Abstract
Objective
Lipoprotein(a) (Lp(a)) is an important genetically determined risk factor for atherosclerotic vascular disease (ASCVD). With the development of Lp(a)-lowering therapies, this study sought to characterise patterns of Lp(a) levels in a global ASCVD population and identify racial, ethnic, regional and gender differences.
Methods
A multicentre cross-sectional epidemiological study to estimate the prevalence of elevated Lp(a) in patients with a history of myocardial infarction, ischaemic stroke or peripheral artery disease conducted at 949 sites in 48 countries in North America, Europe, Asia, South America, South Africa and Australia between April 2019 and July 2021. Low-density lipoprotein cholesterol (LDL-C) and Lp(a) levels were measured either as mass (mg/dL) or molar concentration (nmol/L).
Results
Of 48 135 enrolled patients, 13.9% had prior measurements of Lp(a). Mean age was 62.6 (SD 10.1) years and 25.9% were female. Median Lp(a) was 18.0 mg/dL (IQR 7.9–57.1) or 42.0 nmol/L (IQR 15.0–155.4). Median LDL-C was 77 mg/dL (IQR 58.4–101.0). Lp(a) in women was higher, 22.8 (IQR 9.0–73.0) mg/dL, than in men, 17.0 (IQR 7.1–52.2) mg/dL, p<0.001. Black patients had Lp(a) levels approximately threefold higher than white, Hispanic or Asian patients. Younger patients also had higher levels. 27.9% of patients had Lp(a) levels >50 mg/dL, 20.7% had levels >70 mg/dL, 12.9% were >90 mg/dL and 26.0% of patients exceeded 150 nmol/L.
Conclusions
Globally, Lp(a) is measured in a small minority of patients with ASCVD and is highest in black, younger and female patients. More than 25% of patients had levels exceeding the established threshold for increased cardiovascular risk, approximately 50 mg/dL or 125 nmol/L.
Trial registration number
Keywords: global burden of disease, hyperlipidemias, atherosclerosis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Most prior studies investigated lipoprotein(a) levels in selected populations in single countries or ethnic groups, mostly in patients without pre-existing atherosclerotic vascular disease (ASCVD).
WHAT THIS STUDY ADDS
The current study reports Lp(a) levels in a population with documented ASCVD and identifies racial, ethnic, regional and gender differences.
Median levels in these patients were higher than reported in prior studies and highest in younger and female patients.
Only 14% of these patients with ASCVD had known Lp(a) levels prior to the study.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
The study identifies the need for global efforts to encourage measurement of Lp(a) so that patients with ASCVD can receive emerging treatments when they become available.
Introduction
Lipoprotein(a) (Lp(a)) has long been recognised as an important genetically determined risk factor for development of atherosclerotic cardiovascular disease (ASCVD).1 Recent European and American guidelines have identified Lp(a) as a risk-enhancing factor for development of ASCVD.2 3 However, Lp(a) is not commonly measured in clinical practice, in part because no currently available treatments exist with established benefits on vascular outcomes. Recent developments in messenger RNA targeted gene silencing therapies have made it possible to lower time-averaged Lp(a) levels as much as 90% in patients with elevated levels.4–6 A large phase III clinical trial, Lp(a)HORIZON is now underway, studying the investigational drug pelacarsen, an antisense oligonucleotide, to determine if lowering Lp(a) can reduce the incidence of major adverse cardiovascular outcomes in patients with pre-existing ASCVD. As a prelude to the initiation of this trial, the current Lp(a)HERITAGE study sought to determine Lp(a) levels in a large global population with ASCVD and identify patients suitable for enrolment in Lp(a)HORIZON.
With the development of therapies effective at lowering Lp(a), it is important to understand the prevalence and patterns of elevated Lp(a) levels in a diverse population with pre-existing ASCVD. Most prior studies have examined either a general population that included both primary and secondary prevention patients,1 moderate-sized cohorts7 or performed measurements in single countries or regions.1 7–10 Currently available studies measured Lp(a) levels primarily in high-income countries, resulting in limited availability of data in a global population that includes both high-income and low-income and middle-income countries within Europe, the Americas, Asia and Africa. To make the current study relevant to emerging treatments, the Lp(a)HERITAGE study included only patients with documented ASCVD. An important goal of this study was to better understand the global prevalence of elevated Lp(a) and identify racial, ethnic, regional and gender differences in Lp(a) levels. Additional goals included understanding how results differ across alternative measurement systems based on Lp(a) mass versus concentration and the relationship between levels of Lp(a) and low-density lipoprotein cholesterol (LDL-C).
Methods
Study design and participants
The Lp(a)HERITAGE study was designed to examine the prevalence of elevated Lp(a) levels in patients with a history of ASCVD. Patients were eligible if they had a history of myocardial infarction (MI) or ischaemic stroke ≥3 months and ≤10 years prior to the initial study visit or symptomatic peripheral artery disease (PAD). Symptomatic PAD was defined as intermittent claudication with ankle-brachial index (ABI) ≤0.90 and/or lower limb amputation or re-vascularisation due to lower limb ischaemia. Patients were excluded if they were currently enrolled in clinical studies with investigational drugs.
Demographic and baseline characteristics were collected on all patients including age, sex, race, ethnicity and category of previous ASCVD diagnosis. Race was self-reported. No information about medication, current or past, was collected. LDL-C and Lp(a) levels were determined using the following requirements: LDL-C values were accepted if obtained ≤1 year before the study visit and Lp(a) values were accepted if tested ≤5 years before the study visit. If results were not available as specified, blood testing was performed by local laboratories for the determination of LDL-C and/or Lp(a). No additional laboratory values regarding safety were collected since there was no investigational product and medical device tested.
The database for the Lp(a)HERITAGE study was maintained by the sponsor. After completion of the study, a copy of the database was transferred to the Cleveland Clinic Center for Clinical Research (C5R). The reported analyses were verified by both statisticians employed by the sponsor and C5R. The lead author and the C5R statistician (KW) had complete access to the data and attest to the accuracy of the data.
Statistical analysis
Assuming that approximately 25% of patients with established ASCVD have Lp(a) levels ≥50 mg/dL with no major heterogeneity in sampling, approximately 45 000 patients were needed to assess a two-sided 95% CI with a half-width of 0.4%, corresponding to approximately 1.6% relative error. These calculations were made using EAST V.6.4. The statistical analysis plan (SAP) prespecified categories of interest, including the number and percentage of patients with Lp(a) 30 mg/dL, 30–50 mg/dL and ≥50 mg/dL and in subgroups with Lp(a) level ≥10 mg/dL, 20 mg/dL, 30 mg/dL, 40 mg/dL, 50 mg/dL, 60 mg/dL, 70 mg/dL, 80 mg /dL, 90 mg/dL and 100 mg/dL. Histograms depicting the distribution of Lp(a) were generated.
The SAP prespecified secondary end point assessment using descriptive summary statistics (mean, median, IQR, minimum and maximum) for Lp(a) for categories of interest (<30 mg/dL, 30–50 mg/dL and ≥50 mg/dL), and subgroups with Lp(a) level ≥60 mg/dL, 70 mg/dL, 80 mg/dL, 90 mg/dL and 100 mg/dL. Lp(a) levels are summarised by region, country and other subgroups of interest. Demographic variables are summarised using descriptive summary statistics (mean, median, SD, IQR, minimum and maximum) for continuous variables and frequency and percentage for categorical variables. A limited number of comparisons were made using the Wilcoxon rank sum test. Because the study is purely descriptive with no randomised comparator groups, p values should be interpreted as exploratory.
Results
A total of 48 992 patients were screened and 48 363 enrolled at 949 sites in 48 countries between April 2019 and July 2021. Of these, 48 135 had sufficient data for inclusion in the study, 29 765 with Lp(a) levels obtained in units of mg/dL and 18 370 with levels obtained in nmol/L. Only 13.9% of patients had Lp(a) measurements prior to enrolment. The mean age and SD for all patients was 62.6 (SD 10.1) years, slightly higher in women 64.3 (SD 9.8) years than men 62.0 (SD 10.2) years. Table 1 shows median Lp(a) and LDL-C levels for all patients including those whose values were measured in mass units, mg/dL, or molar concentration, nmol/L. For patients with Lp(a) measured in mg/dL, the median level was 18.0 mg/dL (IQR 7.9–57.1). For patients with levels obtained in nmol/L, the median value was 42.0 nmol/L (IQR 15.0–155.4). The median LDL-C level for the entire population was 77 mg/dL (IQR 58.4–101.0). Female patients represented 25.9% of the population and males 74.1%. Lp(a) levels in women, measured in mg/dL were higher, 22.8 (IQR 9.0–73.0) mg/dL, than levels in men, 17.0 (IQR 7.1–52.2) mg/dL, p<0.001. Similarly, for patients measured using molar concentration, women had higher levels, 54 nmol/L (IQR 18.2–185.7) vs 37.8 (IQR 13.7–143.2) for men, p<0.001. Women also had higher LDL-C levels 82.0 mg/dL (IQR 62.0–111.0) compared with 75.0 (IQR 58.0–98.0) for men, p<0.001.
Table 1.
Lipoprotein(a) (Lp(a)) and low-density lipoprotein cholesterol (LDL-C) by gender, age and race or ethnicity (n=48 135)
| Category | Number (%) | Lp(a) mg/dL Median (IQR) (n=29 765) |
Lp(a) nmol/L Median (IQR) (n=18 370) |
LDL-C mg/dL Median (IQR) (n=47 988) |
| All patients (both genders) | 48 129* | 18.0 (7.9–57.1) | 42.0 (15.0–155.4) | 77.0 (58.4– 101.0) |
| Male, n (%) | 35 670 (74.1) | 17.0 (7.1–52.2) | 37.8 (13.7–143.2) | 75.0 (58.0– 98.0) |
| Female, n (%) | 12 459 (25.9) | 22.8 (9.0–73.0) | 54 (18.2–185.7) | 82.0 (62.0– 111.0) |
| P values for gender comparison | <0.001 | <0.001 | <0.001 | |
| Age category (years) | 48 135 | |||
| <65, n (%) | 25 579 (53.1) | 19.0 (7.9–61.1) | 44.0 (15.0–161.0) | 78.0 (59.1– 104.0) |
| ≥65, n (%) | 22 556 (46.9) | 17.0 (7.9–53.0) | 40.0 (15.0–150.3) | 75.0 (58.0– 98.6) |
| P value for ages ≥65 <65 | <0.001 | 0.04 | <0.001 | |
| <75, n (%) | 42 472 (88.2) | 18.0 (7.8–58.6) | 42.0 (14.9–157.0) | 77.0 (58.8– 101.7) |
| ≥75, n (%) | 5663 (11.8) | 17.0 (8.0–49.5) | 40.7 (16.0–144.5) | 75.0 (58.0– 97.0) |
| P value for ages ≥75 or <75 | 0.01 | 0.92 | <0.001 | |
| Race or ethnicity | 48 135 | |||
| White, n (%) | 32 339 (67.2) | 19.0 (8.0– 71.0) | 37.0 (14.0–152.0) | 77.0 (58.0– 102.5) |
| Black/African-American, n (%) | 2023 (4.2) | 60.0 (22.2–93.0) | 125.8 (53.9–229.6) | 81.2 (60.4– 110.0) |
| Asian, n (%) | 11 286 (23.5) | 17.1 (7.3–39.8) | 37.2 (15.0–102.0) | 74.6 (58.0– 94.7) |
| Other, n (%) | 2458 (5.1) | 13.0 (5.6–44.2) | 25.9 (10.0–89.0) | 83.0 (63.0– 114.0) |
| Hispanic/Latino, n (%) | 6014 (12.8) | 20.0 (8.0–68.1) | 34.5 (11.7–118.0) | 80.0 (61.0– 109.0) |
| P value for black patients compared with other races or ethnicities | <0.001 | <0.001 | <0.001 | |
*Six patients with missing/unknown gender.
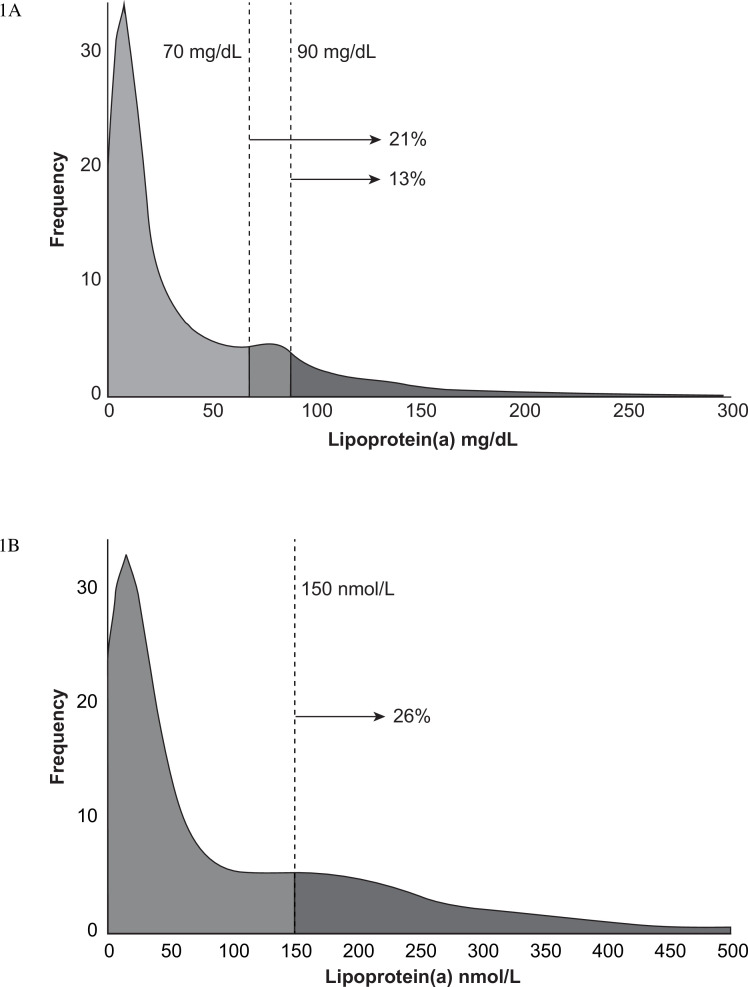
Table 1 also shows levels of Lp(a) and LDL-C by race, age category and ethnicity. Older patients had slightly lower values for both Lp(a) and LDL-C, although these differences were small. Lp(a) levels in white, Hispanic and Asian patients were similar, however, as reported in previous studies, levels were substantially higher in black or African-American patients, p<0.001. The associations between Lp(a) value and gender or race were similar across region. The higher levels observed in younger patients was primarily driven by differences observed in North America and Europe (online supplemental eTable 1). Figure 1A shows a histogram displaying the frequency of Lp(a) for patients with values obtained in mg/dL and figure 1B shows the results for patients with levels obtained in nmol/L. The fraction of patients with levels exceeding thresholds for increased cardiovascular risk indicate that 27.9% of patients had Lp(a) levels (equal or above) ≥50 mg/dL, 20.7% had levels (equal or above) ≥70 mg/dL and 12.9% had (equal or above ≥90 mg/dL, whereas 26% of patients exceeded 150 nmol/L.
Figure 1.
(A) Frequency of lipoprotein(a) (Lp(a)) measurements for patients whose values were measured in mg/dL. The fraction of patients with values f ≥70 and 90 mg/dL (21% and 13%, respectively) are indicated by vertical lines. (B) Frequency of Lp(a) measurements for patients whose values were measured in nmol/L. The fraction of patients whose values were equal or above the commonly used threshold of 150 nmol/L (26%) is indicated by a vertical line.
openhrt-2022-002060supp001.pdf (437.3KB, pdf)
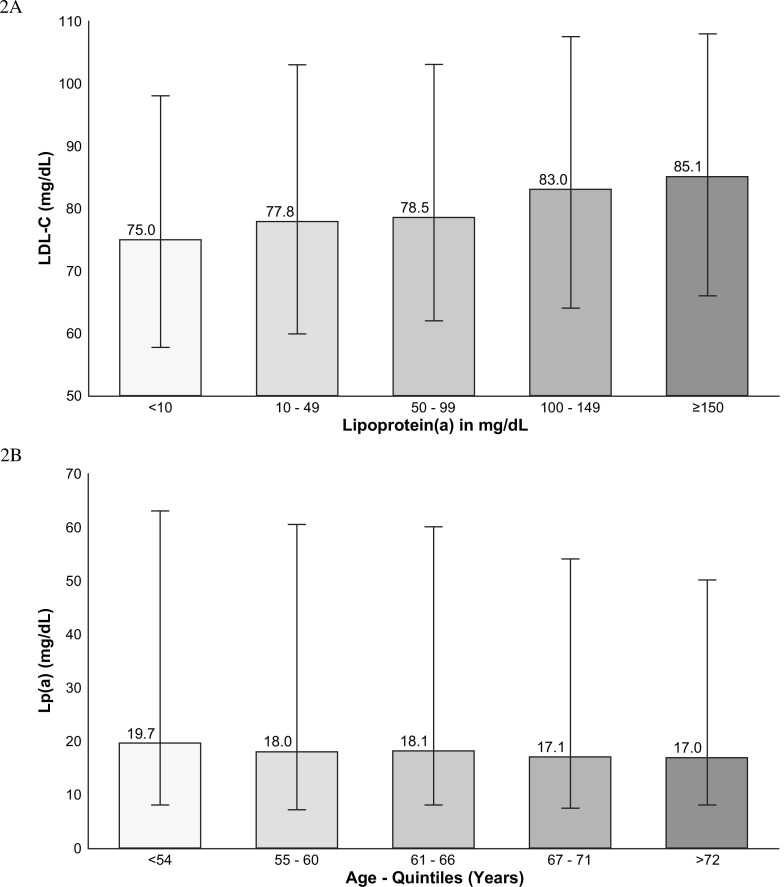
Online supplemental eTable 2 reports age and median Lp(a) for men and women at, or exceeding, the 70 mg/dL and 90 mg/dL thresholds. Women at, or exceeding, the 70 mg/dL threshold were slightly older than men and their median levels were higher, similar to the overall population. Figure 2A shows the relationship between Lp(a) and LDL-C levels with median LDL-C levels consistently trending higher with increasing Lp(a) levels. The median LDL-C was 85.1 mg/dL in patients with a Lp(a) level ≥150 mg/dL and 75.0 mg/dL for patients with an Lp(a) level <10 mg/dL, p value for trend <0.001. Figure 2B shows that younger patients had higher levels than older patients, a median of 19.7 mg/dL (IQR 8–62) for individuals in the bottom age quintile (ages 18–54 years) compared with 17.0 mg/dL (IQR 8.0–50.2) in the top age quintile (ages 72–90 years), p<0.001.
Figure 2.
(A) Relationship between lipoprotein(a) (Lp(a)) and low-density lipoprotein cholesterol (LDL-C) measurements. The bar charts demonstrate the increase in LDL-C related to lipid content of Lp(a) particles. IQR are shown for each category, <10 mg/dL, IQR 57.6–98.0; 10–49 mg/dL, IQR 60.0–103.0; 50–99 mg/dL, IQR 61.9–103.1; 100–150 mg/dL, IQR 64.6–107.5; ≥150 mg/dL, IQR 66.0–108.3. (B) Relationship between age and Lp(a) levels. Patients in the youngest quintile for age tended to have higher levels than patients in the oldest quintile. IQR, age 18–54 years, IQR 8.0–62.6; 55–60, IQR 7.1–60.6; 61–66, IQR 8.0–59.5; 67–71, IQR 7.7–54.0; 72–90, IQR 8.0–50.2.
Table 2 shows Lp(a) levels in the prespecified categories, <30 mg/dL, 30–50 mg/dL and ≥50 mg/dL and subgroups with Lp(a) level ranging from ≥10 mg/dL to ≥100 mg/dL. Online Supplemental eFigure 1 illustrates deciles of Lp(a) for patients with values expressed in mg/dL and nmol/L. Determination of the deciles was based on patients with values measured in mg/dL rather than nmol/L. The top decile of patients had median Lp(a) levels of 134 mg/dL and 329 nmol/L, respectively. The lowest decile of patients had median Lp(a) levels of 2.8 mg/dL and 7.0 nmol/L.
Table 2.
Lipoprotein(a) (Lp(a) in range and threshold categories for patients with values measured in mg/dL
| Lp(a) category (mg/dL) | Number (%) patients N=29 765 |
Median Lp(a) value mg/dL (IQR) |
| <30 | 18 326 (61.6) | 9.5 (5.0–15.6) |
| ≥30–<50 | 3127 (10.5) | 38.4 (33.9–44.0) |
| ≥50 | 8312 (27.9) | 86.4 (69.0–119.0) |
| ≥10 | 20 336 (68.3) | 36.7 (17.1–79.0) |
| ≥20 | 14 117 (47.4) | 60.9 (34.0–93.9) |
| ≥30 | 11 439 (38.4) | 73.5 (47.9–103.3) |
| ≥40 | 9697 (32.6) | 81.5 (59.0–111.7) |
| ≥50 | 8312 (27.9) | 86.4 (69.0–119.0) |
| ≥60 | 7183 (24.1) | 93.0 (76.5–126.0) |
| ≥70 | 6145 (20.7) | 100.0 (83.9–132.6) |
| ≥80 | 5017 (16.9) | 110.0 (90.4–141.0) |
| ≥90 | 3824 (12.9) | 124.0 (103.0–153.0) |
| ≥100 | 3098 (10.4) | 132.0 (114.0–163.0) |
Table 3 reports results for patients based on their cardiovascular disease history. The largest number of patients, 35 088 (72.9%) qualified for participation based on a history of MI, whereas 6037 (12.5%) had experienced a prior ischaemic stroke. Another 4433 (9.2%) qualified due to a known history of PAD. A smaller number of patients 2577 (5.4%) reported having multiple event types. Table 4 reports Lp(a) and LDL-C levels for patients by geographic region. Western Europe contributed the largest number of patients 12 309 (25.6%) followed by Eastern Europe 9639 (20.0%) and North America 9245 (19.2%). Lp(a) levels were generally similar in most regions except North America where levels were higher, 48.0 (IQR 11.0–104.0) mg/dL and 52.3 (IQR 15.6–168.9) nmol/L compared with all other regions combined, p<0.001 for both. The median levels measured in mg/dL were more than twice as high in North America compared with Western Europe, but only moderately higher in patients measured using nmol/L. LDL-C values showed the opposite directional patterns with the lowest median LDL-C in North America, 69.2 mg/dL (IQR 53.0–93.0), and highest in Eastern Europe, 88.2 mg/dL (IQR 68.1–117.9), both p<0.001 compared with all other regions.
Table 3.
Lp(a) levels by atherosclerotic disease history
| Type of event | Patients N=48 135 |
Lp(a) mg/dL Median (IQR) (n=29 765) |
Lp(a) nmol/L Median (IQR) (n=18 370) |
LDL-C mg/dL Median (IQR) (n=47 988) |
| Cardiovascular history | ||||
| MI, n (%) | 37 383 (77.7) | 18.2 (8.0–58.5) | 40.9 (14.8–155.0) | 75.0 (58.0–98.0) |
| Ischaemic stroke, n (%) | 7568 (15.7) | 14.3 (7.0–45.8) | 41.0 (15.0–150.7) | 83.0 (62.0– 112.1) |
| Any PAD | 5925 (12.3) | |||
| Limb artery revascularisation, n (%) | 3475 (7.2) | 19.6 (7.9–62.9) | 54.0 (18.0–175.0) | 77.7 (59.0– 102.0) |
| Leg amputation, n (%) | 421 (0.9) | 26.8 (8.6–80.8) | 41.1 (18.4–162.3) | 78.0 (57.5– 101.0) |
| Symptomatic PAD with ABI ≤0.9, n (%) | 3557 (7.4) | 21.2 (8.0–70.0) | 57.3 (17.9–185.0) | 83.0 (62.0– 111.0) |
| Medical history (n=48 135) | ||||
| Prior MI, n (%) | 35 088 (72.9) | 18.2 (8.0–57.9) | 40.0 (14.5–152.8) | 75.0 (58.0– 97.8) |
| Prior ischaemic stroke, n (%) | 6037 (12.5) | 14.0 (7.0–41.9) | 40.0 (15.0–142.0) | 85.0 (63.8– 114.0) |
| Prior PAD, n (%) | 4433 (9.2) | 21.0 (8.0–66.5) | 50.0 (16.8–171.8) | 83.0 (62.0– 110.0) |
| Multiple events, n (%) | 2577 (5.4) | 18.3 (7.7–69.0) | 54.3 (16.9–182.0) | 76.2 (57.6– 102.5) |
P<0.001 compared with patients with history of MI.
ABI, ankle brachial index; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); MI, myocardial infarction; PAD, peripheral artery disease.
Table 4.
Lipoprotein(a) (Lp(a)) level by geographic region
| Region | Patients with Lp(a) measured in mg/dL N=29 765 N (%) |
Lp(a) mg/dL N=29 765 Median (IQR) |
Patients with Lp(a) measured in nmol/L N=18 370 N (%) |
Lp(a) nmol/L Median (IQR) |
Patients with LDL-C values N=47 988 N (%) |
LDL-C mg/dL Median (IQR) |
| East Asia | 6730 (22.6) | 14.1 (6.0–31.3) | 209 (1.1) | 35.1 (14.0–100.8) | 6936 (14.5) | 75.8 (60.7– 94.0) |
| South Asia | 3347 (11.2) | 24.4 (10.8–56.2) | 418 (2.3) | 30.5 (10.8–92.0) | 3762 (7.8) | 74.0 (56.0– 97.0) |
| Eastern Europe | 5467 (18.4) | 15.0 (7.0–58.0) | 4172 (22.7) | 31.7 (12.0–128.2) | 9626 (20.1) | 88.2 (68.1– 117.9) |
| Western Europe | 8676 (29.2) | 19.4 (8.1–74.0) | 3633 (19.8) | 42.0 (19.0–169.0) | 12 221 (25.5) | 73.0 (56.0– 95.0) |
| Latin America | 3761 (12.6) | 19.7 (8.0–67.0) | 1272 (6.9) | 29.8 (10.0–101.0) | 5019 (10.5) | 82.0 (62.2– 112.0) |
| North America | 1208 (4.1) | 48.0 (11.0–104.0) | 8037 (43.8) | 52.3 (15.6–168.9) | 9228 (19.2) | 69.2 (53.0– 93.0) |
| Other | 576 (1.9) | 17.8 (8.0–49.2) | 629 (3.4) | 59.0 (22.0–150.0) | 1196 (2.5) | 82.0 (61.9–108.3) |
LDL-C, low-density lipoprotein cholesterol.
Online supplemental eTable 3 reports the number of patients included in the study from each participating country and levels of Lp(a) and LDL-C for each country. Median Lp(a) levels varied widely among countries with no specific patterns. In countries with high enrolment, LDL-C levels were lowest in the USA, Canada, the UK, France, India and Italy, and highest in Eastern European countries such as the Russian Federation, Slovakia, Hungary and Bulgaria.
Discussion
There were several goals of the Lp(a)HERITAGE study, including characterisation of Lp(a) patterns in a large global secondary prevention population and increasing awareness of the importance of Lp(a) as a risk factor for ASCVD. The study also sought to identify patients potentially suitable for enrolment in the pivotal phase III Lp(a)HORIZON trial that is studying cardiovascular outcomes for the investigational drug pelacarsen in patients with elevated Lp(a) and pre-existing ASCVD. Identification of patients with elevated Lp(a) eligible for participation in clinical trials of emerging therapies is challenging because this biomarker is not commonly measured even in patients with premature ASCVD. To our knowledge, this is the first large study to report Lp(a) and LDL-C levels in patients with ASCVD in an ethnically and regionally diverse global population. Several pioneering Danish studies included 2527 patients with a history of ASCVD and a recent UK Biobank study included 22 401 patients with incident ASCVD, but both were single country studies and not regionally or ethnically diverse.10 11
In the Lp(a)HERITAGE study, median Lp(a) levels were 18 mg/dL, IQR (7.9–57.1) and 42.0 (IQR 15.0–155.4) nmol/L. Although historical values were permitted by the study protocol, 86.1% of Lp(a) levels were acquired after informed consent was obtained. Slightly >10% of patients exhibited very high Lp(a) levels with values exceeding 100 mg/dL (table 2). The median Lp(a) levels in this top decile were 134 mg/dL and 329 nmol/L (online supplemental eFigure 1). For patients with values expressed in mg/dL, approximately 21% exceeded 70 mg/dL and 13% exceeded 90 mg/dL (figure 1A). In the Lp(a)HORIZON study, the 70 mg/dL threshold is used as an entry criterion and the 90 mg threshold is defined as a subpopulation of interest. The levels of 70 and 90 mg/dL represent approximately the 75th and 90th percentiles in the general population. Levels >70 and 90 mg/dL are associated with an approximately 40% and 70% greater risk of major adverse cardiovascular events. 1 8 11 For patients measured in nmol/L, 26% exceeded 150 nmol/L (figure 1B). These levels were generally similar to other studies in secondary prevention populations. In the UK Biobank study, median Lp(a) levels were moderately lower in patients with ASCVD, 29 nmol/L, but levels in the Copenhagen City Heart Study were very similar to the current study, 22 (IQR 9–54) mg/dL in women and 17 (6–41) mg/dL in men.9 11 However, direct comparisons between studies are challenging because the selection criteria and specific assays used in prior studies varied widely. Most other large datasets, such as the Emerging Risk Factor Collaboration studied Lp(a) levels in pooled community-based prospective cohort studies without pre-existing ASCVD to examine the relationship between baseline levels and subsequent events.1
Several additional insights emerged from the Lp(a)HERITAGE study. As illustrated in table 1 table 2, globally, Lp(a) was measured more often in mass units (mg/dL), 62% of patients, than units of molar concentration units (nmol/L). Because there is no reliable conversion factor for values obtained with these two unit systems, there is a need to harmonise measurement methods used globally. Despite recommendations to measure Lp(a) levels in units of molar concentration, conversion to this measurement approach varies by region and individual laboratories.12 An additional issue in standardisation is measurement variability related to the antibody used in ELISA methods.13
Approximately three-fourths of enrolled patients were male and both Lp(a) and LDL-C levels were higher in women compared with men (table 1 and online supplemental eTable 1). This finding most likely reflects the lower incidence of ASCVD in women and the greater importance of both Lp(a) and LDL-C as risk-enhancing factors in women. Similarly, younger patients had higher Lp(a) and LDL-C levels, again emphasising the importance of both lipoproteins as drivers of premature ASCVD (table 1). As noted in all prior studies, expressed in either unit system, black patients had substantially higher Lp(a) levels, approximately threefold higher than white, Hispanic or Asian patients (table 1). LDL-C levels were similar among patients of differing race and ethnicity with exception of Asian patients who had lower levels.
No clear differences in Lp(a) levels were evident based on the qualifying atherosclerotic event (table 3). Although Lp(a) levels appeared similar, LDL-C levels were higher in patients with ischaemic stroke 85.0 mg/dL (IQR 63.8–114.0) or PAD 83.0 mg/dL (IQR 62.0–110.0) compared with MI 75.0 mg/dL (IQR 58.0–98.0), all comparisons p<0.001. The higher Lp(a) levels in North American patients (table 4) likely reflect a higher fraction of black patients or selection bias in enrolment of these patients. LDL-C levels were lower in North American, 69.1 mg/dL, likely reflecting the effectiveness of guideline-directed care in recent years. LDL-C levels in Eastern Europe were higher, a finding commonly observed in other trials of lipid-modifying therapies. Both Lp(a) and LDL-C levels were mostly similar in individual countries (online supplemental eTable 2), although Lp(a) levels were higher in countries with a larger population of black patients (USA, South Africa and Brazil).
LDL-C levels were progressively higher in patients with increasing Lp(a) levels (figure 2A). This relationship has been previously reported, but the magnitude of LDL-C increase in the current study was smaller than prior studies, which had suggested that about 30% of Lp(a) mass is measured in current LDL-C assays.14 In the Lp(a)HERITAGE study, if we assume that the extent of LDL-C lowering is similar irrespective of Lp(a) levels, the increase in LDL-C linked to higher Lp(a) levels suggests that on average <10% of Lp(a) is comeasured in LDL-C assays (figure 2A). However, we cannot exclude that the patients with the highest Lp(a) levels had the most severe ASCVD and therefore were treated more intensively with LDL-C-lowering therapies. Another recent study in patients with elevated Lp(a) showed the effect of Lp(a) on LDL-C measurement may range from 6% to 55% with median values of approximately 10%–20%.15 The current study also provides some evidence regarding the relationship between measurements of Lp(a) using the two different unit systems (mg/dL and nmol/L). The overall ratio of nmol/L to mg/dL measurements was 2.33:1, similar to the previously reported ratio of 2.4:1.16 However, in the current study, this ratio varied from 1.7:1 in the lowest decile to 2.45:1 in the highest decile (online supplemental eFigure1). Another recent study also reported that this ratio rises with increasing Lp(a) levels.17
Global awareness of the need to measure Lp(a) in patients with ASCVD was an important goal of the Lp(a)HERITAGE study. The development of RNA-based therapies to lower Lp(a) makes it critically important for physicians to begin determining Lp(a) in high-risk patients. However, nearly 90% of Lp(a) values obtained in the Lp(a)HERITAGE study were collected after the informed consent was signed. Accordingly, it seems apparent that globally, physicians are managing the vast majority of patients with pre-existing ASCVD without knowledge of their Lp(a) levels. This finding suggests that major global educational programmes will be required to encourage physicians to measure Lp(a) in routine clinical practice. Development of effective therapies can only influence outcomes if physicians and patients are aware of Lp(a) levels.
The Lp(a)HERITAGE study has several limitations. First, the study was purely descriptive in design, reducing the reliability of statistical comparisons between subgroups. Second, local laboratory values were used and differing methods for measurement of Lp(a) may have influenced the results.18 Third, a prior cardiovascular event or PAD was a key inclusion criterion and historical data were used for both Lp(a) and LDL-C, both of which may have introduced referral bias. Fourth, both mass and molar concentration units were permitted, precluding uniform comparison of subgroups of interest.
Summary
Despite these limitations, several useful insights emerged from the Lp(a)HERITAGE study. Most patients with ASCVD are currently managed without knowledge of their Lp(a) levels. Approximately one-quarter of the global population with ASCVD have elevated Lp(a) levels with 10% of the population exhibiting levels >100 mg/dL. Women and younger patients tended to have higher levels of both LDL-C and Lp(a) reflecting the influence of both lipoproteins on premature ASCVD. Lp(a) represents one of the few untreatable ASCVD risk factors, an observation that may soon change if current gene-silencing therapies are successful.
Footnotes
Correction notice: This article has been corrected since it was first published to correct data errors in Table 4.
Contributors: SEN served as the principal Investigator, wrote the first draft of the manuscript and guarantees the accuracy of the data and fidelity to the statistical analysis plan. KW served as the lead statistician for analysis of the data. LC, SN, JJPK, EL, VL, MB, AML, RM, ST and BN served as advisors to the study, reviewed and provided critical comments for the manuscript. BM, PK, AL and TT assisted with management of the study and reviewed the manuscript on behalf of the sponsor. JL provided statistical oversight in the design of the study. TS, FM, FSS, AM, AB, VV and NEB were among the leading enrollers in the study, represented the investigators and provided review and comments on the manuscript.
Funding: This study was supported by Novartis Pharmaceuticals.
Competing interests: SEN reports receiving clinical trial support from Novartis and Silence Theapeutics; BN reports consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion and Silence Therapeutics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study was approved by regional ethics committees. This study complied with the Declaration of Helsinki. Written, informed consent was obtained from all patients.
References
- 1. Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–23. 10.1001/jama.2009.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–350. 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 3. The task force for the management of dyslipidaemias of the European Society of cardiology (ESC) and European atherosclerosis Society (EAS). 2019 ESC/EAS guidelines for themanagement of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2020;41:188. [Google Scholar]
- 4. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med 2020;382:244–55. 10.1056/NEJMoa1905239 [DOI] [PubMed] [Google Scholar]
- 5. Nissen SE, Wolski K, Balog C, et al. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals With Elevated Plasma Lipoprotein(a) Levels. JAMA 2022;327:1679–87. 10.1001/jama.2022.5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koren MJ, Moriarty PM, Baum SJ, et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat Med 2022;28:96–103. 10.1038/s41591-021-01634-w [DOI] [PubMed] [Google Scholar]
- 7. Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2012;125:241–9. 10.1161/CIRCULATIONAHA.111.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu H-H, Cao Y-X, Jin J-L, et al. Association of lipoprotein(a) levels with recurrent events in patients with coronary artery disease. Heart 2020;106:1228–35. 10.1136/heartjnl-2020-316586 [DOI] [PubMed] [Google Scholar]
- 9. Kamstrup PR, Benn M, Tybjaerg-Hansen A, et al. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation 2008;117:176–84. 10.1161/CIRCULATIONAHA.107.715698 [DOI] [PubMed] [Google Scholar]
- 10. Madsen CM, Kamstrup PR, Langsted A, et al. Lipoprotein(a)-Lowering by 50 mg/dL (105 nmol/L) May Be Needed to Reduce Cardiovascular Disease 20% in Secondary Prevention: A Population-Based Study. Arterioscler Thromb Vasc Biol 2020;40:255–66. 10.1161/ATVBAHA.119.312951 [DOI] [PubMed] [Google Scholar]
- 11. Patel AP, Wang (汪敏先) M, Pirruccello JP, et al. Lp(a) (Lipoprotein[a]) Concentrations and Incident Atherosclerotic Cardiovascular Disease: New Insights From a Large National Biobank. Arterioscler Thromb Vasc Biol 2021;41:465–74. 10.1161/ATVBAHA.120.315291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcovina SM, Koschinsky ML, Albers JJ, et al. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: recent advances and future directions. Clin Chem 2003;49:1785–96. 10.1373/clinchem.2003.023689 [DOI] [PubMed] [Google Scholar]
- 13. Marcovina SM, Albers JJ, Lipoprotein AJJ. Lipoprotein (a) measurements for clinical application. J Lipid Res 2016;57:526–37. 10.1194/jlr.R061648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viney NJ, Yeang C, Yang X, et al. Relationship between "LDL-C", estimated true LDL-C, apolipoprotein B-100, and PCSK9 levels following lipoprotein(a) lowering with an antisense oligonucleotide. J Clin Lipidol 2018;12:702–10. 10.1016/j.jacl.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 15. Yeang C, Witztum JL, Tsimikas S. Novel method for quantification of lipoprotein(a)-cholesterol: implications for improving accuracy of LDL-C measurements. J Lipid Res 2021;62:100053. 10.1016/j.jlr.2021.100053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015;386:1472–83. 10.1016/S0140-6736(15)61252-1 [DOI] [PubMed] [Google Scholar]
- 17. Tsimikas S, Fazio S, Viney NJ, et al. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein(a) thresholds and apolipoprotein(a) isoform size. J Clin Lipidol 2018;12:1313–23. 10.1016/j.jacl.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 18. Scharnagl H, Stojakovic T, Dieplinger B, et al. Comparison of lipoprotein (a) serum concentrations measured by six commercially available immunoassays. Atherosclerosis 2019;289:206–13. 10.1016/j.atherosclerosis.2019.08.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002060supp001.pdf (437.3KB, pdf)
Data Availability Statement
Data are available on reasonable request.