank Dr Hui Li from MSD China Medical Affair oncology team for critical comments on the manuscript.
Footnotes
SZ, MZ and DN contributed equally.
Contributors: SZ,MZ and DN contributed equally. SZ, MZ, DN, and FH wrote the manuscript; HT, MW, SZ, MZ, and DN collected related literature and extracted the data; SZ, MZ, DN, and LX performed the data analysis; WL and ZF helped in drafting figures; FH designed and guided this work. All authors reviewed and approved the manuscript.
Funding: This work was supported by the Scientific and Technological Developing Scheme of Ji Lin Province (20200201603JC) and Jilin Province Medical and Health Talents Special Project (JLSWSRCZX2021-110).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data were extracted from the references and were included in this article.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 2.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1B trial. Lancet Oncol 2016;17:956–65. 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 3.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol 2017;35:1542–9. 10.1200/JCO.2016.70.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seiwert TY, Fayette J, Cupissol D, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol 2014;25:1813–20. 10.1093/annonc/mdu216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lizotte PH, Hong R-L, Luster TA, et al. A high-throughput Immune-Oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol Res 2018;6:1511–23. 10.1158/2326-6066.CIR-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris RL, Licitra L, Fayette J, et al. Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by prior cetuximab use. Clin Cancer Res 2019;25:5221–30. 10.1158/1078-0432.CCR-18-3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacco AG, Chen R, Worden FP, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol 2021;22:883–92. 10.1016/S1470-2045(21)00136-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung CH, Li J, Steuer CE, et al. Phase II multi-institutional clinical trial result of concurrent cetuximab and nivolumab in recurrent and/or metastatic head and neck squamous cell carcinoma. Clin Cancer Res 2022;28:2329–38. 10.1158/1078-0432.CCR-21-3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in Biomarker-Unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 2016;34:3838–45. 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machiels J-PH, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol 2015;16:583–94. 10.1016/S1470-2045(15)70124-5 [DOI] [PubMed] [Google Scholar]
- 15.Fayette J, Wirth L, Oprean C, et al. Randomized phase II study of Duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN study). Front Oncol 2016;6:232. 10.3389/fonc.2016.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochanny SE, Worden FP, Adkins DR. A randomized phase II trial of the Met inhibitor tivantinib + cetuximab versus cetuximab alone in patients with recurrent/ metastatic head and neck cancer. Clin Oncol 2015;33. [Google Scholar]
- 17.Rosenthal DI, Harari PM, Giralt J, et al. Association of human papillomavirus and p16 status with outcomes in the IMCL-9815 phase III registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without cetuximab. J Clin Oncol 2016;34:1300–8. 10.1200/JCO.2015.62.5970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermorken JB, Psyrri A, Mesía R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III extreme trial. Ann Oncol 2014;25:801–7. 10.1093/annonc/mdt574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (spectrum): an open-label phase 3 randomised trial. Lancet Oncol 2013;14:697–710. 10.1016/S1470-2045(13)70181-5 [DOI] [PubMed] [Google Scholar]
- 20.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck Cancers, Version 1.2021 - November 9, 2020 [DOI] [PubMed]
- 21.Kao H-F, Liao B-C, Huang Y-L, et al. Afatinib and pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma (alpha study): a phase II study with biomarker analysis. Clin Cancer Res 2022;28:1560–71. 10.1158/1078-0432.CCR-21-3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 2016;108:djv403. 10.1093/jnci/djv403 [DOI] [PubMed] [Google Scholar]
- 23.Steinberg KK, Smith SJ, Stroup DF, et al. Comparison of effect estimates from a meta-analysis of summary data from published studies and from a meta-analysis using individual patient data for ovarian cancer studies. Am J Epidemiol 1997;145:917–25. 10.1093/oxfordjournals.aje.a009051 [DOI] [PubMed] [Google Scholar]
- 24.European Medicines Agency . Amsterdam: European Union; c1995-2022 [cited 2022 Aug 20]. Assessment report EMA/543713/2018. Available: https://www.ema.europa.eu/en/documents/variation-report/keytruda-h-c-3820-ii-0042-epar-assessment-report-variation_en.pdf
- 25.Anderson JR, Bernstein L, Pike MC. Approximate confidence intervals for probabilities of survival and quantiles in life-table analysis. Biometrics 1982;38:407–16. 10.2307/2530454 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data were extracted from the references and were included in this article.